Abstract
Background
Evidences support the view that central obesity is an independently cardiovascular risk. It is thought that leptin contributes to autonomic dysfunction and cardiovascular risks in type 1 and type 2 diabetes mellitus (T1DM and T2DM). This raises the possibility that leptin might mediate the relationship between central obesity and the severity of cardiovascular autonomic neuropathy (CAN) in patients with well-controlled T2DM and prediabetes.
Methods
The complete cardiovascular reflex tests and biomarkers were assessed for each patient. The severity of CAN was assessed using composite autonomic scoring scale (CASS). A single-level three-variable mediation model was used to investigate the possible relationships among central obesity [as indicated by waist circumference (WC)], leptin level, and severity of CAN (as indicated by CASS value).
Results
A total of 107 patients were included in this study: 90 with diabetes and 17 with prediabetes. The results demonstrate that increased WC is associated with increased severity of CAN (r = 0.242, P = 0.017). We further discovered that leptin level is positively correlated with WC (r = 0.504, P < 0.0001) and the CASS value (r = 0.36, P < 0.0001). Further mediation analysis shows that leptin level serves as mediators between higher WC and higher CASS.
Conclusions
Our results highlighted the relationship among leptin, central obesity, and severity of CAN. As the leptin level serves as mediator between central obesity and severity of CAN, a longitudinal study is needed to confirm that control of WC can decrease leptin levels and can be effective in reducing CAN progression.
Similar content being viewed by others
Background
The prevalence of obesity has increased worldwide over the past several decades to become a global health problem, and it poses an increased risk of multiple serious conditions [1, 2], including type 2 diabetes (T2DM), cardiovascular diseases, nonalcoholic fatty liver disease, chronic kidney disease, and different types of cancer [3].
Currently, adipose tissue is recognized as a complex and dynamic endocrine organ in the body that not only stores energy, but also regulates energy homeostasis, cellular reactions, and metabolic homeostasis [4]. The adipocytes are metabolically active and potent secretory cells, capable of releasing many adipocytokines, and are involved in the regulation of appetite, inflammatory and immune functions, glucose and lipid metabolism, long-term energy balance, insulin sensitivity of insulin responsive tissues, cardiovascular homeostasis and reproduction, and other important biological and physiological functions [5]. Leptin is an adipocyte-secreted hormone with a key role in energy homeostasis, and it can also stimulate the secretion of several cytokines via inflammatory cells. Additionally, serum leptin levels are positively correlated with insulin resistance (IR) [6].
The pathophysiological mechanism of cardiovascular autonomic neuropathy (CAN) is multifactorial, and there is sufficient evidence that it may precede diabetes mellitus (DM) [7]. MetS is associated with multiple risk factors, including central obesity that may increase the risk of cardiovascular events in individuals with type 2 DM [8]. MetS with central obesity is associated with an imbalance of homeostatic mechanisms, leading to adipose tissue dysfunctionality characterized by altered secretion of adipokines. This condition is also associated with a special upregulation in the expression of pro-inflammatory adipokines [9] and increase in the generation of free radicals and other reactive species, leading to increased oxidative stress, production of cell adhesion molecules, and microcirculation dysfunction [10].
The prevalence of CAN is variable and is dependent on the definition and criteria used for the diagnosis. Toronto Consensus recommends using four cardiovascular reflex tests (CARTs) and frequency-domain tests as a sensitive and specific method in assessing the presence of CAN in patients with DM [11]. Furthermore, the American Academy of Neurology’s summary of evidence-based guidelines for clinicians recommends that a combination of autonomic screening tests and composite autonomic scoring scales (CASS) should be considered to achieve the highest diagnostic accuracy of CAN [12]. These two diagnostic methods and scoring systems are commonly used in research and clinical practice.
CAN has a strong influence on various cardiovascular diseases and leads to severe morbidity and mortality in patients with DM [13, 14]. To our knowledge, only a few studies have demonstrated that leptin is associated with autonomic dysfunctions in type 1 diabetes mellitus (T1DM) and T2DM [2, 15,16,17]. To date, there is a paucity of information that focuses on the relationship among central obesity, leptin level and severity of CAN in patients withT2DM.
According to our hypotheses, central obesity is associated with upregulation in the expression of leptin, and leptin might affect autonomic function by acting both centrally and peripherally and thus leads to a decline in CAN. Our results may be beneficial for the development of therapeutic strategies for patients with DM and may help improve the quality of life of patients with T2DM and prediabetes.
