Abstract
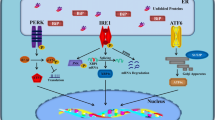
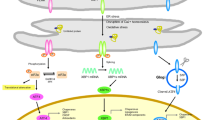
The endoplasmic reticulum (ER) serves many crucial cellular functions. However, when misfolded or unfolded proteins accumulated in the ER, the stress of ER will be induced. Meanwhile, the intracellular signaling network, which is called unfolded protein response, will also be activated to cope with. Those unfolded proteins can be recognized by three kinds of stress sensors which are IRE1, PERK, and ATF6. Based on lots of medical reports, ER stress in postmortem brains from Alzheimer’s disease (AD) patients, animals, and vitro models have indicated that ER dysfunction might work as an important part in causing AD. In this review, we demonstrated that the effect of ER stress contributed to the pathogenesis of AD. ER stress associates almost the whole brain pathology processes which can be observed in AD, such as gene mutation of presenilin1, the abnormal clipped mRNA of presenilin2, β-amyloid production, tau phosphorylation, and cell death. The status of ER stress and unfolded protein response in the pathogenesis of AD also suggests they can be used as potential therapeutic agents.




Similar content being viewed by others
References
2011 Alzheimer’s disease facts and figures (2011) Alzheimer’s & dementia 7 (2):208-244 doi:10.1016/j.jalz.2011.02.004
Jiang T, Yu JT, Tian Y, Tan L (2013) Epidemiology and etiology of Alzheimer’s disease: from genetic to non-genetic factors. Curr Alzheimer’s Res 10(8):852–867
Jiang T, Yu JT, Tan L (2012) Novel disease-modifying therapies for Alzheimer’s disease. J Alzheimer’s Dis JAD 31(3):475–492. doi:10.3233/JAD-2012-120640
Thomas PJ, Qu B-H, Pedersen PL (1995) Defective protein folding as a basis of human disease. Trends Biochem Sci 20(11):456–459. doi:10.1016/S0968-0004(00)89100-8
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334(6059):1081–1086. doi:10.1126/science.1209038
Smith MH, Ploegh HL, Weissman JS (2011) Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science 334(6059):1086–1090. doi:10.1126/science.1209235
Haas IG, Wabl M (1983) Immunoglobulin heavy chain binding protein. Nature 306(5941):387–389
Sanderson TH, Deogracias MP, Nangia KK, Wang J, Krause GS, Kumar R (2010) PKR-like endoplasmic reticulum kinase (PERK) activation following brain ischemia is independent of unfolded nascent proteins. Neuroscience 169(3):1307–1314. doi:10.1016/j.neuroscience.2010.05.076
Marciniak SJ, Garcia-Bonilla L, Hu J, Harding HP, Ron D (2006) Activation-dependent substrate recruitment by the eukaryotic translation initiation factor 2 kinase PERK. J Cell Biol 172(2):201–209. doi:10.1083/jcb.200508099
Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ (2013) Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol 12(1):105–118. doi:10.1016/S1474-4422(12)70238-7
Park SW, Ozcan U (2013) Potential for therapeutic manipulation of the UPR in disease. Semin Immunopathol 35(3):351–373. doi:10.1007/s00281-013-0370-z
Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA (2003) Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol 23(20):7198–7209
Iwawaki T, Akai R, Yamanaka S, Kohno K (2009) Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc Natl Acad Sci U S A 106(39):16657–16662. doi:10.1073/pnas.0903775106
Tsuru A, Fujimoto N, Takahashi S, Saito M, Nakamura D, Iwano M, Iwawaki T, Kadokura H, Ron D, Kohno K (2013) Negative feedback by IRE1beta optimizes mucin production in goblet cells. Proc Natl Acad Sci U S A 110(8):2864–2869. doi:10.1073/pnas.1212484110
Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415(6867):92–96. doi:10.1038/415092a
