Abstract
Introduction
Real-world studies on neoadjuvant dual anti-HER2 therapy combined with chemotherapy for breast cancer (BC) are scarce in China. This study aimed to evaluate the efficacy and safety of neoadjuvant dual anti-HER2 therapy combined with chemotherapy in a real-world setting. Moreover, differences in estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and proliferation cell nuclear antigen (Ki-67) expression pre- and post-neoadjuvant therapy were analyzed.
Methods
Clinical and pathological data of patients with HER2-positive BC who received neoadjuvant dual anti-HER2 therapy combined with chemotherapy at Liaoning Cancer Hospital & Institute, China, between September 2021 and September 2023, were retrospectively reviewed.
Results
Among 179 included patients, a pathologic complete response (pCR) was achieved in 109 patients (60.9%). The univariate analysis results indicated that the hormone receptor (HR) status (P = 0.013), HER2 status (P = 0.003), and cycles of targeted treatment (P = 0.035) were significantly correlated with pCR. Subsequent multivariable analysis showed that HR negative and HER2 status 3 + were independent predictive factors of pCR. Anemia was the most common adverse event (62.0%), and the most common grade 3–4 adverse event was neutropenia (6.1%). The differences in HER2 (34.5%) and Ki-67 (92.7%) expression between core needle biopsy and the residual tumor after neoadjuvant therapy were statistically significant, whereas the differences were insignificant in terms of ER or PR status.
Conclusions
The combination of neoadjuvant trastuzumab and pertuzumab with chemotherapy showed good efficiency, and the toxic side effects were tolerable in patients with BC. In cases where pCR was not achieved after neoadjuvant therapy, downregulation of HER2 and Ki-67 expressions was observed.
Similar content being viewed by others
Introduction
Breast cancer (BC) has emerged as the most common cancer worldwide, surpassing lung cancer [1]. Approximately 15% ~ 20% of patients with BC experienced an overexpression of human epidermal growth factor receptor 2 (HER2), which is related to aggressive biological behavior and poor prognosis [2]. HER2-targeted drugs have significantly improved the prognosis of patients with HER2-positive BC [3]. Neoadjuvant therapy (NAT) can effectively reduce the risk of micrometastases and downstage tumor load to increase surgical opportunity [4]. Importantly, clinicians can formulate subsequent treatment strategies based on the efficacy of NAT [5]. Pathologic complete response (pCR) has been demonstrated to be associated with long-term clinical benefits [6, 7], and the pCR rate is the most reliable indicator to predict the efficacy of NAT in BC [4].
The NOAH trial [8] demonstrated that the addition of anti-HER2 treatment to neoadjuvant chemotherapy significantly improved the pCR rate and reduced the risk of recurrence and progression in patients with BC. The NeoSphere trial [9] and its 5-year analysis [6] showed that neoadjuvant chemotherapy combined with trastuzumab and pertuzumab achieved better pCR, progression-free survival (PFS), and disease-free survival (DFS) than trastuzumab plus chemotherapy, thus launching a new era of dual-HER2 blockade for HER2-positive BC. Presently, neoadjuvant dual-target therapy is the preferred treatment for locally advanced HER2-positive BC.
Compared with core needle biopsy, changes of estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki-67 expressions in residual tumor after neoadjuvant HER2-targeted treatment were reported [10,11,12,13,14]. However, there is no consensus on whether testing should be repeated on the residual tumor and the follow-up treatment plan adjusted accordingly.
The use of pertuzumab was limited in China until it was covered by medical insurance in 2019; as a result, there are few real-world studies on neoadjuvant dual anti-HER2 therapy combined with chemotherapy in China [15, 16]. To address this gap, this single-center, retrospective study aimed to evaluate the efficacy and safety of neoadjuvant dual anti-HER2 therapy combined with chemotherapy in a real-world setting, and to examine the pathological characteristics of the residual disease after NAT.
Methods
Patients and data collection
This study enrolled patients who received neoadjuvant dual anti-HER2 therapy after being diagnosed with HER2-positive BC by core needle biopsy at Liaoning Cancer Hospital & Institute, China, between September 2021 and September 2023. Their clinical and pathological data, including age, menopausal status, BMI, TNM stage, status of immunohistochemical (IHC) markers (ER, PR, HER2, and Ki-67) in the core needle biopsy and surgical specimen, neoadjuvant regimens, adverse events (AEs) during treatment, the time interval to surgery, type of surgery, and pathological data post-surgery, were collected.
