Abstract
Gastrointestinal stromal tumor (GIST) is the most common sarcoma located in gastrointestinal tract and derived from the interstitial cell of Cajal (ICC) lineage. Both ICC and GIST cells highly rely on KIT signal pathway. Clinically, about 80-90% of treatment-naive GIST patients harbor primary KIT mutations, and special KIT-targeted TKI, imatinib (IM) showing dramatic efficacy but resistance invariably occur, 90% of them was due to the second resistance mutations emerging within the KIT gene. Although there are multiple variants of KIT mutant which did not show complete uniform biologic characteristics, most of them have high KIT expression level. Notably, the high expression level of KIT gene is not correlated to its gene amplification. Recently, accumulating evidences strongly indicated that the gene coding, epigenetic regulation, and pre- or post- protein translation of KIT mutants in GIST were quite different from that of wild type (WT) KIT. In this review, we elucidate the biologic mechanism of KIT variants and update the underlying mechanism of the expression of KIT gene, which are exclusively regulated in GIST, providing a promising yet evidence-based therapeutic landscape and possible target for the conquer of IM resistance.
Video Abstract
Similar content being viewed by others
Summary
Imatinib resistance is the major obstacle for the cure of GIST, mostly due to second mutations within KIT gene for its reactivation. Hence, drug development during the past decades was focused on kinase inhibitors, aiming to broader the spectrum of KIT kinase mutations effectively inhibited, including sunitinib, regorafenib and ripertinib, this benefit, however, is modest compared with imatinib. The reason is the emergence of secondary resistant mutations showing highly heterogeneous, and these TKIs are active against only a subset of the KIT secondary mutational spectrum, which constitutes the main determinant for treatment failure in imatinib-resistant GIST. However, one of the most important characteristics of GIST cells in both treatment-naive or TKIs-resistant cells is that its high dependency on KIT signal that necessitate the recapitulation of the underlying biology or mechanism of KIT mutations and expression, with the purpose of developing novel drugs, alone or combined with current KIT-TKIs, to prevent or reverse resistance via the complete elimination of KIT oncogenesis.
Introduction
The tyrosine kinase(TK) KIT was described for the first time in 1987 as the human cellular homologue of the feline sarcoma viral oncogene v-kit [1]. It located on chromosome 4q12 and contains 976 amino acids, which encodes a transmembrane protein belonging to the type III family of RTK (receptor tyrosine kinases) [2, 3]. The extracellular segment contains five immunoglobulin-like structural domains (D1-D5), of them D1-D2-D3 are stem cell factor (SCF) binding regions, and D4-D5 are important units for KIT dimer formation [4]. KIT is a transmembrane glycoprotein with ligand-induced TK activity [5]. Its ligand SCF was identified in 1990 and it exists both as a membrane-bound and soluble form [6]. Upon binding of SCF, dimerization of neighboring KIT receptors is mediated by homotypic interactions at the D4-D5 interface. This is followed by asymmetric arrangements of the cytoplasmic region of the KIT dimers associated to trans-autophosphorylation and final kinase activation [7]. Physiologically, in addition to interstitial cells of Cajal (ICC), KIT receptor is expressed in germ cells, bone marrow stem cells, melanocytes, and mast cells. In response to SCF stimulation, KIT propagates the signal of survival and proliferation to ICC, maintaining the function of gut motility [8,9,10].
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal neoplasm located in the gastrointestinal tracts. The pathogenesis of GIST involve gain-of-function mutation in KIT (accounts for 75–80%), platelet-derived growth factor receptorα(PGFRA) (< 10%), or gene abnormalities including succinate dehydrogenase (SDH)-deficiency, the mutations of neurofibromin 1 (NF1), Kirsten rat sarcoma viral oncogene homolog (KRAS) and Harvey rat sarcoma viral oncogene homolog (HRAS) pro-oncogenes [11,12,13]. GIST cells arise from the musculature of ICC or their precursor cells and highly rely on the KIT expression for survival [14, 15]. KIT mutations is an early step for the development of GIST from ICC, meaning KIT necessitates the survival of GIST cells [16]. KIT mutations, usually occur at the intracellular juxtamembrane (JM) WW domain (encoded by exon 11), or in the membrane-proximal extracellular domain (encoded by exons 8 or 9), accounting for approximately 85–90% of KIT-mutant GIST, they endows KIT gene an oncogenic capacity to proliferate and form tumors via the intermediates of PI3K-AKT, JAK-STAT and RAS-RAF-MEK-ERK (MAPK) cascades [9, 12, 17,18,19].
With the discovery of this druggable KIT mutations, KIT-targeted inhibition with first line Imatinib (IM) become standard of care and offers meaningful clinical benefit in metastatic GIST patients [14, 20]. However, the primary KIT variants showing different sensitivity to IM, treatment resistance is common and occurs between 18–24 months of imatinib treatment due to resistant clones, warranting a switch to second and beyond-second lines of tyrosine kinase inhibitors (TKIs) including sunitinib, regorafenib and repritinib as per the guideline, but only with modest efficacy [21,22,23]. Furthermore, almost all of the patients will progress presented with the metastasis to multiple organs and each of these metastases, or even in the same primary lesion can have different genomic mutations with KIT [24, 25]. These range from point mutations or insertions to large indel variants, which exhibit variable biological traits, including intracellular mis-location, and downstream target molecules, which possibly as the determinants to the response to IM [26, 27]. Moreover, the sole transcription factors were identified in the regulation of these KIT mutants, showing much more different from that of wild type (WT) KIT [28, 29]. These distinct regulation of KIT in GIST could also be causative for its blocked degradation. In fact, KIT expression was observed in 95% of GIST, as a diagnostic algorithm using upfront immunohistochemistry (IHC) markers of KIT (CD117) positivity in GIST [30], and with IM treatment, KIT transcript or/ and protein derived from multiple heterogeneous KIT mutations in IM-resistant cells is higher than that in pre-IM counterparts [31,32,33], implying the compensatory up-regulation of KIT possibly contributing to secondary resistance to IM. Here, we summarize various of KIT variants and its core regulated network, focus on the process of gene regulation, transcription and protein translation, with emphasis on the therapeutical vulnerability and clinical strategy for targeting oncogenic KIT kinase dependency in GIST.
KIT mutations and expression in GIST
KIT mutations
Within the larger group of KIT-mutated GIST, different mutations’ hotspots have been reported, of which the vast majority of KIT mutations are found in exon 11 coding for JM (66–71%), exon 9 coding for extracellular domain (13%), exon 13 coding TK domain I (ATP binding pocket) (1–3%), and exon 17 coding for TK domain II (activation loop) (1–3%). The ATP-binding pocket, encoded by exon 13 and 14, whose mutations directly interfere with IM binding, or the activation ring, where mutations can stabilize the KIT's active conformation [12, 34]. The gain-of-function of KIT mutations resulting in the constitutive activation of the protein, genomic context and alternative signaling pathways, also largely affect the efficacy of IM, as well as sunitinib, and regrifinib and ripritinib, which mostly due to the different transcriptional programs observed in GIST with various genotypes that influenced the protein active structures, dimerization affinity, and cellular localization [35]. Indeed, clinically, most GIST show strong, diffuse cytoplasmic staining, whereas nearly half show concurrent dot-like (bGolgiQ pattern) staining and occasionally, only dot-like or even membranous staining is seen [36, 37]. In vitro GIST cell lines, different KIT mutations were detected, such as classical GIST-T1 (primary mutation in KIT exon 11—Δ560–578) and GIST-882 (primary mutation in KIT exon 13—K642E) cell models, the biological effects upon imatinib treatment can be significantly different [38,39,40]. Notably, as shown in Table 1, the involved down-streams varied across different KIT genotypes, and which also be compelling factors impact tumor behavior and IM sensitivity.
KIT expression
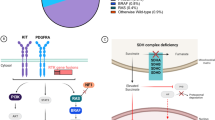
Generally, DNA expression is regulated by the cis-regulatory elements (CREs), which consists of enhancers and super-enhancers transcription factors which comprise of transcription factors and the recruited co-activators and RNA Polymerase II (RNA Pol II). Over-expression of KIT in GIST results from the dysregulation of a large enhancer domain in the DNA strand, but rarely related to its DNA amplification [61]. CHIP-seq of GIST tumor samples and cell lines identified the enhancer domain to be driving KIT gene expression, which is unique and essential for KIT gene expression and cell viability in GIST. Accordingly, exclusive transcription factors model the expression of GIST-type mutant KIT gene which facilitates tumorgenesis and progression [62]. Moreover, GIST cells are highly dependent on KIT expression, which was due to epigenetic regulation rather than amplification in KIT oncogene [61]. However, as more epigenetic mechanisms elucidated in mesenchymal tumors, reversible epigenetic changes can be identified and appears to be an important novel approach to understand the formation, prognosis and therapeutic strategies of various types of mesenchymal tumors [63,64,65]. Figure 1 shows KIT mutations and expression in GIST and its role in epigenetics and multifaceted biological modifications.
Gene and epigenetic regulations of KIT expression in GIST. KIT extracellular segment contains five immunoglobulin-like structural domains (D1-D5), D1-D2-D3 are stem cell factor (SCF) binding regions, and D4-D5 are important units for KIT dimer formation. In the absence of ligand stimulation, two adjacent KIT protein D4 structures remain independent due to charge repulsion. KIT mutations usually arise in the extracellular domains (exon 9), the juxtamembrane domain (exon 11) and in the kinase I and II domains (exons 13 and 17). The SCF binding to KIT and then changes the conformation of KIT and promote its dimerization, thereby activating tyrosine kinase activity in the intracellular segment by recruiting and phosphorylating substrates, thus forming signal transduction. But once KIT mutated, it disrupts its self-inhibition mechanism, resulting in continuous activation of signaling pathways such as Ras-Erk within the cell. FOXF1, ETV1 and HIC1 together form the core TF network in GIST binding enhancer and/or promoter and then promoting KIT expression. BRD4 (function as “readers”), HAND1 and BRAX1 positive regulate the core TF network. Transcription factor MITF and BIRC5 promote KIT transcription. MiRNA-20a, miRNA-17–92, miRNA200b-3p, miRNA-494, miRNA-148-3p are tumor suppressors downregulate KIT transcription and the majority of them directly bind to the 3’-UTR domain of relevant mRNA; the first three mentioned miRNAs can inhibit ETV1 mRNA levels, miRNA-494 can inhibit BIRC5 expression suggesting a negative feedback mechanism. SH3BP2 promotes the expression of mutant KIT by up-regulation of the expression of MITF and ETV1. FTO, functions as a “eraser”, promoting m6A demethylation increases the expression of KIT. Normal KIT chromosomal has CTCF insulator creating a topological boundary. Once CTCF insulator displaced by DNA methylation, allowing the super-enhancer to contact and induce KIT
Regulation of KIT coding gene
The regulations of gene are mainly connected with transcription factor (TF), which directly interpret the genome, and no exception in the change of KIT gene [35, 66, 67]. It seems that TFs forming the regulatory network exclusively act on the enhancers of KIT gene in GIST, showing its rudiment [62, 68,69,70]. In addition, the study of how TF linked to essential chromatin regulators will also provide important insights into the gene regulation and epigenetic mechanisms of GIST [71].
Forkhead box F1 (FOXF1) is a member of the forkhead box (FOX) family, which contains a highly conserved DNA binding region (DBD) [72]. As one of the key TFs, FOXF1 is required for the lineage differentiation of ICCs, as well as the growth of GIST cells. FOXF1 functions as a pioneer factor that modulates the chromatin accessibility for ETS translocation variant 1 (ETV1), after which they accumulate in the enhancer domain of the mutant KIT gene, promoting KIT expression [73, 74]. ETV1, an ETS family transcription factor, is located on chromosome 7p21 [75]. It is involved in the tumorigenesis of multiple cancer types, including prostate cancer and melanoma, where it regulates distinct transcriptional programs [76]. ETV1 is also a master regulator of the ICC lineage, and it is essential for the development of the subtypes of ICCs which are sensitive to oncogenic KIT-mediated transformation. Moreover, ETV1 directly binds to the enhancer and promoter regions of KIT gene and thus forming a positive feedback regulation that advances their expression [70]. Notably, the protein of ETV1 is highly unstable, and active mitogen-activated protein kinase (MAPK) can increase its stability. KIT mutants activate the downstream, MAPK, leading to the stabilization of the ETV1 protein and oncogenic ETS transcript program. Co-activators cyclic AMP-responsive element-binding protein (CREB)-binding protein (CBP) and p300 interact with an oncoprotein ETS translocation variant 1(ETV1), which directly acetylates ETV1, thus enhancing its stability, DNA-binding capacity, and transcriptional activity in vitro [77].
