Abstract
Background
Torpor is an energy saving strategy achieved by substantial reductions of metabolic rate and body temperature that enables animals to survive periods of low resource availability. During hibernation (multiday torpor), the frequency of periodic rewarming—characterised by high levels of oxidative stress—is associated with shortening of telomeres, a marker of somatic maintenance.
Objectives
In this study, we determined the impact of ambient temperature on feeding behaviour and telomere dynamics in hibernating garden dormice (Eliomys quercinus) over winter. This obligate hibernator prepares for hibernation by accumulating fat stores but can also feed during hibernation.
Methodology
Food intake, torpor pattern, changes in telomere length, and body mass change were assessed in animals housed at experimentally controlled temperatures of either 14 °C (i.e., a mild winter) or 3 °C (i.e., a cold winter) over 6 months.
Results
When hibernating at 14 °C, dormice experienced 1.7-fold more frequent and 2.4-fold longer inter-bout euthermia, and spent significantly less time torpid, compared to animals hibernating at 3 °C. Higher food intake enabled individuals to compensate for increased energetic costs when hibernating at milder temperatures (14 °C vs. 3 °C), to buffer body mass loss and thus increase winter survival. Interestingly, we observed a significant increase of telomere length over the entire hibernation period, irrespective of temperature treatment.
Conclusion
We conclude that higher temperatures during winter, if associated with sufficient food availability, can have a positive effect on the individual’s energy balance and somatic maintenance. These results suggest that winter food availability might be a crucial determinant for the survival of the garden dormouse in the context of ever-increasing environmental temperatures.
Similar content being viewed by others
Background
Torpor is an energy saving strategy used by small heterothermic mammals and birds, characterised by a controlled reduction of metabolic rate and body temperature (Tb), which enables animals to survive energetic bottlenecks [1]. During hibernation, i.e., torpor lasting over several days to weeks, animals can reach a minimum torpid metabolic rate of ~ 4% of basal metabolic rate, in association with a pronounced reduction of their Tb [2]. Importantly, hibernation is not a continuous torpid state. Instead, hibernation in most species is structured by successive torpor bouts interspersed by periodic arousals [3,4,5,6,7]. During those arousals, metabolic rate increases drastically, causing Tb to return to normothermic levels for a few hours [4, 8] during phases called inter-bout euthermia (IBE). Arousals represent the highest proportion of energy expended during the hibernation process, e.g., 70–80% of expended energy in temperate species [9].
Periodic arousals occur more frequently at higher ambient temperature (Ta) [10], which increases energy expenditure during hibernation [11, 12]. Most heterotherms rely on body fat stores or food caches over winter that are accumulated prior to hibernation [13,14,15,16,17,18]. Hence, higher winter temperatures invariably lead to the risk that small hibernators will deplete their fat or food stores before the end of hibernation [19, 20].
Based on models of global climate change, the predicted increase of winter temperatures will act in synergy with other factors and, in the worst case, lead to countless species extinctions [21]. Hibernators are of particular concern, emphasising the need to determine how small hibernators will be affected by warmer winter temperatures. Despite the fact that many seasonal hibernators will likely face negative consequences as a result of the predicted increase in environmental temperature [22], the ability of species to use short bouts of torpor opportunistically seems to be rather beneficial when it comes to surviving temperature extremes and associated increase in food shortages [23].
To date, most studies addressing the impacts of climate change on heterothermy are centred at the level of the whole organism. There is, however, a clear lack of studies investigating cellular or molecular aspects in seasonal hibernators facing events associated with climate change. A reliable measure of somatic maintenance is the change in relative telomere length (RTL). Telomeres are non-coding, repetitive sequences, located at the end of the chromosomes in eukaryotic cells. Telomeres prevent degradation of coding DNA sequences and shorten during mitosis after each cell division [24,25,26,27,28,29]. During torpor, mitosis and telomere degradation are arrested or drastically reduced [27, 30], whereas at normothermic Tb, such as during periodic arousals, mitosis is reactivated and telomeres can possibly shorten [30, 31]. Furthermore, periodic arousals, caused by a drastic increase in metabolic rate, are associated with high levels of oxidative stress and substantial increased production of reactive oxygen species (ROS) that can cause accelerated telomere shortening via DNA breaks [4, 32,33,34,35]. Telomere length can be restored by active repair mechanisms, e.g., via telomerase [36, 37] or alternative lengthening [38] but these mechanisms are likely energetically costly [33]. Thus, while shortened telomere length is often associated with physiological ageing, studies reporting lengthening of telomeres in relation to improved environmental conditions and lower levels of life-history stress [33, 39,40,41,42], suggest that telomeres are rather a biomarker of the current status of individual’s somatic maintenance.
We have recently shown that garden and edible dormice hibernating without food at 14 °C experienced less telomere attrition than individuals hibernating at 3 °C, despite a higher arousal frequency and greater body mass loss [43]. These findings suggest that lower levels of ROS might be produced and/or energy-costly repair mechanisms might be (more) active during hibernation at warmer temperatures. The garden dormouse (Eliomys quercinus) sustains its energy requirements during hibernation by relying on its body fat stores, but can also actively forage during the winter [44, 45]; a behaviour that could provide the advantage of buffering telomere attrition and body mass loss during hibernation in winter. To further determine the impact of winter environmental conditions on the somatic maintenance and survival of the individuals, this study built on the previous results and investigated how mild winter Ta affects hibernation patterns, telomere dynamics, and energy balance of the garden dormouse when food is available. Thus, torpor patterns, telomere dynamics, body mass changes, and food intakes were recorded in garden dormice hibernating with food, at either 3 °C or 14 °C. Specifically, we hypothesised (1) that garden dormice fed ad libitum are able to partly or fully compensate for telomere attrition during hibernation at low temperatures, i.e., 3 °C, and (2) that ad-libitum access to food enables individuals to compensate for higher energy requirements and to limit body mass loss when hibernating at warm temperatures (14 °C),.