Patients and methods
Study population
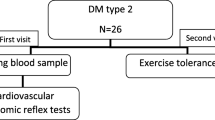
A total of 107 patients (≥ 20 years of age) who visited the outpatient diabetic clinic at Kaohsiung Chang Gung Memorial Hospital (CGMH) in Taiwan were included in this study: 90 subjects with type 2 diabetes and 17 subjects with prediabetes. Exclusion criteria were moderate-to-severe heart failure (NYHA class III and IV), presence of any type of arrhythmia that prevents analysis of heart rate variability (HRV), or pacemaker implantation. This study was approved by the Ethics Committee of Chang Gung Memorial Hospital Institutional Review Board (201800388B0C501 and 201901363B0).
Baseline clinical measurements
All patients underwent complete neurological and physical examinations on enrollment and on follow-up visits at the outpatient clinic. A detailed medical history regarding prior use of medications was obtained from every patient and their families through standardized questions. Demographic data, including age, sex, duration of diabetes (years), body mass index (BMI), systolic and diastolic blood pressure (SBP and DSP, respectively), waist circumference (WC) during autonomic function testing, underlying diseases (hypertension, coronary artery disease, ischemic stroke, and diabetic retinopathy [DR]), and laboratory parameters, were obtained at baseline. MetS was evaluated according to the updated National Cholesterol Education Program/Adult Treatment Panel III criteria [18]. A subject who had at least three of the following components was defined as having MetS: (1) central obesity: WC ≥ 90 cm for men and ≥ 80 cm for women; (2) hypertension: SBP ≥ 130 mmHg or DBP ≥ 85 mmHg or drug treatment for hypertension; (3) fasting blood glucose ≥ 100 mg/dL or diagnosed diabetes; (4) abnormal high-density lipoprotein (HDL) level: HDL cholesterol level < 40 mg/dL for men and < 50 mg/dL for women or drug treatment for low HDL cholesterol (HDL-C); and (5) abnormal triglyceride (TG) level: TG level ≥ 150 mg/dL or drug treatment for high TGs. The cut-off values for obesity, based on BMI, should be much lower in Taiwan than in Western countries. We determined that the optimal cut-off values for our study were BMIs of 23.6 in men and 22.1 in women, and WCs of 80.5 cm in men and 71.5 cm in women may be more appropriate to define adult overweight or obesity in Taiwan [19].
Laboratory measurements
Blood samples were obtained by antecubital vein puncture in a fasting, non-sedative state between 09:00 and 10:00 a.m in the control and study groups to exclude the possible influence of circadian variations. All blood samples were collected into Vacutainer SST tubes (BD, Franklin Lakes, NJ) and centrifuged at 3000 rpm for 10 min; subsequently, serum samples were collected and stored at − 80 °C in multiple aliquots, prior to biochemical measurement. Serum levels of TGs, total cholesterol, HDL-C, low-density lipoprotein cholesterol, blood sugar, glycohemoglobin (HBA1c), and high-sensitive C-reactive protein (hs-CRP) were analyzed by the hospital’s central laboratory. The homeostasis model assessment of insulin resistance (HOMA-IR index) was calculated by fasting glucose (in mmol/L) × fasting insulin (in mU/ml)/22.5. In our study, we defined IR as ≥ 2, based on the Taiwanese population [20]. Additionally, we used the TG/HDL ratio as a surrogate marker for IR [20].
The estimated glomerular filtration rate (eGFR) in each patient was calculated using an equation for Chinese subjects, as previously described [21]. The normal rate of albumin excretion is less than 30 mg/day; therefore, persistent albumin excretion between 30 and 300 mg/day is classified as microalbuminuria and albumin excretion above 300 mg/day is considered macroalbuminuria [22].
Biomarkers for oxidative stress
We evaluated the oxidative stress condition in all subjects by measuring the serum thiobarbituric acid-reactive substance (TBARS) and thiol levels. Serum TBARS levels were measured using a well-established method for detecting lipid peroxidation with a commercially available assay kit (Cayman Chemical, Ann Arbor, MI, USA, cat. no. 10009055). The Assay Kit was used according to the manufacturer’s instructions, as previously described [23]. The values for the samples were calculated using a linear calibration curve, which was prepared using pure malondialdehyde-containing samples (range, 0–50 μmol/L).