The inclusion criteria were as follows: 1) patients diagnosed with HER2-positive BC by core needle biopsy; 2) patients who received at least four cycles of neoadjuvant dual anti-HER2 therapy; 3) patients with no other treatment performed previously; and 4) patients with an Eastern Cooperative Oncology Group performance status ≤ 1. The exclusion criteria were as follows: 1) the presence of distant metastasis; 2) other concurrent cancers; 3) incomplete clinicopathological data; 4) incomplete surgical treatment; and 5) multifocal BC.
Neoadjuvant therapy and surgery
The NAT regimens were formulated in accordance with the National Comprehensive Cancer Network (NCCN) and Chinese Society of Clinical Oncology (CSCO) guidelines. Patients underwent blood and biochemical tests before administration of each cycle, and NAT was implemented in the absence of contraindication. Echocardiography was used to assess patients' left ventricular ejection fraction (LVEF) pre- and post-NAT. After completing NAT, the patients were assessed surgically and histopathologically. The surgical plan was based on the clinical tumor size, breast size, and patient preference. The typical surgical procedures included total mastectomy (TM) with axillary lymph node dissection (ALND), TM with sentinel lymph node biopsy (SLNB), breast-conserving surgery (BCS) with SLNB, and breast reconstruction surgery.
Judgment criteria
Clinical staging was based on the 8th American Joint Committee on Cancer staging. The ER, PR, and HER2 statuses were assessed in accordance with the American Society of Clinical Oncology/College of American Pathologists guidelines (2020). AEs were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE, version 5.0). The Miller–Payne grading system was used to classify the treatment response. Additionally, pCR was defined as no invasive disease in the breast (ypTis/0) and axillary lymph nodes (ypN0) irrespective of ductal carcinoma in situ.
Statistical analysis
SPSS version 26.0 (IBM, Armonk, NY, USA) was used for data analysis. Univariate and multivariate analyses were performed to analyze the efficacy and influencing factors of NAT. Pre- to post-NAT changes in quantitative biomarkers were tested using the Wilcoxon signed rank test, and qualitative changes were analyzed using chi-square tests. A P-value of < 0.05 was considered statistically significant. Graphs were generated using GraphPad Prism 7 (GraphPad Prism Software, Inc.CA, USA).
Results
General information and treatments of the patients
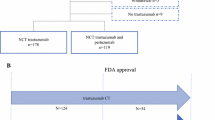
Initially, A total of 280 patients were selected,101 patients were excluded after the application of the exclusion criteria, including 16 cases with distant metastasis, 1 with thyroid cancer, 11 with incomplete clinical data, 63 with incomplete surgical treatment (11 cases were lost to follow-up during treatment, 51 had not yet completed treatment at the cut-off date, 1 refused surgery after neoadjuvant treatment), and 8 with multifocal breast cancer. Finally, 179 patients who underwent neoadjuvant dual-target therapy and surgery were enrolled (Fig. 1).
Table 1 shows the clinicopathologic characteristics of the included patients. Notably, the median age of the patients who received neoadjuvant dual-target treatment was 53 (range, 25–75) years, and 57.8% of them were postmenopausal. Among the 179 patients, 97 (54.2%) were HR-positive (defined as ER or PR positive), and the Ki-67 expression in 172 (96.1%) patients was ≥ 20%.
Among 130 (72.6%) patients who received carboplatin plus taxane in combination with pertuzumab and trastuzumab (TCbHP), carboplatin was removed from the regimen of six patients due to intolerable AEs. Therefore, only 124 (69.3%) patients completed the TCbHP regimen. Forty-six patients (25.7%) received anthracycline (doxorubicin/doxorubicin) plus cyclophosphamide followed by paclitaxel or docetaxel plus pertuzumab and trastuzumab (AC/EC-THP) every 3 weeks, and nine patients (5%) received other neoadjuvant regimens (2 received THP, 1 received capecitabine combined with THP, and 6 patients from the TCbHP regimen were changed to THP due to AEs).
The time interval from completion of neoadjuvant chemotherapy to surgery was 11–63 days, and the median interval was 24 days (interquartile range, IQR 19–31). Regarding the surgical procedure performed, 143(79.9%) received TM with ALND, 24 (13.4%) underwent TM with SLNB, 8 (4.5%) received BCS with SLNB and 4 (2.2%) received immediate breast reconstruction surgery.