Material and methods
Animals
We examined 32 (15 females and 17 males) adult garden dormice (2.2 ± 0.2 years old) during hibernation. The animals were born in captivity and raised under natural climatic conditions in outdoor aviaries at the Research Institute of Wildlife Ecology (FIWI) of the University of Veterinary Medicine Vienna, Austria (48° 15′ N, 16° 22′ E). For individual identification all animals were marked at birth with subcutaneous PIT tags (Tierchip Dasmann, Tecklenburg, Germany). In autumn 2017, animals were transferred from the aviaries to the laboratory. The mean body mass of individuals at hibernation onset was 140.0 ± 18.7 g.
Experimental design
Given their natural duration of hibernation of approximately 6 months [46], experiments were carried out between 21st of September 2017 and 15th of March 2018. Dormice were placed in ventilated cooling units (Liebherr GKv 5730). In each cooling unit, eight animals (with balanced sex ratio) were housed in “holding cages” consisting of a standard laboratory mouse cage (36L x 20T x 14H cm) and a metal grid-lid. Each holding cage was connected to a nest box with a wooden-lid through a connector as previously described in Nowack et al. [43]. Garden dormice were divided into two temperature groups: 16 animals were kept at about 3 °C to mimic a cold winter [mean Ta: 3.70 ± 0.67 °C (SE: 0.003 °C) and 3.60 ± 0.63 °C (SE: 0.003 °C)] and 16 other animals at 14 °C [mean Ta: 14.00 ± 1.73 °C (SE: 0.01 °C) and 14.19 ± 1.63 °C (SE: 0.01 °C)] mimicking a mild winter. Experiments were split into four periods (period 1: 21.09.–09.11.2017; period 2: 09.11.–14.12.2017; period 3: 14.12.2017–08.02.2018; period 4: 08.02–15.03.2018). Period 1 represents the beginning-, period 2 and 3 the main- and finally period 4 the end of the hibernation season. The first period lasted 7 weeks (49 days), the second and fourth period lasted 5 weeks (35 days) and the third period was 8 weeks (56 days). Each period began and ended with the recording of body mass and the sampling of buccal swabs (see below), resulting in five sampling points. After the third period, one female of the 3 °C group was excluded from the experiments due to low body mass (70.1 g; despite ad libitum food). This animal was transferred into a warm room kept at approximately 22 °C, receiving ad libitum access to food (see below) and water for the rest of the winter, before returning to the colony. Hibernating animals had also ad-libitum access to food and water, and food intake was measured during the whole duration of experiments (see below). At the end of the winter experiments, all animals were returned to the colony.
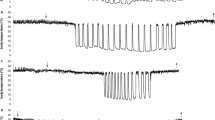
Temperature recording and torpor pattern
We used nest temperature as a proxy for Tb to estimate torpor use, as described by Willis et al. [47] and used in previous studies in garden dormice [43, 48,49,50]. In brief, the nest box was equipped with a customized temperature data logger (FIWI, Vienna, Austria; resolution: 0.2 °C, accuracy: ± 0.06 °C), which measured the temperature of the nest box every minute. Nest boxes were big enough for one dormouse to fit completely inside but small enough that it had to sit directly on the thermologger. The bottom of the nest boxes were covered with a thin layer of hay to provide familiar nesting conditions but to still ensure the contact between the animal and data loggers. To mimic normal conditions during hibernation, animals were kept under constant darkness and at a near stable temperature in the cooling units. Torpor bout duration, arousal frequency and IBE duration were computed with a self-written R script [51], using a threshold of 5 °C for the 3 °C group and a threshold of 16 °C for the 14 °C group to compute arousal (> 5 or > 16 °C) and torpor periods (< 5 or < 16 °C). These two thresholds were chosen as a conservative approach to avoid false data due to slight temperature variations.
Food intake
Individual food intake was measured over the entire duration of the experiment. Each cage was equipped with a bowl of food pellets for rodents (ssniff®HA, ssniff GmbH, Soest, Germany) and a cushion-formed piece of jelly-agar (10 g Agar–Agar in 1 l of water) for water availability. Food pellets were dried for ~ 14 h at 50 °C and weighted before as well as after each period. Pellets and water cushions were exchanged every 1–3 weeks during cage exchange. To prevent animals from disturbance the cooling units were opened under special care, using red light and fresh cages with fresh food and water-cushions were mounted while the hibernating animals inside the nest were left untouched. Under these conditions, animals were undisturbed and continued their hibernation normally (S.G. and M-T.R. Pers. Obs.). To calculate individual food intake (expressed in grams), the amount of food pellets that were left by the animal was subtracted from the amount that was supplied to the animal.
Telomere length
DNA samples were collected during all 5 sampling points. Cells were collected with gynobrush brushes (Heinz Herenz Medizinalbedarf, Hamburg, Germany) from the inner cheeks of animals that subsequently rewarmed from torpor. The brushes were gently twisted for 1 min inside each cheek. This method is considered minimally-invasive. The brushes were placed in 1 ml BC buffer and stored in the fridge at 4 °C. The DNA extraction in the laboratory was carried out with the DNeasy Blood and Tissue Kit (Qiagen). RTL was measured with the real-time PCR approach [52], adapted for garden dormice.
