Abstract
Background
The Registry of Adult and Paediatric Patients Treated with Cystadane® – Homocystinuria (RoCH) is a non-interventional, observational, multi-centre, post-authorization safety study that aimed to identify safety of betaine anhydrous (Cystadane®) in the treatment of patients with inborn errors of homocysteine metabolism (homocystinuria) in order to minimise the treatment associated risks and establish better knowledge on its clinical use. The registry included patients of all ages with homocystinuria who were treated with betaine anhydrous in conjunction with other therapies. Clinical data were collected retrospectively from 2007 to 2013, then prospectively up to February 2014. All adverse events (AEs) reported during the study were recorded. The clinical and biological status of patients was monitored at least once a year.
Results
A total of 125 patients with homocystinuria (adults [> 18 years]: 50; paediatric [≤18 years]: 75) were enrolled at 29 centres in France and Spain. Patients were treated with betaine anhydrous for a mean duration of 7.4 ± 4.3 years. The median total daily dose of betaine anhydrous at the first and last study visits was 6 g/day for cystathionine β-synthase (CBS)-deficient vitamin B6 responders and 9 g/day for methylenetetrahydrofolate reductase-deficient patients, while the median daily dose increased in CBS-deficient B6 non-responders (from 6 to 9 g/day) and cobalamin metabolism-defective patients (from 3 to 6 g/day) between the first and last visits. Treatment caused a mean overall reduction of 29% in plasma homocysteine levels in the study population. A total of 277 AEs were reported during the study, of which two non-serious AEs (bad taste and headache) and one serious AE (interstitial lung disease) were considered to be drug related. Overall, betaine anhydrous was well tolerated with no major safety concerns.
Conclusions
Data from the RoCH registry provided real-world evidence on the clinical safety and efficacy of betaine anhydrous in the management of homocystinuria in paediatric and adult patients.
Similar content being viewed by others
Background
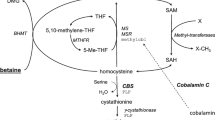
Homocystinuria is an autosomal recessive disorder of homocysteine metabolism leading to increased plasma, urine and tissue accumulation of homocysteine and its metabolites [1, 2]. Under normal conditions, the amino acid methionine is transmethylated to homocysteine, a sulphur amino acid not used for protein synthesis [1]. Homocysteine is either irreversibly degraded to cysteine by the vitamin B6-dependent enzymes cystathionine β-synthase (CBS) and cystathionine-γ-lyase or remethylated back to methionine by methionine synthase (MS) [3]. Inherited disorders of homocysteine metabolism therefore include disorders of the trans-sulphuration pathway with CBS deficiency, known as classical homocystinuria, and remethylation disorders [1, 2]. The clinical manifestations of classical homocystinuria include skeletal abnormalities, osteoporosis, ectopia lentis and/or severe myopia, cognitive impairment, developmental delay/intellectual disability, seizures, psychiatric disorders, atherosclerosis and thromboembolism [4]. If untreated, homocystinuria is a serious, life-threatening disease [3, 5].
The two phenotypic variants of CBS deficiency are B6-responsive and B6-non-responsive homocystinuria, where mutations in the CBS gene cause a varying response to vitamin B6 [6,7,8,9,10]. CBS deficiency results in increased plasma and tissue homocysteine and methionine levels [11]. Remethylation disorders include 5,10-methylenetetrahydrofolate reductase (MTHFR) deficiency and errors of cobalamin (Cbl, vitamin B12) metabolism [2]. MTHFR converts 5,10-methyltetrahydrofolate (5,10-MTHF) into 5-MTHF, the most biologically active form of vitamin B9, which acts as a methyl donor and functions together with vitamin B12 in the conversion of homocysteine to methionine [12]. Defects in the steps of Cbl metabolism include abnormalities in dietary intake, intestinal absorption, blood transport of Cbl by transcobalamin (TC), cellular uptake and intracellular metabolism (cblF, cblJ, cblC, cblD, cblE and cblG defects) [13,14,15].