The efficacy of neoadjuvant dual anti-HER2 therapy and its influencing factors
The results of the postoperative pathological examination showed that the pCR rate was 60.9% (109/179), of which 65.3% (81/124) had received TCbHP and 50% (23/46) received AC/EC-THP. Table 1 summarizes the results of the univariate analysis of pCR-associated factors, in which the patients with HR-negative (pCR: 70.7%, P = 0.013), HER2 IHC (3 +) (pCR: 65.0%, P = 0.003), and more than four cycles of targeted treatment (pCR: 65.9%, P = 0.035) had a high pCR rate. The pCR rate was not significantly associated with the patient’s age (P = 0.974), T stage (P = 0.110), N stage (P = 0.067), clinical stage (P = 0.320), or Ki-67 level (P = 0.851). Moreover, no statistically significant difference was observed in the pCR of patients who had a ≤ 21 and > 21-day interval of neoadjuvant chemotherapy to surgery.
Twenty-two patients were HER2 IHC (2 +) / FISH-positive. However, only 18 patients had complete records. The median (P25, P75) of HER2/cell number ratio was 8.2 (6.0825–8.8250), the ratio of chromosome 17 centromere /cell number (CEP17/cell) was 2.75 (2.0500–3.9925), and HER2/CEP17 ratio was 2.85 (2.3375–3.8500). Statistical analyses showed that none of the three ratios were associated with the pCR rate of neoadjuvant HER2-targeted treatment (P = 0.402, P = 0.825, P = 0.757).
Variables with P < 0.1 in the univariate analysis were subjected to the multivariable analysis. Table 2 shows that the HR negative (OR = 2.252, 95% CI: 1.172 ~ 4.323) and HER2 (IHC)3 + (OR = 4.576, 95% CI: 1.682 ~ 12.449) were independent predictive factors of pCR (Fig. 2).
Changes in the ER, PR, HER2, and Ki-67 expression after neoadjuvant HER2-targeted treatment
pCR was not achieved in 70 patients after neoadjuvant dual anti-HER2 therapy. Moreover, only 55 patients had sufficient residual tumor tissue to re-evaluate the expression of the immunohistochemical indicators.
Quantitative changes of IHC indicators
Figure 3 shows a comparison of the quantitative changes in the ER, PR, HER2, and Ki-67 expressions between core needle biopsy and residual lesions. Additionally, 25.4% (14/55) and 16.3% (9/55) of the patients had an increased and decreased ER expression, respectively. The PR expression was upregulated in 16.3% (9/55) and downregulated in 18.1% (10/55) of the patients. Both ER and PR expression changes were not statistically significant (P = 0.139, P = 0.344) (Fig. 3A, B). More patients had decreased Ki-67 expression post-treatment (81.8%) compared to those with consistent (7.3%) or increased (10.9%) expression (P < 0.001) (Fig. 3C). Compared with core needle biopsy results, the HER2 expression of 32.7% (18/55) of the patients changed from 3 + to 2 + ; whereas, 65.5% (36/55) of the patients showed no change (P < 0.001) (Fig. 3D).
Qualitative changes of IHC indicators
Figure 4 shows the qualitative changes in the status of ER, PR, and Ki-67. Following treatment, a difference in ER, PR, and Ki-67 expression was observed in 7.3% (4/55), 23.6% (13/55), and 60% (33/55) cases, respectively. None of the patients with ER-positive at core needle biopsy turned to ER-negative post-treatment. Nevertheless, the ER status of 19.0% (4/21) changed from positive to negative (P = 0.125) (Fig. 4A). There were 23.1% (6/26) cases with PR positive-to-negative conversion and 24.1% (7/29) cases with a PR negative-to-positive conversion (P = 1.000) (Fig. 4B). Among patients whose Ki-67 expression was ≥ 20% pre-treatment, 56.6% (30/53) had < 20% Ki-67 expression in the residual lesions post-treatment (P < 0.001) (Fig. 4C). Four HER2-positive patients became HER2-negative after treatment, and the other 21 patients with HER2 IHC 2 + post-treatment did not undergo FISH testing. Hence, their qualitative analysis of changes in HER2 status was incomplete.
Safety of neoadjuvant HER2-targeted treatment
Based on the patient’s age, underlying disease tolerance and other aspects, the initial dose was adjusted for some patients. During NAT, 17 (13.1%) patients experienced a carboplatin dose reduction due to AEs among the 130 patients treated with TCbHP, and 6 patients discontinued carboplatin due to intolerable toxicity after reducing the dose (4 patients had bone marrow suppression, 1 had high creatinine, and 1 had severe diarrhea). Among the 46 patients who received AC/EC-THP regimen treatment, only 1 required a docetaxel dose reduction, and no patient had the drugs discontinued.