A 54 bp portion of the IRBP (inter-photoreceptor retinoid-binding) gene-proto-oncogene was used as the non-variable copy number (non-VCN) gene. Primer sequences for the non-VCN gene were 5′-TGG AAG CAG CTC ATG GGC AC-3′(IRBP_Eq_F1) and 5′-GTG GTG GTA TTG GAG GGG CG-3′ (IRBP_Eq_R1), and telomeric primer sequences were 5′-CGG TTT GTT TGG GTT TGG GTT TGG GTT TGG GTT TGG GTT-3′ (tel 1b) and 5′-GGC TTG CCT TAC CCT TAC CCT TAC CCT TAC CCT TAC CCT-3′ (tel 2b) as in Hoelzl et al. [40]. The following procedure as described by Hoelzl et al. [40] was respected. Non-VCN gene and telomere PCRs were carried out in separate runs with 20 ng DNA per reaction, 400 nmol l−1 of each primer in a final volume of 20 μl containing 10 μl of Promega Cybergreen GoTaq® qPCR Master Mix (Cat. Nr. A6001/2; Promega, Madison, USA). PCR conditions for IRBP were 10 min at 95 °C followed by 40 cycles of 10 s at 95 °C, 20 s at 63 °C and 20 s at 72 °C. PCR conditions for the telomere primers were 10 min at 95 °C followed by 40 cycles of 10 s at 95 °C, 20 s at 56 °C and 20 s at 72 °C. In each run, a final melting step was included with the temperature ramping from 65 to 95 °C, at 1 °C steps. Two reference standard samples (standard A and standard B) were included in every run and compared with all ratios of telomere to non-VCN gene. A non-template control was included as well in every run. To minimize pipetting errors, reactions were prepared using the Qiagility PCR robot (Qiagen, Germany). Cycling was conducted on a Rotorgene Q quantitative thermocycler (Qiagen, Germany). For analysis of the non-baseline corrected raw qPCR data, the software LinRegPCR (2012.0) was used. RTL was calculated using a modified formula from Ruijter et al. [53], where E is the qPCR efficiency and Ct the cycle threshold. The subscript ST refers to the telomere reaction of the standard sample, SC to the control gene reaction of the standard sample, T to the telomere reaction of the target sample and C to the control gene (IRBP) reaction of the target sample: RTL = (ETCtT/ESTCtST)/(EcCtC/ESCCtSC).
The mean qPCR efficiency was calculated via the amplification plot method [54] which gives lower but more accurate estimates of efficiency than standard curve based methods [55, 56]. For the non-VCN gene and telomere reactions, mean qPCR efficiencies were 91.0% and 78.6%, respectively.
Reference standard samples were included in every run to provide the relative telomere ratios to the non-VCN gene. Liver tissue was used for standard sample one (S1) (RTL = 1) and standard sample two (S2) which was used to control for run-to-run variability. A negative control was included in every run to control for possible contaminants in the reagents.
The intraclass correlation coefficient (ICC) was calculated as a measure of reliability within and between the runs, as suggested by Koo and Li [57]. ICC estimates and their 95% confident intervals for sample triplicates were calculated in R Version 3.5.1 [51]. Intra-rater ICC was calculated on all included data points based on a single-rating, absolute-agreement, 2-way mixed-effects model (ICC in library ‘irr’, Gamer et al. [58]). Intra-assay ICC for Ct values for telomere assay was 0.99 [p < 0.0001, 95% (CI 0.98–0.991)] and for cMYC 0.94 [p < 0.0001, 95% (CI 0.92–0.96)] showing an excellent degree of reliability. The ICC for inter-assay was calculated for the standard samples based on a mean rating (k = 3), agreement, 2-way mixed-effects model. Interrater ICC for Ct values for telomere assay was 0.72 [p < 0.0001, 95% (CI 0.26–1.0)] and for cMYC 0.99 [p < 0.0001, 95% (CI 0.91–1.0)] showing a moderate-to good degree of reliability and excellent degree of reliability respectively. As all samples per individual were run on the same plate, inter-assay variability should have minimal effect on our longitudinal results.
The intra-assay coefficient of variation among replicates (intra-assay variation), an estimate of system precision, was further used to assess reproducibility. Mean intra-assay CV for Ct values of the non-VCN gene and telomere assay were 0.39 and 0.67%, respectively.
Considering the correlation between initial RTL and telomere shortening RTL0 was included, as a correcting factor in all models that were run to identify factors that influenced the change in RTL. Including initial RTL as a covariate in statistical models corrects for the RTL-typical “regression to the mean”, i.e., changes tend to be larger when the initial value is extremely high or low [40].
Statistical data analyses
Due to a low body mass one animal was excluded from the experiments after period 3. Thus, for the statistical analysis of body mass change, torpor patterns, food intake and RTL change of period 4 the sample size was reduced to 15 individuals in the 3 °C group. Moreover, one sample (RTL0) of one animal and two further samples of another animal (RTL1 and RTL2) of the 14 °C group were excluded due to qPCR reaction failures, reducing the RTL sample size (of all five sampling times) of the 14 °C group to 77. Thus, the total RTL sample size (of both groups) was 156. Data are presented as mean ± standard deviation.
