Abstract
Background
Low-grade papillary Schneiderian carcinoma (LGPSC) is a relatively new entity of the sinonasal tract and is characterized by a bland morphology simulating sinonasal papilloma, invasive growth pattern with pushing borders, and aggressive clinical behavior with multiple recurrences and metastatic potential. Recently, DEK::AFF2 fusions were identified in LGPSC. However, some LPGSCs lack DEK::AFF2 fusion, and the molecular features of these tumors have not been clarified.
Case presentation
A 69-year-old man presented with a discharge of pus from his left cheek. Computed tomography revealed a mass involving the left maxillary sinus, ethmoid sinus, and nasal cavity with the destruction of the orbital wall. The biopsy specimens showed that the tumor had a predominantly exophytic, papillary growth and did not have an apparent stromal invasion. The tumor was composed of multilayered epithelium that showed bland morphology with a round to polygonal shape, abundant eosinophilic cytoplasm, and uniform nuclei. Dense neutrophilic infiltrates were focally present. Immunohistochemically, CK5/6 was strongly and diffusely positive, and p16 was negative. p63 was mainly positive in the basal layer, and EMA was predominantly expressed in the outermost cell layer. DNA-based targeted sequencing showed TP53 R175H mutation, whereas neither EGFR nor KRAS mutation was identified. Reverse transcription polymerase chain reaction and fluorescence in situ hybridization revealed no DEK::AFF2 fusion.
Conclusions
We describe the first case of TP53-mutant LGPSC and review the literature. LGPSC is a genetically heterogeneous entity, and the recognition of this rare entity and comprehensive assessment of clinicopathological and molecular findings are crucial for the correct pathological diagnosis and clinical management.
Similar content being viewed by others
Background
Low-grade papillary Schneiderian carcinoma (LGPSC) was first described by Lewis et al. in 2015, as a bland, benign-looking papillary carcinoma, which in almost all aspects resembled a sinonasal (Schneiderian) papilloma clinically and pathologically but which recurred locally and metastasized to lymph nodes, resulting in the death of the patient [1]. Since its first description, only 17 cases have been described in the literature so far [1,2,3,4,5,6,7,8,9,10]. Histologically, the tumors show predominantly exophytic and inverted papillary lesions and lack malignant cytological features. Tumor epithelia are multilayered and arranged in an orderly pattern without cilia. Most previous reports indicated that the original diagnoses were benign sinonasal tumors, including exophytic and inverted papilloma. However, during the clinical course, the tumors extended into the adjacent structures, such as the nasopharynx, middle ear, temporal bone, cheek soft tissue, and orbit [6]; the diagnoses were subsequently revised to LGPSC. In terms of pathogenesis, LGPSC lacks either virus infection or activating mutations of the MAPK pathway, which are common in sinonasal carcinoma [11] and carcinoma associated with sinonasal papilloma [12], respectively. In 2021, DEK::AFF2 fusions were identified in LGPSC [8]. However, some LPGSCs lack DEK::AFF2 fusion, and the molecular features of these tumors have not been clarified.
Here, we describe a case of LGPSC which involved the sinonasal region and invaded to the skin and orbital wall. Subsequent molecular analysis revealed TP53 missense mutation, but DEK::AFF2 fusion, EGFR, and KRAS mutation were not identified. Invasive growth by clinical and radiological findings and distinctive morphology with p53 immunopositivity provided diagnostic clues for this rare disease. Recognizing this rare entity is important for the correct pathological diagnosis and appropriate clinical management.
Case presentation
A 69-year-old man presented with swelling of his left cheek and discharge of pus from the skin of his cheek. He also a had persistent nasal obstruction and purulent rhinorrhea. A papillary mass was noted in the left maxillary and ethmoid sinuses on nasal endoscopy (Fig. 1A). The mass protruded to the oral cavity through the hard palate. Computed tomography and magnetic resonance imaging revealed a tumor of the left maxillary sinus extending to the left nasal cavity, ethmoid sinus, and orbital floor, measuring about 90 × 50 mm (Fig. 1B). Biopsy was performed from the maxillary sinus.