Betaine anhydrous (Cystadane®, Orphan Europe) is a methylating agent approved for the treatment of homocystinuria involving CBS, MTHFR or cbl defects [16, 17]. Betaine anhydrous acts as a methyl donor for remethylation of homocysteine to methionine, increasing the plasma and tissue concentrations of methionine while reducing homocysteine levels [16]. The efficacy of betaine anhydrous in the treatment of homocystinuria has been well established [3, 7, 8, 18,19,20]. The Registry of Adult and Paediatric Patients Treated with Cystadane® - Homocystinuria (RoCH) was a post-authorization safety study (PASS) conducted in Europe to identify adverse events (AEs) associated with the use of betaine anhydrous in clinical practice, with an aim of minimizing potential risks with treatment and establishing better clinical knowledge about its use in patients with homocystinuria. The results of the RoCH registry study are presented here.
Methods
Registry design
This open-label, non-comparative and non-interventional study included patients of all ages with homocystinuria who were treated with betaine anhydrous in conjunction with other therapies and had available clinical data from at least two study visits (before and after betaine anhydrous treatment). Patients who were not treated with betaine anhydrous were excluded, along with those who did not wish to participate. Patients provided verbal approval prior to entry into the registry.
Clinical data of all patients included in the study were assessed by the treating physician participating in the registry. At the inclusion visit and at each follow-up visit, all ocular, skeletal, cardiovascular, neurological, psychiatric and other miscellaneous abnormalities (Additional file 1: Table S1) were specified on the clinical observation form and assessed for each patient, along with brain and spinal cord nuclear magnetic resonance imaging. Plasma levels of total homocysteine, methionine, vitamin B12, free homocysteine and folates, and urinary levels of methylmalonic acid were also recorded at each visit. Severe homocystinuria was defined as total plasma homocysteine level of > 50 μmol/L.
Data on betaine anhydrous treatment (start and stop dates, dose and frequency), methionine-restricted diet and other relevant concomitant medications were also collected at each visit. Safety data were recorded on specific safety reporting forms and submitted to the pharmacovigilance department of Orphan Europe, where it was entered in the global safety database and evaluated according to the European guidelines [1, 2] and legislation in pharmacovigilance [21].
Statistical analysis
Descriptive statistics were used. For quantitative variables, the sample size, mean, median, standard deviation (SD), standard error of the mean, coefficient of variation, minimum and maximum values, and quartiles were provided. For qualitative variables, sample size and absolute and relative frequencies per class were provided.
Results
All data included in the study were collected retrospectively from 2007 to 2013, after which it was prospectively collected up to 28 February 2014. The clinical and biological status of each patient was monitored at least once a year for most patients. A total of 623 visit forms were collected.
Patients
A total of 125 patients (56.8% male; mean age 16.80 ± 14.4 years) were enrolled from 29 European centres (22 in France and 7 in Spain) during the study period (Table 1). The most common diagnosis of type of homocystinuria at baseline was B6 non-responsive CBS deficiency (n = 49, 39.2%), followed by Cbl metabolism defects (n = 45, 36.0%), MTHFR deficiency (n = 21, 16.8%), and B6 responsive CBS deficiency (n = 9, 7.2%; Table 1). The majority of patients in all diagnostic categories were male, except for an approximately 1:1 male:female ratio observed in patients with Cbl metabolism defects and MTHFR deficiency (Table 1).
Of the 45 patients with Cbl metabolism defects, 42 patients had known Cbl deficiency type at the first visit: 88.1% were cblC-defective (n = 37), 9.5% were cblE/G-defective (n = 4), and 2.4% (n = 1) had TC deficiency. A 46-year-old female patient was reported with a double diagnosis (CBS-deficient vitamin B6 non-responder and MTHFR-deficient).
