Abstract
Background
Structural and functional changes of the choroid plexus (ChP) have been reported in Alzheimer’s disease (AD). Nonetheless, the role of the ChP in the pathogenesis of AD remains largely unknown. We aim to unravel the relation between ChP functioning and core AD pathogenesis using a unique proteomic approach in mice and humans.
Methods
We used an APP knock-in mouse model, APPNL-G-F, exhibiting amyloid pathology, to study the association between AD brain pathology and protein changes in mouse ChP tissue and CSF using liquid chromatography mass spectrometry. Mouse proteomes were investigated at the age of 7 weeks (n = 5) and 40 weeks (n = 5). Results were compared with previously published human AD CSF proteomic data (n = 496) to identify key proteins and pathways associated with ChP changes in AD.
Results
ChP tissue proteome was dysregulated in APPNL-G-F mice relative to wild-type mice at both 7 and 40 weeks. At both ages, ChP tissue proteomic changes were associated with epithelial cells, mitochondria, protein modification, extracellular matrix and lipids. Nonetheless, some ChP tissue proteomic changes were different across the disease trajectory; pathways related to lysosomal function, endocytosis, protein formation, actin and complement were uniquely dysregulated at 7 weeks, while pathways associated with nervous system, immune system, protein degradation and vascular system were uniquely dysregulated at 40 weeks. CSF proteomics in both mice and humans showed similar ChP-related dysregulated pathways.
Conclusions
Together, our findings support the hypothesis of ChP dysfunction in AD. These ChP changes were related to amyloid pathology. Therefore, the ChP could become a novel promising therapeutic target for AD.
Similar content being viewed by others
Background
Alzheimer’s disease (AD) is characterized by the accumulation of amyloid-beta (Aβ) plaques, followed by the accumulation of neurofibrillary tangles [1,2,3]. Increasing evidence suggests choroid plexus (ChP) dysfunction in AD [4, 5]. The ChP is a highly vascularized structure, located inside all four brain ventricles, and composed of a monolayer of tight-junction-bound epithelial cells on a basement membrane [6,7,8], which expresses amyloid precursor protein (APP). The ChP is involved in the production of CSF, transport of ions, proteins, lipids, nutrients and metabolic precursors across the epithelium to the CSF, and clearance of proteins such as Aβ, toxic substances, and metabolites from the CSF. It is also a gateway for immune cell entry into the brain [4, 6,7,8,9,10,11,12]. However, the involvement of the ChP in AD pathophysiology remains largely unclear.
Morphological and functional changes of the ChP have been reported in both AD patients and mouse models [4, 5]. Morphological changes in AD include flattening and atrophy of epithelial cells and thickening of the basement membrane and the vessel wall [5, 13,14,15,16]. Decreased CSF production and turnover by the ChP have also been reported in AD patients [4, 17], which might lead to impaired CSF Aβ clearance [18,19,20]. Dysregulation of protein synthesis by the ChP is also observed in AD patients, such as increased production of Aβ [4, 21, 22] and decreased production of transthyretin (TTR) [14], which is protective against cortical Aβ toxicity [23]. Several ChP transcriptomic and proteomic studies in AD patients have been performed, which have indicated dysregulated CSF production and barrier integrity [24], alongside changes in metabolic, immune, and lipids-related pathways [25, 26]. CSF proteomic analysis in AD patients has shown post-mortem abnormal inflammatory signals and protein accumulations, associated with significant remodeling of the ChP [27]. A recent in vivo CSF proteomic study identified a subgroup of persons with AD showing mainly ChP dysfunction [28].
Animal models of AD are critical to understanding disease pathogenesis and pathophysiology, and can offer insights into early stages of disease. Several years ago, new AD knock-in (KI) mouse models were generated including the APPNL-G-F model [29, 30]. These AD KI models offer a new opportunity to study AD pathology in vivo as they closely represent the physiological accumulation of Aβ, without the potential risk of artificial phenotypes associated with the transgenic overexpression of the Aβ precursor protein (APP) present in the first-generation AD models [31]. This APPNL-G-F mouse model presents early and severe Aβ pathology, but does not manifest neurofibrillary tangles or neurodegeneration [32], which makes it an excellent model to study the earliest stages of AD. Moreover, proteomics allows the identification and quantification of proteins in tissues or biological fluids and is a core technique to study the pathophysiological mechanisms underlying a disease [33, 34]. Currently, there are no reports available investigating the ChP tissue proteomic profile in an AD mouse model, while this would be relevant for understanding the mechanisms underlying ChP changes in relation to amyloid pathology in early stages of AD.
The primary aim of the current study was to investigate the ChP changes in relation to AD pathogenesis using ChP tissue proteomics in the APPNL-G-F mouse model. Our secondary aim was to examine how proteomic changes in the mouse ChP were mirrored in the CSF and to compare this to human CSF proteomics findings in AD participants with amyloid but without tau pathology (A+T−) or with amyloid and tau pathology (A+T+) across the clinical spectrum.
Methods
Mice
Female APPNL-G-F mice (n = 10), a KI mouse model carrying Arctic, Swedish, and Beyreuther/Iberian mutations [29], and female C57BL/6J mice (wild-type (WT) control; n = 10) were bred in the animal house of the VIB-UGent Center for Inflammation Research and were maintained in ventilated cages, under specific pathogen-free conditions, with ad libitum access to food and water, and a 14-h light/10-h dark cycle. APPNL-G-F and WT mice were sacrificed at 7 or 40 weeks old. The 7 weeks old APPNL-G-F mice represent an early stage of AD; amyloid plaques, microgliosis and astrocytosis start to develop [29]. The 40 weeks old APPNL-G-F mice represent a more advanced stage of AD with amyloid plaques, synaptic loss, microgliosis and astrocytosis [29]. Animal studies were conducted in compliance with governmental and EU guidelines and were approved by the ethical committee of the Faculty of Sciences, Ghent University, Belgium.
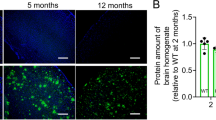
AD pathology in our mouse model was confirmed by immunohistochemistry and 3D image analysis (Additional file 1—Results and Additional Fig. 1A–E; Protocols in Additional file 1—Methods and materials) [29, 31, 35].
Mice CSF and tissue sample isolation
CSF was collected just before sacrifice via the cisterna magna puncture method as described previously [15, 16, 36] and in the Additional file 1—Methods.
To isolate the ChP tissue, mice were transcardially perfused with D-PBS/heparin [0.2% heparin (5.000 IU/ml, Wockhardt)]. Next, both lateral and fourth ventricular ChPs were isolated, snap-frozen in liquid nitrogen and stored at -80 °C until further use [37].
Mass spectrometry
For proteomic analysis, 5 µl of CSF per mouse and pooled lateral and fourth ventricular ChPs were processed using the PreOmics iST Sample preparation kit (PreOmics Gmbh, Germany), as described by the manufacturer. Peptides were re-dissolved in 20 µl loading solvent A [0.1% trifluoroacetic acid in water/acetonitrile (ACN) (98:2, v/v)] of which 2 µl was injected for LC–MS/MS analysis on an Ultimate 3000 RSLCnano system in-line connected to a Q Exactive HF mass spectrometer (Thermo). More details on the mouse proteomic method can be found in the Additional file 1—Methods.