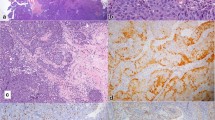
Microscopically, the tumor was composed of an exophytic, papillary growth of multilayered epithelium (Fig. 2A). Some areas showed inverted, anastomosing ribbons with pushing borders. A palisading pattern of columnar cells with reverse polarity was observed in the basal layer (Fig. 2B). The tumor cells were composed of uniformly round and polygonal cells with abundant, eosinophilic cytoplasm and monomorphic round nuclei with fine chromatin and occasional small nucleoli. Discontinuous flattened cells were found in the outermost layer of multilayered epithelium (Fig. 2C). Neutrophilic infiltrates were focally present in both tumor nests and stroma. A focal peculiar acantholytic change was seen. A few scattered mucous cells were found in the middle layer. There was focal ciliated epithelium from residual respiratory mucosa (Fig. 2D), while no dysplasia-carcinoma sequence was observed. There was no overt stromal invasion, lymphovascular invasion, or perineural invasion. Keratinization and necrosis were not found through the lesion. The mitotic rate was relatively low, although increased mitotic activity (up to 38/10 high-power fields) was observed focally.
Microscopic findings. A The tumor was composed of exophytic, papillary growth of multilayered epithelium. Scale bar 1 mm. B A palisading pattern of columnar cells with reverse polarity was observed in the basal layer. C The tumor cells were composed of uniformly round and polygonal cells with abundant, eosinophilic cytoplasm and monomorphic round nuclei with fine chromatin. Discontinuous flattened cells were found in the outermost layer of multilayered epithelium (arrowheads). D Focal ciliated epithelium from residual respiratory mucosa was found (arrows) while no dysplasia-carcinoma sequence was observed
By immunohistochemistry, the tumor was diffusely and strongly positive for CK5/6 (Fig. 3A) and high molecular weight cytokeratin. p63 was mainly positive in the basal layer, and EMA was predominantly expressed in the outermost cell layer (Fig. 3B). CK7 and p16 were negative in tumor cells. p53 was strongly positive in almost all tumor cells (Fig. 3C). The Ki67 labeling index was about 90% (Fig. 3D). Based on the radiological and pathological findings, a diagnosis of LGPSC was rendered.
DEK::AFF2 fusion was analyzed by reverse transcription polymerase chain reaction (RT-PCR) as described previously [8] and confirmed the absence of DEK::AFF2 fusion transcripts (data not shown). Fluorescence in situ hybridization (FISH) using a DEK break-apart FISH probe (CytoTest, Rockville, MD, USA) revealed no DEK::AFF2 fusion (Fig. 4). DNA-based targeted sequencing was performed using Ion AmpliSeq™ Cancer Hotspot Panel v2 (Thermo Fisher Scientific, Waltham, MA), targeting mutation hotspots of 50 cancer-related genes. The sequencing confirmed TP53 R175H mutation (69.1% variant allele frequency, with sequencing depth ~ 1000 ×). There were no hotspot mutations in EGFR, KRAS, and CDKN2A genes.
Because the primary tumor was not surgically resectable, he underwent radiotherapy with 66 Gy in 33 fractions and concomitant intra-arterial chemotherapy. Twelve months after the initiation of treatment, no residual tumor was observed by positron emission tomography.
Discussion and conclusions
LGPSC is a unique type of sinonasal carcinoma that is considerably distinct from conventional sinonasal papilloma and other carcinomas in the sinonasal region and shows a propensity for multiple recurrences, lymph node metastasis, and ultimate mortality [1]. LGPSC is a relatively new entity, and only eighteen cases of LGPSC were clinicopathologically described in the literature, including our case [1,2,3,4,5,6,7,8,9,10]. In the current WHO Classification of Tumours (5th ed.) [13], LGPSC is newly cited in the section of non-keratinizing squamous cell carcinoma of the nasal cavity, paranasal sinuses, and skull base. DEK::AFF2 squamous carcinoma, the newly described subtype of non-keratinizing squamous cell carcinoma in the current WHO classification shows substantial morphologic overlap with tumors reported as LGPSC. Recent data suggest that some LGPSCs also harbor DEK::AFF2 fusions; however, the genetic alterations other than DEK::AFF2 fusions remain unknown. We examined genetic changes in the case of LGPSC using DNA-based targeted sequencing, RT-PCR, and FISH and discovered the new genetic change of TP53 R175H but no DEK::AFF2 fusion.