The median age of patients was 39 years for CBS-deficient B6 responders, 18 years for CBS-deficient B6 non-responders and 20 years for MTHFR-deficient patients (Table 1). Cbl metabolism-defective patients were the youngest, with a median age of 6 years, and were diagnosed with homocystinuria the earliest, at a mean age of 3.5 ± 9.2 years (median in the first year of life) and 3.02 ± 8.3 years after the first onset of symptoms (Table 1). CBS-deficient B6 responders were diagnosed at a much later age compared with the other diagnostic categories, with a mean age of 28.1 ± 17.7 years (median 31 years) (Table 1).
Treatment
All patients included in the study received betaine anhydrous treatment for a mean duration of 7.4 ± 4.3 years (median 7 years [0.0–22.8]). Prior to being treated with betaine anhydrous, 26 patients had received treatment with other formulations of betaine, all of which were discontinued immediately upon prescription of betaine anhydrous.
The median total daily dose of betaine anhydrous was 6 g/day for CBS-deficient B6 responders recorded at both the first and last visits. MTHFR-deficient patients were treated with a higher dose, with a median value of 9 g/day at first and last visits. The median total daily dose between the first and last visits increased from 6 to 9 g/day in CBS-deficient vitamin B6 non-responders, and from 3 to 6 g/day in Cbl metabolism-defective patients.
To exclude the impact of increasing body weight on the prescribed dose of betaine anhydrous in paediatric patients, the median total daily dose was recalculated as mg/kg/day and varied between 108 and 167 mg/kg/day in the overall population. The median total daily dose of betaine anhydrous varied from 107 to 181 mg/kg/day in the paediatric population (n = 75). Paediatric patients received a higher dose of betaine anhydrous during the first year of treatment compared with later years. Specifically, paediatric patients with MTHFR and Cbl metabolism defects received higher doses of betaine anhydrous compared with the other two diagnostic groups (data not shown). Mean levels of total plasma homocysteine in the paediatric subgroup varied between 52.9 and 105.7 μmol/L (Fig. 1a).
The median total daily dose of betaine anhydrous in adult population (n = 50) varied between 92 and 184 mg/kg/day, and the mean levels of total homocysteine were within a range of 63.8–131.0 μmol/L. After 9 years of treatment, adult patients received higher doses of betaine anhydrous (Fig. 1b), which could probably be due to decrease in body weight of these patients, as the dose of betaine anhydrous was not adjusted according to change in weight in these patients during the treatment period.
All CBS-deficient B6 responders were treated with vitamin B6 and folic acid in addition to betaine anhydrous, and 88.9% of these patients were also treated with vitamin B12. CBS-deficient B6 non-responders were mainly supplemented with vitamin B6 (91.8%), folic acid (83.7%) and oral vitamin B12 (61.2%), and 87.8% followed a methionine-restricted diet. MTHFR-deficient patients were mainly supplemented with folinic acid (76.2%) and oral vitamin B12 (57.1%), and Cbl metabolism-defective patients were mostly supplemented with intramuscular vitamin B12 (93.3%), carnitine (80.0%) and folinic acid (71.1%; Table 2).
Disease characteristics
A broad range of clinical presentations associated with homocystinuria were observed during the study period (Additional file 2: Figure S1). CBS-deficient vitamin B6 non-responders mainly reported ocular (74.0%), skeletal (72.0%), neurologic (66.0%) and cardiovascular (46.0%) symptoms. Skeletal and cardiovascular issues were reported by 77.8% of CBS-deficient B6 responders, followed by ocular (66.7%) and neurologic (55.6%) abnormalities. The majority of Cbl metabolism-defective patients (80.0%) reported neurologic disorders, followed by ocular and hematologic disorders in 66.7 and 64.4% of patients, respectively. All MTHFR-deficient patients had neurologic impairment, over half (52.4%) had psychiatric disturbances, and skeletal disorders were reported in 38.1% of these patients (Additional file 2: Figure S1).