Chen Y, Brandizzi F (2013) IRE1: ER stress sensor and cell fate executor. Trends Cell Biol 23(11):547–555. doi:10.1016/j.tcb.2013.06.005
Upton JP, Wang L, Han D, Wang ES, Huskey NE, Lim L, Truitt M, McManus MT, Ruggero D, Goga A, Papa FR, Oakes SA (2012) IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science 338(6108):818–822. doi:10.1126/science.1226191
Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR (2009) IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138(3):562–575. doi:10.1016/j.cell.2009.07.017
Malhotra JD, Kaufman RJ (2007) The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 18(6):716–731. doi:10.1016/j.semcdb.2007.09.003
Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL (2000) ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 6(6):1355–1364
Patil C, Walter P (2001) Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol 13(3):349–355. doi:10.1016/S0955-0674(00)00219-2
Hoozemans JJ, Stieler J, van Haastert ES, Veerhuis R, Rozemuller AJ, Baas F, Eikelenboom P, Arendt T, Scheper W (2006) The unfolded protein response affects neuronal cell cycle protein expression: implications for Alzheimer’s disease pathogenesis. Exp Gerontol 41(4):380–386. doi:10.1016/j.exger.2006.01.013
O’Connor T, Sadleir KR, Maus E, Velliquette RA, Zhao J, Cole SL, Eimer WA, Hitt B, Bembinster LA, Lammich S, Lichtenthaler SF, Hebert SS, De Strooper B, Haass C, Bennett DA, Vassar R (2008) Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron 60(6):988–1009. doi:10.1016/j.neuron.2008.10.047
Nijholt DA, de Graaf TR, van Haastert ES, Oliveira AO, Berkers CR, Zwart R, Ovaa H, Baas F, Hoozemans JJ, Scheper W (2011) Endoplasmic reticulum stress activates autophagy but not the proteasome in neuronal cells: implications for Alzheimer’s disease. Cell Death Differ 18(6):1071–1081. doi:10.1038/cdd.2010.176
Lee JH, Won SM, Suh J, Son SJ, Moon GJ, Park UJ, Gwag BJ (2010) Induction of the unfolded protein response and cell death pathway in Alzheimer’s disease, but not in aged Tg2576 mice. Exp Mol Med 42(5):386–394. doi:10.3858/emm.2010.42.5.040
Hamos JE, Oblas B, Pulaski-Salo D, Welch WJ, Bole DG, Drachman DA (1991) Expression of heat shock proteins in Alzheimer’s disease. Neurology 41(3):345–350
Kulczycki J, Bertrand E, Lojkowska W, Dowjat W, Wisniewski T, Lyczywek-Zwierz M (2001) Familial Alzheimer’s disease connected with mutation in presenilin gene 1 (P117L). Neurol Neurochir Pol 35(2):213–224
Katayama T, Imaizumi K, Sato N, Miyoshi K, Kudo T, Hitomi J, Morihara T, Yoneda T, Gomi F, Mori Y, Nakano Y, Takeda J, Tsuda T, Itoyama Y, Murayama O, Takashima A, St George-Hyslop P, Takeda M, Tohyama M (1999) Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat Cell Biol 1(8):479–485. doi:10.1038/70265
Milhavet O, Martindale JL, Camandola S, Chan SL, Gary DS, Cheng A, Holbrook NJ, Mattson MP (2002) Involvement of Gadd153 in the pathogenic action of presenilin-1 mutations. J Neurochem 83(3):673–681
Hoyer-Hansen M, Jaattela M (2007) Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ 14(9):1576–1582. doi:10.1038/sj.cdd.4402200
Nixon RA, Yang DS (2011) Autophagy failure in Alzheimer’s disease—locating the primary defect. Neurobiol Dis 43(1):38–45. doi:10.1016/j.nbd.2011.01.021
Salminen A, Kaarniranta K (2009) Regulation of the aging process by autophagy. Trends Mol Med 15(5):217–224. doi:10.1016/j.molmed.2009.03.004
Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, Schmidt SD, Wesson D, Bandyopadhyay U, Jiang Y, Pawlik M, Peterhoff CM, Yang AJ, Wilson DA, St George-Hyslop P, Westaway D, Mathews PM, Levy E, Cuervo AM, Nixon RA (2011) Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain J Neurol 134(Pt 1):258–277. doi:10.1093/brain/awq341
