Abstract
Background
The co-existence of meningioma and craniofacial fibrous dysplasia (CFD) is rare. Due to the similar radiological characteristics, it is challenging to differentiate such co-existence from solitary hyperostotic meningioma resulting in a dilemma of prompt diagnosis and appropriate intervention.
Method
We conducted a retrospective review of the data from 21 patients with concomitant meningioma and CFD who were treated at Beijing Tiantan Hospital from 2003 to 2021. We summarized their clinicopathological features and performed a comprehensive literature review. Additionally, we tested the characteristic pathogenic variants in exon 8 and 9 of GNAS gene and the expression of corresponding α-subunit of the stimulatory G protein (Gαs) related to CFD to explore the potential interactions between these two diseases.
Results
The cohort comprised 4 men and 17 women (mean age, 45.14 years). CFD most commonly involved the sphenoid bone (n = 10) and meningiomas were predominantly located at the skull base (n = 12). Surgical treatment was performed in 4 CFD lesions and 14 meningiomas. Simpson grade I-II resection was achieved in 12 out of the 14 resected meningiomas and almost all of them were classified as WHO I grade (n = 13). The mean follow-up duration was 56.89 months and recurrence was noticed in 2 cases. Genetic study was conducted in 7 tumor specimens and immunohistochemistry was accomplished in 8 samples showing that though GNAS variant was not detected, Gαs protein were positively expressed in different degrees.
Conclusions
We presented an uncommon case series of co-diagnosed meningioma and CFD and provided a detailed description of its clinicopathological features, treatment strategy and prognosis. Although a definite causative relationship had not been established, possible genetic or environmental interplay between these two diseases could not be excluded. It was challenging to initiate prompt diagnosis and appropriate treatment for concomitant meningioma and CFD because of its similar radiological manifestations to meningioma with reactive hyperostosis. Personalized and multi-disciplinary management strategies should be adopted for the co-existence of meningioma and CFD.
Similar content being viewed by others
Background
Meningiomas, primarily arising from meningothelial arachnoid cells, are the most common intracranial tumors at present, accounting for almost one third of all primary central nervous system tumors [1]. Its incidence rate varies from 1.28 to 8.81 per 100,000 persons in different studies around the world [2, 3]. Fibrous dysplasia (FD) is an uncommon mosaic disorder resulting in replacement of normal bone with fibro-osseous tissue. The actual incidence of FD is once reported to be 10–30 in 1,000,000 persons, representing as many as 7% of benign bone tumors [4, 5]. It may occur in one single bone (monostotic FD), in multiple bones (polyostotic FD) or in combination with extra-skeletal disease. Craniofacial bones are the most common location affecting as many as 87% of patients with polyostotic FD [6,7,8,9].
The co-existence of meningiomas and craniofacial fibrous dysplasia (CFD) is a fairly uncommon condition which has only been described in a few case reports [10,11,12,13,14,15,16]. However, the clinical and radiological characteristics of this condition have not been well-demonstrated and the actual interactions between these two entities still remain unclear. Sporadic activating variants in the GNAS locus not only result in replacement of normal bone with fibro-osseous tissue in CFD lesions [4], but also is mutationally activated in various cancer types, such as growth hormone-secreting pituitary tumors, pancreatic cancer and colorectal cancer [17, 18]. It remains highly concerned whether GNAS gene is the common genetic predisposition between CFD and meningiomas.
Craniofacial FD typically demonstrates dense and sclerotic lesions or appears as an area of radiolucent ground glass matrix. Relevant differential diagnoses of CFD should consider meningiomas, Paget’s disease of the skull bone, and benign osteosclerotic lesions like osteoma [19]. Since meningioma itself could inflict the adjacent bones resulting in bone destruction with similar radiological manifestations to CFD [5, 20], differential diagnosis between bone-invasive meningiomas and concomitant meningiomas and CFD is clinically problematic.
This article was designed to describe a seldom seen series of coexisting meningiomas and CFD, demonstrate their clinical characteristics, explore the underlying interactions and pathogenesis, and discuss the difference between concomitant meningiomas and CFD and single hyperostotic meningiomas in order to facilitate diagnosis and improve treatment.