For the ChP tissue and CSF proteomic analysis respectively, 8519 and 1358 proteins were identified. For further analysis, only the proteins that had at least 3 observations per group [38] were included resulting in 7696 proteins for the ChP tissue proteomics and 319 proteins for the CSF proteomic analyses.
Classification of ChP protein expression
We labelled the significantly dysregulated proteins in the mouse ChP tissue and CSF proteomic comparisons as being highly expressed in the ChP using published transcriptomic data providing expression levels of genes transcribed in ChP from adult normal mice under physiological conditions [39]. We defined gene expression levels above the 90th percentile as high expression [40].
Pathway enrichment analysis
Pathway enrichment analyses were performed separately for the decreased and increased significant proteins. QIAGEN Ingenuity Pathway Analysis (IPA) software (QIAGEN Inc., https://digitalinsights.qiagen.com/IPA) [41] was used to find the canonical pathways associated with the significant proteins. Gene Ontology (GO) enrichment analysis was performed using PANTHER (Protein ANalysis THrough Evolutionary Relationships, version 15.0, Los Angeles, CA, USA) [42] in order to identify the biological processes, cellular components and molecular functions related to the significant proteins. The GO enrichment results were validated using ClueGO, a Cytoscape plug-in [43]. All tools use Fisher’s exact test with false discovery rate (FDR; Benjamini–Hochberg procedure [34, 44]) and report only pathways with a FDR corrected p-value < 0.05. To reduce redundancy and facilitate interpretation, we clustered related canonical and GO pathways in broader categories. Further investigation on the functions of specific proteins were also performed using Uniprot [45] and the Human Protein Atlas (proteinatlas.org) [46].
Human CSF proteomics
To compare mouse findings to human CSF protein changes, we examined data from 496 participants (mean age 68.0 (SD 8.4) years, 54% women) from the European Medical Information Framework for Alzheimer’s Disease Multimodal Biomarker Discovery study (EMIF-AD MBD, n = 346 from 7 cohorts) [47], the Washington University Knight Alzheimer Disease Research Center (ADRC, n = 98) study [48] and the Maastricht BioBank Alzheimer Center Limburg cohort (BB-ACL, n = 52) memory clinic study [49]. We included individuals with availability of CSF Aβ42 (A) and phosphorylated tau (p-tau, T) data, and centrally analysed CSF proteomics (3102 proteins identified; tandem mass tag (TMT) technique). Methods are described previously [34, 50, 51] and provided in Additional File 1. Participants were classified as controls if they had normal cognition (NC) with normal A and T (n = 141). We included individuals across the clinical spectrum with AD pathology, defined as abnormal CSF Aβ1-42 (A+), with either abnormal p-tau (T+) or normal p-tau (T−), resulting in the following groups: NC A+T− [n = 65], mild cognitive impairment (MCI) A+T− [n = 40], Dementia A+T− [n = 17], NC A+T+ [n = 55], MCI A+T+ [n = 114], Dementia A+T+ [n = 64] (more details on participant classification are provided in the Additional File 1—Methods). We tested whether the significant proteins in the human proteomic comparisons were enriched for expression in the ChP using the online database Allen Brain Atlas [52] through Harmonizome [53]. Additionally, we performed expression enrichment analysis using the R package ABAEnrichment [34, 54].
Statistical analysis
For the mouse study, ChP tissue and CSF protein levels were normalized according to the mean and standard deviation of the respective WT group and compared between groups using ANOVA.
For the human study, CSF protein levels were normalized according to the mean and standard deviation of the control group and compared between groups using ANCOVA corrected for age and sex. In addition, we used linear regression to study associations between human CSF Aβ42 levels (predictors) and CSF levels of proteins associated with the ChP (outcome measures). To this end, Z-scores of local CSF Aβ42 levels were calculated for each centre.
Statistical analyses were performed using R 3.6.2, GraphPad Prism 8.0 and IBM SPSS Statistics version 26.
Results
Choroid plexus tissue proteomic profile of the APP NL-G-F mouse model
To investigate how the ChP changes in relation to AD pathogenesis, we conducted ChP tissue proteomic analysis in the APPNL-G-F mouse model at two distinct ages, i.e., 7 weeks and 40 weeks old.
ChP tissue proteome analysis of 7 weeks old APPNL-G-F mice showed 184 decreased proteins and 119 increased in the ChP compared to the 7 weeks old WT mice (Fig. 1A, Additional Table 2). The decreased proteins were associated with pathways linked with lipids, mitochondria and the energy metabolism, epithelial cells, immune system (complement), metabolism, lysosomes, and protein transport (Fig. 1B, C). Of the 184 decreased proteins, 25 proteins had a high expression in the ChP based on published transcriptomic data [39] (Fig. 1A). The increased proteins were related to pathways associated with endocytosis, actin, protein formation and modification, extracellular matrix (ECM), and epithelial cells (Fig. 1D, E). Of the 119 increased proteins, 14 proteins had a high expression in the ChP (Fig. 1A). The top 10 proteins with the lowest p-values and their main functions can be found in Table 1.
Choroid plexus (ChP) proteomics in APPNL-G-F versus wild-type (WT) mice. A Volcano plot displaying the log2 fold-change against the -log10 statistical P-value for the 7 weeks old AppNL-G-F compared to their respective WT. Significantly different proteins are red. Significantly different proteins highly expressed by the ChP are green. The top 10 proteins are named. B Selected canonical pathways from Ingenuity pathway analysis (IPA) for the decreased proteins in the 7 weeks old APPNL-G-F compared to their respective WT. C Selected Gene Ontology (GO) terms including biological process for the decreased proteins in the 7 weeks old APPNL-G-F compared to their respective WT. D Selected canonical pathways from IPA for the increased proteins in the 7 weeks old APPNL-G-F compared to their respective WT. E Selected GO terms including biological process for the increased proteins in the 7 weeks old APPNL-G-F compared to their respective WT. F Volcano plot displaying the log2 fold-change against the −log10 statistical P-value for the 40 weeks old APPNL-G-F compared to their respective WT. Significantly different proteins are red. Significantly different proteins highly expressed by the ChP are green. The top 10 proteins are named. G Selected canonical pathways from Ingenuity pathway analysis (IPA) for the decreased proteins in the 40 weeks old APPNL-G-F compared to their respective WT. H Selected Gene Ontology (GO) terms including biological process for the decreased proteins in the 40 weeks old APPNL-G-F compared to their respective WT. I Selected canonical pathways from IPA for the increased proteins in the 40 weeks old APPNL-G-F compared to their respective WT. J Selected GO terms including biological process for the increased proteins in the 40 weeks old APPNL-G-F compared to their respective WT. Pathways linked with lipids are yellow, pathways related to mitochondria and energy metabolism are light green, epithelial cells-linked pathways are light purple, immune system-related pathways are pink, metabolism/signaling-linked pathways are grey, lysosome-related pathways are dark purple, protein-linked pathways are brown, pathways linked with nervous system are blue, vascular-related pathways are red, ECM-related pathways are dark green, endocytosis-related pathways are turquoise and actin-related pathways are orange
ChP tissue proteome analysis of 40 weeks old APPNL-G-F mice showed 130 decreased and 107 increased proteins compared to their respective controls (40 weeks old WT mice; Fig. 1F, Additional Table 2). The decreased proteins were associated with pathways linked with nervous system, immune system (interleukins and chemokines), lipids, vascular system and endothelial cells, ECM, as well as signalling (Fig. 1G, H). Of the 130 decreased proteins, 17 proteins had a high expression in the ChP (Fig. 1F). The increased proteins were related to pathways associated with lipids, mitochondria and the energy metabolism, protein modification and degradation, immune system (neutrophils), epithelial cells, and metabolism (Fig. 1I, J). Twenty-two increased proteins were highly expressed in the ChP (Fig. 1F). The top 10 proteins with the lowest p-values and their main functions can be found in Table 2.