The ability of anti-oxidative defense in response to increased oxidative damage was evaluated by measuring the serum levels of total reduced thiols because serum thiols are physiologic free radical scavengers. Serum total protein thiols were estimated by directly reacting thiols with 5,5-dithiobis 2-nitrobenzoic acid to form 5-thio-2-nitrobenzoic acid (TNB). The number of thiols in the sample was calculated based on absorbance, which was determined using the extinction coefficient of TNB (A412 = 13,600/M/cm).
ELISA Analysis for biomarkers of endothelial dysfunction and leptin
Serum sICAM-1 and sVCAM-1 levels were assessed using commercially available ELISA kits (R&D Systems, Minneapolis, USA; sICAM-1: cat. no. DCD 540; sVCAM-1: cat.no. DVC00), as previously described [24]. Leptin and adiponectin levels were evaluated by enzyme-linked immunosorbent assay in the quality-controlled central laboratory of the CGMH.
Assessment of cardiovascular autonomic functions
All subjects underwent a standardized evaluation of cardiovascular autonomic function. CARTs are considered gold-standard measures of autonomic function in patients with DM [11]. Parameters, which were computed as Ewing’s methods, including heart rate responses to deep breathing (E:I ratio), to standing (30:15 ratio), and to the Valsalva maneuver and blood pressure responses to standing [25], were often used by diabetologists. CAN was defined with the presence of at least two abnormal test results [11].
Scoring of severity in cardiovascular autonomic neuropathy
The severity of CAN in our study was assessed using the cardiovagal and adrenergic sub-scores of the CASS [26]. The test battery comprised heart rate response to deep breathing (HR_DB), Valsalva ratio (VR), and 5-min head-up tilt (HUT) tests, as described by Low [27]. The detailed methodology for computing HR_DB and VR were based on a previous study [27]. The CASS had a scale from 0 to 7 points in this study [28].
Statistical analysis
Data are expressed as means ± standard derivations or medians (interquartile ranges). Categorical variables were compared using Chi-square or Fisher’s exact tests. Continuous variables that were not normally distributed by Kolmogorov–Smirnov test were logarithmically transformed to improve normality and compared. Three separate statistical analyses were performed. First, patients were stratified into two groups (diabetes and prediabetes, and presence or absence of CAN) and compared. Second, correlation analysis was used to evaluate the relationship between the leptin and variables that included age, diabetes duration, BMI, WC, SPB, and DSP, peripheral blood studies for vascular risks and autonomic parameters. Finally, a single-level three-variable mediation model [29], illustrated in Fig. 1, was used to investigate the causal relationships between leptin (mediating variable), central obesity (wrist circumference, independent variable), and severity of CAN (CASS, dependent variable). Mediation analysis tests whether the direct effect of an independent variable on a dependent variable can be explained by the indirect influence of the mediating variable. A significant mediator is one whose inclusion as an intermediate variable significantly affects the relationship between the independent and dependent variables. The statistical significance threshold in Sobel test was set at 0.05 for all the relevant paths [30]. All statistical analyses were conducted using the SAS software package, version 9.1 (2002, SAS Statistical Institute, Cary, North Carolina).
Results
General characteristics of the patients with diabetes and prediabetes
A total of 107 patients were included in this study: 90 with diabetes and 17 with prediabetes. Patient characteristics and baseline underlying diseases at assessment are presented in Table 1. The mean diabetes duration in the diabetes group was 11.5 ± 8.6 years. The significant differences between the diabetes and prediabetes groups included HDL-C (mmol/L) (P = 0.017), HbA1c (%) (P < 0.0001), eGFR (P = 0.002), and UACR (P < 0.0001), BMI (p = 0.001), WC (p = 0.001), presence of chronic kidney diseases (p = 0.002), and proteinuria (p = 0.001).
Parameters of cardiovascular autonomic study between patients with and without CAN
Patients with CAN had higher CASS values and lower levels of parasympathetic parameters, which include HR_DB (beats/min), VR, E:I ratio, 30:15 ratio, and sympathetic parameters, such as blood pressure responses to standing (Tables 2, 3).