This mechanism of ETV1 in GIST differs from that in the other ETS-dependent tumors, such as genomic translocation or amplification in prostate cancer [78]. FOXF1 loss results in decreased ETV1 protein, and global loss of ETV1 chromatin binding. Therefore, FOXF1 inhibition causes the reduction of KIT approximately by 2-to-eightfold than ETV1 inhibition does, while ETV1 knockdown showed no impact on FOXF1 expression. These data indicate that the regulation of FOXF1 in GIST may be pre-determined in ICCs precursors [73].
Heart and Neural Crest Derivatives Expressed 1 (HAND1) is a key TF involved in the placentation and morphogenesis of the heart [79], and its deficiency during embryogenesis can cause congenital cardiac defects or adult heart failure [80]. Similarly, HAND1 is involved in the transcriptional amplification of the KIT oncogene via its influence on the expression or protein interactions of the core TF network of KIT [35], including ETV1, HIC1 (hypermethylated in cancer 1), FOXF1 and other GIST-correlated protein, and G protein-coupled receptor 20(GPR20) [81]. Physiologically, Homeobox protein BarH-like 1 (BARX1) controls the development of gastric smooth muscle and spleen, as well as intestinal rotation [35]. Similar to HAND1, BARX1 is a TF localized in the nucleus, which is closely correlated with indolent and micro-GIST, albeit the underlying mechanism remaining unclear [82].
The c-Abl Src homology 3 domain-binding protein-2 (SH3BP2) has 561 amino acids, containing an SH3-binding proline-rich region, an N-terminal pleckstrin homology (PH) domain, and a C-terminal SH2 domain [83, 84]. It serves as a cytoplasmic adaptor protein, which is generally expressed in GIST cells. The epigenetic silencing of SH3BP2 leads to decreased oncogenic KIT and PDGFRA expression at both mRNA and protein levels [84]. SH3BP2 promotes the expression of mutant KIT via up-regulation of the expression of microphthalmia-associated transcription factors (MITF) and ETV1. Conversely, KIT can expedite the expression of SH3BP2 and MITF, demonstrating a positive feedback loop that exists in these molecules [85]. Silencing of SH3BP2 induces miRNAs (miR-1246 and miR-5100) expression which targets MITF and ETV1, thus decreasing KIT expression [86]. A recent study found that reconstitution/recombination of MITF restored KIT expression levels in SH3BP2-silenced cells and restored cell viability in mesenchymal tumors, while also reducing MITF and ETV1 expressions [87]. Meanwhile, SH3BP2 silence can also attenuate PI3K activation induced by KIT kinase [84]. In GIST, TFs such as HAND and the core TF network constitutes a large positive feedback system for KIT mutant gene expression, and this positive feedback promotes the development of mesenchymal tumors. The common regulatory mechanism of these transcription factors in the regulation of KIT-encoding genes is unclear warranted to be further investigated.
Epigenetic modification
Epigenetic modifications are closely associated with genome-wide transcriptional regulation in cancer, and it has also been extensively studied in GIST pathogenesis, including progression and drug resistance. It refers to a stable heritable change in biological phenotype or gene expression without a change in nucleotide sequence [88, 89]. The key molecular mechanisms of epigenetic modification of DNA and chromatin can be divided into four main categories: DNA and RNA methylation [90, 91]; non-coding RNA; covalent post-translational histone modifications and non-covalent mechanisms [92]. All epigenetic changes and regulation are mediated through epigenetic enzymes (EEs), the functions of which can be divided into three categories: writers (responsible for modifications); erasers (remove modifications); and readers (identify and direct these modifications to the correct location) [93]. In KIT-mutated GIST, chromosomes are frequently lost at 1q, 13q, 14q, 22q, etc., involving Protein phosphatase 1A (PPM1A), kinesin family member 1B (KIF1B) and neurofibromin 2 (NF2) gene, but this loss does not occur in WT GIST, which show the alternative epigenetic alterations [28]. Therefore, in this section we explore the role of several epigenetic modifications in relation to KIT and the possible implications for future development.
DNA and RNA methylation
DNA methylation is generally the 5-methylcytosine that occurs on the CpG islands [94] and is often described as a "silent" epigenetic marker [91] that plays important role in the maintenance of imprinting, genomic stability, development, and gene regulation in cancer [63, 95, 96]. Endoglin (ENG, CD105), a transmembrane glycoprotein expressed on activated vascular endothelium and other cells, is a co-protein of the transforming growth factor-h (TGF-h) receptor system. High expression of the ENG gene has been demonstrated in human as well as in mouse model of GIST[96, 97]. And the elevated ENG expression in KIT oncogenic mutants appears to be indirectly mediated by DNA hypomethylation, but the mechanism of this DNA methylation regulation is not fully understood [98]. The DNA-binding insulator protein CCCTC-binding factor (CTCF) and cohesion define the boundaries of chromosomal domains, also called topologically associated domains (TADs) [99,100,101]. It is reported that the super-enhancer was shared by Anoctamin 1(ANO1), which encodes the GIST clinical biomarker also known as DOG-1 (‘Discovered on GIST-1’) and FGF3/4, which reside in a ~ 250 kb TAD flanked by boundaries that contain CTCF binding sites, and the adjacent TAD on the 11q side contains a large cluster of super enhancer (SE), was recently observed in SDH-defcient GIST. In SDH-defcient GIST, DNA CpG methylation replaced CTCF binding in the FGF and KIT locus leading to abnormal contact between the starter switch and oncogenes [102]. So, it is postulated that in GIST, continuation of IM-based therapy for IM-resistant GIST might facilitate disease progression by promoting the malignant behavior of tumors in an FGF2-dependent manner.
Epi-transcriptional modifications are emerging as promising therapeutic targets in cancers. The most important classification of RNA methylation is the modification of RNA [103], including N6-methyladenosine (m6A) and 5-Methylcytosine, etc. [104]. As the most important classification of RNA methylation, the mechanism of M6A modification has been described in the development and treatment of different tumors, such as liver cancer, breast cancer and non-small cell lung cancer [105,106,107]. N6-methyladenosine of messenger RNA (mRNA), mainly occurring around the coding region or the stop codon of the 3´UTR, is the most abundant internal modification in mRNA. The levels of methyltransferase-like 3 (METTL3) is elevated and confer to Imatinib resistance in GIST patients, because METTL3 mediate the m6A modification on the 5’UTR of the multidrug transporter MRP1 mRNA, which facilitating MRP1 mRNA translation [108]. Besides, METTL3-mediated N6-methyladenosine (m6A) modifications facilitate miR-25-3p maturation which progression of GIST [109]. The fat mass- and obesity-associated (FTO) gene, termed as obesity-related gene, is located on chromosome 16q12.2 and is repurposed as a mRNA N6-methyladenosine (m6A) demethylase. A series of small-molecule compounds targeting FTO is developing therapeutic option in acute myeloid leukemia (AML) [110].
Histone modification
Epigenetic modifications of histones, including methylation, phosphorylation, ubiquitination and acetylation, are the key processes for genes expression in GIST oncogenesis. Lysine (K)-specific demethylase 4D (KDM4D), as a histone demethylase, is overexpressed in GIST and promotes the progression of GIST via HIF1β/VEGFA signaling [111]. Bromodomain-containing protein 4 (BRD4) is a member of bromodomain and extra-terminal domain-containing (BET) family that include the proteins of BRD2, BRD3, BRD4, and BRDT. These proteins function as “readers” that tether acetylated lysine residues to both histone and non-histone proteins such as TF, and control genes expression. Accumulating evidences showed that BRD4 can regulate the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) signaling, as a determinant in the progression of various cancers [112]. In GIST, BRD4 binds the acetylated lysine in histones or TF in enhancer or recruited pTEFb to initiate NFκB-dependent acetylated histones, promoting the transcription of c-KIT and C–C motif chemokine ligand 2 (CCL2), which is a chemokine recruiting macrophages to tumor functions as an immunosuppressor [113].
Monocytic zinc finger (MOZ) is a histone acetyltransferase that mediate the activation of histone acetylation in a complex with other molecules [114]. Using Genome-scale CRISP screening, the chromatin modifying enzymes, KAT6A/MOZ and KMT2A/MLL1 was found to be co-dependent and co-localized with GIST-associated genes and regulate oncogenic transcription and cell cycle progression by regulating transcription factor gene expression programs [115, 116]. KMT2A/MLL1 combined with Menin-MLL complex, is responsible for H3K4 methylation and transcription activation. Small molecules against MOZ or Menin remarkably reduced GIST tumor growth with synergistic toxicity in vivo and in vitro with the combination of KIT inhibitors [117].
Non-coding RNAs on KIT gene expression
Non-coding RNAs (ncRNAs) are functional RNAs that is not be translated to proteins but as messager RNAs(mRNAs) functioning as regulators [118]. Micro-RNAs (miRNAs), a subtype of ncRNAs, are small endogenous RNAs of 19 ~ 22 nucleotides (nt) that regulate post-transcriptional silencing of target genes, usually in the 3′UTR [119]. Long non-coding RNAs (lncRNAs) are another important ncRNAs with more than 200nt transcripts. LncRNAs can fine-tune the expression of neighbor genes in a distinct context-dependent way via multifaceted mechanisms [120]. The largest study on miRNAs on primary tumors and metastases highlighted perpetuation of miRNAs features in metastatic lesions and that the primary origin appears to be the main determinant of the metastases miRNA profile [121].
miRNAs
miR-218 which is a tumor suppressor directly bind to the 3’-UTR domain of relevant mRNA in lung cancer [122]. Similarly, in KIT-mutant GIST cells, miR-218 can exert its silencing effect on KIT expression by the direct bond to the 3′UTR of the KIT mRNA [123]. The Imatinib-resistant cell line, GIST430, can be re-sensitized to imatinib when the cells are transfected with miR-218, which possibly targets the PI3K/AKT pathway, KIT, or/and STAT3 molecules [124, 125]. MiR-148b-3p was largely described in the constraints of neoplastic transformation [126]. By binding to the nucleotides 1378–1393 and 1639–1656 of the 3’-UTR of KIT mRNA, miR-148b-3p can directly down-regulate the level of KIT transcript. However, a negative feedback loop causing KIT overexpression with an unknown mechanism offset the inhibitory role of miR-148b-3p. Therefore, miR-148b-3p alone shows no impact on the GIST cells, although it can synergize with IM to suppress the invasion and proliferation of GIST cells[48]. The correlation between downregulation of miRNA-221/222 and KIT expression was reported in several studies, indicating a tumor suppressor-miR in GIST [127, 128]. In vitro, the over-expression of miRNA-221/222 causes cell cycle arrest, apoptosis induction, and the inhibition of cell proliferation via decreasing KIT expression in erythroleukemic cells and erythropoiesis [129]. It binds to 3’UTR of KIT mRNA, where the rs17084733 variant interrupts the binding site of miR-221/222 in GIST cells [47, 130]. In addition, the expression of other miRNAs, including miR-142-5p [131], miR-9, miR-370, miR-494, and miR-501 [132], was negatively associated with the expression of the KIT gene in GIST cells. MiR-494 overexpression caused KIT downregulation and the reduction of its downstream p-AKT and p-STAT3 proteins via decreasing the expression of survivin (BIRC5), an important TF for KIT, which phenotype the KIT inhibition in GIST 882 [124, 133]. Additionally, miR-17–92, miR-200b-3p and miR20a cluster strongly downregulate the mRNA levels of the ETV1, which is the key TF in the regulation of the KIT gene [134, 135].