Statistical analyses were conducted using R (Version 3.5.0) [51]. Normal distribution of model residuals and homogeneity of variances were tested using Shapiro–Wilk test, qqPlot and Levene’s test, respectively (Shapiro Wilk test in library ‘stats’; qqPlot and Levene’s test in library ‘car’, Fox and Weisberg [59]. Linear mixed-effects models (lme) were used to test effects of time (time points 1–5) or period (1–4), group (3 °C or 14 °C), as well as the interaction between time and group, or period and group on body mass, total and mean IBE durations, arousal frequency, total and mean torpor durations, and food intake, with animal ID as a random factor. A linear mixed-effects model was also used to test the effect of group, all hibernation parameters (i.e., IBE, arousal frequency, torpor bout duration), body mass, and food intake on the overall RTL change. “Period” was used for data recorded over time (total and mean IBE durations, arousal frequency, total and mean torpor durations and food intake) and “Time” was used for data recorded only at discrete sampling dates (body mass and RTL). Further, we ran a type III sum-of-squared ANOVA followed, when necessary, by a post-hoc Tukey-like all comparisons test (glht in library ‘multcomp’, Hothorn et al. [60]) to determine significances between groups and detailed information on time-course significances within groups. We also conducted regression analyses between food intake and IBE duration by using spearman’s rank correlation (function ‘corr.test’). In all models, “Group” and “Period” were entered as factors. “Time” was used as a factor in linear mixed-effects model on body mass, and as a continuous variable in the linear mixed-effects model on RTL. For statistical analyses of RTL, the linear mixed-effects model included RTL0 (initial RTL) as a covariate to correct for the “regression to the mean” phenomenon [40]. Possible collinearity of variables was tested by using the Variance Inflation Factor (VIF) [61], and when needed, the number of variables was reduced. This was the case for the food intake, which was then excluded from the model, due to high correlation with IBE duration. The following variables were included in the model: “Group”, “RTL0”, “body mass change”, “IBE duration” and “arousal frequency”. We then performed a model selection based on Akaike’s information criterion corrected for small sample size (AICc; Akaike [62]) and an ANOVA type II, with the variables of the best model only (dredge in library ‘MuMIn’, Barton [63]; Anova in library ‘car’). To test the effect of “Time” on RTL, we further applied a linear mixed-effects model (including “RTL0” and a “Group” and “Time” interaction) with a model selection followed by an ANOVA type II on the best model (∆ AIC = 0) only (lme in library ‘nlme’ Pinheiro et al. [64]; dredge function in library ‘MuMIn’ Barton [63]; Anova in library ‘car’). To examine the significance of group difference of initial RTL and the overall RTL change (between RTL0 and RTL4), we applied linear mixed-effects models (lme in library ‘nlme’, Pinheiro et al. [64]) with animal ID as random factor, followed by an ANOVA type III.
Results
Body mass loss and food intake
Dormice of both groups had a similar body mass at the beginning of the experiment (3 °C group vs. 14 °C group: 140.9 ± 22.9 g vs. 139.0 ± 14.1 g, t = 0.72519, df = 25, p = 0.48) and showed a linear loss of body mass over winter (3 °C: − 43.3 ± 8.8 g vs. 14 °C: − 47.3 ± 12.0 g). There was a significant interaction of group and time for body mass loss (Table 1), however, post-hoc tests did not reveal significant differences between groups at any of the timepoints (Fig. 1).
Body mass change of garden dormice hibernating with food at different temperatures during winter. Body mass loss of animals hibernating at 3 °C (blue line) and 14 °C (orange line) over all four periods (weeks). Time was considered as a continuous variable in the model. Post-hoc analyses showed no significant differences between groups at different sampling times: W0 = Week 0, W7 = Week 7, W12 = Week 12, W20 = Week 20, W25 = Week 25
Individuals kept at 14 °C showed a significant higher food intake in all periods than animals hibernating at 3 °C (Fig. 2). For 14 °C animals, no significant period differences in food intake could be detected (Fig. 2). Animals hibernating at 3 °C displayed a significantly lower food intake in period 3, compared to periods 1 and 4 (Fig. 2). Total food intake was 124.4 ± 165.2 g at 3 °C versus 360.8 ± 118.0 g for 14 °C animals.
Food intake in hibernating garden dormice according to temperatures during winter. Total food intake (grams per week) for all four periods. Significant differences (p < 0.05) are denoted by different superscripts. Period-differences for the 3 °C group and 14 °C group are indicated by lower-case letters and upper-case letters, respectively. Stars indicate group-differences within periods. ***p < 0.001; **p < 0.01
Torpor pattern
We found significant differences in total and mean torpor durations between both groups (total torpor duration: 157.3 ± 9.4 days vs. 129.3 ± 10.4 days; mean torpor duration: 7.1 ± 2.2 days vs. 3.4 ± 1.1 days; see Table 2 for values for each period), caused by increased arousal frequency and longer IBE in individuals hibernating at 14 °C. Individuals hibernating at 14 °C showed a 2.4-fold higher total arousal frequency than individuals hibernating at 3 °C and there was a significant group and period interaction for arousal frequency (Table 1; Fig. 3C). While there was no significant group difference at period 1, periods 2, 3, and 4 showed significantly higher arousal frequencies for 14 °C animals versus 3 °C animals (Fig. 3C).
Torpor patterns of garden dormice hibernating with food at different temperatures during winter. A Total interbout euthermia (IBE) duration (hours per week), B mean IBE duration (h), and C arousal frequency (bouts per week) for all four periods. Significant differences (p < 0.05) are denoted by different superscripts. Period-differences for the 3 °C group and 14 °C group are indicated by lower-case letters and upper-case letters, respectively. Stars indicate group-differences within periods. ***p < 0.001; **p < 0.01
Mean and total IBE durations were 1.5-fold and 2.4-fold higher, respectively, in individuals hibernating at 14 °C than in the 3 °C group (Mean IBE duration: 21.8 ± 8.6 h vs. 14.2 ± 7.1 h; Total IBE duration: 874.8 ± 138.5 h vs. 360.8 ± 219.8 h). All periods showed a significant group-difference (Table 1; see post-hoc tests on Fig. 3A). Individuals hibernating at 3 °C spent a similar amount of time in IBE in all four periods (Fig. 3A), whereas total IBE duration was highest at periods 1 and 4 for the 14 °C animals (Fig. 3A). Food intake and total IBE duration positively correlated across both groups (S = 29,084, ρ = 0.91, p = < 0.001, Fig. 4).