Takuma K, Yan SS, Stern DM, Yamada K (2005) Mitochondrial dysfunction, endoplasmic reticulum stress, and apoptosis in Alzheimer’s disease. J Pharmacol Sci 97(3):312–316
Kishimoto Y, Kirino Y (2013) Presenilin 2 mutation accelerates the onset of impairment in trace eyeblink conditioning in a mouse model of Alzheimer’s disease overexpressing human mutant amyloid precursor protein. Neurosci Lett 538:15–19. doi:10.1016/j.neulet.2013.01.025
Sato N, Imaizumi K, Manabe T, Taniguchi M, Hitomi J, Katayama T, Yoneda T, Morihara T, Yasuda Y, Takagi T, Kudo T, Tsuda T, Itoyama Y, Makifuchi T, Fraser PE, St George-Hyslop P, Tohyama M (2001) Increased production of beta-amyloid and vulnerability to endoplasmic reticulum stress by an aberrant spliced form of presenilin 2. J Biol Chem 276(3):2108–2114. doi:10.1074/jbc.M006886200
Manabe T, Ohe K, Katayama T, Matsuzaki S, Yanagita T, Okuda H, Bando Y, Imaizumi K, Reeves R, Tohyama M, Mayeda A (2007) HMGA1a: sequence-specific RNA-binding factor causing sporadic Alzheimer’s disease-linked exon skipping of presenilin-2 pre-mRNA. Genes Cells Devoted Mol Cell Mech 12(10):1179–1191. doi:10.1111/j.1365-2443.2007.01123.x
Manabe T, Katayama T, Sato N, Gomi F, Hitomi J, Yanagita T, Kudo T, Honda A, Mori Y, Matsuzaki S, Imaizumi K, Mayeda A, Tohyama M (2003) Induced HMGA1a expression causes aberrant splicing of Presenilin-2 pre-mRNA in sporadic Alzheimer’s disease. Cell Death Differ 10(6):698–708. doi:10.1038/sj.cdd.4401221
Ferreira ST, Klein WL (2011) The Abeta oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol Learn Mem 96(4):529–543. doi:10.1016/j.nlm.2011.08.003
Resende R, Ferreiro E, Pereira C, Oliveira CR (2008) ER stress is involved in Abeta-induced GSK-3beta activation and tau phosphorylation. J Neurosci Res 86(9):2091–2099. doi:10.1002/jnr.21648
Fonseca AC, Ferreiro E, Oliveira CR, Cardoso SM, Pereira CF (2013) Activation of the endoplasmic reticulum stress response by the amyloid-beta 1-40 peptide in brain endothelial cells. Biochim Biophys Acta 1832(12):2191–2203. doi:10.1016/j.bbadis.2013.08.007
Costa RO, Ferreiro E, Cardoso SM, Oliveira CR, Pereira CM (2010) ER stress-mediated apoptotic pathway induced by Abeta peptide requires the presence of functional mitochondria. J Alzheimer’s Dis JAD 20(2):625–636. doi:10.3233/JAD-2010-091369
Costa RO, Ferreiro E, Oliveira CR, Pereira CMF (2013) Inhibition of mitochondrial cytochrome c oxidase potentiates Aβ-induced ER stress and cell death in cortical neurons. Mol Cell Neurosci 52:1–8. doi:10.1016/j.mcn.2012.09.005
Lai CS, Preisler J, Baum L, Lee DH, Ng HK, Hugon J, So KF, Chang RC (2009) Low molecular weight Abeta induces collapse of endoplasmic reticulum. Mol Cell Neurosci 41(1):32–43. doi:10.1016/j.mcn.2009.01.006
Lee do Y, Lee KS, Lee HJ, Kim do H, Noh YH, Yu K, Jung HY, Lee SH, Lee JY, Youn YC, Jeong Y, Kim DK, Lee WB, Kim SS (2010) Activation of PERK signaling attenuates Abeta-mediated ER stress. PloS One 5(5):e10489. doi:10.1371/journal.pone.0010489
Schapansky J, Olson K, Van Der Ploeg R, Glazner G (2007) NF-kappaB activated by ER calcium release inhibits Abeta-mediated expression of CHOP protein: enhancement by AD-linked mutant presenilin 1. Exp Neurol 208(2):169–176. doi:10.1016/j.expneurol.2007.04.009
Casas-Tinto S, Zhang Y, Sanchez-Garcia J, Gomez-Velazquez M, Rincon-Limas DE, Fernandez-Funez P (2011) The ER stress factor XBP1s prevents amyloid-beta neurotoxicity. Hum Mol Genet 20(11):2144–2160. doi:10.1093/hmg/ddr100
Hoozemans JJ, van Haastert ES, Nijholt DA, Rozemuller AJ, Eikelenboom P, Scheper W (2009) The unfolded protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. Am J Pathol 174(4):1241–1251. doi:10.2353/ajpath.2009.080814