Next, we identified age-dependent proteomic changes at the ChP by comparing the proteomic results of the 7 weeks old APPNL-G-F mice to the ones of the 40 weeks old APPNL-G-F mice. Pathways linked with epithelial cells, mitochondria, protein modification, ECM and lipids were dysregulated at both ages (Fig. 1B–E, G–J). However, only ~ 5% of the dysregulated proteins overlapped in both 7 and 40 weeks old comparisons (Additional Table 2). More specifically, pathways associated with lysosomes, endocytosis, protein formation, actin and complement were uniquely dysregulated in the 7 weeks old APPNL-G-F mice, while pathways associated with the nervous system, immune system (neutrophils, interleukins, chemokines), protein degradation and vascular system were uniquely dysregulated in the 40 weeks old APPNL-G-F mice.
Comparison of ChP tissue with CSF proteomic profiles of the APP NL-G-F mouse model
Next, we performed CSF proteomic analysis to test whether pathological changes at the ChP are mirrored in the CSF of the APPNL−G−F mouse model (Fig. 2). Results of the mouse CSF proteomic analysis are described in Additional file 1—Results, Fig. 2 and Additional Table 3.
Cerebrospinal fluid (CSF) proteomic profiles of in APPNL-G-F versus wild-type (WT) mice. A Volcano plot displaying the log2 fold-change against the −log10 statistical P-value for the 7 weeks old APPNL-G-F compared to their respective WT. Significantly different proteins are red. Significantly different proteins highly expressed by the ChP are green. The top 10 proteins are named. B Selected canonical pathways from Ingenuity pathway analysis (IPA) for the decreased proteins in the 7 weeks old APPNL-G-F compared to their respective WT. C Selected Gene Ontology (GO) terms including biological process for the decreased proteins in the 7 weeks old APPNL-G-F compared to their respective WT. D Selected canonical pathways from IPA for the increased proteins in the 7 weeks old APPNL-G-F compared to their respective WT. E Selected GO terms including biological process for the increased proteins in the 7 weeks old APPNL-G-F compared to their respective WT. F Volcano plot displaying the log2 fold-change against the −log10 statistical P-value for the 40 weeks old APPNL-G-F compared to their respective WT. Significantly different proteins are red. Significantly different proteins highly expressed by the ChP are green. The top 10 proteins are named. G Selected canonical pathways from Ingenuity pathway analysis (IPA) for the decreased proteins in the 40 weeks old APPNL-G-F compared to their respective WT. H Selected Gene Ontology (GO) terms including biological process for the decreased proteins in the 40 weeks old APPNL-G-F compared to their respective WT. I Selected canonical pathways from IPA for the increased proteins in the 40 weeks old APPNL-G-F compared to their respective WT. J Selected GO terms including biological process for the increased proteins in the 40 weeks old APPNL-G-F compared to their respective WT. Vascular-related pathways are red, actin-related pathways are orange, ECM-related pathways are dark green, immune system-related pathways are pink, pathways associated with oxidative stress are light blue, pathways linked with lipids are yellow, protein-linked pathways are brown, endocytosis/phagocytosis-related pathways are turquoise and lysosome-related pathways are dark purple
We investigated the overlap between ChP tissue and CSF dysregulated proteins for the 7 and 40 weeks old mice comparisons with controls. Two hundred eighty-nine proteins were identified in both ChP tissue and CSF (8% showed correlation between ChP and CSF). However, there was no overlap in dysregulated proteins in ChP tissue and CSF at both ages. When we compared processes associated with the dysregulated proteins in ChP tissue and CSF, the 7 weeks old APPNL-G-F mouse showed overlap in dysregulated pathways associated with ECM, lysosomes, protein processing, actin, lipids and complement, while the 40 weeks old APPNL-G-F mouse showed only overlap in dysregulated pathways linked to ECM and the vascular system.
Comparison of mouse ChP and CSF proteomic results with human CSF proteomics
To further understand how ChP-related changes in the proteomes of the APPNL-G-F mouse model reflect those observed in human patients, we next compared the mouse proteomic results (both CSF and ChP tissue) to the CSF proteomic results in humans with AD. In A+T− and A+T+ individuals with NC, MCI or AD dementia, we first selected CSF proteins that differed between AD patients and controls. Next, we tested which of the proteins had a high expression in the ChP according to the Human Brain Atlas, to define the proteins involved in ChP functioning (Additional Table 4). Among AD CSF proteins with an increased concentration relative to controls, a significant number of proteins were highly expressed by the ChP in NC A+T− (56%, ABAenrichment p ≤ 0.001, Fig. 3A) and MCI A+T− (38%, ABAenrichment p = 0.017, Fig. 3F), but not in AD dementia (33%, ABAenrichment p = 0.817, Additional Fig. 2A). The decreased proteins were not enriched for expression in the ChP. The ChP-enriched dysregulated proteins in persons with A+T− were different along the clinical spectrum (Additional Fig. 3, Additional Table 4). Nonetheless, in NC and MCI A+T−, the increased proteins highly expressed by the ChP were associated with lysosomes, vascular system, ECM, oxidative stress and protein processing or degradation (Fig. 3D-E and I-J). In individuals with A+T+, we did not find significant enrichment for expression in the ChP (15 to 35% of significant proteins highly expressed by the ChP, Additional Fig. 4A–C). Further analysis therefore focused on the A+T− groups. More details on the results of the human CSF proteomics analysis can be found in Additional file 1—Results.