Correlation analysis between leptin levels and parameters of biomarkers and cardiovascular autonomic functions
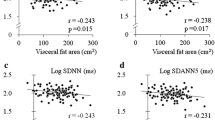
Correlation analysis parameters that are used to test the relationship between leptin level and parameters of biomarkers and cardiovascular autonomic functions are listed in Table 4. The significant statistical results (correlation coefficient, P-value) between leptin level and parameters of biomarkers were as follows: BMI (r = 0.588, P < 0.0001), WC (r = 0.504, P < 0.0001), UACR (r = 0.351, P = 0.001), hs-CRP (r = 0.291, P = 0.006), TBARS (r = 0.259, P = 0.008), thiols (r = -0.25, P = 0.011), TG/HDL-C ratio (r = 0.346, P < 0.0001), and HOMA-IR (r = 0.304, P = 0.008). Further, significant statistical results (correlation coefficient, P-value) between leptin levels and parameters of cardiovascular autonomic functions were as follows: HR_DB (r = -0.30, P = 0.002), Valsalva ratio (r = −0.31, P = 0.002), E:I ratio (r = −0.26, P = 0.008), and 30/15 ratio (r = −0.18, P = 0.07) (Table 3).
Mediation analysis for central obesity, severity of cardiovascular autonomic neuropathy and leptin level
The primary hypothesis of this analysis concerns whether the effect of central obesity (WC, independent variable) on the severity of CAN (CASS, dependent variable) was explained indirectly by leptin level (mediator) with significant group main effect. The path model jointly tested three effects of interest that are required if leptin level links WC with the CASS: (a) the effect of the independent variable (WC value) on the mediator (leptin level) (indirect effect, path a); (b) the effect of the mediator on the dependent variable (CASS) by controlling the effect for the leptin level (indirect effect, path b); and (c) the mediation effect a × b, which is defined as the reduction of the relationship between the independent and dependent variables (WC and CASS) (total relationship, path c) by including the mediator into the model (direct path, path c′). For simplicity, we report a full list of the results from the present study that fulfill the three criteria cited previously. The mediation relationship was significant (p = 0.011 in Sobel test) (Fig. 1) (Tables 4).
Discussion
Major findings of our study
Consistent with our hypothesis and in line with the extant literature, patients with T2DM and prediabetes experienced higher WC, higher leptin level and worse cardiovascular autonomic function. All vascular risk factors, including blood pressure, blood glucose, and lipid profiles, were well-controlled, except for central obesity, a modified risk factor, which was not corrected in our study. Our study also showed that leptin level is significantly correlated with BMI and WC. Thus, our results highlighted the relationship among leptin, central obesity, and severity of CAN.
Pathogenesis of leptin and inflammation, oxidative stress, and endothelial dysfunction
Visceral adipose tissue is a bioactive organ that secretes several adipokines, and is a source of proinflammatory and proatherogenic cytokines [31]. The rapid expansion of adipocyte size in obese individuals occurs in an uncoordinated manner, which leads to altered secretion of adipocytokines with a special upregulation in the expression of pro-inflammatory adipocytokines [9], as well as impaired angiogenesis, endothelial dysfunction, and microvascular complications [10].
Leptin and its receptors structurally resemble proinflammatory cytokines and their receptors [32]. In addition, proinflammatory mediators increase leptin secretion, and leptin levels increase considerably during inflammation [33]. Leptin may also increase oxidative stress via activation of the Rho and Rac family of small GTPase [34, 35]. Due to the proinflammatory and prooxidant properties of leptin, hyperleptinemia produces systemic endothelial dysfunction [34]. Our study results showed a positive association between leptin levels and inflammation (hs-CRP), increased oxidative stress (TBARs), and decreased antioxidative capacity (thiol). It also showed that leptin levels are positively associated with biomarkers of microvascular complications of diabetes (e.g., diabetic kidney diseases and CAN).
Association among leptin, obesity, IR, and cardiac autonomic function in diabetes and pre-diabetes
Leptin is a hormone predominantly synthesized by adipocytes and enterocytes in the small intestine that helps regulate energy balance and acts on cell receptors in the arcuate nucleus of the hypothalamus [6]. Although the regulation of fat stores is deemed to be the primary function of leptin, it also plays a role in other physiological processes. Leptin levels are generally elevated in obesity, and obese individuals are often leptin resistant, as seen in the failure of recombinant leptin to cause weight loss. Hyperleptinemia shunts excess free fatty acids ectopically to non-adipose tissue and diverts fatty acids in those tissue to storage rather than to oxidative consumption [36]. The lipotoxicity, ensuing from this ectopic accumulation of intracellular TGs, contributes to the dysfunction of these organs, and is a critical determinant of IR [37].