Methods
Patients’ selection
In the period 2003–2021, a total of 1176 patients diagnosed with CFD at Beijing Tiantan Hospital were retrospectively screened. The study finally enrolled 21 cases that were reported to have concomitant CFD and cerebral meningiomas. All patients underwent computed tomography (CT) and magnetic resonance imaging (MRI) for diagnosis and evaluation. CFD and meningiomas were diagnosed in accordance with histological examinations in patients managed with surgery, while for patients received conservative treatment, diagnoses were made according to typical radiological characteristics. Demographic characteristics, clinical manifestations, radiological and pathological features, treatment procedures and outcomes were recorded.
Follow-up was accomplished via telephone or at the clinic. CT and MRI was carefully evaluated and whether there was disease recurrence or progression was recorded.
This study was approved by the Institutional Review Board. Due to the retrospective nature of our study, the board waived the need for written consent.
Immunohistochemistry and genetic analysis
Immunohistochemistry was performed to detect α-subunit of the stimulatory G-protein (Gαs) protein expression in meningioma specimens. The tissue sections were incubated with primary Gαs antibody (1:100, sc-365855, Santa Cruz). Each stained slide was individually reviewed and independently scored by two neuropathologists. Genomic DNA was extracted from paraffin-embedded meningioma specimens using the Wizard Genomic DNA Purification kit following the manufacturer’s instructions (Promega, Madison, WI). The Gαs encoding exons 8 and 9 of GNAS were amplified by PCR and sequenced by conventional Sanger sequencing (BigDye Terminator Cycle Sequencing Ready reaction kit, Applied Biosystems).
Literature review
In addition, we searched 3 medical database, PubMed, EMBASE and Cochrane Library up to 2021 for published studies focusing on the coexistence of CFD and meningioma. The following combined terms ([MESH] “fibrous dysplasia” AND [MESH] “meningioma”) were used. A manual researching on the reference of identified studies was performed for more related studies.
Results
Clinical and radiological characteristics
Among the 1176 CFD patients evaluated, concurrent meningiomas were found in 21 patients (17 females, mean age 45.14 years old). Only 1 patient was adolescent. The teenage boy had a heavy disease burden of CFD (Fig. 1a1) and a meningioma located at tuberculum sellae (Fig. 1a2). Tables 1 and 2 presented the distribution of CFD and meningiomas. The majority (57.14%) of meningiomas located at the skull base and most (47.62%) of the CFD lesions affected the sphenoid bone.
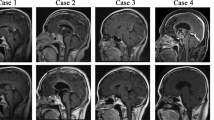
Radiological manifestations. a1, a2 radiology of case 1 shows diffuse CFD in right maxilla, ethmoid sinus and sphenoid bone and tuberculum sellae meningioma; b1–b2 radiology of case 19 shows CFD in left sphenoid bone and left frontal-parietal parafalx meningioma; c1, c2 radiology of case 9 shows CFD in bilateral sphenoid, temporal and occipital bones and left frontal parasagittal meningioma
Surgical intervention was executed in 14 meningiomas. The extent of resection was considered gross-total in 12 patients (Simpson grade I-II) and subtotal in 2 patients (Simpson grade III). No postoperative complications was noticed in the 14 surgically treated patients and none of them received any adjuvant therapy postoperatively. During the average 56.64-month follow-up after surgery, the radiological examinations showed 2 recurrences. Among the 7 unresected meningiomas, 6 opted for watchful waiting, while 1 was treated with Gamma Knife radiosurgery. For instance, case 19 was a 60-year-old female with CFD involving the left sphenoid bone led to the diagnosis of CFD (Fig. 1b1) and a parafalx meningioma at left frontal-parietal lobe (Fig. 1b2). The meningioma showed any sign of progression during the 84 months of “watchful waiting”. These unresected meningiomas showed no progression during the mean 57.60-month follow-up. Referring to the included 21 CFD lesions, only 4 were managed with operation. The mean follow-up time was 56.89 months, and no recurrence or progression was observed in any of the CFD lesions. For example, case 5 who were diagnosed with CFD involving the left frontal bone and left orbit (Fig. 1c1) and meningioma in the right parasellar region (Fig. 1c2). The unresected CFD lesion stayed stable during 120-month follow-up.