Cerebrospinal fluid (CSF) proteomic profiles and associated ChP pathways in A+T− individuals with normal cognition (NC) and mild cognitive impairment (MCI). (A) Volcano plot displaying the log2 fold-change against the −log10 statistical P-value for the comparison NC A+T− vs controls. Significantly different proteins are red. Significantly different proteins highly expressed by the ChP are green. The top 10 proteins highly expressed by the ChP are named. The number of proteins highly expressed by the ChP, as well as the gene expression enrichment in the ChP (ABAenrichment) p-value, are displayed. B Selected canonical pathways from Ingenuity pathway analysis (IPA) for the decreased proteins highly expressed by the ChP in the comparison NC A+T− vs controls. C Selected Gene Ontology (GO) terms including biological process for the decreased proteins highly expressed by the ChP in the comparison NC A+T− vs controls. D Selected canonical pathways from IPA for the increased proteins highly expressed by the ChP in the comparison NC A+T− vs controls. E Selected GO terms including biological process for the increased proteins highly expressed by the ChP in the comparison NC A+T− vs controls. F Volcano plot displaying the log2 fold-change against the −log10 statistical P-value for the comparison MCI A+T− vs controls. Significantly different proteins are red. Significantly different proteins highly expressed by the ChP are green. The top 10 proteins highly expressed by the ChP are named. The number of proteins highly expressed by the ChP, as well as the gene expression enrichment in the ChP (ABAenrichment) p-value, are displayed. G- Selected canonical pathways from Ingenuity pathway analysis (IPA) for the decreased proteins highly expressed by the ChP in the comparison MCI A+T− vs controls. H Selected Gene Ontology (GO) terms including biological process for the decreased proteins highly expressed by the ChP the comparison MCI A+T− vs controls. I Selected canonical pathways from IPA for the increased proteins highly expressed by the ChP in the comparison MCI A+T− vs controls. J Selected GO terms including biological process for the increased proteins highly expressed by the ChP in the comparison MCI A+T− vs controls. Immune-related pathways are pink, vascular-related pathways are red, pathways associated with lysosomes are dark purple, pathways associated with oxidative stress are light blue, pathways related to ECM are dark green, pathways linked with lipids are yellow, pathways related to energy metabolism and mitochondria are light green and protein-linked pathways are brown
Selected Ingenuity pathway analysis (IPA) canonical pathways or Gene Ontology (GO) biological/cellular processes enriched for proteins in the different comparisons of the paper with decreased (blue) or increased (red) concentrations relative to controls. The comparisons include the choroid plexus (ChP) proteomic analysis of 7 weeks old APPNL-G-F mice versus\wild-type (WT), the ChP proteomic analysis of the 40 weeks old APPNL-G-F mice versus their relative WT, the cerebrospinal fluid (CSF) proteomic analysis of the 7 weeks APPNL-G-F mice versus their relative WT, the CSF proteomic analysis of the 40 weeks old APPNL-G-F mice versus their relative WT, the CSF proteomic analysis of human with normal cognition (NC) and abnormal amyloid-β 42 (A) levels and normal phosphorylated tau (T) levels (A+T−) versus controls, the CSF proteomic analysis of individuals with mild cognitive impairment (MCI) A+T− versus controls and the CSF proteomic analysis of Alzheimer’s dementia (AD) A+T− versus controls. P-values are presented and scaled based on the scale in the right of the graphs. ECM-related pathways are dark green, lysosome-related pathways are dark purple, protein-linked pathways are brown, pathways linked with lipids are yellow, immune system-related pathways are pink, vascular-related pathways are red and pathways related to mitochondria and energy metabolism are light green
ChP changes in AD in both mouse (ChP tissue and CSF) and human (CSF) proteomes
Figure 4 presents an overview of the overlap in dysregulated ChP-associated pathways identified in AD mouse CSF, mouse ChP tissue and human CSF proteomes. These analyses showed ChP involvement in AD with protein changes related to the ECM, lysosomes, protein processing, lipids, complement, vascular system and mitochondria.
To investigate the similarity of CSF protein changes associated with ChP functioning in AD across species, we compared human and mouse CSF proteomics. There were 215 proteins commonly identified in both datasets. Seventeen CSF proteins were dysregulated in both mice and humans and relevant for ChP functioning (Fig. 5; 5 proteins decreased, 3 proteins increased, and 9 proteins in opposite direction), which were associated with lysosomes, ECM, immune system (complement, T cells, B cells, immunoglobulins, cytokines), cell adhesion, lipids, actin and microtubule and hemostasis (Table 3). Out of those 17 proteins, 5 were highly expressed by the ChP (Fig. 5, Table 3).
Choroid plexus (ChP)-related proteins dysregulated in both mice (ChP tissue or CSF) and humans (CSF). The comparisons include the ChP tissue proteomic analysis of the 7 weeks old APPNL-G-F mice versus their relative wild-type (WT), the ChP proteomic analysis of the 40 weeks old APPNL-G-F mice versus their relative WT, the cerebrospinal fluid (CSF) proteomic analysis of the 7 weeks old APPNL-G-F mice versus their relative WT, the CSF proteomic analysis of the 40 weeks old APPNL-G-F mice versus their relative WT, the CSF proteomic analysis of human with normal cognition (NC) and abnormal amyloid-β 42 (A) levels and normal phosphorylated tau (T) levels (A+T−) versus controls, the CSF proteomic analysis of individuals with mild cognitive impairment (MCI) A+T− versus controls and the CSF proteomic analysis of Alzheimer’s dementia (AD) A+T− versus controls. P-values are presented and scaled based on the dot scale in the right of the graphs. A blue dot means decreased concentrations relative to controls and a red dot means increased concentrations relative to controls. AD Alzheimer’s dementia, Adam22 ADAM metallopeptidase domain 22, C3 Complement C3, Cacna2d1 Calcium voltage-gated channel auxiliary subunit alpha2delta 1, Cadm4 Cell adhesion molecule 4, Cluh clustered mitochondria protein homolog, CSF cerebrospinal fluid, ChP choroid plexus, Ctsd cathepsin D, Dcn decorin, Enpp2 Autotaxin, Gm2a Ganglioside GM2 activator, Gsn Gelsolin, Icoslg Inducible T cell costimulator ligand, Igkc Immunoglobulin kappa constant, Krt10 Keratin 10, Ldhb Lactate dehydrogenase B, Man1b1 Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase protein, Marcks Myristoylated alanine rich protein kinase C substrate, MCI Mild cognitive impairment, NC Normal cognition, Ntm Neurotrimin, Opcml Opioid binding protein/cell adhesion molecule like, Plod1 procollagen-lysine,2-oxoglutarate 5-dioxygenase 1 protein, Ptprg Receptor-type tyrosine-protein phosphatase gamma, Ptprn2 Protein tyrosine phosphatase receptor type N2, Sema7a Semaphorin 7A, Serpinf2 Serpin family F member 2, Serpini1 Serpin family I member 1, Sirpa Signal regulatory protein alpha, Ube2v1 Ubiquitin conjugating enzyme E2 V1, Vgf Vgf nerve growth factor inducible
Next, to understand to what extent proteomic changes in AD mice ChP tissue are present in AD human CSF, we compared human CSF proteomics to mice ChP tissue proteomics. This also allowed us to identify relevant ChP related proteins in humans beyond those highly expressed by the ChP. There were 691 proteins commonly identified in both datasets. Eleven proteins were dysregulated in both mice and humans (Fig. 5; 4 proteins decreased and 7 proteins in opposite direction), which were associated with mitochondria and energy metabolism, nervous system, complement, ECM, protein formation, folding and modification, cell–cell and cell–matrix interactions and actin (Table 3). Six proteins were highly expressed by the ChP (Fig. 5, Table 3).
Together, we identified 28 proteins associated with ChP functioning and dysregulated in both AD mouse and human proteomes (17 in mouse versus human CSF proteomes; 11 in mouse ChP tissue versus human CSF proteomes; see above). Next, we investigated the association between the levels of those 28 proteins and CSF Aβ42 in the overall human dataset (Table 3). Globally, reduced levels of 17 proteins were associated with lower, thus more abnormal, Aβ42. Those proteins were associated with the nervous system, energy metabolism, protein formation, folding and modification, lipids, cell–cell adhesion and immune system (complement, T cells). We further observed, for 7 proteins, that increased levels were associated with more abnormal Aβ42 levels. Those proteins were linked to the lysosomes, ECM and collagen, mitochondria, immunoglobulins and cytoskeleton. Four proteins were not associated with Aβ42 levels.