Biochemical analysis
Laboratory values were available for patients who had data for at least two visits to the registry. Mean total plasma homocysteine levels at the last visit were decreased compared with the first visit in all diagnostic groups (79.07 ± 46.51 μmol/L versus 111.29 ± 70.31 μmol/L), with a mean overall reduction of 29.0%. Among the subgroups, mean decrease was highest in the CBS-deficient B6 responders (57.9%; Fig. 2a). Total homocysteine levels decreased after treatment with betaine anhydrous regardless of age group and gender (Fig. 2b and c).
In patients with severe homocystinuria, total plasma homocysteine levels decreased from 129.3 μmol/L (n = 93) at the first visit to 96.3 μmol/L (n = 82) at the last visit, at which the mean total homocysteine levels were < 100 μmol/L.
As some patients started treatment with betaine anhydrous only after the first visit, methionine levels were analysed at the second visit to ensure that all patients were on treatment. Mean methionine levels were increased in both sub-types of CBS deficiencies (273.2 μmol/L in B6 responders and 374.9 μmol/L in B6 non-responders) compared with the other two diagnostic groups (Fig. 3a and b). A slight increase was observed in B6 non-responders (428.7 μmol/L) at the last visit, but values remained well below the safety threshold of 1000 μmol/L. As expected, methionine levels were low in MTHFR- and Cbl metabolism-defective patients (18.6 and 23.4 μmol/L, respectively [normal values 16–30 μmol/L]); a slight increase was observed at the last visit (Fig. 3b).
Evolution of clinical symptoms
Overall, clinical symptoms of disorders associated with homocystinuria improved in 28.7% of patients, stabilised in 69.7% of patients and worsened in 1.6% of patients at the end of the study period. Among patients with clinical improvement of symptoms, an improvement of 33.0, 31.5, 26.6 and 13.6% was observed in Cbl metabolism-defective, MTHFR-deficient, CBS-deficient B6 non-responders, and CBS-deficient B6 responders, respectively. Clinical improvement of symptoms was determined by the treating physician at each visit as no change, improvement or worsening compared with previous visits.
Safety
A total of 277 AEs were reported by System Organ Class (SOC) during the study period, of which 109 were serious. The most frequently reported AEs were: injury, poisoning and procedural complications (16.1%), infections and infestations (15.5%), gastrointestinal disorders (9.75%), nervous system disorders (9.0%), musculoskeletal and connective tissue disorders (7.2%), and respiratory, thoracic and mediastinal disorders (7.2%). Only two non-serious AEs (bad taste and headache) and one serious AE (interstitial lung disease) were assessed as being possibly related to betaine anhydrous.
Interstitial lung disease was reported as a serious AE in a 2-year-old cblC-defective male patient treated with betaine anhydrous (2 g/day) who experienced respiratory distress related to pulmonary hypertension and interstitial lung disease leading to death.
Two other deaths occurred during the registry. A 9-year-old CBS-deficient B6 non-responder male patient treated with 6 g/day of betaine anhydrous (initiated after the start of the event) experienced brain vein thrombosis which led to brain oedema and intracranial hypertension. The patient was diagnosed 2 days prior to the start of treatment and methionine levels were 433 μmol/L a day prior to death. A 15-month old MTHFR-deficient female patient treated with 3 g/day betaine anhydrous died of respiratory distress. These deaths were not considered as related to treatment.
There were eight cases of betaine anhydrous exposure during pregnancy: five resulted in the delivery of healthy babies and three resulted in spontaneous abortion, none of which were presumed to be related to betaine anhydrous.
Discussion
The results of this registry provide real-world information on the use of betaine anhydrous in the management of homocystinuria in clinical practice. Betaine anhydrous was associated with decreased total plasma homocysteine, increased methionine, and improvement/stabilisation of the clinical symptoms of different disorders associated with homocystinuria in the treated population. Overall, betaine anhydrous was well tolerated, with interstitial lung disease being the only serious AE reported in one patient that was considered likely to be associated with treatment.