CFD typically demonstrated dense, sclerotic lesions and was often associated with the term “ground glass bone matrix”. However, a smooth outer cortical contour always maintained (Fig. 2a). Although meningioma related hyperostosis (Fig. 2b) and intraosseous meningiomas (Fig. 2c) were also evaluated as sclerotic lesions, these lesions exhibited irregular and spiculated borders. Figure 2d demonstrated a co-existing meningioma adjacent to the CFD lesion, the involvement of the lamina interna cranii caused by the meningioma could complicate and interfere with the identification of CFD, making it challenging to differentiate the co-occurrence from the bone-invasive meningioma.
Differential diagnosis between bone invasive meningioma and concomitant meningioma and CFD. a1, 2 typical CFD showing asymmetric expansive lesion at the left frontal bone with typical ground-glass matrix; b1, b2 hyperostosis caused by meningioma revealing a sclerotic lesion of the left greater sphenoid wing with spiculated margins; c1, c2 hyperostotic intraosseous meningioma with irregular inner table; d1, d2 concomitant meningioma and CFD
Pathological characteristics and genetic results
Among the 14 surgically resected and pathologically examined meningiomas, transitional meningiomas were the most common type (6, 42.86%), and almost all the meningiomas (92.86%) were reported to be WHO I grade (Table 3) (Additional file 1). DNA sequencing was accomplished in 7 cases with no GNAS variant detected. In addition, 8 meningiomas were immunohistochemically examined and Gαs expression was positive (grade 1 and grade 2) in 6 specimens (Fig. 3).
Both of the meningioma samples with grade 2 Gαs expression were transitional meningiomas (case 1 and case 6). Case 1 was a suprasellar meningioma co-existed with diffuse CFD involving right maxilla, ethmoid sinus and sphenoid bone and case 6 was a left olfactory groove meningioma concomitant with a CFD lesion inflicting left maxilla (Additional file 2). Although these 2 CFD lesions showing strongly positive Gαs expression both involved maxilla, no definite correlation between the level of Gαs expression and the location of CFD lesions could be drawn because of the limited sample size.
The two recurrent meningiomas (case 3 and case 12) were both WHO I grade. Case 3 was a meningothelial meningioma in the right cerebellopontine angle co-diagnosed with sphenoid bone CFD. Although without GNAS mutation, the meningioma revealed grade 1 Gαs expression. Co-occurrence of CFD inflicting the parietal bone and a transitional meningioma in the right petroclival region was seen in case 12 (Additional file 2). Immunohistochemistry and genetic analysis was missing for this recurrent meningioma. No relation was found between recurrence and pathological characteristics.
Literature review
Only 8 studies met the inclusion criteria and all of them were case reports. Detailed information of these 4 articles were described in Table 4. The mean age of the included patients was 29.38 years old with 6 males and only 2 females. Operations were reported in 5 meningiomas and 4 FD lesions. None of them explored GNAS variant and Gαs expression in meningioma specimens and no hypothesis was put forward to explain the co-occurrence.
Discussion
This article reported an infrequent series of concomitant meningiomas and CFD. To the best of our knowledge, this study was the largest case series highlighting the clinicopathologic features, treatment modalities and prognosis. In addition, we also reviewed the literature and discussed the challenges to differentiate such co-existence with solitary bone-invasive meningioma.
The actual mechanism of co-existed meningiomas and CFD remains unclear. There are several possible explanations: (1) genetic predisposition; (2) a purely coincidental event; (3) environmental influence as an irritating agent for the local proliferation and growth of the other [21,22,23]. Sporadic CFD is reported to be most common in children and adolescents and barely have any gender difference [24], which varies a lot from the age (mean age 45.14 years old) and gender profile (marked female: male ratio up to 4:1) of the included 21 CFD co-diagnosed with meningiomas. What’s more, the incidence rate of meningioma in the general population varies from 1.28 to 8.81 per 100,000 persons [2, 3, 25], however, the present study found 21 meningiomas in the 1176 CFD patients indicating much higher incidence of meningioma (1.8%). Therefore, it is reasonable to hypothesize a possible link between meningiomas and CFD. However, radiological imaging is more regularly performed in CFD patients increasing the chance of incidental findings of other intracranial lesions including meningiomas, which should also be taken into account when evaluating the actual mechanism of the co-existence. Additionally, previous case reports show quite different demographic and radiological characteristics. There were 8 cases included in the literature review. When compared with regular meningioma patients, their mean age was much younger (29.38 years old) and the female predominance was absent since male patients outnumbered female by a ratio of 3:1 [10,11,12,13,14,15,16, 26]. And inconsistent with the present findings showing only two meningiomas in the same side of CFD, most of meningiomas included in the previous reports were found to be adjacent to CFD lesions. Such random demographic and radiological profile also provides further evidence for the possibility that the co-existence might be coincidental.