The clinicopathological and molecular findings of previously reported cases of LGPSC and our case are summarized in Table 1. Briefly, 13 patients were female, 4 were male, and one was unknown, with a median age of 56 years (range 18–82 years). Although nasal obstruction is common, the presenting symptoms of LGPSC are variable and non-specific, depending on the involved sites of the tumor. Some patients experienced facial pain, sensory or auditory neuropathies, loss of swelling, tinnitus, or significant facial swelling [3, 4, 6, 7], indicating the infiltrative nature of this aggressive tumor. As shown here, most tumors were initially diagnosed as benign neoplasms, such as inverted papilloma and oncocytic papilloma. However, the revision of the original samples documented peculiar histopathological features distinct from conventional sinonasal papillomas. Four of the 18 cases developed nodal metastases, one distant metastasis, and two died of progressive disease. Although the Ki67 labeling index was very high in our case, other clinicopathological features were similar to those of other LGPSCs.
Thirteen out of the 18 cases of LGPSC were analyzed genetically and revealed no EGFR and KRAS hotspot mutations, which are known as the driver mutations in inverted and oncocytic sinonasal papillomas, respectively, and their associated carcinomas. This indicates that LGPSC is not associated with benign sinonasal papillomas genetically, despite the morphologic resemblance.
DEK::AFF2 fusion was first reported in the literature in a patient with skull base squamous cell carcinoma who was an exceptional responder to programmed cell death protein 1 inhibitor therapy [14]. Since this case report, about 30 cases of sinonasal carcinoma with DEK::AFF2 fusion were described in the literature using RNA sequencing, FISH, or RT-PCR [8, 9, 15,16,17]. About 40% of DEK::AFF2 carcinoma showed high-grade morphology, whereas the others were low-grade with morphological overlap with LGPSC [8, 17]. Indeed, six out of the 18 patients of LGPSC were tested for DEK::AFF2 fusion and five patients other than our case showed DEK::AFF2 fusion (Cases 1, 9, 10, 15, and 16). More recently, Kuo et al. described that 68.6% (11/16 patients) of sinonasal tumors showing features of LGPSC had DEK::AFF2 fusion; however, they did not show the other genetic alterations in DEK::AFF2-negative LGPSC [17], which indicates that LGPSC is a genetically more heterogeneous entity.
TP53 is one of the most mutated genes in human cancers, including head and neck carcinomas. TP53 is frequently mutated in HPV-negative head and neck squamous cell carcinomas but not in HPV-positive tumors [18]. In the LGPSCs, five cases were examined for TP53 mutation by targeted sequencing, and none of them had TP53 mutations [7]. Moreover, none of the previously reported cases of LGPSC showed strong, diffuse expression or complete absence, suggesting missense and nonsense TP53 mutation, respectively. Therefore, our case is the first case of LGPSC with TP53 mutation.p53 immunohistochemistry is the most sensitive marker for TP53 mutation, and diffuse expression of p53 was observed in our case. In cases without TP53 mutations, LGPSC showed TP53 positivity in 10–50% of tumor cells (Table 1), which is generally higher than those of sinonasal papilloma. Therefore, p53 immunohistochemistry is very useful for the differential diagnosis of sinonasal papillary tumors.
The TP53 R175 hotspot is located at the zinc-binding site near the DNA binding interface, which is essential to maintaining structural stability. The p53-R175H mutation causes global conformational changes leading to indirect disruption of p53-DNA interaction [19]. Moreover, the p53-R175H gains function by binding to some DNA sequences which are different from the wild-type p53 and transactivating its target genes [20], leading to promote cancer cell proliferation, migration, invasion, initiation, metabolic reprogramming, and angiogenesis. However, the function of p53-R173H is highly dependent on the cellular context. Therefore, the reason why TP53 mutation causes the same histomorphology as LGPSC with DEK::AFF2 is unclear. The limitation is that we did not perform whole-genome sequence or RNA-based sequencing, so the possibility of another novel fusion may have been missed in our case.