To ensure the safety of NAT, pegylated granulocyte colony-stimulating factor was prophylactically used for patients with intermediate or high-risk of febrile neutropenia according to the guidelines of CSCO. However, 23.5% of patients still developed leukopenia. The most common AE was anemia (62.0%), and the highest incidence of grade 3–4 AE was neutropenia (6.1%). The common AEs among patients with TCbHP and AC/EC-THP were anemia (69.3% vs. 41.3%), leukopenia (26.9% vs. 13.0%), and neutropenia (23.8% vs. 10.9%). The common grade 3–4 AE was neutropenia (6.9%) in patients who received TCbHP and transaminase increase (4.3%) in those with AC/EC-THP (Table 3).
Discussion
The pCR rate of patients with HER2-positive BC who received neoadjuvant dual-target combination chemotherapy ranges from 39%~68% [9, 17,18,19,20,21,22]. There is limited data on neoadjuvant dual-target therapy in China. A domestic study named CSBrS-015 has reported the pCR rate of neoadjuvant dual-target combination chemotherapy to be 57.9% (324/560) [15]. Another multi-center study observed that 46.8% (88/188) of patients with HER2-positive BC achieved pCR [16]. In our study, the pCR rate was 60.9% (109/179), which confirmed the effectiveness of neoadjuvant trastuzumab plus pertuzumab combined with neoadjuvant chemotherapy in treating HER2-positive BC in China.
Existing studies have reported that the pCR rate of NAT in HER2-positive BC was related to the status of HR and HER2 [2, 23,24,25]. Our results indicate that the status of HR and HER2 were significantly correlated with the pCR rate. Previous clinical trials revealed that patients with HR-negative BC had a higher pCR rate than those with HR-positive BC[18, 19, 21]. The NeoSphere trial reported that 63.2% (36/57) of HR-negative patients with BC achieved pCR, whereas the value was only 26.0% (13/50) for HR-positive patients [9]. This phenomenon may be attributed to the bidirectional crosstalk between ER and HER2 signaling pathways; when HR-positive patients receive anti-HER2 therapy, ER signaling provides cell survival and proliferation stimuli, thereby leading to anti-HER2 resistance [26]. Additionally, compared to patients with IHC (2 +)/FISH-positive, those with HER2 IHC (3 +) had a higher pCR rate, which was consistent with the results of previous studies [27,28,29,30]. Some scholars believe that the poor response to anti-HER2 therapy in IHC (2 +)/FISH-positive patients was associated with higher intratumoral heterogeneity [31]. Choi JH et al. [32] and Singer CF et al. [33] found that the pCR rate of neoadjuvant dual anti-HER2 therapy was correlated with the HER2/CEP17 ratio. However, in our study, the pCR rate was not associated with the HER2/CEP17 ratio or HER2 /cell number, which may be attributed to the smaller number of patients who underwent FISH testing (n = 22). Our study also found that patients with more than four targeted treatment cycles had a higher pCR rate. However, Zhou M et al. [16] believed that the number of cycles of targeted treatment was not statistically correlated with the pCR rate.
No consensus was reached on the optimal time interval from completion of NAT to surgery for BC. A retrospective study by Sanford RA et al. [34] reported that a prolonged time interval (> 8 weeks) may affect survival outcomes. Omarini C et al. [35] analyzed a shorter time interval (21 days) and showed that patients who underwent surgery within 21 days after the completion of NAT experienced improvements in OS and DFS. However, Arciero C believed that the time interval has no impact on postoperative complications and prognosis [36]. Presently, clinicians believe that it is appropriate to perform surgery within 2–4 weeks of completing NAT and that it is considered safe within 8 weeks [37]. The time of surgery should be determined according to the clinical situation, and clinicians should seek a balance between recovery from adverse reactions and disease progression control. In our study, 95.5% (171/179) patients underwent surgery within 8 weeks after NAT, but only 36.9% (66/179) patients underwent surgery within 21 days, which may be related to the delayed operation caused by the coronavirus disease 2019 (COVID-19) pandemic.