FD is caused by a mosaic activating pathogenic variant in GNAS gene [27, 28], and the development of sporadic meningiomas also has genetic predisposition including NF2, TRAF7, KLF4, AKT1 and TERT [29,30,31]. GNAS pathogenic variants have been previously found in various systems and has been reported to be associated with many extra-skeletal diseases such as thyroid hyperfunction, hormone-secreting pituitary tumors, pancreatic cancer, breast cancer and colorectal cancer [6,7,8,9, 17, 18, 32, 33].Furthermore, GNAS pathogenic variant is also detected in an endothelial meningioma with multiple recurrences recently [34]. However, the present results did not find any pathogenic GNAS variant in the 7 meningiomas analyzed, consistent with the study of Eun who examined 13 meningioma samples [35]. To date there is no evidence concerning the definite role of GNAS variants in the co-occurrence of meningioma and CFD. The current study also tested the Gαs protein encoded by GNAS. Though there was no association between Gαs expression and the histology of meningiomas, the different expression levels of Gαs in the meningioma specimens delineated the possibility that the development of these two diseases might share a common molecular pathway. Case 1 showed a 15-year-old transitional meningioma with strong positive Gαs expression. The relatively young age of meningioma onset and the multiple surgeries of CFD provided some evidence for the hypothesis that CFD might have environmental influence as an irritating agent on the occurrence and development of meningiomas.
Bone involvement is a major concern in meningioma [36], which is documented in 20–68% of meningiomas by histopathological studies [37] and is proved to influence tumor recurrence and prognosis [38]. Bone invasive meningiomas are associated with NF2 and TRAF7 variants [39]. Radiographic evidence of bone involvement includes hyperostosis, bone sclerosis and osteolytic lesions [40]. Both characterized by an increased bone density involving the craniofacial bones, meningioma associated hyperostosis and CFD can be confounded easily resulting in the dilemma to differentiate concomitant meningioma and CFD from meningioma with hyperostotic bone involvement. Seen in 25–49% of meningiomas [41], meningioma associated hyperostosis most frequently affects the convexity and sphenoid wing [5, 42] and is featured by irregular inner surface margins and diffuse “hairy spicules” trabecular hyperostosis without the destruction of trabecular structures [43,44,45]. Additionally, as a special condition of meningioma restricted in bone (accounting for about 2%) [46, 47], intraosseous meningiomas are readily evaluated as sclerotic lesions with irregular and spiculated borders [48,49,50]. However, CFD prototypically appears as an area of radiolucent homogeneous ground glass matrix with a smooth cortical contour [51,52,53]. Therefore, it can be inferred that the key to diagnose CFD is the regular contours of cortical table, but when the co-exist meningioma was adjacent to CFD, the intact lamina interna cranii could be destroyed, making the differential diagnosis more complicated.
Misdiagnosis may influence the treatment preferences and patients’ prognosis. The management strategy should be based on the accurate diagnosis. If it is considered to be meningioma with reactive hyperostosis or intraosseous meningioma, complete resection might be recommended to reduce recurrence and improve prognosis [39, 54]. However, if the patient is diagnosed with co-existed meningioma and CFD, “watchful waiting” treatment of the bone lesion may be acceptable especially when there is no CFD related symptom since FD turns to be stable after adolescence. This managemeng strategy is further proved by the current series of co-existed meningioma and CFD. Although only 4 CFD was surgically resected, most patients had favorable prognosis without any obvious CFD progression suggesting that the co-existence of CFD may not influence the prognosis of meningiomas. However, if important structures are compressed causing complaints, surgical resection should be considered. In addition, when meningioma is located in close juxtaposition of CFD, the bone lesions caused by CFD will make the exposure laborious for the resection of meningioma. In this situation, surgical resection of CFD can be recommended. However, whether these two diseases should be managed at one session ought to be evaluated carefully [55, 56]. Therefore, interdisciplinary and more personalized management should be adopted for patients diagnosed with concomitant CFD and meningioma.
