Abstract
Background
Brainstem metastases (BSM) are associated with a poor prognosis and their management represents a therapeutic challenge. BSM are often inoperable and, in absence of randomized trials, the optimal radiation treatment of BSM remains to be defined. We evaluated the efficacy and toxicity of linear accelerator (linac)-based stereotactic radiosurgery (SRS) and hypofractionated steretotactic radiotherapy (HSRT) in the treatment of BSM in a series of patients treated in different clinical centers.
Methods
We conducted a multicentric retrospective study of patients affected by 1–2 BSM from different histologies who underwent SRS/HSRT. Freedom from local progression (FLP), cancer-specific survival (CSS), overall survival (OS), and treatment-related toxicity were evaluated. In addition, predictors of treatment response and survivals were evaluated.
Results
Between 2008 and 2021, 105 consecutive patients with 111 BMS who received SRS or HSRT for 1–2 BSM were evaluated. Median follow-up time was 10 months (range 3–130). One-year FLP rate was 90.4%. At the univariate analysis, tumor volume ≤ 0.4 cc, and concurrent targeted therapy were associated with longer FLP, with combined treatment that remained a significant independent predictor [0.058, HR 0.139 (95% CI 0.0182–1.064]. Median OS and CSS were 11 months and 14.6 months, respectively. At multivariate analysis, concurrent targeted therapy administration was significantly associated with longer OS [HR 0.514 (95%CI 0.302–0.875); p = 0.01]. Neurological death occurred in 30.4% of patients, although this was due to local progression in only 3 (2.8%) patients.
Conclusion
Linac-based SRS/HSRT offers excellent local control to patients with BSM, with low treatment-related toxicity and no apparent detrimental effects on OS. When treated with ablative intent, BSM are an uncommon cause of neurological death. The present results indicates that patients with BSM should not be excluded a priori from clinical trials.
Similar content being viewed by others
Introduction
Stereotactic radiosurgery (SRS) given in a single fraction using doses of 18–24 Gy is the current standard treatment for patients with a limited number of brain metastases (1–4), and its use has progressively replaced the use of whole-brain radiotherapy (WBRT) 1,2,3]; however, patients with brainstem metastases (BSM) [4] are often excluded from prospective trials of SRS because the fear of excessive toxicity caused by exposure of the brainstem to high doses of radiation and its potential negative impact on survival. Therefore, there is a lack of evidence-based recommendations on the optimal radiation treatment of BSM in terms of techniques and dose-fractionation, and the management of these patients in clinical practice remains controversial.
The reluctance to use SRS as treatment of BSM is derived, at least in part, from historical studies demonstrating a maximum tolerated radiation dose for the brainstem of 12–12.5 Gy given as a single fraction [5, 6]. SRS for BMS was described for the first time in 1993 [7]. Since then, several authors reported their retrospective experience, usually small and monoinstitutional 8,9,10,11,12,13,14,15,16,17,18]. These studies did not show an increased risk of toxicity but provided inhomogeneous indication regarding dose prescription and clinical results, also due to heterogeneity in patients’ selection [11, 11,18,19,20,21]. Moreover, the majority of these patients were treated with gamma-knife SRS, with few data available for frameless linear accelerator (linac)-based SRS. A recent systematic review and meta-analysis of those retrospective studies demonstrated the efficacy of SRS in the treatment of BSM; however, only a few predictive parameters of treatment response were identified [22]. Therefore, it is hard to date to infer specific recommendations on dose prescription, as well as the identification of predictive factors of outcome because of the limited available data.
This multicentric retrospective study aims to evaluate the efficacy and safety of frameless linac-based SRS or hypofractionated stereotactic radiotherapy (HSRT) to BSM and to identify factors predictive of tumor control and survival.