Plasma homocysteine levels generally determine the severity of homocystinuria [22]. One of the aims of betaine anhydrous treatment is to keep plasma total homocysteine levels as low as possible [23]. As per the current guidelines, additional treatment such as betaine and/or dietary modifications should be considered in CBS-deficient B6 responders in whom total homocysteine levels remain above 50 μmol/L [1]. These guidelines also highlight that patients are unlikely to develop complications if plasma homocysteine levels are maintained below 120 μmol/L, with the recommendation to maintain levels below 100 μmol/L to allow for natural homocysteine fluctuations [1]. In this RoCH registry study, betaine anhydrous treatment decreased total plasma homocysteine levels below the recommended 100 μmol/L in the total study population, regardless of the nature of the deficiency (79.07 ± 46.51 μmol/L vs 111.29 ± 70.31 μmol/L at baseline), and the clinical symptoms of disorders associated with homocystinuria either improved or stabilised in the majority of patients during the study period.
In the present study population, betaine anhydrous was well tolerated, with manageable AEs. Bad taste and headache were the main non-serious AEs associated with treatment, and interstitial lung disease was the only serious AE leading to death. Interstitial lung disease occurred after 1 year of betaine anhydrous treatment and was considered to be treatment-related as no other reason for its occurrence could be determined. However, since only one case of interstitial lung disease was reported during the study period, it could be idiopathic, but has been included in the risk management plan as a potential important risk with betaine anhydrous treatment due to its seriousness.
The main safety issue with the use of betaine anhydrous is the possibility of elevated methionine levels formed by homocysteine remethylation, which may lead to cerebral oedema [3, 24]. Elevated methionine levels (> 1000 μmol/L) are a major concern in CBS-deficient patients, and cerebral oedema has previously been reported in these patients when treated with betaine anhydrous [6, 25,26,27]. Since the majority of patients in the present study followed a methionine-restricted diet along with treatment, methionine levels of CBS-deficient patients remained well below the critical threshold of 1000 μmol/L, which is an important safety-monitoring parameter for the treatment of this diagnostic group. The slight increase in methionine levels in MTHFR- and Cbl metabolism-defective patients may reflect the efficacy of treatment in these subgroups.
CBS deficiency is generally characterised by developmental delay, intellectual disability, ocular complications, skeletal abnormalities and thromboembolism, with the expression of these clinical signs being extremely varied [3, 10, 28]. During the present study, clinical presentations of homocystinuria were wide-ranging and varied depending on the specific genetic defect. The majority of B6 non-responders presented ocular and skeletal disorders, while CBS-deficient B6 responders presented skeletal and cardiovascular disorders, followed by ocular complications. Although all these deficiencies resulted from increased homocysteine levels, patients in each diagnostic subgroup presented different symptoms and required different disease management.
The median age of patients varied across diagnostic groups, Cbl metabolism-defective patients being the youngest, and all diagnostic groups except the CBS-deficient B6 responders were diagnosed in childhood. Furthermore, the delay between the onset of first symptoms and diagnosis among Cbl-deficient patients was the shortest compared with other deficiencies. In Cbl metabolism-defective patients, clinical features included acute neurologic deterioration, developmental delay, lethargy, hypotonia and feeding problems in neonatal (< 1 month old) and early onset patients (< 1 year of life). The severity of initial manifestations in this subgroup may explain the early diagnosis as well as the short delay between the onset of symptoms and diagnosis.
Currently, newborn screening programs allow the diagnosis of CBS deficiency, detected by elevated methionine and homocysteine levels [29, 30]. However, this approach only allows the detection of the B6 non-responsive form, and rarely detects B6-responsive CBS-deficient newborn babies as increase in methionine is not obvious in these infants [30]. Very often the diagnosis of B6 responders is still made only after the development of complications that are mainly irreversible [4, 6]. This could explain the higher median age of diagnosis, and the delay between the onset of symptoms and diagnosis of homocystinuria in CBS-deficient B6 responders in the present study.