LncRNAs
The lncRNA coiled-coil domain-containing 26 (CCDC26) was identified as a retinoic acid-dependent modulator and plays an important role in the pathogenesis of glioblastoma [136]. CCDC26 can inhibit cell proliferation via directly decreasing the KIT expression in myeloid leukemia [137]. It also interacts with c-KIT as shown by the assay of RNA pull-down. CCDC26 knockdown increases the level of c-KIT mRNA, up-regulates insulin-like growth factor 1 (IGF-1R) expression, resulting in IM resistance, whereas IGF-1R inhibition reversed IM resistance [138, 139]. H19 and FOXF1 adjacent non-coding developmental regulatory RNA (FENDRR) are oncogenic lncRNAs that are associated with cancer invasion, proliferation, and migration in various types of cancers [140]. Their expressions vary in GIST tissue, where H19 is 25.8-fold while FENDRR is 4.7-fold, both were in comparison to normal adjacent tissues [141]. Highly positive correlations between H19 and ETV1 were found in GIST cells, maybe via MEK and ERK pathways, as detected in colorectal cancer [142], and the mechanism underlining the regulation of FENDRR on the expression of KIT is not yet fully explored (Table 2).
Translation and post-translation regulation
The vast majority (95%) of GIST express KIT protein which is usually higher in the GIST with KIT mutations than those without KIT mutations [143]. Upon the IM treatment, c-KIT oncoprotein was substantially up-regulated, hinting as a protective mechanism for GIST cells to escape from TKIs, which was widely accepted as one of the major resistance mechanisms [144] (Fig. 2).
Regulations in KIT protein and signaling pathway. LMTK3 directly promotes KIT protein translation, but also inhibit the expression of PKC (PKC-θ) and KIT phosphorylation. LIX1 controls MAPK pathway. Hedgehog pathway has two activate models: with ligands, HHs binding to the receptors, Patched-1/2, initiate the Hh pathway and inhibit SMO; without ligands, SMO starts to activate different GLIs, GLI1/2 up-regulate the KIT mRNA level, while GLI3 down-regulates KIT mRNA levels via the proteasome pathway. There are three classic intracellular signaling pathways for KIT activation, respectively Ras-Erk pathway, PI3K-AKT pathway and JAK-STAT pathway. Ras-Erk pathway and PI3K-AKT pathway have crosstalk with Hh pathway. FGF2 binds to and activates its receptors FGFR (FGFR3), mediating the reactivation of mutant KIT and JAK-STAT pathway. AMPD3 also promotes the JAK-STAT pathway. FGFR3 and AMPD3 both form a positive protein–protein feedback loop with mutant KIT. MiRNA-218 can inhibit JAK-STAT pathway and P55PIK can promote PI3K-AKT pathway. ACK1 activates the MAPK pathway and the PI3K pathway, whereas lix1 may only regulates the MAPK pathway
Protein translation rate
Protein translation was another rate-limiting process of KIT expression. Lemur tyrosine kinase-3 (LMTK3) is a kind of serine/threonine kinase that regulates genes transcription, translation, and the stability of proteins via chromatin condensation and binding of the chromatin to the nuclear periphery, or tethers to DNA region for its function as tumorigenesis-promoting [145, 146]. Intriguingly, LMTK3 specifically modulates KIT gene-specific translation in GIST and melanoma cells, but not in the mast cell line and primary leukemia. LMTK3 also accelerates the translation rate of the KIT gene, resulting in secondary mutations that further leads to KIT resistance to IM [147, 148].
Protein translocation and stabilization
The auto-phosphorylation of KIT mutant is spatiotemporally regulated. The fully transformed products of KIT mutants, KIT onco-proteins were usually in the endoplasmic reticulum, transferring to the Golgi apparatus and activated as an immature KIT protein form [149,150,151]. Protein kinase C (PKC) is a superfamily characteristic of phospholipid-dependent serine-threonine kinases. It physiologically regulates cell stability and differentiation. As one member of PKC, Protein kinase C-θ (PKC-θ) is highly expressed in the ICCs of the digestive tracts of guinea pigs, and in GIST ranging from 72 to 98% [152]. PKC-θ promotes the Golgi complex retention of mutant-KITs and blocks its proteasomal degradation, thus sustains the activation of abnormally localized intracellular activation of MT-KITs [151, 153]. PKC-θ over-expression was significantly positively correlated with KIT expression and poor clinicopathological characteristics and worse prognosis [154].
Signal pathways involved in KIT oncogenesis and expression
Mutations on KIT often result in abnormal activation of kinases that activate downstream MAPK, STATs and PI3K pathways, promoting tumorigenesis and malignant progression [155, 156].
Hedgehog signaling pathway
The genes correlated with the Hedgehog (Hh) pathway are robustly expressed in ICC stem cells and mature cells of human and murine intestines, indicating its indispensable role in the development of epithelium and mesenchymal cells in GI tract. Physiologically, the ligands (HHs), sonic Hedgehog (Shh), Indian Hedgehog (Ihh), and Desert Hedgehog (Dhh), bind to the receptors, Patched-1 (Ptch-1) and Patched-2 (Ptch-2), respectively, thereby initiating the Hh pathway [157, 158]. Loss of the 7-pass transmembrane protein Smoothened (SMO) inhibition leads to the regulation of Hh pathway which controls the expression levels of KIT mRNA via its downstream glioma-associated oncogene homolog isoform1, 2, and 3(GLI1, 2, or 3) [159]. In the absence of ligand, SMO starts to activate the hedgehog pathway [160]. Various GLI1, 2, and 3 have different roles in the expression of KIT mRNA. GLI1/2 up-regulate the KIT mRNA level, while GLI3 is a transcriptional repressor on KIT mRNA levels via the proteasome pathway [161]. Besides, the Hh pathway has crosstalk with PI3K/AKT/mTOR and RAF/MAPK/ERK signal cascades involved in the KIT regulation [162]. In vivo, targeting the Hh pathway can reduce KIT mRNA and re-enhance the sensitivity of GIST cells to TKIs.
PI3K signaling pathway
PI3K pathway is the dominant signal directly engaged by mutant KIT oncogenic cascade in GIST, therefore, PI3K inhibitors showed meaningful efficacy when combined with IM is ongoing in the clinical trials for the treatment of GIST [163]. In mutant KITs, they direct active themselves and engaging PI3K pathway to promote imatinib resistance. For example, in double-mutant KitV558Δ; Y567F/Y567F knock-in mice which lack the SRC family kinase-binding site on KIT (pY567) exhibited attenuated MAPK signaling, and engagement of the PI3K pathway for tumor growth [164], as shown in Table 1. Phosphoinositide-3-kinase, regulatory subunit 3 (gamma) (PIK3R3, p55PIK) is a regulatory subunit of phosphoinositide 3-kinase (PI3K) and is involved in ICC hyperplasia which is prone to the tumorigenesis of GIST [32, 165]. Over-expression of p55PIK in GIST882 cells bind the promoter and increase the expression of NF-κB p65 (Ser536), leading to resultant KIT upregulation. Similar mechanism of p55PIK activating the NF-κB signal was also observed in the colorectal cancer cells [166]. Non-RTK activated CDC42 associated kinase 1 (ACK1), was colocalized and form complex with KIT protein in GIST cells and ACK1 activation is in a partially KIT and CDC42 dependent manner. Treatment with a specific ACK1 inhibitor AIM-100 or ACK1 siRNA markedly inhibits cell migration in imatinib sensitive and in imatinib resistant GIST cell lines, which is associated with inactivation of PI3K/AKT/mTOR and RAF/MAPK signaling pathways [167]. Recently, a study of PD-1/PD-L1 blockade rescue exhausted CD8 + T cells via the PI3K/Akt/mTOR signaling pathway in GIST revealing the involvement of PI3K in immunotherapy in GIST [19].
FGF2- FGFR3 and JAK/ STAT signaling pathway
Fibroblast growth factors (FGFs) and their receptors (FGFR1-4) are universally expressed in human tissues and have an important effect on various cell physiologic processes ranging from cell proliferation, survival, and migration. The dysregulation of the FGFs signal pathway is extensively involved in several types of cancers [168]. FGF2 is highly expressed in Imatinib-resistant GIST cells and the tumor tissue from the patients who progressed on Imatinib [169]. FGF2 binds to and activates its receptors FGFR, mediating the reactivation of KIT and MAPK pathways [170]. The opening of CTCF binding in the chromosome topology of FGF and KIT mentioned above leads to the increase of their expression, which may demonstrate the close relationship between FGF and KIT in the development of resistance in GIST. The Janus kinase/signal transducers and activators of transcription (JAK/ STAT) pathway is one of the important downstream pathways by KIT activating [171]. KIT D816V and KIT N882 both are situated closely on the KIT receptor activation loop activating the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway, but in KIT N822K it is also the downstream activation of the MAPK [172]. The V558Δ; V653A mutant mislocalization of Golgi, and lead to the enhanced activation of STAT3 and STAT5, although no differences were seen in MAPK or PI3K pathway activation, therefore, contributing to the increased tumor oncogenesis, compared to control mice with a single V558Δ Kit mutation. Blocking KIT’s localization to the Golgi from the endoplasmic reticulum (ER) can inhibit oncogenic signaling [173].
MAPK signaling pathway
AMPD3, belonging to the adenosine monophosphate deaminases (AMPDs), functions as a main catalyzer which hydrolytically deaminates AMP to inosine monophosphate. This is a vital step in nucleotide metabolism aiding and energy balance in cells. AMPD3 is extensively expressed in tumor tissues, and its knockdown may favor the activation of AMPK, and subsequently result in the inhibition of the anabolic pathways which is prerequisite for the survival of cancer cell [174]. AMPD3 is significantly related to KIT expression in GIST. Interestingly, when the expression of KIT or AMPD3 was suppressed by siRNA, respectively, the expression of AMPD3 or KIT was also comparably decreased. These results indicate that KIT and AMPD3 may form a positive protein–protein feedback loop to promote their reciprocal expression, albeit their underlying mechanism remaining unknown [175]. Limb Expression 1 (LIX1) is a unique marker of digestive mesenchyme immaturity. It regulates mesenchymal progenitor proliferation and differentiation by controlling the Hippo effector Yes-associated protein 1 (YAP1), which is constitutively activated in many sarcomas [176, 177]. In GIST, upon LIX1 inactivation in GIST cells, YAP1/TAZ activity is reduced, KIT, as the GIST signature, is down-regulated via reducing YAP1/TAZ protein level, and cells acquire smooth muscle lineage features [178]. Moreover, LIX1 can controls MAPK signaling pathway [179]. Upon the condition of hypoxia during IM treatment in GIST, hypoxia inducible factor 1 alpha (HIF-1α) can increase the transcription level of MET gene via its bind to the MET promoter [180]. Ligation of upregulated MET by hepatocyte growth factor (HGF) expand the activation of the downstream of mitogen-activated protein kinase (MAPK). And the latter then stabilizes ETV1 for promoting KIT expression [181, 182]. These results strongly suggested that reactivation of MAPK by bypass signal may represent a therapeutic vulnerability for targeting KIT expression.
Targeting KIT expression and mutation in GIST
In screening a compound library enriched for U.S FDA-approved chemotherapeutic agents, GIST cells displayed high sensitivity to transcriptional inhibitors, and mechanistically, these compounds exploited the cells dependency on continuous KIT expression. For example, Mithramycin A inhibits the TF, SP1, which is also a major transcriptional activation of the KIT gene, thus explaining why Mithramycin A induces apoptosis [183]. These indicated that it is plausible to target KIT abnormal regulatory-circus, together with kinase activity-inhibition in GIST treatment (Fig. 3).