Patient and methods
The present multi-institutional study was conducted on a retrospective cohort of patients with1-2 BSM who received SRS or HSRT between April 2008 and July 2021. Data were anonymously collected in an internal review board (IRB)-approved database. Inclusion criteria were: (a) age > 18 years; (b) diagnosis of BSM confirmed by contrast-enhanced MRI acquired no more than 4 weeks before radiation treatment; (c) Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2. Patients treated with concomitant systemic therapy were included in the study. SRS could have been administered concomitantly to systemic therapy (within one months from the last administration). The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the IRB of participating Institutions.
Treatment characteristics
Patients underwent a CT simulation without contrast media (1-mm slice thickness) for radiation therapy planning with a thermoplastic mask. A co-registration of volumetric CT and the T1 sequences of the MRI (3-dimensional spoiled gradient series with 1-mm slice thickness) no older than 2 weeks was used to define organs at risk (OARs) and target volumes. Gross tumor volume (GTV) encompassed the macroscopic contrast-enhancing lesion on T1-MRI and was assumed equal to the clinical target volume (CTV). The planning target volume (PTV) was obtained from the GTV plus an isotropic margin of 0–2 mm. We performed a risk-adapted dose prescription. SRS with a dose of 16–18 Gy was generally administered for lesions ≤ 10 mm, while HSRT with total doses of 14–32 Gy given in 2–5 fractions was reserved for lesions > 10 mm. The dose was usually prescribed at 80% isodose line and dose optimization was performed to cover 95% of PTV with the prescription dose. Treatment was administered with a linac using either volumetric-modulated arc therapy (VMAT), or multiple dynamic arcs (DCAT) or fixed beams.
Follow-up
Physical examination, toxicity assessment, and radiological response with MRI were performed after 45–60 days following the first treatment. Subsequently, follow-up was performed every 3 months for the first 2 years and every 4–6 months for the next 3 years. Tumor response was evaluated using the Response Assessment in Neuro-Oncology (RANO) criteria [23]. MR response assessment was based on contrast enhanced T1-w and fluid attenuated inversion recovery (FLAIR) sequences. Toxicity was assessed during and after radiotherapy according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Acute toxicity was defined as an adverse event occurring within 90 days from the beginning of treatment, whereas late toxicity after 90 days.
Study end-points and statistics
The primary end-point was freedom from local progression (FLP). Secondary endpoints were overall survival (OS), cancer-specific survival (CSS), neurological death (ND), and toxicity. Survivals were defined as follows: FLP as the time between SRS administration and the occurrence of in-field or marginal regrowth of the disease; OS was defined as the time to death or last follow-up; CSS as the time to death due to tumor progression or last follow-up, and ND as the death due to brain disease progression.
The univariate analysis was performed with the Kaplan–Meier method. The log-rank test was applied to determine differences between the corresponding curves. The following covariates were evaluated for survival end-points: sex, age, lesion volume (cc), PTV margin, BSM site (midbrain, pons, medulla oblongata) biological effective dose (BED), previous WBRT, primary tumor histology, concomitant therapy (target therapy, immunotherapy, chemotherapy), fractionation. Univariate and multivariate analyses were performed by the Cox proportional hazards model. Clinically relevant variables with a p < 0.1 at univariate analysis were included in the multivariate analysis. The threshold of tumor volume related to SRS response and/or survival was determined with the ROC curve method, calculating the highest product of (sensitivity*specificity). BED calculations for different radiation schedules was determined by linear-quadratic model according to an α/β ratio of 10 Gy for tumors. Statistical analysis was performed using the SPSS statistical software package version 26.0 (SPSS Inc, Chicago, IL). A p-value ≤ 0.05 indicated a significant association.