Our study has some limitations. Firstly, due to the rarity, the sample size of qualified cases is limited. More cases are needed to strengthen the reliability. Secondly, no definite mechanism concerning the coexisting meningioma and CFD is clarified which still needs further exploration and verification. Technologies such as whole exome sequencing can be considered to study the common molecular pathway of meningioma and CFD in future researches.
Conclusion
We reported a seldom seen case series of co-diagnosed meningioma and CFD and provided a detailed description of their clinicopathological features, treatment strategy and prognosis. Although a definite causative relationship is still undefined, possible genetic or environmental interplay between these two diseases cannot be excluded and requires further investigations. It can be quite intriguing to be differentiated from bone invasive meningiomas. The comprehensive assessment of this seldom seen and challenging condition in the present study can provide more profound understanding of this co-occurrence thus facilitating the diagnosis and helping with the determination of the appropriate treatment strategy.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to individual privacy of the patients included but are available from the corresponding author on reasonable request.
References
Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro-Oncol. 2022;24:v1–95. https://doi.org/10.1093/neuonc/noac202.
Ogasawara C, Philbrick BD, Adamson DC. Meningioma: a review of epidemiology, pathology, diagnosis, treatment, and future directions. Biomedicines. 2021. https://doi.org/10.3390/biomedicines9030319.
Huntoon K, Toland AMS, Dahiya S. Meningioma: a review of clinicopathological and molecular aspects. Front Oncol. 2020;10:579–99. https://doi.org/10.3389/fonc.2020.579599.
Javaid MK, Boyce A, Appelman-Dijkstra N, Ong J, Defabianis P, Offiah A, et al. Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: a consensus statement from the FD/MAS international consortium. Orphanet J Rare Dis. 2019;14:139. https://doi.org/10.1186/s13023-019-1102-9.
Van de Voorde N, Mortier GR, Vanhoenacker FM. Fibrous dysplasia, paget’s disease of bone, and other uncommon sclerotic bone lesions of the craniofacial bones. Semin Musculoskelet Radiol. 2020;24:570–8. https://doi.org/10.1055/s-0039-3400292.
Belsuzarri TA, Araujo JF, Melro CA, Neves MW, Navarro JN, Brito LG, et al. McCune-Albright syndrome with craniofacial dysplasia: clinical review and surgical management. Surg Neurol Int. 2016;7:S165–9. https://doi.org/10.4103/2152-7806.178567.
Collins MT, Singer FR, Eugster E. McCune-Albright syndrome and the extraskeletal manifestations of fibrous dysplasia. Orphanet J Rare Dis. 2012;7 Suppl 1:S4. https://doi.org/10.1186/1750-1172-7-S1-S4.
Boyce AM, Chong WH, Shawker TH, Pinto PA, Linehan WM, Bhattacharryya N, et al. Characterization and management of testicular pathology in McCune-Albright syndrome. J Clin Endocrinol Metab. 2012;97:E1782–90. https://doi.org/10.1210/jc.2012-1791.
Gaujoux S, Salenave S, Ronot M, Rangheard AS, Cros J, Belghiti J, et al. Hepatobiliary and Pancreatic neoplasms in patients with McCune-Albright syndrome. J Clin Endocrinol Metab. 2014;99:E97-101. https://doi.org/10.1210/jc.2013-1823.
Fehlow P, Walther F. McCune-Albright syndrome in association with meningioma and mental and psychological retardation. Klin Padiatr. 1992;204:447–52. https://doi.org/10.1055/s-2007-1025387.
Bayas A, Naumann M, Wever S. Meningioma associated with McCune-Albright syndrome. J Neurol. 1999;246:1199–200. https://doi.org/10.1007/s004150050544.
Frankel J, Ianotti F, Powell M. Meningioma-an unrecognised complication of fibrous dysplasia of the skull? J Neurol Neurosurg Psychiatry. 1989;52:546–7. https://doi.org/10.1136/jnnp.52.4.546.