Patients with residual disease after NAT have a worse prognosis than those who achieved pCR [38]. Hurley J et al. [13] had reported changes in the expression of IHC indicators in residual disease. In the study by Mittendorf EA [12], 20% of patients experienced an ER-negative to ER-positive switch. In another retrospective study, 5.9% of ER-positive patients turned to ER-negative after NAT [39]. A previous study suggested that the upregulated ER expression after anti-HER2 treatment is related to the bidirectional crosstalk between ER and the HER2 signaling pathway [40], and patients with increased ER expression were more likely to develop anti-HER2 resistance [26]. Niikura N et al. [41] reported that the negative conversion rate of PR (18.7%) was significantly higher than its positive conversion rate (9.3%), and the loss of PR expression was related to an improved survival rate. The PR expression changes were not statistically significant in the study performed by Wang RX [39]. The differences in ER and PR expression were not statistically significant in both the quantitative and qualitative analyses in our study. Some studies have shown that Ki-67 expression is significantly reduced after treatment, demonstrating that low Ki-67 levels after neoadjuvant targeted therapy were associated with better prognosis [42, 43].
Interestingly, a decreased expression of HER2 is more common than an increased expression in our study. However, a qualitative analysis of HER2 status changes from positive to negative could not be carried out in this study. A decreased HER2 expression was observed in approximately 8.3–43% of patients with HER2-positive BC after receiving neoadjuvant targeted therapy in previous studies [12,13,14]. Ignatov T et al. [44] reported that neoadjuvant dual anti-HER2 therapy had a higher HER2-negative conversion rate than single-targeted therapy. Branco FP [11] suggested that HR-negative patients were more likely to have reduced HER2 expression. Wang RX et al. [39] found that patients with a loss of HER2 expression tend to have a higher risk of recurrence.
Presently, there is no definite conclusion on whether the subsequent treatment for BC should be adjusted according to the differences. A further subgroup analysis based on the KATHERINE study showed that adjuvant T-DM1 was beneficial for patients with HER2 absent residual lesions [14]. Giugliano F et al. [45] believed that the bystander effect can partially compensate for the impairment of drug efficacy in the inconsistency of HER2 expression, and the novel antibody–drug conjugates with bystander effect, for example, trastuzumab deruxtecan (T-DXd), may produce more benefits for patients with absent HER2 expression. Since increased ER expression is associated with anti-HER2 resistance, endocrine therapy is recommended for patients whose HR changes from negative to positive after treatment [26].
This retrospective study had incomplete records of subjective AEs such as nausea, vomiting, diarrhea, rash, hair loss, and fatigue. Therefore, only adverse reactions that can be monitored by laboratory tests were emphasized. The widely used granulocyte colony-stimulating factor may exert an impact on our observation of AEs. Anemia was the most common AE in our study, similar to a previous study [23]. No patient experienced a significant decrease in LVEF (LVEF decreased by ≥ 10% from baseline or was between 40–50%), which was consistent with the results of the NeoSphere [9] and TRYPHAENA [46] trials. The balance between efficacy and safety is crucial in clinical settings. For patients with serious AEs, adjusting the treatment dose and strengthening management is necessary. Lv M et al. [30] had proposed that a treatment regimen without carboplatin is recommended for elderly patients with poor tolerance.
This study had some limitations. Firstly, it is a retrospective study without a completion date, making it challenging to evaluate the occurrence and severity of all AEs during NAT. Secondly, this study was conducted during the COVID-19 epidemic, which could have led to delays in the treatment of the patients. The impact of COVID-19 infections on BC development was not considered. Thirdly, the follow-up time was insufficient to analyze long-term survival. Lastly, our study was a single-center study with a limited sample size.
In conclusion, our results show that neoadjuvant dual anti-HER2 therapy combined with chemotherapy has good efficacy, and the toxic side effects are tolerable. The expression of HER2 and Ki-67 in the residual lesions was decreased, and more research on individualized treatment for patients who did not achieve pCR needs to be conducted. We believe that dual anti-HER2 therapy is not the end of HER2-positive BC, but just the beginning.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- BC:
-
Breast cancer
- HER2:
-
Human epidermal growth factor receptor 2
- NAT:
-
Neoadjuvant therapy
- pCR:
-
Pathologic complete response
- PFS:
-
Progression-free survival
- DFS:
-
Disease-free survival
- ER:
-
Estrogen receptor
- RP:
-
Progesterone receptor
- Ki67:
-
Proliferation cell nuclear antigen
- HR:
-
Hormone receptor
- IHC:
-
Immunohistochemistry
- FISH:
-
Fluorescence in-situ hybridization
- AEs:
-
Adverse events
- NCCN:
-
National Comprehensive Cancer Network
- CSCO:
-
Chinese Society of Clinical Oncology
- LVEF:
-
Left ventricular ejection fraction
- TM:
-
Total mastectomy
- ALND:
-
Axillary lymph node dissection
- SLNB:
-
Sentinel lymph node biopsy
- BCS:
-
Breast-conserving surgery
- CEP17:
-
Chromosome 17 centromere
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
Loibl S, Gianni L. HER2-positive breast cancer. The Lancet. 2017;389(10087):2415–29.