The mechanism of action of existing drugs targeting KIT expression and mutations. In this figure we describe several inhibitors targeting KIT expression and function. Therapeutic strategies of TKIs targeting KIT mutations in gastrointestinal stromal tumors: fist-line therapy-Imatinib; second-line therapy-Sunitinib; third-line therapy-Regorafenib; fourth-line therapy-Ripretinib. Other new drugs targeting KIT mutations in gastrointestinal stromal tumors: Avapritinib, Larotrectinib, Entrectinib, Bezuclastinib, Carbozantinib, Sorafenib. The c-KIT-Hsp90Β-Apaf-1 complex inhibits the ubiquitination degradation of mutant KIT. Bortezomib binds to Cbl, destabilizing the c-KIT-Hsp90Β-Apaf-1 complex and releasing Apaf-1, and then KIT proteins are internalized and degraded in GIST cells. IPI-504, IPI-493, TAS-116, AT13387 and NVP-AUY922 are HSP90 inhibitors. HDAC inhibitors, SAHA and LBH589, attenuate the activity of HSP90 by acetylating on HSP90 gene. For Hh pathway, HHs, SMO and GI1/2 have their own inhibitors. PI3K/mTOR inhibitor voxtalisib, the pan-PI3K inhibitor pilaralisib, and the PI3K-restricted inhibitor alpelisib all reducing GIST cell proliferation. TAT-N24 and the emerging PI3K or P55PIK inhibitors, such as Copanlisib, both inhibit NF -κB. BGJ398, PD173074 and nintedanib are FGFR inhibitor targeting FGFR1-4. ACK1 inhibitor AIM-100 or ACK1 siRNA inhibits ACK1. CS-1 and CS-2 are functioned as FTO inhibitors preventing KIT m6A mRNA demethylation. BBIs can reverse the transcription abnormalities of KIT gene which are induced by BRD4. Mithramycin A inhibits the TF, SP1, and HZ1 decreases the transcriptions of OSR1
Targeting special transcriptional factors
Clinically, both HAND and BARX1 can be positive predictors for the progression or relapse events in the patients with metastatic GIST or post-operation GIST, respectively. HAND was enriched in small intestine ICCs while BARX1 was enriched in micro-GIST [35], thereby offering site-dependent targets for GIST treatment. In vivo, ETV1 is a critical survival factor for the growth of Imatinib-sensitive and Imatinib-resistant GIST cell lines [70]. Double inhibition of MAP kinase and KIT signaling can synergistically destabilize ETV1 by interrupting the positive feedback loop of KIT-MEK-ETV1-KIT. Recent Phase II clinical trial showed an overall response of 69.0% and median progression free survival of 29.9 ms in the treatment-naïve patients with advanced GIST using MEK inhibitor Binimetinib plus Imatinib [184, 185]. In addition, SHBP2 and FOXF1 could be an ideal target for being further explored therapeutically in the treatment of GIST.
Phenothiazine is a cytotoxic drug that induces apoptosis and autophagy that showed synergistic role with ERK-inhibitor in the treatment of GIST [186]. CDK7 mRNA and protein levels are elevated in high-risk GIST and indicated of poor clinical outcomes, in which CDK7 is a key activator of RNAPII that preferentially dysregulate RNAPII CTD phosphorylation. THZ1 blocks the role of CDK7 on RNAPII, and thus decrease the transcriptions of odd-skipped related transcription factor 1(OSR1), which is one of super enhancer (SE) of KIT gene in GIST [187]. Similarly, KIT-regulated enhancer domain in GIST could be targeted by BRD4, a crucial activator of RNAPII transcription at active chromatin marks, and the BBIs can reverse the transcription abnormalities of targeted genes which are induced by BRD4 [67, 188].
Targeting epigenetic modification molecules
The combination of BBI and TKI led to superior cytotoxic effects in vitro and in vivo, with the advantage of preventing tumor growth in TKI-resistant GIST xenografts. The expression profiles of GIST cells treated with BBIs were akin to those treated with KIT inhibition, indicating the KIT expression be the target of BBIs [113]. Also, BET bromodomain is becoming an attractive target for KIT-mutant GIST as shown in clinical trials. A synthetic analogue of cerulenin, JQ1, has been shown to potently inhibit BRD4 and exhibited synergy with imatinib and induced apoptosis and autophagy in vitro [188].
Epi-transcriptional modifications on mRNA mainly influence its stability and expression, several molecules function as “writers” or “erasers” for the regulation of mRNA. Our previous study showed that FTO inhibition can dramatically inhibit cell proliferation and increase sensitivity of GIST cells to IM-induced apoptosis. CS-1 and CS-2 were recently repurposed as a FTO inhibitor and exhibited the synergistic effect with IM in KIT-mutated GIST [189, 190].
Targeting ncRNA
With the successful development of mRNA COVID-19 vaccines and the approval of a number of novel RNA-based drugs, RNA has jumped to the forefront of drug research [191]. In addition to mRNA's role in producing antigens or therapeutic proteins, different types of RNA have a variety of functions and play important regulatory roles in cells and tissues. One type of these RNAs, lncRNAs, has the potential to be used as new therapies, and the lncRNA itself can be used as both drugs and targets. To date, no studies have associated lncRNA treatment to altered expression of KIT gene and mutations in GIST, despite the fact that some lncRNAs that have been shown to regulate KIT expression in GIST and are associated with imatinib resistance [132, 192]. For example, miRNA sponges is a kind of artificial transcripts that contains multiple miRNA binding sites to trap and sequester it [193]. Although this tool can simultaneously inhibit miR-221/miR-222 in tumour cells such as breast cancer cells [194] and recently a PhaseI study of the frst-in-class locked nucleic acid (LNA) miR-221 selective inhibitor shows good safety and SD + PR in refractory advanced cancer patients [195]. The above suggests promising therapeutic ability in targeting ncRNA and thereby modulating KIT expression warranted future investigation. And intriguingly, use of an aptamer-based method for KIT expression targeted detection of GIST was successfully executed in vitro and in vivo, and intracellular delivery of TKIs to signaling terrace via anti-KIT DNA aptamer or modified RNA aptamers to inhibit the activity of KIT kinase, heralding a novel avenue in GIST treatment [196, 197].
Targeting protein translation and post-translation process
LMTK3 increases KIT expression via the speedup of translation rate of KIT transcripts. LMTK3 knockdown not only directly decreases KIT protein translation, but also inhibit the expression of PKC and KIT phosphorylation, indicating LMTK3 as a druggable target. Mutated KIT protein is usually detained within intracellular organelle and chemical inhibition for intracellular transport to Golgi or specific organelles-targeting TKIs by chemical modifications seems a promising therapeutic strategy [198]. Intracellular delivery of TKIs to signaling terrace via anti-KIT DNA aptamer or modified RNA aptamers to inhibit the activity of KIT kinase, heralding a novel avenue in GIST treatment [196, 199, 200]. Homoharringtonine (HHT) is a first-in-class inhibitor of protein biosynthesis and is FDA-approved for the treatment of chronic myeloid leukemia (CML) that is resistance to at least two lines of treatment with TKIs. In vivo and in vitro experiment, HHT showed significant anti-proliferation and apoptosis-induction with a notable reduction of KIT protein and subsequent decrease of KIT activation and downstream signaling, while KIT mRNA levels were slightly affected [201].
Bortezomib is a dipeptide boronic acid inhibitor of the 20S proteasome. It synergistically augmentes the efficacy of TKIs in multiple myeloma and mantle cell lymphoma. Bortezomib can bind to Cbl, an E3 ubiquitin-protein ligase, destabilizing the c-KIT-Hsp90Β-Apaf-1 complex and releasing Apaf-1; then KIT was unleashed from the complex, and be internalized and degraded in the cytoplasm of GIST cells. Treatment with bortezomib can enhance dasatinib-induced apoptosis in GIST T1 cells and increase the inhibition effect of IM on cell proliferation and invasion in GIST 882 cells[144, 202]. HSP (heat-shock protein) is a multi-protein chaperone, acting as a key mediator for the correct folding, intracellular disposition, and proteolytic turnover of the proteins which are responsible for cell growth and survival. The fundamental chaperoning role of HSPs is subverted, especially heat-shock protein of 90 KD (HSP 90), leading to the maintenance of mutant proteins with its gain-of-function of protecting cancer cells from onco-stress [203].
HSP 90 inhibitors, IPI-504 and AT13387 can decrease the KIT protein level, showing obvious antitumor activity in GIST as a single agent, and they are more potent when in combination with IM or sunitinib [54, 204]. HSP90AA1, one of the client proteins of HSP90, is a major chaperone protein for KIT oncoprotein with a protective role from its degradation in GIST. Other HSP90 inhibitors, including 7-allylamino-17- demethoxygeldanamycin (17-AAG) and IPI-493, also exhibit the inhibition effect in cell growth due to the degradation of mutant KIT protein via both proteasome- and autophagy-dependent pathways [53]. Novel HSP90 inhibitors, NVP-AUY922 and TAS-116, can downregulate both total and phosphorylated KIT proteins, and mTOR inhibitors can enhance the inhibitory role of NVP-AUY922 in GIST cells [205,206,207]. Intriguingly, the HDAC inhibitors (HDACi), SAHA and LBH589 can epigenetically attenuate the activity of HSP90 by its acetylating on HSP90 gene, with the resultant degradation of KIT protein. In vitro and vivo experiments, HDAC inhibitors presented with a synergistic effect in the IM-treated GIST cells [208]. Although there are many drugs directly targeting HSP 90 on its co-factors, challenges remain in clinical translation for its endurable toxicity [209]. Genome-wide functional screening identifies CDC37 as a crucial HSP90-cofactor for KIT oncogenic expression in gastrointestinal stromal tumors [210]. PBOX-15, a novel microtubule-targeting agent (MTA) reduced CKII expression, an enzyme which regulates the expression of CDC37, which downregulate KIT expression via CDC37-HSP90 mediated KIT degradation [211]. These findings indicate the potential of PBOX-15 to improve the apoptotic response of IM in GIST cells and provide a more effective treatment option for GIST patients.
Targeting signal pathways regulating KIT expression
Targeting the Hh pathway can reduce KIT mRNA and re-enhance the sensitivity of GIST cells to TKIs in in vivo experiments. GI1/2 inhibitor, arsenic trioxide, decreases KIT expression and reduces cell viability by significantly increasing the bonding of GLI3 to the KIT promoter, demonstrating efficacy in the Imatinib-sensitive and Imatinib-resistant GIST cells [157]. The p55PIK specific inhibitor, TAT-N24, can abrogate the resistance of GIST cells to Imatinib [32] and dramatically down-regulated KIT expression and enhanced the Imatinib effectiveness in an NF-κB -dependent manner as validated in the PDX tissue from IM-resistance-GIST patients. Inhibitors of the PI3K pathway have already made significant contributions to GIST, with the dual PI3K/mTOR inhibitor voxtalisib, the pan-PI3K inhibitor pilaralisib, and the PI3K-restricted inhibitor alpelisib all reducing GIST cell proliferation [164]. The emerging PI3K or P55PIK inhibitors, including Copanlisib, showed high efficacy and low toxicity in IM-resistant GIST [165, 212].
KIT and FGFR3 have a direct interaction in GIST cells, and BGJ398, a selective FGFR1-4 inhibitor, can re-sensitize GIST to IM in in vitro and in vivo experiments [170]. With the combination of BGJ398 and sunitinib, SDH-GIST patients may get better outcomes [102]. Nintedanib, first approved by FDA for idiopathic pulmonary fibrosis, overcame not only resistance induced by KIT mutations, but also ERK-reactivation-mediated resistance induced by FGF upregulation [43]. The combination of FGFR inhibitor PD173074 and IM showed highly synergistic effect in IM-resistant GIST cells [213]. The phase Ib study of BGJ398 and imatinib in the treatment of IM-refractory advanced GIST showed that the primary endpoint was achieved in which approximately 25% (3/12) of patients sustained stable disease for more than 32 weeks [214]. That suggests that FGF inhibitors like BGJ398 might be a promising treatment strategy combination with TKIs after imatinib resistance.
Targeting KIT mutations
Different genotypes of KIT mutations are associated with diversified responses to specific TKIs. Primary mutations often appear in KIT Exon 9 and 11, and the latter is more sensitive to Imatinib with better progression-free survival (PFS), and median overall survival (OS). However, IM showed almost ineffective for secondary KIT mutations including exon 13, 14 and exon 17, 18 [215, 216]. In the setting of second and beyond, sunitinib had better inhibitory effect on KIT mutants with exon 9 or 11/13 or 14 double mutations than IM, but regorafenib, dasatinib, nilotinib and sorafenib was largely effective to inhibit the phosphorylation of KIT with secondary exon 17 mutation [217,218,219]. In animal model, compared to control mice with a single V558Δ Kit mutation, mice with a double V558Δ; V653A Kit mutation had increased tumor oncogenesis and associated KIT-dependent STAT activation, while cabozantinib was more effective in overcoming resistance than sunitinib[125]. In vitro, V654A mutation encoded by KIT exon 13 was intermediately sensitive to anlotinib. Moreover, anlotinib was able to partly suppress the activation loop mutation D820A from exon 17 while another activation loop mutation N822K, also from exon 17, was resistant to anlotinib [220]. BLU-263 (avapritinib), targeting KIT D816V, is currently being evaluated in the phase 2/3 HARBOR study of patients with ISM [221]. Recently, PLX-9486 (Bezuclastinib), an active-state TK inhibitor with activity against mutations in KIT exons 9, 11, 17, and 18, including D816V, has several clinical trials and shows great clinical benefit with an acceptable safety profile either using alone or in combination with other TKIs [222, 223].