Ghosal N, Furtado SV, Santosh V, Sridhar M, Hegde AS. Co-existing fibrous dysplasia and atypical lymphoplasmacyte-rich meningioma. Neuropathology. 2007;27:269–72. https://doi.org/10.1111/j.1440-1789.2007.00753.x.
Taşar M, Örs F, Yetişer S, Uğurel MŞ, Üçöz T. Multiple globoid meningiomas associated with craniomandibular fibrous dysplasia. Clin Imaging. 2004;28:20–2. https://doi.org/10.1016/s0899-7071(03)00008-1.
Alves RV, Souza AR, Silva Ados S, Cardim VL. Co-existing fibrous dysplasia and meningothelial meningioma. Arq Neuropsiquiatr. 2009;67:699–700. https://doi.org/10.1590/s0004-282x2009000400025.
Settecase F, Nicholson AD, Amans MR, Higashida RT, Halbach VV, Cooke DL, et al. Onyx embolization of an intraosseous pseudoaneurysm of the middle meningeal artery in a patient with meningiomatosis, McCune-Albright syndrome, and gray platelet syndrome. J Neurosurg Pediatr. 2016;17:324–9. https://doi.org/10.3171/2015.9.PEDS15267.
O’Hayre M, Vazquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S, et al. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer. 2013;13:412–24. https://doi.org/10.1038/nrc3521.
Lemos MC, Thakker RV. GNAS mutations in Pseudohypoparathyroidism type 1a and related disorders. Hum Mutat. 2015;36:11–9. https://doi.org/10.1002/humu.22696.
Daffner RH, Yakulis R. Intraosseous meningioma. Skeletal Radiol. 1998;27:108–11. https://doi.org/10.1007/s002560050347.
Butscheidt S, Ernst M, Rolvien T, Hubert J, Zustin J, Amling M, et al. Primary intraosseous meningioma: clinical, histological, and differential diagnostic aspects. J Neurosurg. 2019. https://doi.org/10.3171/2019.3.JNS182968.
Lee JS, FitzGibbon E, Butman JA, Dufresne CR, Kushner H, Wientroub S, et al. Normal vision despite narrowing of the optic canal in fibrous dysplasia. N Engl J Med. 2002;347:1670–6. https://doi.org/10.1056/NEJMoa020742.
Erdem H. Collision tumor of meningioma and non hodgkin malignant lymphoma of cerebellum. Brain Disord Therapy. 2012;1:1. https://doi.org/10.4172/2168-975x.1000103.
Zhu H, Miao Y, Shen Y, Guo J, Xie W, Zhao S, et al. The clinical characteristics and molecular mechanism of pituitary adenoma associated with meningioma. J Transl Med. 2019;17:354. https://doi.org/10.1186/s12967-019-2103-0.
Lee JS, FitzGibbon EJ, Chen YR, Kim HJ, Lustig LR, Akintoye SO, et al. Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet J Rare Dis. 2012;7 Suppl 1:S2. https://doi.org/10.1186/1750-1172-7-S1-S2.
Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol. 2020;22:iv1–96. https://doi.org/10.1093/neuonc/noaa200.
Gao H, Zhang JL, ST Q. Fibrous dysplasia of the skull complicated with meningioma: report of 2 cases. Di Yi Jun Yi Da Xue Xue Bao. 2002; 22:664
Hartley I, Zhadina M, Collins MT, Boyce AM. Fibrous dysplasia of bone and Mccune-Albright syndrome: a bench to bedside review. Calcif Tissue Int. 2019;104:517–29. https://doi.org/10.1007/s00223-019-00550-z.
Riminucci M, Saggio I, Robey PG, Bianco P. Fibrous dysplasia as a stem cell disease. J Bone Miner Res. 2006;21(Suppl 2):P125–31. https://doi.org/10.1359/jbmr.06s224.
Pereira BJA, Oba-Shinjo SM, de Almeida AN, Marie SKN. Molecular alterations in meningiomas: literature review. Clin Neurol Neurosurg. 2019;176:89–96. https://doi.org/10.1016/j.clineuro.2018.12.004.
Cordova C, Kurz SC. Advances in molecular classification and therapeutic opportunities in meningiomas. Curr Oncol Rep. 2020;22:84. https://doi.org/10.1007/s11912-020-00937-4.