Ho YS, Yang X, Lau JC, Hung CH, Wuwongse S, Zhang Q, Wang J, Baum L, So KF, Chang RC (2012) Endoplasmic reticulum stress induces tau pathology and forms a vicious cycle: implication in Alzheimer’s disease pathogenesis. J Alzheimer’s Dis JAD 28(4):839–854. doi:10.3233/JAD-2011-111037
Kins S, Crameri A, Evans DR, Hemmings BA, Nitsch RM, Gotz J (2001) Reduced protein phosphatase 2A activity induces hyperphosphorylation and altered compartmentalization of tau in transgenic mice. J Biol Chem 276(41):38193–38200. doi:10.1074/jbc.M102621200
Nijholt DA, van Haastert ES, Rozemuller AJ, Scheper W, Hoozemans JJ (2012) The unfolded protein response is associated with early tau pathology in the hippocampus of tauopathies. J Pathol 226(5):693–702. doi:10.1002/path.3969
Abisambra JF, Jinwal UK, Blair LJ, O’Leary JC 3rd, Li Q, Brady S, Wang L, Guidi CE, Zhang B, Nordhues BA, Cockman M, Suntharalingham A, Li P, Jin Y, Atkins CA, Dickey CA (2013) Tau accumulation activates the unfolded protein response by impairing endoplasmic reticulum-associated degradation. J Neurosci Off J Soc Neurosci 33(22):9498–9507. doi:10.1523/JNEUROSCI.5397-12.2013
Ferreiro E, Pereira CM (2012) Endoplasmic reticulum stress: a new playER in tauopathies. J Pathol 226(5):687–692. doi:10.1002/path.3977
Song J, Park KA, Lee WT, Lee JE (2014) Apoptosis signal regulating kinase 1 (ASK1): potential as a therapeutic target for Alzheimer’s disease. Int J Mol Sci 15(2):2119–2129. doi:10.3390/ijms15022119
Sano R, Reed JC (2013) ER stress-induced cell death mechanisms. Biochim Biophys Acta (BBA) Mol Cell Res 1833(12):3460–3470. doi:10.1016/j.bbamcr.2013.06.028
Akterin S, Cowburn RF, Miranda-Vizuete A, Jimenez A, Bogdanovic N, Winblad B, Cedazo-Minguez A (2006) Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer’s disease. Cell Death Differ 13(9):1454–1465. doi:10.1038/sj.cdd.4401818
Gupta S, Giricz Z, Natoni A, Donnelly N, Deegan S, Szegezdi E, Samali A (2012) NOXA contributes to the sensitivity of PERK-deficient cells to ER stress. FEBS Lett 586(22):4023–4030. doi:10.1016/j.febslet.2012.10.002
Katayama T, Imaizumi K, Manabe T, Hitomi J, Kudo T, Tohyama M (2004) Induction of neuronal death by ER stress in Alzheimer’s disease. J Chem Neuroanat 28(1–2):67–78. doi:10.1016/j.jchemneu.2003.12.004
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403(6765):98–103. doi:10.1038/47513
Martinez JA, Zhang Z, Svetlov SI, Hayes RL, Wang KK, Larner SF (2010) Calpain and caspase processing of caspase-12 contribute to the ER stress-induced cell death pathway in differentiated PC12 cells. Apoptosis Int J Program Cell Death 15(12):1480–1493. doi:10.1007/s10495-010-0526-4
Ferreiro E, Oliveira CR, Pereira CM (2008) The release of calcium from the endoplasmic reticulum induced by amyloid-beta and prion peptides activates the mitochondrial apoptotic pathway. Neurobiol Dis 30(3):331–342. doi:10.1016/j.nbd.2008.02.003
Vaux DL (2011) Apoptogenic factors released from mitochondria. Biochim Biophys Acta 1813(4):546–550. doi:10.1016/j.bbamcr.2010.08.002
Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A (2009) Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ 16(7):966–975. doi:10.1038/cdd.2009.33
Tooze SA, Schiavo G (2008) Liaisons dangereuses: autophagy, neuronal survival and neurodegeneration. Curr Opin Neurobiol 18(5):504–515. doi:10.1016/j.conb.2008.09.015
Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, Duff K, Uchiyama Y, Naslund J, Mathews PM, Cataldo AM, Nixon RA (2005) Macroautophagy—a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol 171(1):87–98. doi:10.1083/jcb.200505082
Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26(24):9220–9231. doi:10.1128/MCB.01453-06