Results
Patients’ characteristics
The patient population was represented by 105 patients with 111 BSM. Patient and treatment characteristics are shown in Table 1. Main primary histology were non-small cell lung cancer (NSCLC, 46.6%), breast cancer (22%), renal cell carcinoma (10.5%), and melanoma (9.5%). Concomitant systemic therapy was administered in 76 patients, and included chemotherapy (26.2%), target therapy (25%), immunotherapy (13.5%), and hormone therapy (2.8%). More specifically, targeted therapies consisted of anti-HER2 agent (n = 12), ALK inhibitor (n = 7), tyrosin-kinase inhibitor (n = 6), and VRAF inhibitor (n = 3). The median follow-up was 11 months (range 3–130). RT treatment consisted of single-fraction SRS for 58 (52.3%) lesions and HSRT (2–5 fractions) for 53 lesions (47.7%). The median administered BED10 was 35.7 Gy (range 23.8–60). The GTV volume threshold for survival analysis was 0.4 cc (AUC 0.69, 95%CI 0.61–0.74; p = 0.02). Median BSM volume was 0.4 cc (range 0.02–23.6).
Freedom from local progression and toxicity
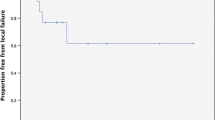
With a median time of 11 months, the 1-year FLP was 90.4%, with 13 lesions that progressed at a median time of 10 months (Fig. 1). Results of univariate and multivariate analysis are shown in Table 2. GTV volume ≤ 0.4 cc, and concurrent targeted therapy were predictive of better FLP. In particular, 1-year FLP rates were 98.1% for a GTV volume ≤ 0.4 cc and 82.9% for a GTV volume > 0.4 cc (p = 0.041). 1-year FLP was 96.5% following combined treatment and 86% after SRS/HSRT alone (p = 0.03), with no difference between SRS and HSRT (Fig. 2; p = 0.51). At the multivariate analysis, pons location was an independent factor of improved FLP [HR 0.202 (95%CI 0.048–0.845); p = 0.02], while concurrent targeted therapy was of borderline significance [0.058, HR 0.139 (95% CI 0.0182–1.064]. No severe acute toxicity occurred. A case of grade 2 pseudoprogression was recorded in one patient 3 months after the treatment, and grade 2 headache in 3 patients, who were successfully treated with steroids. For these patients, perfusion MRI changes were suggestive of symptomatic radiation necrosis. No grade 3 or higher acute toxicity occurred.
Overall Survival and prognostic factors
The median OS time was 11 months (range 9–17.4) and 1-year OS rate was 49.5% (Fig. 3). The median CSS time was 14.6 months (range 10–22.3). The 1-year CSS rate was 55.6%. In total, 32 patients (30.4%) had ND. The 1-year ND rate was 77.5%. At the last follow-up, 84 patients (80%) died. Forty-eight (45.7%) died of systemic progression and 32 (30.4%) died of intracranial progression; however, this was due to local BSM progression only in 3 patients. In four (3.8%) patients, death resulted from noncancer causes.
At the univariate analysis, previous WBRT was associated with worse 1-year OS (30.5% versus 52.6%, p = 0.01), CSS (35.6% versus 58.6%, p = 0.03), and ND rates (57.9% versus 80.6%, p = 0.001). Concurrent target therapy was a predictor of better OS (67.5% versus 45.8%, p = 0.01) and CSS (70.2% versus 48.2%, p = 0.03) rates at 1 year. Similarly, SRS was associated with improved OS (p = 0.03). At the multivariate analysis, concurrent targeted therapy remained significantly associated with improved OS [HR 0.514 (95%CI 0.302–0.875); p = 0.01] and CSS [HR 0.573 (95%CI 0.331–0.992); p = 0.04]. Previous WBRT was an independent factor of lower CSS [HR 2.055 (95%CI 1.023–4.128); p = 0.04] and ND [HR 3.381 (95%CI 1.287–8.879); p = 0.01].
Discussion
Brainstem metastases in cancer patients are usually associated with poor survival and quality of life. The presence of vital structures justifies the rapid onset of symptoms even when the lesion dimension is small. For these inoperable lesions, radiotherapy has historically been the most widely used therapeutic option. The use of SRS was generally limited by the fear of side effects; however, an accumulating body of literature in the last two decades is demonstrating its efficacy in patients with BSM.