Discussion
We aimed to investigate the changes of the ChP in relation to the pathogenesis of AD using ChP tissue proteomics in APPNL-G-F mice, and compared this to CSF proteomic profiles in both AD mice and humans. In ChP tissue of mice at both 7 and 40 weeks old, pathways linked with epithelial cells, mitochondria, protein modification, extracellular matrix and lipids were dysregulated, while pathways associated with lysosome, endocytosis, protein formation, actin and complement were mainly seen at 7 weeks, and pathways associated with nervous system, interleukins and neutrophils, protein degradation and vascular system were mainly found at 40 weeks. Similar results were observed in the CSF of APPNL-G-F mice, as well as of human AD patients with amyloid but without tau pathology. Our findings highlight ChP dysfunction in relation to amyloid pathology, which is relevant for AD treatment strategies.
A high number of dysregulated proteins were found in the ChP tissue of the APPNL-G-F AD mouse model, already at early disease stages (7 weeks old). The ChP protein changes were linked to multiple dysregulated pathways of which several showed consistency across ages, while some differed across ages. Findings are consistent with a previous ChP transcriptomic study in another AD mouse model (J20), in which they found a significant number of dysregulated genes already at an early AD stage (3 months), with differences across ages [55]. This suggests a dynamic and complex process underlying ChP dysfunction in AD.
The dysregulated pathways observed in AD and linked with epithelial cells, vascular system, ECM, lysosome, mitochondria and protein processing can be associated with changes in the morphology of the ChP. Flattening and atrophy of ChP epithelial cells, as well as a decline of epithelial tight junctions, in mice and humans with AD have been reported previously, and might be linked with increased Aβ deposits [5, 13, 14, 56]. Changes in the ChP basement membrane, a thin layer of ECM, in AD has also been previously reported, with increased thickness (due to an accumulation of collagen) and irregularity, which reduce the permeability, plasma ultrafiltration, ChP epithelial oxygenation and CSF formation [4, 14, 57]. A high number of vesicles with lysosomal characteristics are present in the ChP cytoplasm [58]. Multiple human and mouse AD studies have reported impairment of autophagy–lysosomal pathway, which is partly responsible for the accumulation of Aβ [59,60,61,62]. A high density of mitochondria, Golgi apparatus and a smooth endoplasmic reticulum can be found in the ChP epithelial cells [58]. In AD, Golgi defects and endoplasmic reticulum stress have been reported, leading to a dysfunction of folding, trafficking, processing, and sorting of proteins [63, 64], while a defect in mitochondrial enzyme activity of the ChP epithelial cells can result in decreased transport across the epithelial cells and thus has implications in Aβ clearance in the ChP of AD patients [65, 66]. On the other hand, Aβ itself can also impair mitochondrial function in the ChP [67].
The dysregulated pathways related to lipids and immune system observed in AD can be associated with functional dysfunction of the ChP. The ChP plays a crucial role in the transport of lipids from the blood to the CSF [68] and acts as a reservoir for multiple types of immune cells [10]. Previous studies on AD patients reported the presence of complement components as well as activation of the complement cascade in the ChP [69, 70].
While the protein changes in tissue were similar to those in CSF on a pathway level, at the protein level, ChP tissue changes were not directly reflected in the CSF in our AD mouse model. This could be linked with the ChP epithelial cell and tight junction alterations that we found in our ChP tissue proteomics analysis, which may indicate changes in blood-CSF barrier permeability [56, 71]. Furthermore, a previous mouse study showed that intracerebroventricular injection of Aβ1-42 oligomers rapidly affected ChP epithelial cells and tight junctions, which were associated with an increase in blood-CSF barrier leakage [15]. Alternatively, as CSF has been isolated in sedated mice while tissue has been extracted after death, this could have resulted in differences in changes in proteins in CSF and ChP tissue. Future studies are needed to further explore the AD-related changes in ChP permeability in relation to changes in epithelial cells, epithelial tight junctions and epithelial transport proteins. Similarly, a small overlap was observed at the protein level between mice and humans for significant CSF proteins associated with ChP functioning.
We found a correlation with CSF amyloid levels for most proteins that were associated with ChP changes in both mouse and human. In line with previous publications, this supports a causal relationship between ChP protein changes and amyloid pathology in AD [4, 17,18,19,20,21,22]. Several studies in both AD patients and AD mouse models have reported Aβ deposits in the ChP epithelial cells and stroma surrounding capillaries in AD [5, 14, 67, 72], which could lead to morphological and functional alterations of ChP [15, 67, 73].
Our study has several strengths and limitations. To the best of our knowledge, this is the first study reporting ChP tissue proteomic analysis in an AD mouse model. We used APPNL-G-F knock-in mice, which is an AD model exhibiting amyloid pathology without the typical APP overexpression artefacts. Another main strength of this study is our translational approach. We compared ChP tissue proteomics in AD mice to CSF proteomics in AD mice and humans to gain novel insights into the role of the ChP in AD pathogenesis. Yet, for comparisons between these findings, this resulted in a smaller set of overlapping proteins that could be studied. This could have led to missing key pathways and proteins associated with the ChP implication in AD. Moreover, we made use of a unique large dataset for the human CSF proteomic analyses which covered the whole clinical spectrum. Yet, for the human dataset, we used ChP expression to define the proteins involved in the functioning of the ChP. While this is the best proxy at hand, this may have resulted in less identified proteins that play a role in the ChP. Future research should validate our findings using post-mortem human ChP samples, from individuals with various extents of AD pathology. Moreover, the exclusive use of female mice may potentially limit the generalizability of our findings to both sexes. Previous publications showed earlier AD pathology onset in female mice compared to male mice, with more profound amyloidosis and a higher percentage of astrocytes in the cortex and hippocampus of 18-month old female APPNL-G-F mice compared to male mice [74]. It could also be that early ChP changes are more pronounced in female mice.
Conclusions
Together, our findings support the hypothesis of dysregulated ChP functioning in AD. These ChP changes were already present at early stages of AD, were related to amyloid pathology, and were related to similar key pathways across the disease trajectory for mice and the clinical trajectory of humans. Key pathways related to the ChP dysfunction in AD are associated with ECM, lysosomes, lipids, protein processing, complement, vascular system and mitochondria. Our results further contribute towards better pathophysiological characterization of the involvement of the ChP in AD. It has implications for drug development, as ChP changes were already present at early stages of AD and associated with amyloid pathology. Addressing fundamental mechanisms linked to ChP functioning, such as ECM-related pathways, lysosomal pathways, or vascular pathways, may hold therapeutic promise.
Availability of data and materials
The mouse mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD052590. The data underlying this article will be shared on reasonable request to the corresponding author. The EMIF-AD MBD mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifiers PXD019910 and https://doi.org/10.6019/PXD019910.