The clinical signs of remethylation defects are known to be mainly neurologic [31,32,33]. The main abnormalities observed in remethylation defective patients at baseline in this registry were indeed neurologic, with 80% of Cbl metabolism-defective and 100% of MTHFR-deficient patients displaying neurologic abnormalities. If untreated, these patients may develop acute or rapidly progressive neurologic deterioration, sometimes leading to death [4]. In the present study, betaine anhydrous treatment improved or stabilised the overall clinical symptoms in the majority of patients, indicating that betaine anhydrous effectively controls the symptoms of homocystinuria when used in conjunction with other relevant medications.
The recommended total daily dose of betaine anhydrous in adult and paediatric patients > 10 years of age is 6 g/day [1]. During the present study, CBS-deficient B6 responders were treated with a median dose of 6 g/day, whereas MTHFR-deficient patients were treated with a higher dose of betaine anhydrous (9 g/day), and the median total daily dose for CBS-deficient B6 non-responders and Cbl-deficient patients increased between the first and last visits. These data are consistent with literature reports, where betaine is administered at a higher dose of 5–20 g/day in adults and 150–250 mg/kg/day in children [4]. Since the majority of patients included in the study were ≤ 18 years of age (n = 75), in whom the adult dose of 6 g/day was to be administered from the age of 10 years, many investigators may have continued to adjust treatment to weight in paediatric patients aged > 10 years, which would explain the increase of total daily dose of betaine anhydrous recorded at the last visit.
The main limitation of the present study is that data was not statistically analysed to determine the significance of changes in different parameters observed during the study period. Also, the study was designed mainly to assess safety of betaine anhydrous and enrolled a highly heterogeneous patient population, which did not allow for statistical confirmation of changes in plasma homocysteine or methionine levels. Furthermore, data were collected retrospectively during most of the study period, which may have introduced bias.
Conclusion
Overall, the RoCH registry provides a better understanding of the clinical management of homocystinuria in different diagnostic subgroups. The results from this registry show that betaine anhydrous is well tolerated in patients with homocystinuria and highlights the importance of maintaining methionine levels below the safety threshold of 1000 μmol/L in patients with CBS defects as these patients have a higher risk of AEs due to hypermethioninemia. Further data characterizing the real-world use of betaine anhydrous in patients with homocystinuria are being collected through partnership with E-HOD (European network and registry for homocystinurias and methylation defects).
Abbreviations
- AE:
-
Adverse events
- Cbl:
-
Cobalamin
- CBS:
-
Cystathionine β-synthase
- MS:
-
Methionine synthase
- MTHF:
-
Methyltetrahydrofolate
- MTHFR:
-
5,10 methylenetetrahydrofolate reductase
- PASS:
-
Post-authorization safety study
- RoCH:
-
Registry of Adult and Paediatric Patients Treated with Cystadane® - Homocystinuria
- SD:
-
Standard deviation
- SOC:
-
System organ class
- TC:
-
Transcobalamin
References
Morris AA, Kozich V, Santra S, Andria G, Ben-Omran TI, Chakrapani AB, et al. Guidelines for the diagnosis and management of cystathionine beta-synthase deficiency. J Inherit Metab Dis. 2017;40:49–74.
Huemer M, Diodato D, Schwahn B, Schiff M, Bandeira A, Benoist JF, et al. Guidelines for diagnosis and management of the cobalamin-related remethylation disorders cblC, cblD, cblE, cblF, cblG, cblJ and MTHFR deficiency. J Inherit Metab Dis. 2017;40:21–48.
Maclean KN. Betaine treatment of cystathionine β-synthase-deficient homocystinuria; does it work and can it be improved? Orphan Drugs Res Rev. 2012;2012:23–33.
Schiff M, Blom HJ. Treatment of inherited homocystinurias. Neuropediatrics. 2012;43:295–304.
Castro R, Rivera I, Blom HJ, Jakobs C, Tavares de Almeida I. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: an overview. J Inherit Metab Dis. 2006;29:3–20.
Walter JH, Wraith JE, White FJ, Bridge C, Till J. Strategies for the treatment of cystathionine beta-synthase deficiency: the experience of the Willink biochemical genetics unit over the past 30 years. Eur J Pediatr. 1998;157(Suppl 2):S71–6.