Although leptin levels are closely associated with adiposity and increased MetS components, the role of leptin levels in diabetes and prediabetes groups is rather controversial [38, 39]. A recent study from Japan shows that plasma leptin level is comparable between diabetic and non-diabetic patients, despite the fact that BMI, visceral fat area, and subcutaneous fat area are significantly higher in patients with diabetes [15]. Another study in relatively lean rural Chinese adults found that plasma leptin levels are associated with IR and prediabetes, which were not totally explained by adiposity [40]. Our study results also shows that leptin levels are significantly correlated with both TG/HDL-C ratio and HOMA-IR index, the biomarkers of IR.
Leptin affects autonomic function by acting both centrally and peripherally. Leptin receptors in hypothalamic brain regions, implicated in cardiovascular control, may exert a stimulatory effect on sympathetic activation, which results in autonomic hyperactivity due to direct effects of neuropeptide systems, such as the melanocortin and corticotropin-releasing hormone [41]. Additionally, leptin receptors are abundantly present in the carotid body (CB) [42]. CBs contain glomus, which are polymodal chemoreceptors, also known as peripheral chemoreceptors that detect multiple chemical changes in oxygen, carbon dioxide, PH, insulin, and blood sugar levels. A recent study showed that leptin increases the carotid sinus nerve activity [43], which transmits chemosensory input from the carotid bodies to the medullary centers (central chemoreceptors) in a mouse model. However, it is not yet clear how plasma leptin regulate autonomic function in patients with diabetes and prediabetes. Obesity triggers inflammation and oxidative stress, which activates the carotid bodies. The overactivation of the carotid bodies can contribute to increased sympathetic system activity and lead to hypertension and IR in T2DM [44].
Risk factors associated with the severity of CAN
The pathophysiological mechanism of CAN development is multifactorial, and several studies have reported the important role of cardiovascular risk factors [7, 45,46,47]. CAN is a length-dependent pattern of disease, and parasympathetic activity can be damaged in the early phase of CAN with autonomic imbalance. As the disease progresses, sympathetic denervation occurs in the late stage of CAN [48]. Our results also shows that all parasympathetic parameters included in our study (e.g., HR_DB, VR, E:I ratio, 30:15 ratio) are significantly lower in patients with CAN than in those without CAN; however, the difference between sympathetic parameters (LF power, orthostatic BP change) is not obvious between groups. This finding is compatible with that of previous reports [2, 15, 16].
Regarding research on the relationship between leptin and CAN, one study from Japan shows that leptin is specifically associated with reduced HRV parameters in patients with DM compared with those without DM, with full adjustment of clinical parameters, which comprise quantitatively determined visceral adiposity [15]. Our results also show that leptin level is negatively correlated with all parasympathetic parameters included in our study (e.g., HR_DB, VR, E:I ratio, and 30/15 ratio). Another study from Korea shows a borderline significant positive correlation between leptin level and CAN score [16]. Autonomic dysfunction is seen early in the course of DM, and it occurs alongside alterations of various inflammatory adipocytokines [2]. Therefore, it highlights the concept of autonomic imbalance in the pathogenesis of CAN. With the progression of CAN, cardiovagal impairment is followed by sympathetic impairment. Increased leptin level could be secondary to autonomic imbalance or obesity-related leptin resistance [2].
Another important issue is whether the medications for modifying vascular risk factors (e.g., antihypertensive, antihyperlipidemic, and antihyperglycemic agents) can decrease leptin levels. One systematic review for meta-analysis of randomized placebo-controlled trials (RCTs) focuses on the impact of statin therapy on plasma leptin levels and shows that results are inconclusive [49]. Another meta-analysis of RCTs investigates the effects of pioglitazone on blood leptin levels in patients with T2DM and showed a significant difference, although relatively few RCTs are included, and a high level of statistical heterogeneity are found. Current studies have demonstrated that some antihypertensive medications may be more relevant than others in terms of reducing leptin levels in obesity. It seems that focus on pharmacologic suppression of the sympathetic nervous system, the renin–angiotensin system, and the blood pressure reduction effect is induced by weight loss. However, the results are also inconclusive [50]. Although our patients were well-controlled and almost all of them received medications to control the vascular risk factors, leptin levels remain significantly associated with the severity of CAN, according to both the CASS and CARTs score.