Relative telomere length
We found a significant increase of RTL over the duration of the study (RTL0-RTL4) (Fig. 5 and Additional file 1: Figure S1; χ2 = 11.23, df = 1, p < 0.001) in individuals of both groups (no group difference: χ2 = 3.09, df = 1, p = 0.08). Total RTL over all four periods during hibernation was best explained by the model including initial RTL and time (Table 3), with a significant effect of both variables on RTL (initial RTL: χ2 = 33.5, p < 0.001; Time: χ2 = 15.9, p < 0.001). Although initial RTL was the only variable significantly affecting RTL change (Table 4), the factor that best explained RTL change (besides initial RTL) was arousal frequency (Table 3).
Changes in telomere length of garden dormice hibernating with food at different temperatures during winter. Relative telomere length (RTL) of dormice hibernating at 3 °C (panel A) or 14 °C (panel B) over the five sampling times during the hibernation trial. Sampling time “0” was at the start of the experiments, and times “1” to “4” correspond to samplings performed at the end of each of the four experimental periods
Discussion
Our study revealed that while individuals hibernating at warmer temperatures had higher arousal rates and spend more time active, they were able to compensate the high energy requirement via increased food intake, as shown by similar body mass loss in both groups. Furthermore, the presence of food during hibernation allowed dormice hibernating at 3 °C and 14 °C to compensate any shortening of telomere length via repair mechanisms, leading to an increase in telomere length during winter. Such maintenance of somatic integrity during winter hibernation would allow individuals to optimize the successive breeding season as available energy can be fully used to maximize body condition at emergence from hibernation and to ensure a successful breeding.
The role of hibernation pattern, food intake, and Ta on telomere length
In contrast to previous work reporting significant RTL attrition over the course of hibernation, notably in relation to the number of periodic arousals and the duration of IBE [33, 49, 65], this study did not find a significant relationship between telomere dynamics, arousal frequency and IBE duration. Moreover, telomere length after hibernation did not differ significantly between animals that hibernated at mild or cold winter temperatures. While our previous study had shown that dormice hibernating at 3 °C without food shortened telomere [43], dormice hibernating at 3 °C with food in this study steadily elongated their telomeres toward the end of the hibernation season. This suggests that telomere elongation is costly, as previously underlined by Hoelzl et al. [33], and therefore high energy availability either in the form of stored fat or via direct food intake can counteract the negative effects of periodic arousals on telomeres (i.e., on somatic maintenance) during hibernation by allocating metabolic resources to somatic maintenance (telomeres elongation).
In our study, arousal frequency, temperature, total IBE duration, and body mass change were included in the best models explaining RTL variations between individuals, although initial RTL was the only significant predictor variable. Still, it is important to mention that more factors must be considered, with which RTL can be stabilised or elongated. For instance, a fluctuating Ta as potentially experienced in a more natural setting may have minimized the cost of arousals and thus the production of ROS and its negative effect on RTL [49].
Alternatively, the availability of food could also have allowed individuals to upregulate their antioxidant defences, leading to dampened concentrations of ROS and to lower oxidative stress level associated with periodic arousals [4, 32], reducing telomere attrition during hibernation. Nowack et al. [43] suggested that the greater extent of telomere attrition in 3 °C animals hibernating without food could be explained by the fact that animals exposed to cold temperatures (3 °C) had to rewarm from deeper torpor than individuals hibernating at mild temperatures (14 °C) resulting in a greater level of oxidative stress. In the present study, dormice from both temperature treatment groups not only prevented telomere attrition during hibernation but also elongated them toward the end of the winter. While telomere elongation was also reported to occur in edible dormice when supplemented with food during the summer [33], the existence of a circannual program aiming at reaching a certain telomere length at emergence from hibernation remains unknown. These findings emphasize that the topic of RTL restoration or elongation is still very complex and that further studies are warranted.
Dormice hibernating at mild temperatures with food spend less time in torpor
In this study, dormice hibernating at mild temperatures (14 °C) spent overall more time in IBE than individuals hibernating at cold temperatures (3 °C) and our data further revealed a positive correlation between food intake and the time animals spent in IBE. This may reflect the necessity for the 14 °C animals to compensate for higher energy expenditure as warmer Ta during hibernation is associated with higher energetic costs, due to Ta limiting further reduction of Tb and metabolism and leading to an increased number of periodic arousals during winter hibernation [2, 66]. Alternatively, the presence of food during winter might have caused animals to be more active (and less torpid) during hibernation. Individuals hibernating with food in the present study had on average 1.7-fold longer IBE compared to garden dormice hibernating without food at the same Ta of 14 °C during winter in our previous study (see Table 1 in [43]). This suggests that food availability reduces the propensity of animals to hibernate, spending less time in torpor and longer time in IBE, underlying the avoidance of torpor use when possible [16, 67, 68]. However, this effect might also have been linked to differences in body energy (fat) reserves, between dormice of the present study and individuals from Nowack et al. [43] that were lighter with ~ 20% lower body mass during winter than individuals from the present study. Previous studies have found that dormice in better body condition, i.e., with more body energy reserves reduce the time spent in torpor (e.g., [68]). Hence, the exact contributions from external food supply and internal energy (fat) reserves to the overall energetics of hibernation in garden dormice still warrant further investigations.