Waks AG, Winer EP. Breast Cancer Treatment: A Review. JAMA. 2019;321(3):288–300.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet. 2014;384(9938):164–72.
Shien T, Iwata H. Adjuvant and neoadjuvant therapy for breast cancer. Jpn J Clin Oncol. 2020;50(3):225–9.
Gianni L, Pienkowski T, Im Y-H, Tseng L-M, Liu M-C, Lluch A, Starosławska E, de la Haba-Rodriguez J, Im S-A, Pedrini JL, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791–800.
Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, Smith BL, Alexander B, Moy B, Isakoff SJ, et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin Cancer Res. 2020;26(12):2838–48.
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–84.
Gianni L, Pienkowski T, Im Y-H, Roman L, Tseng L-M, Liu M-C, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im S-A, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32.
Guarneri V, Dieci MV, Barbieri E, Piacentini F, Omarini C, Ficarra G, Bettelli S, Conte PF. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann Oncol. 2013;24(12):2990–4.
Branco FP, Machado D: Loss of HER2 and disease prognosis after neoadjuvant treatment of HER2+ breast cancer. Am J Transl Res 2019;11(9):6110–6116.
Mittendorf EA, Wu Y, Scaltriti M, Meric-Bernstam F, Hunt KK, Dawood S, Esteva FJ, Buzdar AU, Chen H, Eksambi S, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009;15(23):7381–8.
Hurley J, Doliny P, Reis I, Silva O, Gomez-Fernandez C, Velez P, Pauletti G, Powell JE, Pegram MD, Slamon DJ. Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J Clin Oncol. 2006;24(12):1831–8.
Loibl S, Huang CS, Mano MS, Mamounas EP, Geyer CE Jr, Untch M, Thery JC, Schwaner I, Limentani S, Loman N, et al. Adjuvant trastuzumab emtansine in HER2-positive breast cancer patients with HER2-negative residual invasive disease in KATHERINE. NPJ Breast Cancer. 2022;8(1):106.
Cheng Y, Xiang H, Xin L, Duan X, Liu Y. Chinese Society of Breast Surgery CSoSoCMA: Neoadjuvant therapy for early human epidermal growth factor receptor 2 positive breast cancer in China: A multicenter real-world study (CSBrS-015). Chin Med J (Engl). 2022;135(19):2311–8.
Zhou M, Wang S, Wan N, Yuan S, Hu X, Zhou W, Qing B, Liu M, Sun W, Fan P, et al. Efficacy and safety of neoadjuvant pertuzumab plus trastuzumab in combination with chemotherapy regimen in Chinese patients with HER2-positive early breast cancer: a real-world retrospective multi-center cohort study. Ann Transl Med. 2022;10(24):1387.
Swain SM, Ewer MS, Viale G, Delaloge S, Ferrero JM, Verrill M, Colomer R, Vieira C, Werner TL, Douthwaite H, et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol. 2018;29(3):646–53.
Gonzalez-Santiago S, Saura C, Ciruelos E, Alonso JL, de la Morena P, Santisteban Eslava M, Gallegos Sancho MI, de Luna A, Dalmau E, Servitja S, et al. Real-world effectiveness of dual HER2 blockade with pertuzumab and trastuzumab for neoadjuvant treatment of HER2-positive early breast cancer (The NEOPETRA Study). Breast Cancer Res Treat. 2020;184(2):469–79.
van Ramshorst MS, van der Voort A, van Werkhoven ED, Mandjes IA, Kemper I, Dezentje VO, Oving IM, Honkoop AH, Tick LW, van de Wouw AJ, et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(12):1630–40.
Shao Z, Pang D, Yang H, Li W, Wang S, Cui S, Liao N, Wang Y, Wang C, Chang YC, et al. Efficacy, Safety, and Tolerability of Pertuzumab, Trastuzumab, and Docetaxel for Patients With Early or Locally Advanced ERBB2-Positive Breast Cancer in Asia: The PEONY Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6(3): e193692.