The differential diagnosis of LGPSC is sinonasal papilloma and carcinoma arising in a sinonasal papilloma. Sinonasal papillomas show respiratory-type columnar-to-squamous gradation with a mixture of immature squamous, ciliated, mucous, and focally more maturing squamous cells. They have no cytological atypia with only rare mitoses and lack destructive or irregular, infiltrative growth [21]. Conventional sinonasal papilloma with associated carcinoma shows prominent architectural abnormalities, moderate to severe cytologic atypia, pleomorphism, high mitotic activity, and/or necrosis [22]. EGFR and KRAS mutations were known as a driver mutation of sinonasal papilloma and carcinoma associated with sinonasal papilloma [12], and TP53 mutations and CKDN2A alterations were reported to be associated with malignant transformation of sinonasal papilloma [23]. In our case, the tumor showed bland cytomorphology, abundant eosinophilic cytoplasm, and the absence of respiratory epithelial cells, keratinization, and glandular differentiation, which were histological diagnostic clues for LGPSC [6]. Although TP53 mutation was identified, no hotspot mutations in EGFR, KRAS, and CDKN2A were identified. There remains a possibility of carcinoma arising in a sinonasal papilloma since only a small biopsy was submitted for histopathological examination.
In summary, we described a case of LGPSC with TP53 mutation but without DEK::AFF2 fusion. Mutation of TP53 may play a crucial role in the pathogenesis of DEK::AFF2-negative LGPSC. LGPSC could easily be underdiagnosed as a sinonasal papilloma at initial presentation due to a deceptively bland morphology without overt stromal invasion, especially in a case of a small biopsy specimen. Given its aggressive nature, an appropriate diagnosis requires a comprehensive assessment of clinical history, radiological imaging, morphology, and ancillary testing for p53, p16/HPV, EGFR/KRAS mutations, and DEK rearrangement.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- LGPSC:
-
Low-grade papillary Schneiderian carcinoma
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- FISH:
-
Fluorescence in situ hybridization
References
Lewis JS Jr, Chernock RD, Haynes W, et al. Low-grade papillary schneiderian carcinoma, a unique and deceptively bland malignant neoplasm: report of a case. Am J Surg Pathol. 2015;39:714–21.
Jeong HJ, Roh J, Lee BJ, et al. Low-Grade Papillary Schneiderian Carcinoma: A Case Report. Head Neck Pathol. 2018;12:131–5.
Brown CS, Abi Hachem R, Pendse A, et al. Low-Grade Papillary Schneiderian Carcinoma of the Sinonasal Cavity and Temporal Bone. Ann Otol Rhinol Laryngol. 2018;127:974–7.
Williamsona A, Sharmaa R, Cooperb L, et al. Low-Grade Papillary Schneiderian Carcinoma; rare or under-recognised? Otolaryngol Case Rep. 2019;11: 100107.
Carnevale S, Ferrario G, Sovardi F, et al. Low-Grade Papillary Schneiderian Carcinoma: Report of a Case with Molecular Characterization. Head Neck Pathol. 2020;14:799–802.
Zhai C, Wang H, Li S, et al. Clinicopathological analysis of low-grade papillary Schneiderian carcinoma: report of five new cases and review of the literature. Histopathology. 2021;79:370–80.
Saab-Chalhoub MW, Guo X, Shi Q, et al. Low Grade Papillary Sinonasal (Schneiderian) Carcinoma: A Series of Five Cases of a Unique Malignant Neoplasm with Comparison to Inverted Papilloma and Conventional Nonkeratinizing Squamous Cell Carcinoma. Head Neck Pathol. 2021;15:1221–34.
Kuo YJ, Lewis JS Jr, Zhai C, et al. DEK-AFF2 fusion-associated papillary squamous cell carcinoma of the sinonasal tract: clinicopathologic characterization of seven cases with deceptively bland morphology. Mod Pathol. 2021;34:1820–30.
Rooper LM, Agaimy A, Dickson BC, et al. DEK-AFF2 Carcinoma of the Sinonasal Region and Skull Base: Detailed Clinicopathologic Characterization of a Distinctive Entity. Am J Surg Pathol. 2021;45:1682–93.