Conclusion and perspective
Collectively, continued KIT-dependency is a typical characteristic of GIST, and complete tumor eradication of non-operable GIST may require a powerful inhibition of the KIT pathway, which is potentially attained by targeting both the tyrosine kinase and the abnormal overexpression of KIT protein. Currently, seven drugs targeting KIT mutations have already been approved by the FDA for the treatment of advanced-stage GIST (imatinib, sunitinib, regorafenib, ripretinib, avapritinib, larotrectinib and entrectinib), all of which are TKIs [22]. Although these agents can be very effective for treating certain GIST subtypes, challenges remain and novel therapeutic approaches are needed [12, 224]. GIST is inherently resistant to radiotherapy and cytotoxic drug [225], albeit some end-stage cases with GIST treated by radiotherapy can achieved palliative pain-relief [226,227,228]. Immunotherapy is a promising hotspot in the treatment of tumors [229, 230], but the efficacy cannot be determined in the existing cases of GIST [231]. Efforts continue to be made in immunotherapy as well as radiation therapy for GIST [232,233,234,235].
It has been speculated that special regulation network exclusively in KIT-driven GIST, on the gene coding, epigenetic modification, and protein level. Available evidence that the decrease of KIT expression via HSP90- chaperone protein for the reversal of IM resistance has become a realistic possibility in clinical application [236, 237]. This fact further improves KIT value not only as a diagnosis marker, but also could be a predictive marker for the therapeutic treatments targeting KIT expression. Thus, the development of standardized approaches to measure KIT expression in different molecule level or various cellular organelles will make it possible.
The emerging molecules which were unravelled recently such as FOFX1, ETV1, which have potentially sculpted the innate link of GIST and its derivation ICC. Because ETV1 also regulates the expression of ICC gene signatures and is crucial for ICC cell survival, as well as its upstream FOFX1 do [74]. So deeply exploring the key gene expression and regulation process of ICC lineage-specific differentiation or the compelling molecules impact its physical function such as gut motility, may offer the possibility of novel promising targets. Additionally, these KIT auto-regulation positive loop mediated by the components of the PI3K or MAPK signal pathways strengthened the interest in the conjoined inhibition of these two pathways as potential therapeutic strategy, thus support the continuous endeavor for the development of novel drugs in this field in the setting of IM-resistant GIST, although how to decrease the toxicity of these combination is a key unanswered question.
The crosstalk between various RTK is the common phenomenon in cell body, this KIT in GIST is not an exception. Evidence chains range from basic research to pre- or early-phase clinical trials consolidated the knowledge that FGF/FGFR signal pathway is a vital target for KIT–mutated GIST via multifaceted mechanism including decreased KIT expression, expected for large-size random clinical trial to confirm and propel FGFR-targeted therapy in GIST patients in the future.
Taken together, there is no doubt that KIT oncoprotein remains a therapeutic target for novel drug or drug-repurpose because the heterogeneous mutations within KIT oncogene generated for the reactivation of KIT signal cascade, contributing to the disease progression in most cases after the failure of IM and even multiple-lines of TKIs. Current research is evaluating the agents target the high level of KIT expression, either alone or in combination with imatinib or other TKIs, aiming to circumvent the high rate of resistance. However, several research questions remain unanswered including (Fig. 4) (Table 3):
-
what is the relationship between KIT activation and KIT upregulation, the rate-limiting processes for abnormal KIT expression in GIST,
-
if the underlying mechanism of KIT expression is exclusive to GIST, but not in WT KIT,
-
imatinib treatment induces the change of tumor microenvironment (TME) [231, 238], if it in turn affected the expression of KIT gene,
-
upon the IM treatment, c-KIT oncoprotein was substantially up-regulated, which was considered as a protective mechanism for GIST cells, if these from the release of negative feedback, such as sprouty homolog 4 (SPRY4) [239],
-
the protein of KIT mutants usually mislocated within intracellular organelles, such as golgi apparatus, how to detect them used for the guidelines of drugs targeting KIT expression?
Perspective of KIT in GIST. KIT expression and its downstream activation pathway regulation mechanism needs to be taken seriously as its mechanism is not fully explained. KIT mutations are the main culprit responsible for the development of GIST and the primary/secondary resistance of imatinib. The epigenetic modification of KIT mutation and the study of new mutations’ targets are worthy of further study. Further treatment options may consider combining TKIs with other drugs that target KIT and their signaling pathway to achieve better efficacy and address drug use in resistant patients
Availability of data and materials
Not applicable.
Abbreviations
- TK:
-
Tyrosine kinase
- RTK:
-
Receptor tyrosine kinases
- SCF:
-
Stem cell factor
- ICC:
-
The interstitial cell of Cajal
- GIST:
-
Gastrointestinal stromal tumor
- PGFRA:
-
Platelet-derived growth factor receptor α
- SDH:
-
Succinate dehydrogenase
- NF1:
-
Neurofibromin 1
- KRAS:
-
Kirsten rat sarcoma viral oncogene homolog
- HRAS:
-
Harvey rat sarcoma viral oncogene homolog
- JM:
-
Juxtamembrane
- IM:
-
Imatinib
- TKIs:
-
Tyrosine kinase inhibitors
- WT:
-
Wild type
- IHC:
-
Immunohistochemistry
- CREs:
-
Cis-regulatory elements
- RNA Pol II:
-
RNA Polymerase II
- TF:
-
Transcription factor
- FOXF1:
-
Forkhead box F1
- DBD:
-
DNA binding region
- ETV1:
-
ETS translocation variant 1
- MAPK:
-
Mitogen-activated protein kinase
- CREB:
-
Cyclic AMP-responsive element-binding protein
- CBP:
-
Cyclic binding protein
- HAND1:
-
Heart and Neural Crest Derivatives Expressed 1
- GPR20:
-
G protein-coupled receptor 20
- BARX1:
-
Homeobox protein BarH-like 1
- SH3BP2:
-
C-Abl Src homology 3 domain-binding protein-2
- PH:
-
Pleckstrin homology
- MITF:
-
Microphthalmia-associated transcription factors
- EEs:
-
Epigenetic enzymes
- PPM1A:
-
Protein phosphatase 1A
- KIF1B:
-
Kinesin family member 1B
- NF2:
-
Neurofibromin 2
- ENG:
-
Endoglin
- TGF-h:
-
Transforming growth factor-h
- CTCF:
-
CCCTC-binding factor
- TADs:
-
Topologically associated domains
- ANO1:
-
Anoctamin 1
- SE:
-
Super enhancer
- m6A:
-
N6-methyladenosine
- mRNA:
-
Messenger RNA
- METTL3:
-
Methyltransferase-like 3
- FTO:
-
Fat mass- and obesity-associated
- AML:
-
Acute myeloid leukemia
- KDM4D:
-
Lysine (K)-specific demethylase 4D
- BRD4:
-
Bromodomain-containing protein 4
- BET:
-
Bromodomain and extra-terminal domain-containing
- NFκB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- CCL2:
-
C–C motif chemokine ligand 2
- BBIs:
-
BET bromodomain inhibitor
- MOZ:
-
Monocytic zinc finger
- ncRNAs:
-
Non-coding RNAs
- miRNAs:
-
Micro-RNAs
- nt:
-
Nucleotides
- LncRNAs:
-
Long non-coding RNAs
- BIRC5:
-
Survivin
- CCDC26:
-
LncRNA coiled-coil domain-containing 26
- FENDRR:
-
FOXF1 adjacent non-coding developmental regulatory RNA
- LMTK3:
-
Lemur tyrosine kinase-3
- PCK:
-
Protein kinase C
- PKC-θ:
-
Protein kinase C-θ
- Hh:
-
Hedgehog
- Shh:
-
Sonic Hedgehog
- Ihh:
-
Indian Hedgehog
- Dhh:
-
Desert Hedgehog
- Ptch-1:
-
Patched-1
- Ptch-2:
-
Patched-2
- SMO:
-
Smoothened
- GLI1, 2, and 3:
-
Homolog isoform1, 2, and 3
- p55PIK:
-
Phosphoinositide-3-kinase, regulatory subunit 3 (gamma)
- ACK1:
-
CDC42 associated kinase 1
- FGFs:
-
Fibroblast growth factors
- JAK/ STAT:
-
Janus kinase/signal transducers and activators of transcription
- AMPDs:
-
Adenosine monophosphate deaminases
- LIX1:
-
Limb Expression 1
- YAP1:
-
Yes-associated protein 1
- HIF-1α:
-
Hypoxia inducible factor 1 alpha
- HGF:
-
Hepatocyte growth factor
- OSR1:
-
Odd-skipped related transcription factor 1
- LNA:
-
Locked nucleic acid
- HHT:
-
Homoharringtonine
- CML:
-
Chronic myeloid leukemia
- 17-AAG:
-
7-Allylamino-17- demethoxygeldanamycin
- HSP90:
-
Heat Shock Protein 90
- Hsp90 Β:
-
HSP90AB
- HDACi:
-
HDAC inhibitors
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- MTA:
-
Microtubule-targeting agent
- TME:
-
Tumor microenviroment
- SPRY4:
-
Sprout homolog 4
References
Yarden Y, et al. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6(11):3341–51.
Mol CD, et al. Structure of a c-kit product complex reveals the basis for kinase transactivation. J Biol Chem. 2003;278(34):31461–4.
Pathania S, Pentikäinen OT, Singh PK. A holistic view on c-Kit in cancer: Structure, signaling, pathophysiology and its inhibitors. Biochim Biophys Acta Rev Cancer. 2021;1876(2):188631.
Lemmon MA, Ferguson KM. A new twist in the transmembrane signaling tool-kit. Cell. 2007;130(2):213–5.
Meng D, Carvajal RD. KIT as an Oncogenic Driver in Melanoma: An Update on Clinical Development. Am J Clin Dermatol. 2019;20(3):315–23.
Zsebo KM, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63(1):213–24.
Liang J, et al. The C-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci. 2013;9(5):435–43.
Tilayov T, et al. Engineering Stem Cell Factor Ligands with Different c-Kit Agonistic Potencies. Molecules. 2020;25(20):4850.
Hirota S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science (New York, NY). 1998;279(5350):577–80.
Lennartsson J, Ronnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev. 2012;92(4):1619–49.
Flavahan WA, et al. Altered chromosomal topology drives oncogenic programs in SDH-deficient GISTs. Nature. 2019;575(7781):229–33.
Blay J-Y, et al. Gastrointestinal stromal tumours. Nat Rev Dis Primers. 2021;7(1):22.
Heinrich MC, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–10.
Al-Share B, et al. Gastrointestinal stromal tumor: a review of current and emerging therapies. Cancer Metastasis Rev. 2021;40(2):625–41.
Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865–78.
Tsai M, Valent P, Galli SJ. KIT as a master regulator of the mast cell lineage. Journal of Allergy and Clinical Immunology. 2022;149(6):1845–54.
Zook P, et al. Combination of Imatinib Mesylate and AKT Inhibitor Provides Synergistic Effects in Preclinical Study of Gastrointestinal Stromal Tumor. Clin Cancer Res. 2017;23(1):171–80.
Neiswender JV, et al. KIT Suppresses BRAF(V600E)-Mutant Melanoma by Attenuating Oncogenic RAS/MAPK Signaling. Cancer Res. 2017;77(21):5820–30.
Zhao R, et al. PD-1/PD-L1 blockade rescue exhausted CD8+T cells in gastrointestinal stromal tumours via the PI3K/Akt/mTOR signalling pathway. Cell Prolif. 2019;52(3):e12571.
Blay J-Y, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(7):923–34.
Poveda A, et al. GEIS guidelines for gastrointestinal sarcomas (GIST). Cancer Treat Rev. 2017;55:107–19.
Klug LR, et al. New treatment strategies for advanced-stage gastrointestinal stromal tumours. Nat Rev Clin Oncol. 2022;19(5):328–41.
Di Vito A, et al. The multifaceted landscape behind imatinib resistance in gastrointestinal stromal tumors (GISTs): A lesson from ripretinib. Pharmacol Ther. 2023;248:108475.
Liegl B, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol. 2008;216(1):64–74.
Serrano C, George S. Gastrointestinal Stromal Tumor: Challenges and Opportunities for a New Decade. Clin Cancer Res. 2020;26(19):5078–85.