Yet, the largest published series is a multi-institutional analysis by Trifiletti et al. [24] including 547 patients with BMS who were treated with Gamma Knife SRS. The 1-year local control and FLP rates were 81.8% and 90.4%, respectively, using a median marginal dose of 16 Gy. In the univariate analysis, a marginal dose < 16 Gy was associated with worse local control; however, the correlation was not confirmed in the multivariate analysis. These results compare with those reported in series of SRS for cerebral and cerebellar metastases, reporting 1-year local control rates of 86.7% to 95% [2, 25].
Differently from other studies, in the current series a significant proportion of patients received HSRT, typically 14–32 Gy given in 2–5 fractions. The 1-year FLP rates were similar, 92.9% and 86.8% after SRS and HSRT, respectively, although fractionated SRS was used more often for larger lesions. Based on our results, both approaches offer similar excellent long-term tumor control, at least for a BED 10 > 35 Gy, with low treatment-related toxicity.
BSM have been generally linked to poor survival due to the rapid onset of symptoms, indicating local tumor progression as a frequent cause of cancer death. However, the reported survival in our and other published SRS series might suggest a different scenario. The median cancer-specific survival observed in our study was 14.6 months, being similar to those reported by others [21]. In the study of Trifiletti et al. [24], median OS time and 1-year survival rates were 5.6 months and 32.7%, respectively. Interestingly, death rate for BSM progression was 0.7%, and ND was 16%. In another large Gamma Knife series by Kawabe et al. [12], the death rate for patients with BSM progression was 2.3% and ND rate was 10.9%. In our series, we observed a similar death rate of 2.8%, confirming that SRS is an effective treatment associated with a low mortality rate due to BSM progression.
A point for discussion comes from the role of WBRT in the management of BM. For patients with a limited number of brain metastases, SRS has become the recommended treatment over WBRT. In addition, recent evidence demonstrated that focal irradiation may have a role also in patients with further intracranial progression after a first course of SRS [26, 27]. In the current study, patients who received a previous WBRT had worse CSS, suggesting that SRS should be always considered in all patients with limited brain disease, even in presence of BSM.
Our study has several limitations due to its retrospective nature and selection biases; patients were treated with different radiation schedules, and a subgroup received targeted therapy which might have influenced clinical outcomes. Nevertheless, this is the largest series of linac-based SRS for BSM, and our data on local control and the cause of death strongly support the use of SRS/HSRT in these patients confirming the excellent results reported in previous smaller mono-institutional series (see Table 3) [10, 11, 21, 28,29,30]. Of note, the rate of local control observed with frameless linac-based SRS/HSRT is in line with other SRS techniques, and our study adds evidence to the role of frameless linac-based SRS in the treatment of BSM.
In conclusion, frameless linac-based SRS/HSRT is a safe and effective treatment associated with excellent local control in patients with BSM, similar to those reported for lesions in other regions of the brain, with no detrimental effect on survival. Death due to BSM is in fact a rare event, and the presence of BSM in cancer patients should not be considered an exclusion criterion from clinical trials.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BED:
-
Biological effective dose
- BSM:
-
Brainstem metastases
- CI:
-
Confidence interval
- CSS:
-
Cancer-specific survival
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- ECOG:
-
Eastern Cooperative Oncology Group
- FLP:
-
Freedom from locap orgression
- GTV:
-
Gross tumor volume
- HR:
-
Hazard ratio
- IRB:
-
Internal review board
- MRI:
-
Magnetic resonance imaging
- ND:
-
Neurological death
- OAR:
-
Organ at risk
- OS:
-
Overall survival
- PTV:
-
Planning target volume
- RECIST:
-
Response evaluation criteria in solid tumors
- SRS:
-
Stereotactic radiosurgery
- HSRT:
-
Hypofractionated stereotactic radiotherapy
- VMAT:
-
Volumetric-modulated arc therapy
- WBRT:
-
Whole-brain radiotherapy
References
Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014. https://doi.org/10.1016/S1470-2045(14)70061-0.