Jiang T, Yu JT, Zhu XC, Tan MS, Wang HF, Cao L, Zhang QQ, Shi JQ, Gao L, Qin H, Zhang YD, Tan L (2014) Temsirolimus promotes autophagic clearance of amyloid-beta and provides protective effects in cellular and animal models of Alzheimer’s disease. Pharmacol Res Off J Ital Pharmacol Soc. doi:10.1016/j.phrs.2014.02.008
Kim DS, Li B, Rhew KY, Oh HW, Lim HD, Lee W, Chae HJ, Kim HR (2012) The regulatory mechanism of 4-phenylbutyric acid against ER stress-induced autophagy in human gingival fibroblasts. Arch Pharm Res 35(7):1269–1278. doi:10.1007/s12272-012-0718-2
Paschen W, Mengesdorf T (2005) Cellular abnormalities linked to endoplasmic reticulum dysfunction in cerebrovascular disease–therapeutic potential. Pharmacol Ther 108(3):362–375. doi:10.1016/j.pharmthera.2005.05.008
Cawley K, Logue SE, Gorman AM, Zeng Q, Patterson J, Gupta S, Samali A (2013) Disruption of microRNA biogenesis confers resistance to ER stress-induced cell death upstream of the mitochondrion. PloS One 8(8):e73870. doi:10.1371/journal.pone.0073870
Yoshida H, Yoshizawa T, Shibasaki F, Shoji S, Kanazawa I (2002) Chemical chaperones reduce aggregate formation and cell death caused by the truncated Machado-Joseph disease gene product with an expanded polyglutamine stretch. Neurobiol Dis 10(2):88–99
Verhoef LG, Lindsten K, Masucci MG, Dantuma NP (2002) Aggregate formation inhibits proteasomal degradation of polyglutamine proteins. Hum Mol Genet 11(22):2689–2700
Winklhofer KF, Reintjes A, Hoener MC, Voellmy R, Tatzelt J (2001) Geldanamycin restores a defective heat shock response in vivo. J Biol Chem 276(48):45160–45167. doi:10.1074/jbc.M104873200
Piper PW (2001) The Hsp90 chaperone as a promising drug target. Curr Opin Investig Drugs 2(11):1606–1610
Cuervo AM, Dice JF (2000) Age-related decline in chaperone-mediated autophagy. J Biol Chem 275(40):31505–31513. doi:10.1074/jbc.M002102200
Lund J, Tedesco P, Duke K, Wang J, Kim SK, Johnson TE (2002) Transcriptional profile of aging in C. elegans. Curr Biol CB 12(18):1566–1573
Tonoki A, Kuranaga E, Tomioka T, Hamazaki J, Murata S, Tanaka K, Miura M (2009) Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process. Mol Cell Biol 29(4):1095–1106. doi:10.1128/MCB.01227-08
Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, Seto E, Bhalla K (2005) Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem 280(29):26729–26734. doi:10.1074/jbc.C500186200
Ricobaraza A, Cuadrado-Tejedor M, Perez-Mediavilla A, Frechilla D, Del Rio J, Garcia-Osta A (2009) Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer’s disease mouse model. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 34(7):1721–1732. doi:10.1038/npp.2008.229
Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J (2005) A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307(5711):935–939. doi:10.1126/science.1101902
Verfaillie T, Garg AD, Agostinis P (2013) Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett 332(2):249–264. doi:10.1016/j.canlet.2010.07.016
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China to L.T. (81171209, 81371406) and J.T.Y. (81000544), the grants from the Shandong Provincial Natural Science Foundation to L.T. (ZR2011HZ001) and J.T.Y. (ZR2010HQ004), the Medicine and Health Science Technology Development Project of Shandong Province to L.T. (2011WSA02018) and J.T.Y. (2011WSA02020), and the Innovation Project for Postgraduates of Jiangsu Province to T.J. (CXLX13_561).
Conflicts of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, JQ., Yu, JT., Jiang, T. et al. Endoplasmic Reticulum Dysfunction in Alzheimer’s Disease. Mol Neurobiol 51, 383–395 (2015). https://doi.org/10.1007/s12035-014-8695-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8695-8