Uraguchi K, Nishida K, Makino T, et al. Nasopharyngeal low-grade papillary schneiderian carcinoma with cervical metastasis. Auris Nasus Larynx. 2022. https://doi.org/10.1016/j.anl.2022.12.010.
Thompson LDR, Franchi A. New tumor entities in the 4th edition of the World Health Organization classification of head and neck tumors: Nasal cavity, paranasal sinuses and skull base. Virchows Arch. 2018;472:315–30.
Maisch S, Mueller SK, Traxdorf M, et al. Sinonasal papillomas: A single centre experience on 137 cases with emphasis on malignant transformation and EGFR/KRAS status in “carcinoma ex papilloma.” Ann Diagn Pathol. 2020;46: 151504.
WHO Classification of Tumours Editorial Board. WHO classification of tumours 5th Edition: Head and Neck Tumours. Lyon: IARC Press; 2022. [Internet; beta version ahead of print]
Yang W, Lee KW, Srivastava RM, et al. Immunogenic neoantigens derived from gene fusions stimulate T cell responses. Nat Med. 2019;25:767–75.
Todorovic E, Truong T, Eskander A, et al. Middle Ear and Temporal Bone Nonkeratinizing Squamous Cell Carcinomas With DEK-AFF2 Fusion: An Emerging Entity. Am J Surg Pathol. 2020;44:1244–50.
Bishop JA, Gagan J, Paterson C, et al. Nonkeratinizing Squamous Cell Carcinoma of the Sinonasal Tract With DEK-AFF2: Further Solidifying an Emerging Entity. Am J Surg Pathol. 2021;45:718–20.
Kuo YJ, Lewis JS Jr, Truong T, et al. Nuclear expression of AFF2 C-terminus is a sensitive and specific ancillary marker for DEK::AFF2 carcinoma of the sinonasal tract. Mod Pathol. 2022;35:1587–95.
Deneka AY, Baca Y, Serebriiskii IG, et al. Association of TP53 and CDKN2A Mutation Profile with Tumor Mutation Burden in Head and Neck Cancer. Clin Cancer Res. 2022;28:1925–37.
Chiang YT, Chien YC, Lin YH, et al. The Function of the Mutant p53–R175H in Cancer. Cancers (Basel). 2021;13:4088.
Quante T, Otto B, Brázdová M, et al. Mutant p53 is a transcriptional co-factor that binds to G-rich regulatory regions of active genes and generates transcriptional plasticity. Cell Cycle. 2012;11:3290–303.
Kaufman MR, Brandwein MS, Lawson W. Sinonasal papillomas: clinicopathologic review of 40 patients with inverted and oncocytic schneiderian papillomas. Laryngoscope. 2002;112:1372–7.
Nudell J, Chiosea S, Thompson LD. Carcinoma ex-Schneiderian papilloma (malignant transformation): a clinicopathologic and immunophenotypic study of 20 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2014;8:269–86.
Brown NA, Plouffe KR, Yilmaz O, et al. TP53 mutations and CDKN2A mutations/deletions are highly recurrent molecular alterations in the malignant progression of sinonasal papillomas. Mod Pathol. 2021;34:1133–42.
Acknowledgements
We thank Naoko Akiyama at the Department of Diagnostic Pathology, Asahikawa Medical University Hospital for her expertise in FISH.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Contributions
All authors were involved in patient management and manuscript conception. SY and MT drafted the manuscript critically. SY, TM, MH, MT, AK, and MT participated in the diagnosis and treatment. RK, YO, and YM contributed to RT-PCR and DNA-based targeted sequencing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Asahikawa Medical University Research Ethics Committee (No. 21055).
Consent for publication
Consent for publication was waived because the submission did not include images that might identify the recipients.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yuzawa, S., Michizuka, T., Kakisaka, R. et al. Low-grade papillary Schneiderian carcinoma with TP53 mutation: a case report and review of the literature. Diagn Pathol 18, 44 (2023). https://doi.org/10.1186/s13000-023-01334-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-023-01334-8