Alongi F, Nicosia L, Figlia V, et al. Long-term disease outcome and volume-based decision strategy in a large cohort of multiple brain metastases treated with a mono-isocentric linac-based Stereotactic Radiosurgery technique. Clin Transl Oncol. 2021. https://doi.org/10.1007/s12094-020-02550-0.
Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009. https://doi.org/10.1016/S1470-2045(09)70263-3.
Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Phys. 2000. https://doi.org/10.1016/S0360-3016(99)00507-6.
Sharma MS, Kondziolka D, Khan A, et al. Radiation tolerance limits of the brainstem. Neurosurgery. 2008. https://doi.org/10.1227/01.NEU.0000325726.72815.22.
Mayo C, Yorke E, Merchant TE. Radiation Associated Brainstem Injury. Int J Radiat Oncol Biol Phys. 2010. https://doi.org/10.1016/j.ijrobp.2009.08.078.
Somaza S, Kondziolka D, Lunsford LD, Kirkwood JM, Flickinger JC. Stereotactic radiosurgery for cerebral metastatic melanoma. J Neurosurg. 1993. https://doi.org/10.3171/jns.1993.79.5.0661.
Kased N, Huang K, Nakamura JL, et al. Gamma knife radiosurgery for brainstem metastases: the UCSF experience. J Neurooncol. 2008. https://doi.org/10.1007/s11060-007-9458-4.
Koyfman SA, Tendulkar RD, Chao ST, et al. Stereotactic radiosurgery for single brainstem metastases: The Cleveland clinic experience. Int J Radiat Oncol Biol Phys. 2010. https://doi.org/10.1016/j.ijrobp.2009.07.1750.
Hatiboglu MA, Chang EL, Suki D, Sawaya R, Wildrick DM, Weinberg JS. Outcomes and prognostic factors for patients with brainstem metastases undergoing stereotactic radiosurgery. Neurosurgery. 2011. https://doi.org/10.1227/NEU.0b013e31821d31de.
Lin CS, Selch MT, Lee SP, et al. Accelerator-based stereotactic radiosurgery for brainstem metastases. Neurosurgery. 2012. https://doi.org/10.1227/NEU.0b013e31823c40fe.
Kawabe T, Yamamoto M, Sato Y, et al. Gamma Knife surgery for patients with brainstem metastases. J Neurosurg. 2012. https://doi.org/10.3171/2012.7.gks12977.
Şengöz M, Kabalay IA, Tezcanli E, Peker S, Pamir N. Treatment of brainstem metastases with gamma-knife radiosurgery. J Neurooncol. 2013. https://doi.org/10.1007/s11060-013-1086-6.
Peterson HE, Larson EW, Fairbanks RK, et al. Gamma knife treatment of brainstem metastases. Int J Mol Sci. 2014. https://doi.org/10.3390/ijms15069748.
Kilburn JM, Ellis TL, Lovato JF, et al. Local control and toxicity outcomes in brainstem metastases treated with single fraction radiosurgery: Is there a volume threshold for toxicity? J Neuro-Oncol. 2014. https://doi.org/10.1007/s11060-014-1373-x.
Voong KR, Farnia B, Wang Q, et al. Gamma knife stereotactic radiosurgery in the treatment of brainstem metastases: the MD anderson experience. Neuro-Oncology Pract. 2015. https://doi.org/10.1093/nop/npu032.
Shuto T, Fujino H, Asada H, Inomori S, Nagano H. Gamma knife radiosurgery for metastatic tumours in the brain stem. Acta Neurochir (Wien). 2003. https://doi.org/10.1007/s00701-003-0034.