Arora S, Gogia DA, Deo S, Sharma D, Mathur SR. Neoadjuvant pertuzumab plus trastuzumab in combination with anthracycline- free chemotherapy regimen in patients with HER2 positive breast cancer-Real-world data from a single center in India. Cancer Treat Res Commun. 2021;29: 100483.
Medina EAG, Caballero BB, Miguel KL, Gutierrez ZA, Fernandez BM, Tul LEA, Rodriguez LEM, Guerrero OV, Varela IGS, Bernardo MCC, et al. Neoadjuvant Trastuzumab and Pertuzumab in Combination with Standard Chemotherapy for HER2-Positive Early Breast Cancer: Real-World Practice in Cuba. Cancer Treat Res Commun. 2023;34: 100670.
Jagiello-Gruszfeld AI, Rosinska M, Meluch M, Pogoda K, Niwinska A, Sienkiewicz R, Grous A, Winter P, Nowecki ZI: Neoadjuvant Pertuzumab Plus Trastuzumab in Combination with Docetaxel and Carboplatin in Patients with HER2-Positive Breast Cancer: Real-World Data from the National Institute of Oncology in Poland. Cancers (Basel). 2022;14(5):1218.
Choi JDW, Hughes TMD, Marx G, Rutovitz J, Hasovits C, Ngui NK. Pathological outcomes of HER2-positive non-metastatic breast cancer patients treated with neoadjuvant dual anti-HER2 therapy and taxane: An Australian experience. Asia Pac J Clin Oncol. 2020;16(3):103–7.
Katayama A, Miligy IM, Shiino S, Toss MS, Eldib K, Kurozumi S, Quinn CM, Badr N, Murray C, Provenzano E, et al. Predictors of pathological complete response to neoadjuvant treatment and changes to post-neoadjuvant HER2 status in HER2-positive invasive breast cancer. Mod Pathol. 2021;34(7):1271–81.
Giuliano M, Hu H, Wang YC, Fu X, Nardone A, Herrera S, Mao S, Contreras A, Gutierrez C, Wang T, et al. Upregulation of ER Signaling as an Adaptive Mechanism of Cell Survival in HER2-Positive Breast Tumors Treated with Anti-HER2 Therapy. Clin Cancer Res. 2015;21(17):3995–4003.
Krystel-Whittemore M, Xu J, Brogi E, Ventura K, Patil S, Ross DS, Dang C, Robson M, Norton L, Morrow M, et al. Pathologic complete response rate according to HER2 detection methods in HER2-positive breast cancer treated with neoadjuvant systemic therapy. Breast Cancer Res Treat. 2019;177(1):61–6.
Wang Y, Singh K, Dizon D, Graves T, Amin A, Yakirevich E. Immunohistochemical HER2 score correlates with response to neoadjuvant chemotherapy in HER2-positive primary breast cancer. Breast Cancer Res Treat. 2021;186(3):667–76.
Ma Y, Zhu M, Lv M, Yuan P, Chen X, Liu Z. Impact of residual disease biomarkers on the prognosis of HER2-positive breast cancer following neoadjuvant therapy. Cancer Med. 2023;12(10):11293–304.
Lv M, Guo H, Wang C, Tian P, Ma Y, Chen X, Luo S. Neoadjuvant docetaxel with or without carboplatin plus dual HER2 blockade for HER2-positive breast cancer: a retrospective multi-center Chinese study. Gland Surg. 2020;9(6):2079–90.
Hou Y, Nitta H, Wei L, Banks PM, Portier B, Parwani AV, Li Z. HER2 intratumoral heterogeneity is independently associated with incomplete response to anti-HER2 neoadjuvant chemotherapy in HER2-positive breast carcinoma. Breast Cancer Res Treat. 2017;166(2):447–57.
Choi JH, Jeon CW, Kim YO, Jung S. Pathological complete response to neoadjuvant trastuzumab and pertuzumab therapy is related to human epidermal growth factor receptor 2 (HER2) amplification level in HER2-amplified breast cancer. Medicine (Baltimore). 2020;99(46): e23053.
Singer CF, Tan YY, Fitzal F, Steger GG, Egle D, Reiner A, Rudas M, Moinfar F, Gruber C, Petru E, et al. Pathological Complete Response to Neoadjuvant Trastuzumab Is Dependent on HER2/CEP17 Ratio in HER2-Amplified Early Breast Cancer. Clin Cancer Res. 2017;23(14):3676–83.