Abbreviations
- A:
-
Amyloid
- Aβ:
-
Amyloid-beta
- AD:
-
Alzheimer’s disease
- ADRC:
-
Alzheimer Disease Research Center
- BB-ACL:
-
BioBank Alzheimer Center Limburg
- ChP:
-
Choroid plexus
- CSF:
-
Cerebrospinal fluid
- ECM:
-
Extracellular matrix
- EMIF-AD MBD:
-
European Medical Information Framework for Alzheimer’s Disease Multimodal Biomarker Discovery
- FDR:
-
False discovery rate
- GO:
-
Gene Ontology
- IPA:
-
Ingenuity pathway analysis
- KI:
-
Knock-in
- MCI:
-
Mild cognitive impairment
- NC:
-
Normal cognition
- P-tau:
-
Phosphorylated tau
- T:
-
Tau
- TMT:
-
Tandem mass tag
- TTR:
-
Transthyretin
- WT:
-
Wild-type
References
Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, Benzinger TL, et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci Transl Med. 2014;6(226):226–30.
Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795–804.
Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8(6):595–608.
Giao T, Teixeira T, Almeida MR, Cardoso I. Choroid plexus in Alzheimer’s disease—the current state of knowledge. Biomedicines. 2022;10(2):224.
Balusu S, Brkic M, Libert C, Vandenbroucke RE. The choroid plexus-cerebrospinal fluid interface in Alzheimer’s disease: more than just a barrier. Neural Regen Res. 2016;11(4):534–7.
Ghersi-Egea JF, Strazielle N, Catala M, Silva-Vargas V, Doetsch F, Engelhardt B. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 2018;135(3):337–61.
Damkier HH, Brown PD, Praetorius J. Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev. 2013;93(4):1847–92.
Redzic ZB, Segal MB. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv Drug Deliv Rev. 2004;56(12):1695–716.
Praetorius J, Damkier HH. Transport across the choroid plexus epithelium. Am J Physiol Cell Physiol. 2017;312(6):C673–86.
Kaur C, Rathnasamy G, Ling EA. The choroid plexus in healthy and diseased brain. J Neuropathol Exp Neurol. 2016;75(3):198–213.
Alvira-Botero X, Carro EM. Clearance of amyloid-beta peptide across the choroid plexus in Alzheimer’s disease. Curr Aging Sci. 2010;3(3):219–29.
Chebli J, Rahmati M, Lashley T, Edeman B, Oldfors A, Zetterberg H, et al. The localization of amyloid precursor protein to ependymal cilia in vertebrates and its role in ciliogenesis and brain development in zebrafish. Sci Rep. 2021;11(1):19115.
Serot JM, Bene MC, Foliguet B, Faure GC. Morphological alterations of the choroid plexus in late-onset Alzheimer’s disease. Acta Neuropathol. 2000;99(2):105–8.
Gonzalez-Marrero I, Gimenez-Llort L, Johanson CE, Carmona-Calero EM, Castaneyra-Ruiz L, Brito-Armas JM, et al. Choroid plexus dysfunction impairs beta-amyloid clearance in a triple transgenic mouse model of Alzheimer’s disease. Front Cell Neurosci. 2015;9:17.
Brkic M, Balusu S, Van Wonterghem E, Gorle N, Benilova I, Kremer A, et al. Amyloid beta oligomers disrupt blood-CSF barrier integrity by activating matrix metalloproteinases. J Neurosci. 2015;35(37):12766–78.
Steeland S, Gorle N, Vandendriessche C, Balusu S, Brkic M, Van Cauwenberghe C, et al. Counteracting the effects of TNF receptor-1 has therapeutic potential in Alzheimer’s disease. EMBO Mol Med. 2018;10(4):e8300.
Silverberg GD, Heit G, Huhn S, Jaffe RA, Chang SD, Bronte-Stewart H, et al. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology. 2001;57(10):1763–6.
Serot JM, Peltier J, Fichten A, Ledeme N, Bourgeois AM, Jouanny P, et al. Reduced CSF turnover and decreased ventricular Abeta42 levels are related. BMC Neurosci. 2011;12:42.
Serot JM, Zmudka J, Jouanny P. A possible role for CSF turnover and choroid plexus in the pathogenesis of late onset Alzheimer’s disease. J Alzheimers Dis. 2012;30(1):17–26.
Wostyn P, Audenaert K, De Deyn PP. Choroidal proteins involved in cerebrospinal fluid production may be potential drug targets for Alzheimer’s disease therapy. Perspect Medicin Chem. 2011;5:11–7.
Kalaria RN, Premkumar DR, Pax AB, Cohen DL, Lieberburg I. Production and increased detection of amyloid beta protein and amyloidogenic fragments in brain microvessels, meningeal vessels and choroid plexus in Alzheimer’s disease. Brain Res Mol Brain Res. 1996;35(1–2):58–68.
Premkumar DR, Kalaria RN. Altered expression of amyloid beta precursor mRNAs in cerebral vessels, meninges, and choroid plexus in Alzheimer’s disease. Ann N Y Acad Sci. 1996;777:288–92.
Sousa JC, Cardoso I, Marques F, Saraiva MJ, Palha JA. Transthyretin and Alzheimer’s disease: where in the brain? Neurobiol Aging. 2007;28(5):713–8.
Kant S, Stopa EG, Johanson CE, Baird A, Silverberg GD. Choroid plexus genes for CSF production and brain homeostasis are altered in Alzheimer’s disease. Fluids Barriers CNS. 2018;15(1):34.
Stopa EG, Tanis KQ, Miller MC, Nikonova EV, Podtelezhnikov AA, Finney EM, et al. Comparative transcriptomics of choroid plexus in Alzheimer’s disease, frontotemporal dementia and Huntington’s disease: implications for CSF homeostasis. Fluids Barriers CNS. 2018;15(1):18.
Leitner DF, Kanshin E, Faustin A, Thierry M, Friedman D, Devore S, et al. Localized proteomic differences in the choroid plexus of Alzheimer’s disease and epilepsy patients. Front Neurol. 2023;14:1221775.
Carna M, Onyango IG, Katina S, Holub D, Novotny JS, Nezvedova M, et al. Pathogenesis of Alzheimer’s disease: involvement of the choroid plexus. Alzheimers Dement. 2023;19(8):3537–54.
Tijms BM, Vromen EM, Mjaavatten O, Holstege H, Reus LM, van der Lee S, et al. Cerebrospinal fluid proteomics in patients with Alzheimer’s disease reveals five molecular subtypes with distinct genetic risk profiles. Nat Aging. 2024. https://doi.org/10.1038/s43587-023-00550-7.
Saito T, Matsuba Y, Mihira N, Takano J, Nilsson P, Itohara S, et al. Single App knock-in mouse models of Alzheimer’s disease. Nat Neurosci. 2014;17(5):661–3.
Nilsson P, Saito T, Saido TC. New mouse model of Alzheimer’s. ACS Chem Neurosci. 2014;5(7):499–502.
Xie J, Gorle N, Vandendriessche C, Van Imschoot G, Van Wonterghem E, Van Cauwenberghe C, et al. Low-grade peripheral inflammation affects brain pathology in the App(NL-G-F)mouse model of Alzheimer’s disease. Acta Neuropathol Commun. 2021;9(1):163.