Delgado-Lopez PD, Cubo-Delgado E, Gonzalez-Bernal JJ, Martin-Alonso J. A practical overview on the molecular biology of meningioma. Curr Neurol Neurosci Rep. 2020;20:62. https://doi.org/10.1007/s11910-020-01084-w.
Farfel Z, Bourne HR. The expanding spectrum of G protein diseases. N Engl J Med. 1999;340:1012–20. https://doi.org/10.1056/NEJM199904013401306.
Majoor BC, Boyce AM, Bovee JV, Smit VT, Collins MT, Cleton-Jansen AM, et al. Increased risk of breast cancer at a young age in women with fibrous dysplasia. J Bone Miner Res. 2018;33:84–90. https://doi.org/10.1002/jbmr.3286.
Hong W, Shan C, Ye M, Yang Y, Wang H, Du F, et al. Case report: identification of a novel GNAS mutation and 1p/22q co-deletion in a patient with multiple recurrent meningiomas sensitive to sunitinib. Front Oncol. 2021;11:737523. https://doi.org/10.3389/fonc.2021.737523.
Je EM, An CH, Chung YJ, Yoo NJ, Lee SH. GNAS mutation affecting codon 201 is rare in most human tumors. Pathol Oncol Res. 2015;21:859–60. https://doi.org/10.1007/s12253-015-9919-6.
Della Puppa A, Rustemi O, Gioffre G, Troncon I, Lombardi G, Rolma G, et al. Predictive value of intraoperative 5-aminolevulinic acid-induced fluorescence for detecting bone invasion in meningioma surgery. J Neurosurg. 2014;120:840–5. https://doi.org/10.3171/2013.12.JNS131642.
Goyal N, Kakkar A, Sarkar C, Agrawal D. Does bony hyperostosis in intracranial meningioma signify tumor invasion? A radio-pathologic study. Neurol India. 2012;60:50–4. https://doi.org/10.4103/0028-3886.93589.
Gabeau-Lacet D, Aghi M, Betensky RA, Barker FG, Loeffler JS, Louis DN. Bone involvement predicts poor outcome in atypical meningioma. J Neurosurg. 2009;111:464–71. https://doi.org/10.3171/2009.2.JNS08877.
Jin L, Youngblood MW, Gupte TP, Vetsa S, Nadar A, Barak T, et al. Type of bony involvement predicts genomic subgroup in sphenoid wing meningiomas. J Neurooncol. 2021;154:237–46. https://doi.org/10.1007/s11060-021-03819-2.
De Jesús O. Surgical management of meningioma en plaque of the sphenoid ridge. Surg Neurol. 2001;55:265–9. https://doi.org/10.1016/s0090-3019(01)00440-2.
Terrier LM, Bernard F, Fournier HD, Morandi X, Velut S, Henaux PL, et al. Spheno-orbital meningiomas surgery: multicenter management study for complex extensive tumors. World Neurosurg. 2018;112:e145–56. https://doi.org/10.1016/j.wneu.2017.12.182.
Honeybul S, Neil-Dwyer G, Lang DA, Evans BT. Sphenoid wing meningioma en plaque: a clinical review. Acta Neurochir Wien. 2001. https://doi.org/10.1007/s007010170028.
Han JJ, Lee DY, Kong SK, Chang KH, Yoon YJ, Kim HJ, et al. Clinicoradiologic characteristics of temporal bone meningioma: multicenter retrospective analysis. Laryngoscope. 2021;131:173–8. https://doi.org/10.1002/lary.28534.
Hamilton BE, Salzman KL, Patel N, Wiggins RH 3rd, Macdonald AJ, Shelton C, et al. Imaging and clinical characteristics of temporal bone meningioma. AJNR Am J Neuroradiol. 2006;27:2204–9.
Pieper DR, Al-Mefty O, Hanada Y. Hyperostosis associated with meningioma of the cranial base: secondary changes or tumor invasion. Neurosurgery. 1999;44:742–6. https://doi.org/10.1097/00006123-199904000-00028.
Chen TC. Primary intraosseous meningioma. Neurosurg Clin N Am. 2016;27:189–93. https://doi.org/10.1016/j.nec.2015.11.011.