Effects of warmer winter temperatures on the survival of the garden dormouse
Global mean surface temperature has increased by approximately 0.8 °C over the last century and is likely to continue to increase by 0.3–4.8 °C, over the twenty-first century [22]. Hibernating species are particularly vulnerable to such critical rise in Tas [69, 70]. Hence, it is crucial to investigate how hibernators, such as the garden dormouse, react to increasing Ta, conferring some phenotypic flexibility for individuals to survive the winter, especially when temperatures during the hibernation period are increasingly mild. Given the garden dormouse’s status as near-threatened [71], and the fact that this species experienced the largest decline among all European rodents over the last 30 years [72], it is of high relevance to determine to which extent the flexibility of hibernation phenotypes would enable this species to survive warmer winters. Our results suggest that torpor pattern of garden dormice can be highly flexible and that garden dormice are able to survive uprising mild Ta during hibernation—as long as food is available. However, Hallmann et al. [73] reports an alarming 75% decline of flying insect biomass over the last 27 years across several natural reserves in Germany. Although the exact causes for the decline of garden dormice still remains unknown the loss of insect biomass could strongly influence animal population that mainly feed on insects, such as the garden dormouse, enhancing species extinction.
Despite the many other advantageous roles of torpor, such as predation avoidance [23, 74, 75], Eliomys quercinus would rather employ less torpor or might even skip hibernation when environmental conditions are good, i.e., high Ta possibly accompanied by high food availability [76]. Additionally, mild Ta can also lead to ecological advantages including extra-time for the breeding season [77] notably the occurrence of litters during winter with mild temperatures [76]. Although there are already many studies examining the impacts of climate change, there is still a need to improve knowledge concerning animal responsiveness to warmer winter, especially at the physiological and ecological level of this hibernating dormouse species. Hence, it will be of high importance to investigate the phenotypic flexibility of garden dormice in the context of ever-increasing climate and global changes.
Conclusion
This study highlights that (1) telomeres can be restored during hibernation and (2) individuals can compensate for the high energy requirement when hibernating at mild temperatures, if food is available during winter. Although dormice from both temperature groups were losing body mass, all individuals were able to survive the entire winter. Surprisingly, telomeres increased during the process of hibernation irrespective of the temperature treatment. Animals hibernating at 3 °C allocated the extra-energy -provided via food intake- to their somatic maintenance, whereas animals kept at 14 °C primarily allocated available energy toward regulating their energy balance to survive the winter, along with directing a part of it to their somatic maintenance. To conclude, this data indicates that temperature and food availability during winter have an important impact on hibernating patterns and cellular maintenance.
Availability of data and materials
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Lyman CP, Willis JS, Malan A, Wang LCH. Hibernation and torpor in mammals and birds. New York: Academic Press; 1982.
Ruf T, Geiser F. Daily torpor and hibernation in birds and mammals. Biol Rev. 2015;90:891–926.
Barnes BM, Kretzmann M, Licht P, Zucker I. The influence of hibernation on testis growth and spermatogenesis in the golden-mantled ground squirrel Spermophilus lateralis. Biol Reprod. 1986;35:1289–97.
Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–81.
French AR. Effects of temperature on the duration of arousal episodes during hibernation. J Appl Physiol. 1982;52:216–20.
French AR. Allometries of the durations of torpid and euthermic intervals during mammalian hibernation: a test of the theory of metabolic control of the timing of changes in body temperature. J Comp Physiol B. 1985;156:13–9.
Twente JW, Twente J, Moy RM. Regulation of arousal from hibernation by temperature in three species of Citellus. J Appl Physiol. 1977;42:191–5.
Heldmaier G, Ortmann S, Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol. 2004;141:317–29.
Wang LCH. Energetic and field aspects of mammalian torpor: the Richardson’s ground squirrel. In: Wang LCH, Hudson JW, editors. Strategies in cold: natural torpidity and thermogenesis. New York: Academic Press; 1978. p. 109–45.
Geiser F, Broome LS. The effect of temperature on the pattern of torpor in a marsupial hibernator. J Comp Physiol B. 1993;163:133–7.
Bieber C, Ruf T. Summer dormancy in edible dormice (Glis glis) without energetic constraints. Naturwissenschaften. 2009;96:165–71.
Geiser F, Kenagy GJ. Torpor duration in relation to temperature and metabolism in hibernating ground squirrels. Physiol Zool. 1988;61:442–9.
Dark J. Annual lipid cycles in hibernators: integration of physiology and behavior. Annu Rev Nutr. 2005;25:469–97.
Florant GL, Healy J. The regulation of food intake in mammalian hibernators: a review. J Comp Physiol. 2012;182:451–67.
French AR. Interdependency of stored food and changes in body temperature during hibernation of the eastern chipmunk, Tamias striatus. J Mammal. 2000;81:979–85.
Humphries MM, Thomas DW, Kramer DL. The role of energy availability in mammalian hibernation: a cost-benefit approach. Physiol Biochem Zool. 2003;76:165–79.
Munro D, Thomas DW, Humphries MM. Extreme suppression of aboveground activity by a food-storing hibernator, the eastern chipmunk (Tamias striatus). Can J Zool. 2008;86:364–70.
Sheriff MJ, Fridinger RW, Tøien Ø, Barnes BM, Buck CL. Metabolic rate and prehibernation fattening in free-living arctic ground squirrels. Physiol Biochem Zool. 2013;86:515–27.