Sasaguri H, Nilsson P, Hashimoto S, Nagata K, Saito T, De Strooper B, et al. APP mouse models for Alzheimer’s disease preclinical studies. EMBO J. 2017;36(17):2473–87.
Noor Z, Ahn SB, Baker MS, Ranganathan S, Mohamedali A. Mass spectrometry-based protein identification in proteomics-a review. Brief Bioinform. 2021;22(2):1620–38.
Delvenne A, Gobom J, Tijms B, Bos I, Reus LM, Dobricic V, et al. Cerebrospinal fluid proteomic profiling of individuals with mild cognitive impairment and suspected non-Alzheimer’s disease pathophysiology. Alzheimers Dement. 2022;19:807–20.
Mehla J, Lacoursiere SG, Lapointe V, McNaughton BL, Sutherland RJ, McDonald RJ, et al. Age-dependent behavioral and biochemical characterization of single APP knock-in mouse (APP(NL-G-F/NL-G-F)) model of Alzheimer’s disease. Neurobiol Aging. 2019;75:25–37.
Liu L, Duff K. A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse. J Vis Exp. 2008. https://doi.org/10.3791/960.
Van Wonterghem E, Van Hoecke L, Van Imschoot G, Verhaege D, Burgelman M, Vandenbroucke RE. Microdissection and whole mount scanning electron microscopy visualization of mouse choroid plexus. J Vis Exp. 2022;190: e64733.
Boeddrich A, Haenig C, Neuendorf N, Blanc E, Ivanov A, Kirchner M, et al. A proteomics analysis of 5xFAD mouse brain regions reveals the lysosome-associated protein Arl8b as a candidate biomarker for Alzheimer’s disease. Genome Med. 2023;15(1):50.
Marques F, Sousa JC, Coppola G, Gao F, Puga R, Brentani H, et al. Transcriptome signature of the adult mouse choroid plexus. Fluids Barriers CNS. 2011;8(1):10.
Booij JC, van Soest S, Swagemakers SM, Essing AH, Verkerk AJ, van der Spek PJ, et al. Functional annotation of the human retinal pigment epithelium transcriptome. BMC Genomics. 2009;10:164.
Kramer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2014;30(4):523–30.
Mi H, Muruganujan A, Huang X, Ebert D, Mills C, Guo X, et al. Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat Protoc. 2019;14(3):703–21.
Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25(8):1091–3.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B (Methodol). 1995;57(1):289–300.
UniProt C. UniProt: the universal protein knowledgebase in 2023. Nucleic Acids Res. 2023;51(D1):D523–31.
Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419.
Bos I, Vos S, Vandenberghe R, Scheltens P, Engelborghs S, Frisoni G, et al. The EMIF-AD multimodal biomarker discovery study: design, methods and cohort characteristics. Alzheimers Res Ther. 2018;10(1):64.
Morris JC, Schindler SE, McCue LM, Moulder KL, Benzinger TLS, Cruchaga C, et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 2019;76(3):264–73.
Bos I, Verhey FR, Ramakers I, Jacobs HIL, Soininen H, Freund-Levi Y, et al. Cerebrovascular and amyloid pathology in predementia stages: the relationship with neurodegeneration and cognitive decline. Alzheimers Res Ther. 2017;9(1):101.
Batth TS, Francavilla C, Olsen JV. Off-line high-pH reversed-phase fractionation for in-depth phosphoproteomics. J Proteome Res. 2014;13(12):6176–86.
Magdalinou NK, Noyce AJ, Pinto R, Lindstrom E, Holmen-Larsson J, Holtta M, et al. Identification of candidate cerebrospinal fluid biomarkers in parkinsonism using quantitative proteomics. Parkinsonism Relat Disord. 2017;37:65–71.
Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489(7416):391–9.
Rouillard AD, Gundersen GW, Fernandez NF, Wang Z, Monteiro CD, McDermott MG, et al. The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database Oxford. 2016;2016:baw100.
Grote S, Prufer K, Kelso J, Dannemann M. ABAEnrichment: an R package to test for gene set expression enrichment in the adult and developing human brain. Bioinformatics. 2016;32(20):3201–3.
Mesquita SD, Ferreira AC, Gao F, Coppola G, Geschwind DH, Sousa JC, et al. The choroid plexus transcriptome reveals changes in type I and II interferon responses in a mouse model of Alzheimer’s disease. Brain Behav Immun. 2015;49:280–92.
Liu R, Zhang Z, Chen Y, Liao J, Wang Y, Liu J, et al. Choroid plexus epithelium and its role in neurological diseases. Front Mol Neurosci. 2022;15: 949231.
Serot JM, Bene MC, Foliguet B, Faure GC. Altered choroid plexus basement membrane and epithelium in late-onset Alzheimer’s disease: an ultrastructural study. Ann N Y Acad Sci. 1997;826:507–9.
Marques F, Sousa JC. The choroid plexus is modulated by various peripheral stimuli: implications to diseases of the central nervous system. Front Cell Neurosci. 2015;9:136.
Whyte LS, Hassiotis S, Hattersley KJ, Hemsley KM, Hopwood JJ, Lau AA, et al. Lysosomal dysregulation in the murine App(NL-G-F/NL-G-F) model of Alzheimer’s disease. Neuroscience. 2020;429:143–55.
Szabo MP, Mishra S, Knupp A, Young JE. The role of Alzheimer’s disease risk genes in endolysosomal pathways. Neurobiol Dis. 2022;162: 105576.
Van Acker ZP, Bretou M, Annaert W. Endo-lysosomal dysregulations and late-onset Alzheimer’s disease: impact of genetic risk factors. Mol Neurodegener. 2019;14(1):20.
Zhang W, Xu C, Sun J, Shen HM, Wang J, Yang C. Impairment of the autophagy-lysosomal pathway in Alzheimer’s diseases: pathogenic mechanisms and therapeutic potential. Acta Pharm Sin B. 2022;12(3):1019–40.
Joshi G, Bekier ME 2nd, Wang Y. Golgi fragmentation in Alzheimer’s disease. Front Neurosci. 2015;9:340.
Li JQ, Yu JT, Jiang T, Tan L. Endoplasmic reticulum dysfunction in Alzheimer’s disease. Mol Neurobiol. 2015;51(1):383–95.
Krzyzanowska A, Carro E. Pathological alteration in the choroid plexus of Alzheimer’s disease: implication for new therapy approaches. Front Pharmacol. 2012;3:75.
Cottrell DA, Blakely EL, Johnson MA, Ince PG, Turnbull DM. Mitochondrial enzyme-deficient hippocampal neurons and choroidal cells in AD. Neurology. 2001;57(2):260–4.
Vargas T, Ugalde C, Spuch C, Antequera D, Moran MJ, Martin MA, et al. Abeta accumulation in choroid plexus is associated with mitochondrial-induced apoptosis. Neurobiol Aging. 2010;31(9):1569–81.
Pifferi F, Laurent B, Plourde M. Lipid transport and metabolism at the blood-brain interface: implications in health and disease. Front Physiol. 2021;12: 645646.
Yin C, Ackermann S, Ma Z, Mohanta SK, Zhang C, Li Y, et al. ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nat Med. 2019;25(3):496–506.