Study Limitations
This study has several limitations. First, visceral adipose tissue is a bioactive organ that secretes several adipokines (e.g., leptin) and proatherogenic cytokines involved in cardiovascular events. A previous clinical study showed that visceral obesity revealed a more significant correlation with IR compared to central obesity, assessed by WC [51]. However, other quantitative approaches to assess visceral obesity, such as dual bioelectrical impedance analysis for visceral fat measurement [52], should be considered for future studies. Second, current evidence supports that aerobic exercise, alone or combined with hypocaloric diet, improves symptoms of MetS, and also alters systemic levels of adipokines [53]. Clinical studies conclude that intensified multifactorial intervention (hyperglycemia, hypertension, dyslipidemia, and microalbuminuria) reduced the risk of CAN progression [54] in T2D and T1D [55]. Although we observed a close relationship among WC, leptin level and severity of CAN in our observational study, a longitudinal study is needed to confirm that control of WC can decrease leptin levels and can be effective in reducing CAN progression.
Conclusions
The coexistence of poor cardiovascular function, higher leptin level, and central obesity is demonstrated in patients with T2DM and prediabetes. It is been highlighted that these presentations are closely related to each other in this study. The results of mediation analysis provide possible pathophysiology for how both high WC and high leptin level adversely impact the severity of CAN. As the leptin level serves as mediator between central obesity and severity of CAN, a longitudinal study is needed to confirm that control of WC can decrease leptin levels and can be effective in reducing CAN progression.
Availability of data and materials
The data from this study can be acquired from the corresponding author upon reasonable request.
Abbreviations
- T1DM and T2DM:
-
Type 1 and type 2 diabetes mellitus
- CAN:
-
Cardiovascular autonomic neuropathy
- CASS:
-
Composite autonomic scoring scale
- CARTs:
-
The cardiac autonomic reflex tests
- TBARS:
-
Thiobarbituric acid-reactive substance
- HDL-C:
-
High-density lipoprotein cholesterol
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- IR:
-
Insulin resistance
- MetS:
-
Metabolic syndrome
- HRV:
-
Heart rate variability
- BMI:
-
Body mass index
- SBP and DSP:
-
Systolic and diastolic blood pressure
- WC:
-
Waist circumference
- DR:
-
Diabetic retinopathy
- TG:
-
Triglyceride
- eGFR:
-
Estimated glomerular filtration rate
- HBA1c:
-
Glycohemoglobin
- hs-CRP:
-
High-sensitive C-reactive protein
- UACR:
-
Urine albumin-creatine ratio
- HR_DB:
-
Heart rate response to deep breathing
- VR:
-
Valsalva ratio
- HUT:
-
Head-up tilt tests
- CB:
-
Carotid body
- RCTs:
-
Placebo-controlled trials
References
Collaboration NCDRF. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–42.
Lieb DC, Parson HK, Mamikunian G, Vinik AI. Cardiac autonomic imbalance in newly diagnosed and established diabetes is associated with markers of adipose tissue inflammation. Exp Diabetes Res. 2011;2012:878760.
Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48(9):e12997.
Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444(7121):847–53.
Rodríguez A, Ezquerro S, Méndez-Giménez L, Becerril S, Frühbeck G. Revisiting the adipocyte: a model for integration of cytokine signaling in the regulation of energy metabolism. Am J Physiol Endocrinol Metab. 2015;309(8):E691–E714.
Brennan AM, Mantzoros CS. Drug Insight: the role of leptin in human physiology and pathophysiology—emerging clinical applications. Nat Clin Pract Endocrinol Metab. 2006;2(6):318–27.
Valensi P, Paries J, Attali J, for Research FG. Cardiac autonomic neuropathy in diabetic patients: influence of diabetes duration, obesity, and microangiopathic complications—the French multicenter study. Metabolism. 2003;52(7):815–20.
Shin JA, Lee JH, Lim SY, Ha HS, Kwon HS, Park YM, et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J Diabetes Investig. 2013;4(4):334–43.
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85.
Cao Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 2013;18(4):478–89.
Spallone V, Bellavere F, Scionti L, et al. Recommendations for the use of cardiovascular tests in diagnosing diabetic autonomic neuropathy. Nutr Metab Cardiovasc Dis. 2011;21(1):69–78.