Pretzlaff I, Dausmann KH. Impact of climatic variation on the hibernation physiology of Muscardinus avellanarius. In: Ruf T, Bieber C, Arnold W, Millesi E, editors. Living in a seasonal world thermoregulatory and metabolic adaptations. Heidelberg: Springer; 2012. p. 85–97.
Reeder DM, Frank CL, Turner GG, Meteyer CU, Kurta A, Britzke ER, Vodzak ME, Darling SR, Stihler CW, Hicks AC, et al. Frequent arousal from hibernation linked to severity of infection and mortality in bats with white-nose syndrome. PLoS ONE. 2012;7: e38920.
Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. Impacts of climate change on the future of biodiversity. Ecol Lett. 2012;15:365–77.
Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM. Climate change 2013: the physical science basis: working group I contribution to the fifth assessment report of the intergovernmental panel on climate change. New York: Cambridge University Press; 2013.
Nowack J, Stawski C, Geiser F. More functions of torpor and their roles in a changing world. J Comp Physiol B. 2017;187:889–97.
Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–73.
Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–62.
Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol. 2007;3:640–9.
Marcand S, Brevet V, Mann C, Gilson E. Cell cycle restriction of telomere elongation. Curr Biol. 2000;10:487–90.
Meyne J, Ratliff RL, Moyzis RK. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci USA. 1989;86:7049–53.
Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci USA. 1994;91:9857–60.
Kruman II, Ilyasova EN, Rudchenko SA, Khurkhulu ZS. The intestinal epithelial cells of ground squirrel (Citellus undulatus) accumulate at G2 phase of the cell cycle throughout a bout of hibernation. Comp Biochem Physiol A. 1988;90A:233–6.
Vinogradova MS. Mitotic activity of stomach epithelium in the ground squirrel, Citellus erythrogenys Brandt. Comp Biochem Physiol A. 1988;91A:235–9.
van Breukelen F, Carey H. Ubiquitin conjugate dynamics in the gut and liver of hibernating ground squirrels. J Comp Physiol B. 2002;172:269–73.
Hoelzl F, Cornils JS, Smith S, Moodley Y, Ruf T. Telomere dynamics in free-living edible dormice (Glis glis): the impact of hibernation and food supply. J Exp Biol. 2016;219:2469–74.
Richter T, von Zglinicki T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp Gerontol. 2007;42:1039–42.
von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–44.
Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in tetrahymena extracts. Cell. 1985;43:405–13.
Harley CB, Vaziri H, Counter CM, Allsopp RC. The telomere hypothesis of cellular aging. Exp Gerontol. 1992;27:375–82.
Neumann AA, Watson CM, Noble JR, Pickett HA, Tam PP, Reddel RR. Alternative lengthening of telomeres in normal mammalian somatic cells. Genes Dev. 2013;27:18–23.
Turbill C, Smith S, Deimel C, Ruf T. Daily torpor is associated with telomere length change over winter in Djungarian hamsters. Biol Lett. 2012;8:304–7.
Hoelzl F, Smith S, Cornils JS, Aydinonat D, Bieber C, Ruf T. Telomeres are elongated in older individuals in a hibernating rodent, the edible dormouse (Glis glis). Sci Rep. 2016;6:36856.
Foley NM, Petit EJ, Brazier T, Finarelli JA, Hughes GM, Touzalin F, Puechmaille SJ, Teeling EC. Drivers of longitudinal telomere dynamics in a long-lived bat species, Myotis myotis. Mol Ecol. 2020;29:2963–77.
Brown TJ, Spurgin LG, Dugdale HL, Komdeur J, Burke T, Richardson DS. Causes and consequences of telomere lengthening in a wild vertebrate population. Mol Ecol. 2021.
Nowack J, Tarmann I, Hoelzl F, Smith S, Giroud S, Ruf T. Always a price to pay: hibernation at low temperatures comes with a trade-off between energy savings and telomere damage. Biol Lett. 2019;15:20190466.
Ambid L, Castan I, Atgié C, Nibbelink M. Food intake and peripheral adrenergic activity in a hibernating rodent, the garden dormouse. Comp Biochem Physiol A. 1990;97A:361–6.
Gil-Delgado JA, Mira Ó, Viñals A, Gómez J, Banyuls N, Vives-Ferrándiz C. Diet of the garden dormouse (Eliomys quercinus Linnaeus 1766) in orange groves: seasonal variation and use of available resources. Mammalia. 2010;74:147–51.
Niethammer G, Krapp F. Handbuch der Säugetiere Europas. Nagetiere I. Wiesbaden: Akadem. Verlagsgesellschaft; 1978.
Willis CKR, Goldzieher A, Geiser F. A non-invasive method for quantifying patterns of torpor and activity under semi-natural conditions. J Therm Biol. 2005;30:551–6.
Giroud S, Turbill C, Ruf T. Torpor use and body mass gain during pre-hibernation in late-born juvenile garden dormice exposed to food shortage. In: Ruf T, Bieber C, Arnold W, Millesi E, editors. Living in a seasonal world thermoregulatory and metabolic adaptations. Berlin: Springer; 2012. p. 481–91.
Giroud S, Zahn S, Criscuolo F, Chery I, Blanc S, Turbill C, Ruf T. Late-born intermittently fasted juvenile garden dormice use torpor to grow and fatten prior to hibernation: consequences for ageing processes. Proc R Soc B. 2014;281:20141131.
Mahlert B, Gerritsmann H, Stalder G, Ruf T, Zahariev A, Blanc S, Giroud S. Implications of being born late in the active season for growth, fattening, torpor use, winter survival and fecundity. Elife. 2018;7:e31225.
R Core Team: R: a language and environment for statistical computing; 2018. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org/.