Serot JM, Bene MC, Faure GC. Comparative immunohistochemical characteristics of human choroid plexus in vascular and Alzheimer’s dementia. Hum Pathol. 1994;25(11):1185–90.
Solar P, Zamani A, Kubickova L, Dubovy P, Joukal M. Choroid plexus and the blood-cerebrospinal fluid barrier in disease. Fluids Barriers CNS. 2020;17(1):35.
Choi JD, Moon Y, Kim HJ, Yim Y, Lee S, Moon WJ. Choroid plexus volume and permeability at brain MRI within the Alzheimer disease clinical spectrum. Radiology. 2022;304(3):635–45.
Dietrich MO, Spuch C, Antequera D, Rodal I, de Yebenes JG, Molina JA, et al. Megalin mediates the transport of leptin across the blood-CSF barrier. Neurobiol Aging. 2008;29(6):902–12.
Masuda A, Kobayashi Y, Kogo N, Saito T, Saido TC, Itohara S. Cognitive deficits in single App knock-in mouse models. Neurobiol Learn Mem. 2016;135:73–82.
Acknowledgements
The present study was supported by Alzheimer Nederland and the Research Foundation Flanders (FWO Vlaanderen; 1295223N, 1157621N and 1195019N), and partly supported by the Memorabel program of ZonMw (the Netherlands Organization for Health Research and Development) grant numbers 733050502 and 7330505021, an anonymous foundation and EMIF-AD. The EMIF-AD project has received support from the Innovative Medicines Initiative Joint Undertaking under EMIF grant agreement n° 115372, resources of which are composed of financial contribution from the European Union's Seventh Framework Program (FP7/2007-2013) and EFPIA companies’ in kind contribution. The DESCRIPA study was funded by the European Commission within the 5th framework program (QLRT-2001-2455). The EDAR study was funded by the European Commission within the 5th framework program (contract # 37670). San Sebastian GAP study is partially funded by the Department of Health of the Basque Government (allocation 17.0.1.08.12.0000.2.454.01.41142.001.H), Provincial Government of Gipuzkoa (124/16), Kutxa Fundazioa, and by the Carlos III Institute of Health (PI15/00919, PN de I+D+I 2013-2016). The Lausanne study was funded by a grant from the Swiss National Research Foundation (SNF 320030_141179). Collection of CSF samples from the Knight ADRC was supported by P30AG06644, P01AG003991, and P01AG026276. HZ is a Wallenberg Scholar and a Distinguished Professor at the Swedish Research Council supported by grants from the Swedish Research Council (#2023-00356; #2022-01018 and #2019-02397), the European Union’s Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), and the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C, #ADSF-21-831377-C, and #ADSF-24-1284328-C). The authors want to thank the VIB BioImaging Core for training, support and access to the instrument park, and the VIB Proteomics Core for performing the mass spectrometry and for support. The authors also thank Takashi SAITO and Takaomi C. SAIDO for the generation of the APPNL-G-F mouse model.
Funding
The present study was supported by Alzheimer Nederland grant number WE.15-2022-01, the Research Foundation Flanders (FWO Vlaanderen; 1295223N, 1157621N and 1195019N), and partly supported by ZonMw (the Netherlands Organization for Health Research and Development) grant numbers 733050502 and 7330505021, an anonymous foundation and EMIF-AD. The EMIF-AD project has received support from the Innovative Medicines Initiative Joint Undertaking under EMIF grant agreement n° 115372, resources of which are composed of financial contribution from the European Union’s Seventh Framework Program (FP7/2007-2013) and EFPIA companies’ in kind contribution.
Author information
Authors and Affiliations
Contributions
AD provided data analyses, statistical analysis, data interpretation and wrote the manuscript. AD, CV, PJV, RV and SJBV led the conception and design of the paper. CV, PJV, RV and SJBV provided supervision of the project and critical revision of the manuscript. CDN and BMT provided substantial help in the statistical analysis. CV, MB and PD were responsible for sample collection and preparation for proteomic analysis for the mice part of the study. AD was responsible for immunochemistry, astrocytes and microglia three-dimensional reconstruction and image analysis for the mice part of the study. CET, SES, FV, IR, PML, MT, RV, JS, SE, EDR, JP, GP, MT, YFL, SL, JS, LB and KB provided data and sample collection for the human part of the study. JG and HZ were responsible for CSF analysis in EMIF-AD MBD, Maastricht BB-ACL and Washington University Knight ADRC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Animal studies were conducted in compliance with governmental and EU guidelines and were approved by the ethical committee of the Faculty of Sciences, Ghent University, Belgium.
All patients provided informed consent for research. All centers approved participation in this study after local medical ethics committee approval.
Consent for publication
Not applicable.
Competing interests
Ms. Delvenne received funding from Alzheimer Nederland (grant number WE.15-2022-01). Dr. Schindler has served on advisory boards for Eisai. The institution of Dr. Vandenberghe has clinical trial agreements (RV as PI) with Alector, Biogen, Denali, EliLilly, J&J, UCB. The institution of Dr. Vandenberghe has consultancy agreements (RV as DSMB member) with AC Immune. Dr. Schaeverbeke is a senior postdoctoral fellow [12Y1623N] of FWO. Dr. Schaeverbeke receives funding from Stichting Alzheimer Onderzoek [SAO-FRA 2021/0022]. Dr. Popp served as a consultant and at advisory boards for the Nestlé Institute of Health Sciences, Ono Pharma, OM Pharma, Schwabe Pharma, Lilly, Roche, and Fujirebio Europe. All his disclosures are unrelated to the present work. The VD cohort was supported by grants from the Swiss National Research Foundation (SNF 320030_204886), Synapsis Foundation—Dementia Research Switzerland (Grant number 2017-PI01). Dr. Blennow has served as a consultant and at advisory boards for AC Immune, Acumen, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. Dr. Zetterberg has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). Dr. Visser received funding from the European Commission, IMI 2 Joint Undertaking (JU), AMYPAD, grant n° 115952; European Commission, IMI 2 JU, RADAR-AD, grant n°806999; European Commission, IMI 2 JU, EPND, grant n°101034344. The IMI JU receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. Dr. Visser received also funding from Zon-MW, Redefining Alzheimer's disease, grant n°733050824736; and Biogen (Amyloid biomarker study group). Grants were paid to the university. Dr. Vos received funding from ZonMW (SNAP VIMP grant n°7330505021), Stichting Adriana van Rinsum-Ponssen, and the EPND project, which received funding from the European Commision, IMI 2 Joint Undertaking (JU) under grant agreement n°101034344. The IMI JU receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. All others authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
This file provides supplemental information on the methods, supplementary results and supplementary figures.
Additional table 2.
Dysregulated proteins for each comparison in the whole list of identified proteins in mouse ChP tissue.
Additional table 3.
Dysregulated proteins for each comparison in the whole list of identified proteins in mouse CSF.
Additional table 4.
Dysregulated proteins for each comparison in the whole list of identified proteins in human CSF.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Delvenne, A., Vandendriessche, C., Gobom, J. et al. Involvement of the choroid plexus in Alzheimer’s disease pathophysiology: findings from mouse and human proteomic studies. Fluids Barriers CNS 21, 58 (2024). https://doi.org/10.1186/s12987-024-00555-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12987-024-00555-3