Obata Y, et al. Golgi retention and oncogenic KIT signaling via PLCgamma2-PKD2-PI4KIIIbeta activation in gastrointestinal stromal tumor cells. Cell Rep. 2023;42(9):113035–113035.
Arock M, et al. Clinical impact and proposed application of molecular markers, genetic variants, and cytogenetic analysis in mast cell neoplasms: Status 2022. Journal of Allergy and Clinical Immunology. 2022;149(6):1855–65.
Nannini M, et al. Integrated genomic study of quadruple-WT GIST (KIT/PDGFRA/SDH/RAS pathway wild-type GIST). Bmc Cancer. 2014;14:685.
Schaefer I-M, Marino-Enriquez A, Fletcher JA. What is New in Gastrointestinal Stromal Tumor? Adv Anat Pathol. 2017;24(5):259–67.
Nishida T, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19(1):3–14.
Theou N, et al. High expression of both mutant and wild-type alleles of c-kit in gastrointestinal stromal tumors. Biochim Biophys Acta. 2004;1688(3):250–6.
Lai S, et al. KIT over-expression by p55PIK-PI3K leads to Imatinib-resistance in patients with gastrointestinal stromal tumors. Oncotarget. 2016;7(2):1367–79.
Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000;13(10):1134–42.
Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382(9896):973–83.
Hemming ML, et al. HAND1 and BARX1 Act as Transcriptional and Anatomic Determinants of Malignancy in Gastrointestinal Stromal Tumor. Clin Cancer Res. 2021;27(6):1706–19.
Guler B, et al. Histopathological Features of Gastrointestinal Stromal Tumors and the Contribution of DOG1 Expression to the Diagnosis. Balkan Med J. 2015;32(4):388–96.
Foo WC, Liegl-Atzwanger B, Lazar AJ. Pathology of gastrointestinal stromal tumors. Clinical medicine insights Pathology. 2012;5:23–33.
Noma K, et al. Effiects of imatinib vary with the types of KIT-mutation in gastrointestinal stromal tumor cell lines. Oncol Rep. 2005;14(3):645–50.
Tarn C, et al. Therapeutic effect of imatinib in gastrointestinal stromal tumors: AKT signaling dependent and independent mechanisms. Can Res. 2006;66(10):5477–86.
Tuveson DA, et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20(36):5054–8.
Chi HT, et al. All-trans retinoic acid inhibits KIT activity and induces apoptosis in gastrointestinal stromal tumor GIST-T1 cell line by affecting on the expression of survivin and Bax protein. J Exp Clin Cancer Res. 2010;29(1):165.
Taguchi T, et al. Conventional and molecular cytogenetic characterization of a new human cell line, GIST-T1, established from gastrointestinal stromal tumor. Lab Invest. 2002;82(5):663–5.
Liu J, et al. Nintedanib overcomes drug resistance from upregulation of FGFR signalling and imatinib-induced KIT mutations in gastrointestinal stromal tumours. Mol Oncol. 2022;16(8):1761–74.
Garner AP, et al. Ponatinib Inhibits Polyclonal Drug-Resistant KIT Oncoproteins and Shows Therapeutic Potential in Heavily Pretreated Gastrointestinal Stromal Tumor (GIST) Patients. Clin Cancer Res. 2014;20(22):5745–55.
Sugase T, et al. SOCS1 gene therapy has antitumor effects in imatinib-resistant gastrointestinal stromal tumor cells through FAK/PI3 K signaling. Gastric Cancer. 2018;21(6):968–76.
Zeng S, et al. Wnt/β-catenin Signaling Contributes to Tumor Malignancy and Is Targetable in Gastrointestinal Stromal Tumor. Mol Cancer Ther. 2017;16(9):1954–66.
Ihle MA, et al. miRNA-221 and miRNA-222 induce apoptosis via the KIT/AKT signalling pathway in gastrointestinal stromal tumours. Mol Oncol. 2015;9(7):1421–33.
Wang Y, et al. miR-148b-3p functions as a tumor suppressor in GISTs by directly targeting KIT. Cell Commun Signal. 2018;16(1):16.
Rossi S, et al. Gastrointestinal stromal turnours overexpress fatty acid synthase. Journal of Pathology. 2006;209(3):369–75.
Bauer S, et al. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway. Oncogene. 2007;26(54):7560–8.
Chen W, et al. Dual Targeting of Insulin Receptor and KIT in Imatinib-Resistant Gastrointestinal Stromal Tumors. Can Res. 2017;77(18):5107–17.
Floris G, et al. High Efficacy of Panobinostat Towards Human Gastrointestinal Stromal Tumors in a Xenograft Mouse Model. Clin Cancer Res. 2009;15(12):4066–76.
Floris G, et al. The Novel HSP90 inhibitor, IPI-493, is highly effective in human gastrostrointestinal stromal tumor xenografts carrying heterogeneous KIT mutations. Clinical Cancer Research : an Official Journal of the American Association For Cancer Research. 2011;17(17):5604–14.
Floris G, et al. The heat shock protein 90 inhibitor IPI-504 induces KIT degradation, tumor shrinkage, and cell proliferation arrest in xenograft models of gastrointestinal stromal tumors. Mol Cancer Ther. 2011;10(10):1897–908.
Kim TS, et al. Increased KIT Inhibition Enhances Therapeutic Efficacy in Gastrointestinal Stromal Tumor. Clin Cancer Res. 2014;20(9):2350–62.
Serrano C, et al. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br J Cancer. 2019;120(6):612–20.
Bauer S, et al. Heat shock protein 90 inhibition in imatinib-resistant gastrointestinal stromal tumor. Can Res. 2006;66(18):9153–61.
Simon S, et al. DOG1 Regulates Growth and IGFBP5 in Gastrointestinal Stromal Tumors. Can Res. 2013;73(12):3661–70.
Cohen NA, et al. Pharmacological Inhibition of KIT Activates MET Signaling in Gastrointestinal Stromal Tumors. Can Res. 2015;75(10):2061–70.
Duensing A, et al. Mechanisms of oncogenic KIT signal transduction in primary gastrointestinal stromal tumors (GISTs). Oncogene. 2004;23(22):3999–4006.
Tabone S, et al. KIT overexpression and amplification in gastrointestinal stromal tumors (GISTs). Biochim Biophys Acta. 2005;1741(1–2):165–72.
Hemming ML, et al. Gastrointestinal stromal tumor enhancers support a transcription factor network predictive of clinical outcome. Proc Natl Acad Sci U S A. 2018;115(25):E5746–55.
Koch A, et al. Analysis of DNA methylation in cancer: location revisited. Nat Rev Clin Oncol. 2018;15(7):459–66.
Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150(1):12–27.
Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12(1):31–46.
Lambert SA, et al. The Human Transcription Factors. Cell. 2018;172(4):650–65.
Hemming ML, et al. Enhancer Domains in Gastrointestinal Stromal Tumor Regulate KIT Expression and Are Targetable by BET Bromodomain Inhibition. Cancer Res. 2019;79(5):994–1009.
Bar-Joseph Z, et al. Computational discovery of gene modules and regulatory networks. Nat Biotechnol. 2003;21(11):1337–42.
Lee TI, et al. Transcriptional regulatory networks in Saccharomyces cerevisiae. Science. 2002;298(5594):799–804.
Chi P, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature. 2010;467(7317):849–53.
Voss TC, Hager GL. Dynamic regulation of transcriptional states by chromatin and transcription factors. Nat Rev Genet. 2014;15(2):69–81.
Katoh M, et al. Cancer genetics and genomics of human FOX family genes. Cancer Lett. 2013;328(2):198–206.
Ran L, et al. FOXF1 Defines the Core-Regulatory Circuitry in Gastrointestinal Stromal Tumor. Cancer Discov. 2018;8(2):234–51.
Lee DM, Duensing A. What’s the FOX Got to Do with the KITten? Regulating the Lineage-Specific Transcriptional Landscape in GIST. Cancer Discov. 2018;8(2):146–9.
Wei G-H, et al. Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo. EMBO J. 2010;29(13):2147–60.
Hsu T, Trojanowska M, Watson DK. Ets proteins in biological control and cancer. J Cell Biochem. 2004;91(5):896–903.
Gu ML, et al. An inhibitor of the acetyltransferases CBP/p300 exerts antineoplastic effects on gastrointestinal stromal tumor cells. Oncol Rep. 2016;36(5):2763–70.
Clark JP, Cooper CS. ETS gene fusions in prostate cancer. Nat Rev Urol. 2009;6(8):429–39.
Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet. 1998;18(3):271–5.
Firulli BA, et al. HAND1 loss-of-function within the embryonic myocardium reveals survivable congenital cardiac defects and adult heart failure. Cardiovasc Res. 2020;116(3):605–18.
Iida K, et al. Identification and Therapeutic Targeting of GPR20, Selectively Expressed in Gastrointestinal Stromal Tumors, with DS-6157a, a First-in-Class Antibody-Drug Conjugate. Cancer Discov. 2021;11(6):1508–23.
Jayewickreme CD, Shivdasani RA. Control of stomach smooth muscle development and intestinal rotation by transcription factor BARX1. Dev Biol. 2015;405(1):21–32.
Deckert M, Rottapel R. The adapter 3BP2: how it plugs into leukocyte signaling. Adv Exp Med Biol. 2006;584:107–14.
Serrano-Candelas E, et al. Silencing of adaptor protein SH3BP2 reduces KIT/PDGFRA receptors expression and impairs gastrointestinal stromal tumors growth. Mol Oncol. 2018;12(8):1383–97.
Ainsua-Enrich E, et al. The adaptor 3BP2 is required for early and late events in FcepsilonRI signaling in human mast cells. J Immunol. 2012;189(6):2727–34.
Proano-Perez E, et al. SH3BP2 Silencing Increases miRNAs Targeting ETV1 and Microphthalmia-Associated Transcription Factor, Decreasing the Proliferation of Gastrointestinal Stromal Tumors. Cancers (Basel). 2022;14(24):6198.
Proano-Perez E, et al. The microphthalmia-associated transcription factor is involved in gastrointestinal stromal tumor growth. Cancer Gene Ther. 2023;30(2):245–55.
Chen, X., et al., Mapping epigenetic modifications by sequencing technologies. Cell Death and Differentiation, 2023.
Zhao L-Y, et al. Mapping the epigenetic modifications of DNA and RNA. Protein Cell. 2020;11(11):792–808.
Boulias K, Greer EL. Biological roles of adenine methylation in RNA. Nat Rev Genet. 2023;24(3):143–60.
Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484–92.
Zaib S, Rana N, Khan I. Histone Modifications and their Role in Epigenetics of Cancer. Curr Med Chem. 2022;29(14):2399–411.
Jasek K, et al. Epigenetics: an alternative pathway in GISTs tumorigenesis. Neoplasma. 2018;65(4):477–93.
Mattei AL, Bailly N, Meissner A. DNA methylation: a historical perspective. Trends Genet. 2022;38(7):676–707.
Greenberg MVC, Bourc’his D. The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol. 2019;20(10):590–607.
Dallas NA, et al. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clin Cancer Res. 2008;14(7):1931–7.
Basilio-de-Oliveira RP, Pannain VL. Prognostic angiogenic markers (endoglin, VEGF, CD31) and tumor cell proliferation (Ki67) for gastrointestinal stromal tumors. World J Gastroenterol. 2015;21(22):6924–30.
Gromova P, et al. ENDOGLIN/CD105 is expressed in KIT positive cells in the gut and in gastrointestinal stromal tumours. J Cell Mol Med. 2012;16(2):306–17.
Dekker J, Mirny L. The 3D Genome as Moderator of Chromosomal Communication. Cell. 2016;164(6):1110–21.
Davidson, I.F., et al., CTCF is a DNA-tension-dependent barrier to cohesin-mediated loop extrusion. Nature, 2023.
Hansen AS, et al. Distinct Classes of Chromatin Loops Revealed by Deletion of an RNA-Binding Region in CTCF. Mol Cell. 2019;76(3):395.
Flavahan WA, et al. Altered chromosomal topology drives oncogenic programs in SDH-deficient GISTs. Nature. 2019;575(7781):229.
Yang B, et al. RNA methylation and cancer treatment. Pharmacol Res. 2021;174: 105937.
Chen X, et al. RNA methylation and diseases: experimental results, databases, Web servers and computational models. Brief Bioinform. 2019;20(3):896–917.