Ilica AT, Mossa-Basha M, Zan E, Vikani A, Pillai JJ, Gujar S, et al. Cranial intraosseous meningioma: spectrum of neuroimaging findings with respect to histopathological grades in 65 patients. Clin Imaging. 2014;38:599–604. https://doi.org/10.1016/j.clinimag.2014.05.013.
Changhong L, Naiyin C, Yuehuan G. Primary intraosseous meningiomas of the skull. Clin Radiol. 1997;52:546–9. https://doi.org/10.1016/s0009-9260(97)80333-9.
Nasi-Kordhishti I, Hempel JM, Ebner FH, Tatagiba M. Calvarial lesions: overview of imaging features and neurosurgical management. Neurosurg Rev. 2021;44:3459–69. https://doi.org/10.1007/s10143-021-01521-5.
Mitra I, Duraiswamy M, Benning J, Joy HM. Imaging of focal calvarial lesions. Clin Radiol. 2016;71:389–98. https://doi.org/10.1016/j.crad.2015.12.010.
Kushchayeva YS, Kushchayev SV, Glushko TY, Tella SH, Teytelboym OM, Collins MT, et al. Fibrous dysplasia for radiologists: beyond ground glass bone matrix. Insights Imaging. 2018;9:1035–56. https://doi.org/10.1007/s13244-018-0666-6.
Atalar MH, Salk I, Savas R, Uysal IO, Egilmez H. CT and MR imaging in a large series of patients with craniofacial fibrous dysplasia. Pol J Radiol. 2015;80:232–40. https://doi.org/10.12659/PJR.893425.
Zelenik K, Hanzlikova P, Blatova B, Formanek M, Kominek P. Temporal bone meningiomas: emphasizing radiologic signs to improve preoperative diagnosis. Eur Arch Otorhinolaryngol. 2021;278:271–3. https://doi.org/10.1007/s00405-020-06110-8.
Terrier LM, Bernard F, Fournier HD, Morandi X, Velut S, Henaux PL, et al. Spheno-orbital meningiomas surgery: multicenter management study for complex extensive tumors. World Neurosurg. 2018;112:e145–56.
Bowers CA, Taussky P, Couldwell WT. Surgical treatment of craniofacial fibrous dysplasia in adults. Neurosurg Rev. 2014;37:47–53. https://doi.org/10.1007/s10143-013-0500-z.
Feller L, Wood NH, Khammissa RA, Lemmer J, Raubenheimer EJ. The nature of fibrous dysplasia. Head Face Med. 2009;5:22. https://doi.org/10.1186/1746-160X-5-22.
Bayas A, Naumann M, Wener S. Meningi oma associated with McCune–Albright syndrome. J Neurol. 1999;246:1199–200. https://doi.org/10.1007/s004150050544.
Fehlow P, Walther F. McCune–Albright syndrome in association with meningioma and mental and psychological retardation. Klin Padiatr. 1992;204:447–52. https://doi.org/10.1055/s-2007-1025387.
Frankel J, Ianotti F, Powell M, Schon F. Meningioma–an unrecognised complication of fibrous dysplasia of the skull? J Neurol Neurosurg Psychiatry. 1989;52:546–7. https://doi.org/10.1136/jnnp.52.4.546.
Acknowledgements
No.
Funding
No.
Author information
Authors and Affiliations
Contributions
SXW analyzed and interpreted the patient data and was a major contributor in writing the manuscript. LZ designed the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University. Due to the retrospective nature of our study, the board waived the need for written consent.
Consent for publication
Due to the retrospective nature of our study, the Institutional Review Board waived the need for written consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. 1
. Representative images of meningioma pathology. (a) Pathological hematoxylin–eosin staining of case 1 tumor specimen indicating transitional meningioma (WHO I grade); (b) Pathological hematoxylin–eosin staining of case 5 tumor specimen showing fibrous meningioma (WHO I grade); (c) Pathological hematoxylin–eosin staining of case 9 tumor specimen reporting metaplastic meningioma with a Ki-67 label index of 3% (WHO I grade).
Additional file 2
: Clinical descriptions and radiological presentations of 21 included cases.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, X., Li, Z. Coexistence of meningioma and craniofacial fibrous dysplasia: a case series of clinicopathological study and literature review. Orphanet J Rare Dis 19, 30 (2024). https://doi.org/10.1186/s13023-024-03032-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-024-03032-0