Sanford RA, Lei X, Barcenas CH, Mittendorf EA, Caudle AS, Valero V, Tripathy D, Giordano SH, Chavez-MacGregor M. Impact of Time from Completion of Neoadjuvant Chemotherapy to Surgery on Survival Outcomes in Breast Cancer Patients. Ann Surg Oncol. 2016;23(5):1515–21.
Omarini C, Guaitoli G, Noventa S, Andreotti A, Gambini A, Palma E, Papi S, Tazzioli G, Balduzzi S, Dominici M, et al. Impact of time to surgery after neoadjuvant chemotherapy in operable breast cancer patients. Eur J Surg Oncol. 2017;43(4):613–8.
Arciero C, Buhariwalla K, Liu Y, Torres MA, Subhedar P. Time from Completion of Neo-adjuvant Chemotherapy to Surgery: Effects on Outcomes in Breast Cancer Patients. Breast J. 2020;26(2):155–61.
Lai V, Hajjaj O, Le D, Shokoohi A, Chia S, Simmons C. Impact of wait time from neoadjuvant chemotherapy to surgery in breast cancer: Does time to surgery affect patient outcomes? : Time from Neoadjuvant Chemotherapy to Surgery. Breast Cancer Res Treat. 2020;184(3):755–62.
von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, Wolmark N, Rastogi P, Schneeweiss A, Redondo A, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019;380(7):617–28.
Wang RX, Chen S, Jin X, Chen CM, Shao ZM. Weekly paclitaxel plus carboplatin with or without trastuzumab as neoadjuvant chemotherapy for HER2-positive breast cancer: loss of HER2 amplification and its impact on response and prognosis. Breast Cancer Res Treat. 2017;161(2):259–67.
Giuliano M, Trivedi MV, Schiff R. Bidirectional Crosstalk between the Estrogen Receptor and Human Epidermal Growth Factor Receptor 2 Signaling Pathways in Breast Cancer: Molecular Basis and Clinical Implications. Breast Care (Basel). 2013;8(4):256–62.
Niikura N, Tomotaki A, Miyata H, Iwamoto T, Kawai M, Anan K, Hayashi N, Aogi K, Ishida T, Masuoka H, et al. Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese breast cancer registry. Ann Oncol. 2016;27(3):480–7.
Ding Y, Ding K, Qian H, Yu X, Zou D, Yang H, Mo W, He X, Zhang F, Qin C, et al. Impact on survival of estrogen receptor, progesterone receptor and Ki-67 expression discordance pre- and post-neoadjuvant chemotherapy in breast cancer. PLoS ONE. 2020;15(4): e0231895.
Fujita N, Enomoto Y, Inakami K, Yanagisawa T, Iguchi C, Aono T, Nomura T, Yamamoto H, Kasugai T, Shiba E. Prognostic Factors in HER2-Positive Primary Breast Cancer Patients Treated Using Neoadjuvant Chemotherapy Plus Trastuzumab. Oncology. 2020;98(1):35–41.
Ignatov T, Gorbunow F, Eggemann H, Ortmann O, Ignatov A. Loss of HER2 after HER2-targeted treatment. Breast Cancer Res Treat. 2019;175(2):401–8.
Giugliano F, Corti C, Tarantino P, Michelini F, Curigliano G. Bystander effect of antibody-drug conjugates: fact or fiction? Curr Oncol Rep. 2022;24(7):809–17.
Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, Tausch C, Seo JH, Tsai YF, Ratnayake J, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278–84.
Acknowledgements
Thanks to all the patients who were included in the research and the support in collecting data by Cancer Hospital of China Medical University.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Dong-Mei Peng was responsible for the conceptualization and study design, and Lin Zhao participated in the study design and supervision. Juan Li and Jia-Xin Qiu contributed to data collection and statistical analysis, Dong-Mei Peng wrote the manuscript and all authors revised the manuscript and gave final approval of final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study approved by the ethics committee of the Ethics Committee of the Liaoning Cancer Hospital & Institute and it was in accordance with the 1964 Helsinki declaration. (NO. KY20231205).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Peng, DM., Li, J., Qiu, JX. et al. Neoadjuvant pertuzumab plus trastuzumab in combination with chemotherapy for human epidermal growth factor receptor 2 positive breast cancer: a real-world retrospective single-institutional study in China. World J Surg Onc 22, 88 (2024). https://doi.org/10.1186/s12957-024-03365-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-024-03365-x