Yin H, et al. M6A RNA methylation-mediated RMRP stability renders proliferation and progression of non-small cell lung cancer through regulating TGFBR1/SMAD2/SMAD3 pathway. Cell Death Differ. 2023;30(3):605–17.
Wu Y, et al. PRMT5 regulates RNA m6A demethylation for doxorubicin sensitivity in breast cancer. Mol Ther. 2022;30(7):2603–17.
Zhang C, et al. YTHDF2 promotes the liver cancer stem cell phenotype and cancer metastasis by regulating OCT4 expression via m6A RNA methylation. Oncogene. 2020;39(23):4507–18.
Xu K, et al. N6-methyladenosine modification regulates imatinib resistance of gastrointestinal stromal tumor by enhancing the expression of multidrug transporter MRP1. Cancer Lett. 2022;530:85–99.
Qian K, et al. Methyltransferase-like 3 (METTL3) mediated N6-methyladenosine (m6A) modifications facilitate mir-25-3p maturation to promote gastrointestinal stromal tumors (GISTs) progression. Genes Genomics. 2022;44(12):1519–30.
Huang Y. et al. Small-Molecule Targeting of Oncogenic FTO Demethylase in Acute Myeloid Leukemia. Cancer Cell. 2019;35(4):677–91.
Hu F, et al. Histone demethylase KDM4D promotes gastrointestinal stromal tumor progression through HIF1β/VEGFA signalling. Mol Cancer. 2018;17(1):107.
Hajmirza A, et al. BET Family Protein BRD4: An Emerging Actor in NFkappaB Signaling in Inflammation and Cancer. Biomedicines. 2018;6(1):16.
Mu J, et al. BRD4 promotes tumor progression and NF-κB/CCL2-dependent tumor-associated macrophage recruitment in GIST. Cell Death Dis. 2019;10(12):935.
Miyamoto R, et al. Activation of CpG-Rich Promoters Mediated by MLL Drives MOZ-Rearranged Leukemia. Cell Rep. 2020;32(13):108200.
Baell JB, et al. Inhibitors of histone acetyltransferases KAT6A/B induce senescence and arrest tumour growth. Nature. 2018;560(7717):253.
Su J, et al. The role of MOZ/KAT6A in hematological malignancies and advances in MOZ/KAT6A inhibitors. Pharmacol Res. 2021;174:105930.
Hemming ML, et al. MOZ and Menin-MLL Complexes Are Complementary Regulators of Chromatin Association and Transcriptional Output in Gastrointestinal Stromal Tumor. Cancer Discov. 2022;12(7):1804–23.
Chen B, et al. Targeting non-coding RNAs to overcome cancer therapy resistance. Signal Transduct Target Ther. 2022;7(1):121.
Lu TX, Rothenberg ME. MicroRNA. Journal of Allergy and Clinical Immunology. 2018;141(4):1202–7.
Kopp F, Mendell JT. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell. 2018;172(3):393–407.
Ravegnini G, et al. miRNA landscape in primary tumors and matched metastases in gastrointestinal stromal tumors. Epigenomics. 2021;13(05):369–77.
Yang Y, et al. MicroRNA-218 functions as a tumor suppressor in lung cancer by targeting IL-6/STAT3 and negatively correlates with poor prognosis. Mol Cancer. 2017;16(1):141.
Fan R, et al. microRNA-218 increase the sensitivity of gastrointestinal stromal tumor to imatinib through PI3K/AKT pathway. Clin Exp Med. 2015;15(2):137–44.
Fan R, et al. MicroRNA-218 inhibits gastrointestinal stromal tumor cell and invasion by targeting KIT. Tumour Biology : the Journal of the International Society For Oncodevelopmental Biology and Medicine. 2014;35(5):4209–17.
Zhang JQ, et al. The V654A second-site KIT mutation increases tumor oncogenesis and STAT activation in a mouse model of gastrointestinal stromal tumor. Oncogene. 2020;39(49):7153–65.
Friedrich M, et al. The role of the miR-148/-152 family in physiology and disease. Eur J Immunol. 2017;47(12):2026–38.
Koelz M, et al. Down-regulation of miR-221 and miR-222 correlates with pronounced Kit expression in gastrointestinal stromal tumors. Int J Oncol. 2011;38(2):503–11.
Haller F, et al. Localization- and mutation-dependent microRNA (miRNA) expression signatures in gastrointestinal stromal tumours (GISTs), with a cluster of co-expressed miRNAs located at 14q32.31. J Pathol. 2010;220(1):71–86.
Felli N, et al. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc Natl Acad Sci USA. 2005;102(50):18081–6.
Ravegnini G, et al. The rs17084733 variant in the KIT 3’ UTR disrupts a miR-221/222 binding site in gastrointestinal stromal tumour: a sponge-like mechanism conferring disease susceptibility. Epigenetics. 2019;14(6):545–57.
Jia N, et al. CeRNA Expression Profiling Identifies KIT-Related circRNA-miRNA-mRNA Networks in Gastrointestinal Stromal Tumour. Front Genet. 2019;10:825.
Choi H-J, et al. MicroRNA expression profile of gastrointestinal stromal tumors is distinguished by 14q loss and anatomic site. Int J Cancer. 2010;126(7):1640–50.
Yun S, et al. Survivin is a novel transcription regulator of KIT and is downregulated by miRNA-494 in gastrointestinal stromal tumors. Int J Cancer. 2018;142(10):2080–93.
Gits CMM, et al. MiR-17-92 and miR-221/222 cluster members target KIT and ETV1 in human gastrointestinal stromal tumours. Br J Cancer. 2013;109(6):1625–35.
Gyvyte U, et al. The Role of miR-375–3p and miR-200b-3p in Gastrointestinal Stromal Tumors. Int J Mol Sci. 2020;21(14):5151.
Enciso-Mora V, et al. Deciphering the 8q24.21 association for glioma. Hum Mol Genet. 2013;22(11):2293–302.
Hirano T, et al. Long noncoding RNA, CCDC26, controls myeloid leukemia cell growth through regulation of KIT expression. Mol Cancer. 2015;14:90.
Cao K, et al. CCDC26 knockdown enhances resistance of gastrointestinal stromal tumor cells to imatinib by interacting with c-KIT. American Journal of Translational Research. 2018;10(1):274–82.
Yan J, et al. Downregulation of lncRNA CCDC26 contributes to imatinib resistance in human gastrointestinal stromal tumors through IGF-1R upregulation. Braz J Med Biol Res. 2019;52(6):e8399.
Weidle UH, et al. Long Non-coding RNAs and their Role in Metastasis. Cancer Genomics Proteomics. 2017;14(3):143–60.
Gyvyte U, et al. Identification of long intergenic non-coding RNAs (lincRNAs) deregulated in gastrointestinal stromal tumors (GISTs). PLoS ONE. 2018;13(12): e0209342.
Yang W, et al. Long non-coding RNA H19 promotes the migration and invasion of colon cancer cells via MAPK signaling pathway. Oncol Lett. 2018;16(3):3365–72.
Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2004;22(18):3813–25.
Dong Y, et al. Bortezomib enhances the therapeutic efficacy of dasatinib by promoting c-KIT internalization-induced apoptosis in gastrointestinal stromal tumor cells. Cancer Lett. 2015;361(1):137–46.
Stebbing J, Filipovic A, Giamas G. Lemur tyrosine kinase-3 (LMTK3) in cancer and evolution. Oncotarget. 2011;2(6):428–9.
Ditsiou A, et al. The structure-function relationship of oncogenic LMTK3. Sci Adv. 2020;6(46):eabc3099.
Giamas G, et al. Kinome screening for regulators of the estrogen receptor identifies LMTK3 as a new therapeutic target in breast cancer. Nat Med. 2011;17(6):715–9.
Klug LR, et al. LMTK3 is essential for oncogenic KIT expression in KIT-mutant GIST and melanoma. Oncogene. 2019;38(8):1200–10.
Edris B, et al. Anti-KIT monoclonal antibody inhibits imatinib-resistant gastrointestinal stromal tumor growth. Proc Natl Acad Sci USA. 2013;110(9):3501–6.
Tabone-Eglinger S, et al. KIT mutations induce intracellular retention and activation of an immature form of the KIT protein in gastrointestinal stromal tumors. Clin Cancer Res. 2008;14(8):2285–94.
Kim WK, et al. Sustained Mutant KIT Activation in the Golgi Complex Is Mediated by PKC-θ in Gastrointestinal Stromal Tumors. Clinical Cancer Research : an Official Journal of the American Association For Cancer Research. 2017;23(3):845–56.
Lee HE, et al. Characteristics of KIT-negative gastrointestinal stromal tumours and diagnostic utility of protein kinase C theta immunostaining. J Clin Pathol. 2008;61(6):722–9.
Kim KM, et al. PKCtheta expression in gastrointestinal stromal tumor. Mod Pathol. 2006;19(11):1480–6.
Park CK, Kim WK, Kim H. Clinicopathological characteristics of KIT and protein kinase C-δ expression in adenoid cystic carcinoma: comparison with chromophobe renal cell carcinoma and gastrointestinal stromal tumour. Histopathology. 2017;71(4):529–42.
Meyer KD, et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell. 2012;149(7):1635–46.
Du H, et al. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016;7:12626.
Mao J, et al. Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development (Cambridge, England). 2010;137(10):1721–9.
Kolterud A, et al. Paracrine Hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology. 2009;137(2):618–28.
Stecca B, Ruiz i Altaba A. Context-dependent regulation of the GLI code in cancer by HEDGEHOG and non-HEDGEHOG signals. J Mol Cell Biol. 2010;2(2):84–95.
Girardi D, et al. Targeting the Hedgehog Pathway in Cancer: Current Evidence and Future Perspectives. Cells. 2019;8(2):153.
Tang C-M, et al. Hedgehog pathway dysregulation contributes to the pathogenesis of human gastrointestinal stromal tumors via GLI-mediated activation of KIT expression. Oncotarget. 2016;7(48):78226–41.
Ruiz i Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17(9):438–47.
Duan Y, Haybaeck J, Yang Z. Therapeutic Potential of PI3K/AKT/mTOR Pathway in Gastrointestinal Stromal Tumors: Rationale and Progress. Cancers. 2020;12(10):2972.
Bosbach B, et al. Direct engagement of the PI3K pathway by mutant KIT dominates oncogenic signaling in gastrointestinal stromal tumor. Proc Natl Acad Sci USA. 2017;114(40):E8448–57.
Hu J, et al. A peptide inhibitor derived from p55PIK phosphatidylinositol 3-kinase regulatory subunit: a novel cancer therapy. Mol Cancer Ther. 2008;7(12):3719–28.
Wang G, et al. p55PIK-PI3K stimulates angiogenesis in colorectal cancer cell by activating NF-κB pathway. Angiogenesis. 2013;16(3):561–73.
He W, et al. Co-targeting of ACK1 and KIT triggers additive anti-proliferative and -migration effects in imatinib-resistant gastrointestinal stromal tumors. Biochim Biophys Acta Mol Basis Dis. 2023;1869(5):166690.
Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10(2):116–29.
Boichuk S, et al. A Novel Receptor Tyrosine Kinase Switch Promotes Gastrointestinal Stromal Tumor Drug Resistance. Molecules. 2017;22(12):2152.
Boichuk S, et al. Targeting of FGF-Signaling Re-Sensitizes Gastrointestinal Stromal Tumors (GIST) to Imatinib In Vitro and In Vivo. Molecules. 2018;23(10):2643.
Park S-Y, et al. The JAK2/STAT3/CCND2 Axis promotes colorectal Cancer stem cell persistence and radioresistance. J Exp Clin Cancer Res. 2019;38(1):399.
Omori I, et al. D816V mutation in the KIT gene activation loop has greater cell-proliferative and anti-apoptotic ability than N822K mutation in core-binding factor acute myeloid leukemia. Exp Hematol. 2017;52:56–64.
Chaix A, et al. KIT-D816V oncogenic activity is controlled by the juxtamembrane docking site Y568–Y570. Oncogene. 2014;33(7):872–81.
Mahnke DK, Sabina RL. Calcium activates erythrocyte AMP deaminase [isoform E (AMPD3)] through a protein-protein interaction between calmodulin and the N-terminal domain of the AMPD3 polypeptide. Biochemistry. 2005;44(14):5551–9.
Wong M, et al. AMPD3 is associated with the malignant characteristics of gastrointestinal stromal tumors. Oncol Lett. 2017;13(3):1281–7.