England J, Gronseth G, Franklin G, Carter G, Kinsella L, Cohen J, et al. Practice parameter: evaluation of distal symmetric polyneuropathy: role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review): report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 2009;72(2):177–84.
Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553–799.
Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115(3):387–97.
Kurajoh M, Koyama H, Kadoya M, Naka M, Miyoshi A, Kanzaki A, et al. Plasma leptin level is associated with cardiac autonomic dysfunction in patients with type 2 diabetes: HSCAA study. Cardiovasc Diabetol. 2015;14(1):117.
Jung CH, Kim BY, Kim CH, Kang SK, Jung SH, Mok JO. Association of serum adipocytokine levels with cardiac autonomic neuropathy in type 2 diabetic patients. Cardiovasc Diabetol. 2012;11(1):24.
El Dayem SMA, Battah AA, El Bohy AEM, El Shehaby A, El Ghaffar EA. Relationship of plasma level of chemerin and vaspin to early atherosclerotic changes and cardiac autonomic neuropathy in adolescent type 1 diabetic patients. J Pediatr Endocrinol Metab. 2015;28(3–4):265–73.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–52.
Lin W, Lee L, Chen C, Lo H, Hsia H, Liu I, et al. Optimal cut-off values for obesity: using simple anthropometric indices to predict cardiovascular risk factors in Taiwan. Int J Obes Relat Metab Disord. 2002;26(9):1232–8.
Yeh WC, Tsao YC, Li WC, Tzeng IS, Chen LS, Chen JY. Elevated triglyceride-to-HDL cholesterol ratio is an indicator for insulin resistance in middle-aged and elderly Taiwanese population: a cross-sectional study. Lipids Health Dis. 2019;18(1):176.
Yeh WC, Tsao YC, Li WC, Tzeng IS, Chen LS, Chen JY. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–44.
Chiu WC, Lai YR, Cheng BC, Huang CC, Chen JF, Lu CH. HbA1C variability is strongly associated with development of macroalbuminuria in normal or microalbuminuria in patients with type 2 diabetes mellitus: a six-year follow-up study. Biomed Res Int. 2020;2020:7462158.
Lu CH, Lin HC, Huang CC, Lin WC, Chen HL, Chang H-W, et al. Increased circulating endothelial progenitor cells and anti-oxidant capacity in obstructive sleep apnea after surgical treatment. Clin Chim Acta. 2015;448:1–7.
Wang HC, Lin WC, Lin YJ, Rau CS, Lee TH, Chang WN, et al. The association between serum adhesion molecules and outcome in acute spontaneous intracerebral hemorrhage. Crit Care. 2011;15(6):R284.
Ewing D, Clarke B. Diagnosis and management of diabetic autonomic neuropathy. Br Med J. 1982;285(6346):916.
Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc. 1993;68(8):748–52.
Low PA. Testing the autonomic nervous system. Semin Neurol. 2003;23(4):407–21.
Huang CC, Lee JJ, Lin TK, Tsai NW, Huang CR, Chen SF, et al. Diabetic retinopathy is strongly predictive of cardiovascular autonomic neuropathy in type 2 diabetes. J Diabetes Res. 2016;2016:6090749.
Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173.
Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–50.
Alexopoulos N, Katritsis D, Raggi P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis. 2014;233(1):104–12.
Sader S, Nian M, Liu P. Leptin: a novel link between obesity, diabetes, cardiovascular risk, and ventricular hypertrophy. Circulation. 2003;108:644.
Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–49.
Sanchez-Margalet V, Martin-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clin Exp Immunol. 2003;133(1):11–9.
Halle M, Persson P. Role of leptin and leptin receptor in inflammation. Am J Physiol Regul Integr Comp Physiol. 2003;284(3):R760–R762762.
Atkinson LL, Fischer MA, Lopaschuk GD. Leptin activates cardiac fatty acid oxidation independent of changes in the AMP-activated protein kinase-acetyl-CoA carboxylase-malonyl-CoA axis. J Biol Chem. 2002;277(33):29424–30.
Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, et al. Assessment of skeletal muscle triglyceride content by 1H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes. 2002;51(4):1022–7.
Tatti P, Masselli L, Buonanno A, Di Mauro P, Strollo F. Leptin levels in diabetic and nondiabetic subjects. Endocrine. 2001;15(3):305–8.