Davidson L, Zada G, Yu C, et al. Delayed toxicity from gamma knife radiosurgery to lesions in and adjacent to the brainstem. J Clin Neurosci. 2009. https://doi.org/10.1016/j.jocn.2009.03.003.
Lorenzoni JG, Devriendt D, Massager N, et al. Brain stem metastases treated with radiosurgery: prognostic factors of survival and life expectancy estimation. Surg Neurol. 2009. https://doi.org/10.1016/j.surneu.2008.01.029.
Leeman JE, Clump DA, Wegner RE, Heron DE, Burton SA, Mintz AH. Prescription dose and fractionation predict improved survival after stereotactic radiotherapy for brainstem metastases. Radiat Oncol. 2012. https://doi.org/10.1186/1748-717X-7-107.
Valery CA, Boskos C, Boisserie G, et al. Minimized doses for linear accelerator radiosurgery of brainstem metastasis. Int J Radiat Oncol Biol Phys. 2011. https://doi.org/10.1016/j.ijrobp.2010.02.028.
Chen WC, Baal UH, Baal JD, et al. Efficacy and safety of stereotactic radiosurgery for brainstem metastases: a systematic review and meta-analysis. JAMA Oncol. 2021. https://doi.org/10.1001/jamaoncol.2021.1262.
Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015. https://doi.org/10.1016/S1470-2045(15)70057-4.
Trifiletti DM, Lee CC, Kano H, et al. Stereotactic radiosurgery for brainstem metastases: an international cooperative study to define response and toxicity. Int J Radiat Oncol Biol Phys. 2016. https://doi.org/10.1016/j.ijrobp.2016.06.009.
Redmond KJ, Gui C, Benedict S, et al. Tumor control probability of radiosurgery and fractionated stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2021. https://doi.org/10.1016/j.ijrobp.2020.10.034.
Nicosia L, Figlia V, Mazzola R, et al. Repeated stereotactic radiosurgery (SRS) using a non-coplanar mono-isocenter (HyperArcTM) technique versus upfront whole-brain radiotherapy (WBRT): a matched-pair analysis. Clin Exp Metastasis. 2020. https://doi.org/10.1007/s10585-019-10004-3.
Nicosia L, Figlia V, Giaj-Levra N, Minniti G, Alongi F. Repeated stereotactic radiosurgery for the treatment of relapsed brain metastases is it time to give up whole-brain radiotherapy? Oncoscience. 2020. https://doi.org/10.18632/oncoscience.500.
Samblás JM, Sallabanda K, Bustos JC, et al. Radiosurgery and whole brain therapy in the treatment of brainstem metastases. Clin Transl Oncol. 2009;11(10):677–80. https://doi.org/10.1007/s12094-009-0423-x.
Kelly PJ, Lin YB, Yu AY, et al. Linear accelerator-based stereotactic radiosurgery for brainstem metastases: the Dana-Farber/Brigham and Women's Cancer Center experience. J Neurooncol. 2011;104(2):553-7. https://doi.org/10.1007/s11060-010-0514-0.
Sugimoto T, Matsuda R, Tamamoto T, et al. Linac-based fractionated stereotactic radiotherapy with a micro-multileaf collimator for brainstem metastasis. World Neurosurg. 132e680-e686, S1878875019322041. https://doi.org/10.1016/j.wneu.2019.08.049.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LN: research plan management, data analysis, manuscript writing; PN: data collection, manuscript review; VP: data collection, manuscript review; MG: table writing, literature review; IR: table writing, literature review; PT: data collection, literature review; NGL: manuscript revision, statistical support; FA: data handling, manuscript review; GM: research conceptualization, data analysis, final manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nicosia, L., Navarria, P., Pinzi, V. et al. Stereotactic radiosurgery for the treatment of brainstem metastases: a multicenter retrospective study. Radiat Oncol 17, 140 (2022). https://doi.org/10.1186/s13014-022-02111-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-022-02111-5