Wilcken DE, Dudman NP, Tyrrell PA. Homocystinuria due to cystathionine beta-synthase deficiency--the effects of betaine treatment in pyridoxine-responsive patients. Metabolism. 1985;34:1115–21.
Wilcken DE, Wilcken B, Dudman NP, Tyrrell PA. Homocystinuria--the effects of betaine in the treatment of patients not responsive to pyridoxine. N Engl J Med. 1983;309:448–53.
Urreizti R, Balcells S, Rodes M, Vilarinho L, Baldellou A, Couce ML, et al. Spectrum of CBS mutations in 16 homocystinuric patients from the Iberian Peninsula: high prevalence of T191M and absence of I278T or G307S. Hum Mutat. 2003;22:103.
Mudd SH, Skovby F, Levy HL, Pettigrew KD, Wilcken B, Pyeritz RE, et al. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet. 1985;37:1–31.
Wu XY, Lu L. Vitamin B6 deficiency, genome instability and cancer. Asian Pac J Cancer Prev. 2012;13:5333–8.
Blom HJ, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75–81.
Quadros EV. Advances in the understanding of cobalamin assimilation and metabolism. Br J Haematol. 2010;148:195–204.
Watkins D, Rosenblatt DS. Inborn errors of cobalamin absorption and metabolism. Am J Med Genet C Semin Med Genet 2011;157C:33–44.
Martinelli D, Deodato F, Dionisi-Vici C. Cobalamin C defect: natural history, pathophysiology, and treatment. J Inherit Metab Dis. 2011;34:127–35.
Orphan Europe. Cystadane (betaine anhydrous for oral solution): highlights of prescribing information. 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020576s010lbl.pdf. Accessed 28 Feb 2018.
Orphan Europe. Cystadane 1 g oral powder: summary of product characteristics. 2007. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000678/WC500037404.pdf. Accessed 28 Feb 2018.
Fischer S, Huemer M, Baumgartner M, Deodato F, Ballhausen D, Boneh A, et al. Clinical presentation and outcome in a series of 88 patients with the cblC defect. J Inherit Metab Dis. 2014;37:831–40.
Diekman EF, de Koning TJ, Verhoeven-Duif NM, Rovers MM, van Hasselt PM. Survival and psychomotor development with early betaine treatment in patients with severe methylenetetrahydrofolate reductase deficiency. JAMA Neurol. 2014;71:188–94.
Smolin LA, Benevenga NJ, Berlow S. The use of betaine for the treatment of homocystinuria. J Pediatr. 1981;99:467–72.
European Medical Agency. Guideline on good pharmacovigilance practices (GVP) Module VI – Management and reporting of adverse reactions to medicinal products (Rev 1). 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/09/WC500172402.pdf. Accessed 26 Mar 2018.
Isherwood DM. Homocystinuria. BMJ. 1996;313:1025–6.
Wilcken DE, Wang XL, Adachi T, Hara H, Duarte N, Green K, et al. Relationship between homocysteine and superoxide dismutase in homocystinuria: possible relevance to cardiovascular risk. Arterioscler Thromb Vasc Biol. 2000;20:1199–202.
Devlin A, Hajipour L, Gholkar A, Fernandes H, Ramesh V, Morris A. Cerebral edema associated with betaine treatment in classical homocystinuria. J Pediatr. 2004;144:545–8.
Sasai H, Shimozawa N, Asano T, Kawamoto N, Yamamoto T, Kimura T, et al. Successive MRI findings of reversible cerebral white matter lesions in a patient with cystathionine beta-synthase deficiency. Tohoku J Exp Med. 2015;237:323–7.
Devlin AM, Hajipour L, Gholkar A, Fernandes H, Ramesh V, Morris AA. Cerebral edema associated with betaine treatment in classical homocystinuria. J Pediatr. 2004;144:545–8.