Thomas P, O’ Callaghan NJ, Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mech Ageing Dev. 2008;129:183–90.
Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, Moorman AF. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37: e45.
Ramakers C, Ruijter JM, Deprez RHL, Moorman AFM. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62–6.
Morinha F, Magalhães P, Blanco G. Standard guidelines for the publication of telomere qPCR results in evolutionary ecology. Mol Ecol Res. 2020;20:635–48.
Spießberger M, Hoelzl F, Smith S, Vetter S, Ruf T, Nowack J. The tarnished silver spoon? Trade-off between prenatal growth and telomere length in wild boar. J Evol Biol. 2022;35:81–90.
Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–63.
Gamer M, Lemon J, Singh IFP: Irr: various coefficients of interrater reliability and agreement; 2019. https://CRAN.R-project.org/package=irr.
Fox J, Weisberg S. An R companion to applied regression. 2nd ed. Thousand Oaks: Sage; 2011.
Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–63.
Naimi B, Hamm NAS, Groen TA, Skidmore AK, Toxopeus AG. Where is positional uncertainty a problem for species distribution modelling? Ecography. 2014;37:191–203.
Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csáki F (eds) Proceedings of the 2nd international symposium on information theory. Tsahkadsor, Armenia, USSR: Akadémiai Kiado, Budapest, Hungary; 1973. p. 267–81.
Barton K: MuMln: multi-model inference. R package version 1.42.1; 2018. http://CRAN.R-project.org/package=MuMln.
Pinheiro J, Bates D, DebRoy S, Sarkar D. R Core team: nlme: linear and nonlinear mixed effects models (R Package Version 3.1–137); 2018. https://CRAN.R-project.org/package=nlme.
Turbill C, Ruf T, Smith S, Bieber C. Seasonal variation in telomere length of a hibernating rodent. Biol Lett. 2013;9:20121095.
Geiser F, Ruf T. Hibernation versus daily torpor in mammals and birds: physiological variables and classification of torpor patterns. Physiol Zool. 1995;68:935–66.
Zervanos SM, Maher CR, Florant GL. Effect of body mass on hibernation strategies of woodchucks (Marmota monax). Integr Comp Biol. 2014;54:443–51.
Bieber C, Lebl K, Stalder G, Geiser F, Ruf T. Body mass dependent use of hibernation: why not prolong the active season, if they can? Funct Ecol. 2014;28:167–77.
Cordes LS, Blumstein DT, Armitage KB, CaraDonna PJ, Childs DZ, Gerber BD, Martin JGA, Oli MK, Ozgul A. Contrasting effects of climate change on seasonal survival of a hibernating mammal. Proc Natl Acad Sci. 2020;117:201918584.
Humphries MM, Thomas DW, Speakman JR. Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature. 2002;418:313–6.
Bertolino S, Amori G, Henttonen H, Zagorodnyuk I, Zima J, Juškaitis R, Meinig H, Kryštufek B. Eliomys quercinus, Garden Dormouse. pp. e.T7618A12835766: The IUCN Red List of Threatened Species Version 2014.2; 2008:e.T7618A12835766.
Bertolino S. Distribution and status of the declining garden dormouse Eliomys quercinus. Mammal Rev. 2017;47:133–47.
Hallmann CA, Sorg M, Jongejans E, Siepel H, Hofland N, Schwan H, Stenmans W, Müller A, Sumser H, Hörren T, et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE. 2017;12: e0185809.
Constant T, Giroud S, Viblanc VA, Tissier ML, Bergeron P, Dobson FS, Habold C. Integrating mortality risk and the adaptiveness of hibernation. Front Physiol. 2020;11:706.
Turbill C, Bieber C, Ruf T. Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc R Soc B. 2011;278:3355–63.
Gil-Delgado JA, Cabaret P, Declercq S, Gómez J, Sánchez I. Winter reproduction of Eliomys quercinus (Rodentia) in the orange groves of Sagunto (Valencia, Spain). Mammalia. 2006;70:76–9.
Moreno S. Reproduction of garden dormouse Eliomys quercinus lusitanicus, in southwest Spain. Mammalia. 1988;52:401–8.
Acknowledgements
We thank Michaela Salaba and Peter Steiger for animal maintenance and Gerhard Fluch for technical support. Further, the authors want to thank Renate Hengsberger for the formatting of the manuscript and her help in the literature search.
Funding
This work was financially supported by the city of Vienna (to SG and TR), the Humboldt Foundation (to JN), and the Austrian Science Fund (FWF, P27267-B25 and P31577-B25 to SG).
Author information
Authors and Affiliations
Contributions
TR and SG conceived the idea for the study. SG, JN and M-TR designed the study. M-TR, AB, JN and SG conducted the experiments; M-TR and SG analyzed the data. M-TR, FH and SS conducted genetic analyses. TR provided statistical support. M-TR and SG wrote the first version of the manuscript. All authors edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experiments in this study were carried out in compliance with the ARRIVE guidelines on animal research and were approved by the Ethics and Animal Welfare Committee of the University of Veterinary Medicine, Vienna, in accordance with the University’s guidelines for Good Scientific Practice (ETK-04/10/2016).
Consent for publication
Consent form is attached to the submission.
Competing interests
We declare we have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1
. Change of relative telomere length (RTL) between groups during the entire hibernation experiments. N3°C = 15; N14°C = 15, time = 25 weeks.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Giroud, S., Ragger, MT., Baille, A. et al. Food availability positively affects the survival and somatic maintenance of hibernating garden dormice (Eliomys quercinus). Front Zool 20, 19 (2023). https://doi.org/10.1186/s12983-023-00498-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12983-023-00498-9