Soliman AT, Omar M, Assem HM, Nasr IS, Rizk MM, El Matary W, et al. Serum leptin concentrations in children with type 1 diabetes mellitus: relationship to body mass index, insulin dose, and glycemic control. Metabolism. 2002;51(3):292–6.
Wang G, Liu X, Christoffel KK, Zhang S, Wang B, Liu R, et al. Prediabetes is not all about obesity: association between plasma leptin and prediabetes in lean rural Chinese adults. Eur J Endocrinol. 2010;163(2):243–9.
Simonds SE, Cowley MA. Hypertension in obesity: is leptin the culprit? Trends Neurosci. 2013;36(2):121–32.
Porzionato A, Rucinski M, Macchi V, Stecco C, Castagliuolo I, Malendowicz LK, et al. Expression of leptin and leptin receptor isoforms in the rat and human carotid body. Brain Res. 2011;1385:56–67.
Shin MK, Eraso CC, Mu YP, Gu C, Yeung BH, Kim LJ, et al. Leptin induces hypertension acting on transient receptor potential melastatin 7 channel in the carotid body. Circ Res. 2019;125(11):989–1002.
Conde SV, Ribeiro MJ, Melo BF, Guarino MP, Sacramento JF. Insulin resistance: a new consequence of altered carotid body chemoreflex? J Physiol. 2017;595(1):31–41.
Rolim L, Sá J, Chacra AR, Dib SA. Diabetic cardiovascular autonomic neuropathy: risk factors, clinical impact and early diagnosis. Arq Bras Cardiol. 2008;90(4):e24–31.
Dafaalla MD, Nimir MN, Mohammed MI, Ali OA, Hussein A. Risk factors of diabetic cardiac autonomic neuropathy in patients with type 1 diabetes mellitus: a meta-analysis. Open Heart. 2016;3(2):e000336.
Lai YR, Huang CC, Chiu WC, Liu RT, Tsai NW, Wang HC, et al. HbA1C variability is strongly associated with the severity of cardiovascular autonomic neuropathy in patients with Type 2 diabetes after longer diabetes duration. Front Neurosci. 2019;13:458.
Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8(5):491–8.
Sahebkar A, Giua R, Pedone C. Impact of statin therapy on plasma leptin concentrations: a systematic review and meta-analysis of randomized placebo-controlled trials. Br J Clin Pharmacol. 2016;82(6):1674–84.
Bravo PE, Morse S, Borne DM, Aguilar EA, Reisin E. Leptin and hypertension in obesity. Vasc Health Risk Manag. 2006;2(2):163.
Kurniawan LB, Bahrun U, Hatta M, Arif M. Body mass, total body fat percentage, and visceral fat level predict insulin resistance better than waist circumference and body mass index in healthy young male adults in Indonesia. J Clin Med. 2018;7(5):96.
Cho D-H, Kim M-N, Joo HJ, Shim WJ, Lim D-S, Park S-M. Visceral obesity, but not central obesity, is associated with cardiac remodeling in subjects with suspected metabolic syndrome. Nutr Metab Cardiovasc Dis. 2019;29(4):360–6.
You T, Nicklas BJ. Effects of exercise on adipokines and the metabolic syndrome. Curr Diab Rep. 2008;8(1):7–11.
Gæde P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353(9153):617–22.
The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia. 1998;41(4):416–23.
Acknowledgements
The authors thank all the subjects who participated in this study.
Funding
This work was supported by grants from Chang Gung Memorial Hospital and Ministry of Science and Technology (Chang Gung Medical Research Project CMRPG8H0501 and CMRPG8J1211 to YR Lai, and NMRPG8H6082 to CH Lu).
Author information
Authors and Affiliations
Contributions
YRL participated in the design of the study and drafted the manuscript. WCC, CCH, NWT, BCC, and JFC participated in the sequence alignment and clinical evaluation of patients. MHC and WCL performed the statistical analysis. CHL and CCH conceived the idea for the study and participated in its design and coordination. They also helped drafting the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
This study conformed to the guidelines of the Declaration of Helsinki, and the study has been approved by the Institutional Review Board of Chang Gung Memorial Hospital (201800388B0C501 and 201901363B0).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lai, YR., Chen, M.H., Lin, W.C. et al. Leptin mediate central obesity on the severity of cardiovascular autonomic neuropathy in well-controlled type 2 diabetes and prediabetes. J Transl Med 18, 396 (2020). https://doi.org/10.1186/s12967-020-02559-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-020-02559-7