Yaghmai R, Kashani AH, Geraghty MT, Okoh J, Pomper M, Tangerman A, et al. Progressive cerebral edema associated with high methionine levels and betaine therapy in a patient with cystathionine beta-synthase (CBS) deficiency. Am J Med Genet. 2002;108:57–63.
Sacharow SJ, Picker JD, Levy HL. Homocystinuria caused by cystathionine beta-synthase deficiency. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mefford HC, Stephens K, Amemiya A, Ledbetter N, editors. GeneReviews®. Seattle: University of Washington; 1993.
Naughten E, Yap S, Mayne P. Newborn screening for homocystinuria: Irish and world experience. Eur J Pediatr. 1998;157:S84–7.
Kaye CI. Newborn screening fact sheets. Pediatrics. 2006;118:e934–63.
Carrillo-Carrasco N, Chandler RJ, Venditti CP. Combined methylmalonic acidemia and homocystinuria, cblC type. I. Clinical presentations, diagnosis and management. J Inherit Metab Dis. 2012;35:91–102.
Ogier de Baulny H, Gerard M, Saudubray JM, Zittoun J. Remethylation defects: guidelines for clinical diagnosis and treatment. Eur J Pediatr. 1998;157(Suppl 2):S77–83.
Rosenblatt DS, Aspler AL, Shevell MI, Pletcher BA, Fenton WA, Seashore MR. Clinical heterogeneity and prognosis in combined methylmalonic aciduria and homocystinuria (cblC). J Inherit Metab Dis. 1997;20:528–38.
Acknowledgements
This study was initiated and funded by Orphan Europe. The authors wish to thank Hélène Ogier De Baulny, Reda Sharobeem, Denys Chaigne, Benoit Funalot, Kaiss Lassoued, Maria Concepcion García Jiménez, Pierre Kieffer, Amaya Belanger-Quintana, Laurence Bouillet and Karine Mention for their participation in the registry, and Elisabeth Brachet and Celine Plisson for their support. The authors would also like to thank Dr. Marie Husson for her invaluable contribution to this study, and for her role in drafting and developing the manuscript. Medical writing assistance was provided by Meryem Güller, PhD, Scinopsis, Fréjus, France, and editorial assistance was provided by ApotheCom Ltd., London. This assistance was funded by Orphan Europe. The authors also thank Nishad Parkar, PhD, of Springer Healthcare Communications for editing and preparing the manuscript for submission. This assistance was funded by Orphan Europe.
Funding
The RoCH registry is a post-authorization safety study for Cystadane® funded by Orphan Europe.
Availability of data and materials
The data that support the findings of this study are available from Orphan Europe, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are, however, available from the authors upon reasonable request and with the permission of Orphan Europe.
Author information
Authors and Affiliations
Contributions
All authors were involved in data collection and interpretation, and reviewing and approving the manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval is not mandatory for non-interventional studies conducted in France and Spain. Data privacy collection approval was obtained. An information sheet was provided to patients and verbal consent was obtained in compliance with French and Spanish law for such a study.
Consent for publication
Not applicable.
Competing interests
All authors except GT, BF and MS received payment from Orphan Europe directly or indirectly through their institution/association for participation in the registry. VV is currently an employee of Sanofi Genzyme. He performed most of this work while he was at Necker-Enfants Malades University Hospital. LD has a conflict of interest with Orphan Europe. FM has received an honorarium from Orphan Europe. The other authors have no conflicts of interest to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Abnormalities recorded at inclusion visit and each follow-up visit among patients included in the study. (TIFF 4325 kb)
Additional file 2:
Figure S2. Distribution of presenting symptoms of homocystinuria during the study. Cbl, cobalamin; CBS, cystathionine β-synthase; MRI, magnetic resonance imaging; MTHFR, 5, 10-methylenetetrahydrofolate reductase. (DOCX 15 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Valayannopoulos, V., Schiff, M., Guffon, N. et al. Betaine anhydrous in homocystinuria: results from the RoCH registry. Orphanet J Rare Dis 14, 66 (2019). https://doi.org/10.1186/s13023-019-1036-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-019-1036-2