Guerin A, et al. LIX1-mediated changes in mitochondrial metabolism control the fate of digestive mesenchyme-derived cells. Redox Biol 2022;56:102431.
Fullenkamp CA, et al. TAZ and YAP are frequently activated oncoproteins in sarcomas. Oncotarget. 2016;7(21):30094–108.
Guerin A, et al. LIX1 regulates YAP activity and controls gastrointestinal cancer cell plasticity. J Cell Mol Med. 2020;24(16):9244–54.
Ruiz-Demoulin S, et al. LIX1 Controls MAPK Signaling Reactivation and Contributes to GIST-T1 Cell Resistance to Imatinib. Int J Mol Sci. 2023;24(8):7138.
Xu K, et al. HIF-1α regulates cellular metabolism, and Imatinib resistance by targeting phosphogluconate dehydrogenase in gastrointestinal stromal tumors. Cell Death Dis. 2020;11(7):586.
Shi X, et al. Hypoxia activated HGF expression in pancreatic stellate cells confers resistance of pancreatic cancer cells to EGFR inhibition. Ebiomedicine. 2022;86:104352.
Zhang T, et al. HGF-mediated elevation of ETV1 facilitates hepatocellular carcinoma metastasis through upregulating PTK2 and c-MET. J Exp Clin Cancer Res. 2022;41(1):275.
Boichuk S, et al. Unbiased compound screening identifies unexpected drug sensitivities and novel treatment options for gastrointestinal stromal tumors. Cancer Res. 2014;74(4):1200–13.
Ran L, et al. Combined inhibition of MAP kinase and KIT signaling synergistically destabilizes ETV1 and suppresses GIST tumor growth. Cancer Discov. 2015;5(3):304–15.
Chi P, et al. Phase II Trial of Imatinib Plus Binimetinib in Patients With Treatment-Naive Advanced Gastrointestinal Stromal Tumor. J Clin Oncol. 2022;40(9):997–1008.
Yen CC, et al. Identification of phenothiazine as an ETV1-targeting agent in gastrointestinal stromal tumors using the Connectivity Map. Int J Oncol. 2019;55(2):536–46.
Sun J, et al. THZ1 targeting CDK7 suppresses c-KIT transcriptional activity in gastrointestinal stromal tumours. Cell Commun Signal. 2022;20(1):138.
Mu J, et al. Bromodomain and extraterminal domain inhibitor enhances the antitumor effect of imatinib in gastrointestinal stromal tumours. J Cell Mol Med. 2020;24(4):2519–30.
Cui Y-H, et al. Autophagy of the m6A mRNA demethylase FTO is impaired by low-level arsenic exposure to promote tumorigenesis. Nat Commun. 2021;12(1):2183.
Su R, et al. Targeting FTO Suppresses Cancer Stem Cell Maintenance and Immune Evasion. Cancer Cell. 2020;38(1):79.
Barbier AJ, et al. The clinical progress of mRNA vaccines and immunotherapies. Nat Biotechnol. 2022;40(6):840–54.
Winkle M, et al. Noncoding RNA therapeutics - challenges and potential solutions. Nat Rev Drug Discovery. 2021;20(8):629–51.
Lima JF, et al. Anti-miRNA oligonucleotides: A comprehensive guide for design. RNA Biol. 2018;15(3):338–52.
Jung J, et al. Simultaneous inhibition of multiple oncogenic miRNAs by a multi-potent microRNA sponge. Oncotarget. 2015;6(24):20370–87.
Tassone P, et al. Safety and activity of the first-in-class locked nucleic acid (LNA) miR-221 selective inhibitor in refractory advanced cancer patients: a first-in-human, phase 1, open-label, dose-escalation study. J Hematol Oncol. 2023;16(1):68.
Banerjee S, et al. Anti-KIT DNA Aptamer for Targeted Labeling of Gastrointestinal Stromal Tumor. Mol Cancer Ther. 2020;19(5):1173–82.
Vijayakumar S, et al. Anti-KIT DNA aptamer-conjugated porous silicon nanoparticles for the targeted detection of gastrointestinal stromal tumors. Nanoscale. 2022;14(47):17700–13.
Obata Y, et al. Oncogenic Kit signalling on the Golgi is suppressed by blocking secretory trafficking with M-COPA in gastrointestinal stromal tumours. Cancer Lett. 2018;415:1–10.
Shraim AS, et al. Developing and Characterization of Chemically Modified RNA Aptamers for Targeting Wild Type and Mutated c-KIT Receptor Tyrosine Kinases. J Med Chem. 2020;63(5):2209–28.
Xiang Z, et al. Neoplasia driven by mutant c-KIT is mediated by intracellular, not plasma membrane, receptor signaling. Mol Cell Biol. 2007;27(1):267–82.
Lee DM, et al. Targeting the translational machinery in gastrointestinal stromal tumors (GIST): a new therapeutic vulnerability. Sci Rep. 2022;12(1):8275.
Bauer S, et al. Proapoptotic activity of bortezomib in gastrointestinal stromal tumor cells. Can Res. 2010;70(1):150–9.
Schopf FH, Biebl MM, Buchner J. The HSP90 chaperone machinery. Nat Rev Mol Cell Biol. 2017;18(6):345–60.
Smyth T, et al. The HSP90 inhibitor, AT13387, is effective against imatinib-sensitive and -resistant gastrointestinal stromal tumor models. Mol Cancer Ther. 2012;11(8):1799–808.
Hsueh Y-S, et al. Autophagy is involved in endogenous and NVP-AUY922-induced KIT degradation in gastrointestinal stromal tumors. Autophagy. 2013;9(2):220–33.
Hsueh Y-S, et al. MTOR inhibition enhances NVP-AUY922-induced autophagy-mediated KIT degradation and cytotoxicity in imatinib-resistant gastrointestinal stromal tumors. Oncotarget. 2014;5(22):11723–36.
Saito Y, et al. TAS-116 inhibits oncogenic KIT signalling on the Golgi in both imatinib-naïve and imatinib-resistant gastrointestinal stromal tumours. Br J Cancer. 2020;122(5):658–67.
Mühlenberg T, et al. Inhibitors of deacetylases suppress oncogenic KIT signaling, acetylate HSP90, and induce apoptosis in gastrointestinal stromal tumors. Can Res. 2009;69(17):6941–50.
Ren X, et al. Targeting Heat-Shock Protein 90 in Cancer: An Update on Combination Therapy. Cells. 2022;11(16):2556.
Mariño-Enríquez A, et al. Genome-wide functional screening identifies CDC37 as a crucial HSP90-cofactor for KIT oncogenic expression in gastrointestinal stromal tumors. Oncogene. 2014;33(14):1872–6.
Kinsella P, et al. The novel pyrrolo-1,5-benzoxazepine, PBOX-15, synergistically enhances the apoptotic efficacy of imatinib in gastrointestinal stromal tumours; suggested mechanism of action of PBOX-15. Invest New Drugs. 2016;34(2):159–67.
García-Valverde A, et al. Preclinical Activity of PI3K Inhibitor Copanlisib in Gastrointestinal Stromal Tumor. Mol Cancer Ther. 2020;19(6):1289–97.
Javidi-Sharifi N, et al. Crosstalk between KIT and FGFR3 Promotes Gastrointestinal Stromal Tumor Cell Growth and Drug Resistance. Can Res. 2015;75(5):880–91.
Kelly CM, et al. A phase Ib study of BGJ398, a pan-FGFR kinase inhibitor in combination with imatinib in patients with advanced gastrointestinal stromal tumor. Invest New Drugs. 2019;37(2):282–90.
Fu, Y., et al., Deep learning predicts patients outcome and mutations from digitized histology slides in gastrointestinal stromal tumor. Npj Precision Oncology, 2023. 7(1).
Debiec-Rychter M, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42(8):1093–103.
Hsueh Y-S, et al. Selecting Tyrosine Kinase Inhibitors for Gastrointestinal Stromal Tumor with Secondary KIT Activation-Loop Domain Mutations. Plos One. 2013;8(6):e65762.
Napolitano A, et al. KIT Exon 9-Mutated Gastrointestinal Stromal Tumours: Biology and Treatment. Chemotherapy. 2022;67(2):81–90.
Venkataraman, V., S. George, and G.M. Cote, Molecular Advances in the Treatment of Advanced Gastrointestinal Stromal Tumor. Oncologist, 2023.
Zhou, Y., et al., Activity of Anlotinib in the Second-Line Therapy of Metastatic Gastrointestinal Stromal Tumors: A Prospective, Multicenter, In Vitro Study. Oncologist, 2023.
Castells M, et al. A Phase 2/3 Study of BLU-263 in Patients with Indolent Systemic Mastocytosis or Monoclonal Mast Cell Activation Syndrome. J Allergy Clin Immunol. 2022;149(2):AB221–AB221.
Wagner AJ, et al. Association of Combination of Conformation-Specific KIT Inhibitors With Clinical Benefit in Patients With Refractory Gastrointestinal Stromal Tumors A Phase 1b/2a Nonrandomized Clinical Trial. JAMA Oncol. 2021;7(9):1343–50.
Gotlib J, et al. A Phase 2 Study of Bezuclastinib (CGT9486), an Oral, Selective, and Potent KIT D816V Inhibitor, in Adult Patients with Advanced Systemic Mastocytosis (AdvSM). Blood. 2021;138:3636.
Huang WK, et al. Systemic Therapy for Gastrointestinal Stromal Tumor: Current Standards and Emerging Challenges. Curr Treat Options Oncol. 2022;23(9):1303–19.
Group, E.S.E.S.N.W., Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol, 2014. 25 Suppl 3: p. iii21–6.
Zhang H, et al. Radiotherapy in the Management of Gastrointestinal Stromal Tumors: A Systematic Review. Cancers (Basel). 2022;14(13):3169.
Casali PG, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(1):20–33.
Joensuu H, et al. Radiotherapy for GIST progressing during or after tyrosine kinase inhibitor therapy: A prospective study. Radiother Oncol. 2015;116(2):233–8.
Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–21.
He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30(8):660–9.
Roulleaux Dugage M, et al. Beyond the Driver Mutation: Immunotherapies in Gastrointestinal Stromal Tumors. Front Immunol. 2021;12: 715727.
Cui H, et al. Escalation of radiotherapy dose in large locally advanced drug-resistant gastrointestinal stromal tumors by multi-shell simultaneous integrated boost intensity-modulated technique: a feasibility study. Radiat Oncol. 2022;17(1):216.
Arshad, J., et al., Immunotherapy Strategies for Gastrointestinal Stromal Tumor. Cancers (Basel), 2021. 13(14).
Ozkan EE. Radiotherapy for Gastrointestinal Stromal Tumors. Chin Med J (Engl). 2018;131(2):235–40.
Li B, et al. Advances in immunology and immunotherapy for mesenchymal gastrointestinal cancers. Mol Cancer. 2023;22(1):71.
Teranishi R, et al. Combination of pimitespib (TAS-116) with sunitinib is an effective therapy for imatinib-resistant gastrointestinal stromal tumors. Int J Cancer. 2023;152(12):2580–93.
Kurokawa Y, et al. Pimitespib in patients with advanced gastrointestinal stromal tumor (CHAPTER-GIST-301): a randomized, double-blind, placebo-controlled phase III trial. Ann Oncol. 2022;33(9):959–67.
Cavnar MJ, et al. KIT oncogene inhibition drives intratumoral macrophage M2 polarization. J Exp Med. 2013;210(13):2873–86.
Li F, et al. FGFR-Mediated Reactivation of MAPK Signaling Attenuates Antitumor Effects of Imatinib in Gastrointestinal Stromal Tumors. Cancer Discov. 2015;5(4):438–51.
Acknowledgements
Not applicable.
Funding
This study was funded by a grant from the Province Natural Science Foundation of Hunan (No. 2021JJ31069).
Author information
Authors and Affiliations
Contributions
Bin Li designed the article; Shishan Zhou and Bin Li drafted the manuscript; Haiyan Zhou, Heli Liu and Zhi Li contributed to literature search and discussion: Omar Abdihamid and Sheng Xiao helped to revise the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, S., Abdihamid, O., Tan, F. et al. KIT mutations and expression: current knowledge and new insights for overcoming IM resistance in GIST. Cell Commun Signal 22, 153 (2024). https://doi.org/10.1186/s12964-023-01411-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-023-01411-x