Abstract
Purpose
Neoadjuvant chemotherapy (NCT) plus anti-HER2 agents are the standard of care for locally advanced HER2-positive breast cancer. The aim of this study was to evaluate the prevalence and prognostic impact of HER2 loss in patients with HER2-positive disease treated with neoadjuvant therapy with or without trastuzumab.
Methods
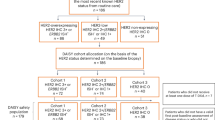
549 consecutive HER2-positive patients were included in this study. 379 patients were treated with paclitaxel, carboplatin, and trastuzumab (PCH cohort) and 170 were treated with paclitaxel and carboplatin only (PC cohort). Conversion of biomarkers before and after NCT was evaluated via immunohistochemistry (IHC) test. Cox regression model was used to investigate prognostic markers to relapse-free survival (RFS).
Results
50.9% patients were considered as pCR responder in PCH cohort, whereas only 25.9% of patients experienced pCR in PC cohort (P < 0.001). HER2 loss were more frequently shown in PCH cohort with a proportion of 19.8%, compared to 9.4% in PC cohort (P = 0.009). In PCH cohort, patients with a loss of HER2 expression tended to have a higher risk of relapse compared to patients with maintained HER2 expression (HR = 2.639, 95% CI 1.103–6.311, P = 0.029). However, it did not correlate to patient outcome in the PC cohort (P = 0.296). Loss of HER2 was also correlated to ER conversion in PCH cohort.
Conclusion
Our study has provided new evidence that anti-HER2 treatment has a significant impact on HER2 loss. Far more importantly, the loss of HER2 amplification could identify non-pCR patients with high risk of disease relapse, which might help in tailoring following systemic treatment.



Similar content being viewed by others
References
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172
Kong X, Moran MS, Zhang N, Haffty B, Yang Q (2011) Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer 47(14):2084–2090
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182
Andrulis IL, Bull SB, Blackstein ME, Sutherland D, Mak C, Sidlofsky S, Pritzker KP, Hartwick RW, Hanna W, Lickley L et al (1998) neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol 16(4):1340–1349
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792
Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH et al (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23(16):3676–3685
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M et al (2010) Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 375(9712):377–384
Mittendorf EA, Wu Y, Scaltriti M, Meric-Bernstam F, Hunt KK, Dawood S, Esteva FJ, Buzdar AU, Chen H, Eksambi S et al (2009) Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res 15(23):7381–7388
Guarneri V, Dieci MV, Barbieri E, Piacentini F, Omarini C, Ficarra G, Bettelli S, Conte PF (2013) Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann Oncol 24(12):2990–2994
van de Ven S, Smit VT, Dekker TJ, Nortier JW, Kroep JR (2011) Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev 37(6):422–430
Niikura N, Tomotaki A, Miyata H, Iwamoto T, Kawai M, Anan K, Hayashi N, Aogi K, Ishida T, Masuoka H et al (2016) Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese breast cancer registry. Ann Oncol 27(3):480–487
Dowsett M, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I, Salter J, Detre S, Hills M, Ashley S et al (2005) Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer–a study from the IMPACT trialists. J Clin Oncol 23(11):2477–2492
Arens N, Bleyl U, Hildenbrand R (2005) HER2/neu, p53, Ki67, and hormone receptors do not change during neoadjuvant chemotherapy in breast cancer. Virchows Arch 446(5):489–496
Chen XS, Nie XQ, Chen CM, Wu JY, Wu J, Lu JS, Shao ZM, Shen ZZ, Shen KW (2010) Weekly paclitaxel plus carboplatin is an effective nonanthracycline-containing regimen as neoadjuvant chemotherapy for breast cancer. Ann Oncol 21(5):961–967
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Zhang N, Moran MS, Huo Q, Haffty BG, Yang Q (2011) The hormonal receptor status in breast cancer can be altered by neoadjuvant chemotherapy: a meta-analysis. Cancer Invest 29(9):594–598
Dieci MV, Barbieri E, Piacentini F, Ficarra G, Bettelli S, Dominici M, Conte PF, Guarneri V (2013) Discordance in receptor status between primary and recurrent breast cancer has a prognostic impact: a single-institution analysis. Ann Oncol 24(1):101–108
Liedtke C, Broglio K, Moulder S, Hsu L, Kau SW, Symmans WF, Albarracin C, Meric-Bernstam F, Woodward W, Theriault RL et al (2009) Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol 20(12):1953–1958
Niikura N, Liu J, Hayashi N, Mittendorf EA, Gong Y, Palla SL, Tokuda Y, Gonzalez-Angulo AM, Hortobagyi GN, Ueno NT (2012) Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol 30(6):593–599
de Azambuja E, Cardoso F, de Castro G Jr, Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M (2007) Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 96(10):1504–1513
Brown JR, Digiovanna MP, Killelea B, Lannin DR, Rimm DL (2013) Quantitative assessment Ki-67 score for prediction of response to neoadjuvant chemotherapy in breast cancer. Lab Invest 94(1):98–106
Matsubara N, Mukai H, Masumoto M, Sasaki M, Naito Y, Fujii S, Wada N (2014) Survival outcome and reduction rate of Ki-67 between pre- and post-neoadjuvant chemotherapy in breast cancer patients with non-pCR. Breast Cancer Res Treat 147(1):95–102
Colleoni M, Bagnardi V, Rotmensz N, Dellapasqua S, Viale G, Pruneri G, Veronesi P, Torrisi R, Luini A, Intra M et al (2009) A risk score to predict disease-free survival in patients not achieving a pathological complete remission after preoperative chemotherapy for breast cancer. Ann Oncol 20(7):1178–1184
Guarneri V, Frassoldati A, Bottini A, Cagossi K, Bisagni G, Sarti S, Ravaioli A, Cavanna L, Giardina G, Musolino A et al (2012) Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol 30(16):1989–1995
Baselga J, Bradbury I, Eidtmann H, di Cosimo S, Aura C, de Azambuja E, Gomez H, Dinh P, Fauria K, Van Dooren V et al (2011) Final Results of the NeoALTTO Trial (BIG01-06/EGF106903): a Phase III, randomized, open-label, neo-adjuvant study of lapatinib, trastuzumab, and their combination plus paclitaxel in women with HER2-positive primary breast cancer. Cancer Res 70:S3
Pernas S, Gil MG, Urruticoechea A, Fernandez-Ortega A, Falo C, Piulats JM, Prieto L, Pla MJ, Moreno A, Germa JR (2006) High pathologic complete response after primary chemotherapy in patients treated with weekly paclitaxel followed by FEC chemotherapy with simultaneous trastuzumab in Her-2 positive operable breast cancer. Ann Oncol 17:96–96
Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, Elledge RM (2005) Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst 97(17):1254–1261
Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R (2004) Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96(12):926–935
Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P et al (2005) Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 11(16):5678–5685
Chen Y, Chen C, Yang B, Xu Q, Wu F, Liu F, Ye X, Meng X, Mougin B, Liu G et al (2011) Estrogen receptor-related genes as an important panel of predictors for breast cancer response to neoadjuvant chemotherapy. Cancer Lett 302(1):63–68
Acknowledgements
This research is supported by the National Natural Science Foundation of China (81302298). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declared no potential conflicts of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Ruo-Xi Wang and Sheng Chen have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, RX., Chen, S., Jin, X. et al. Weekly paclitaxel plus carboplatin with or without trastuzumab as neoadjuvant chemotherapy for HER2-positive breast cancer: loss of HER2 amplification and its impact on response and prognosis. Breast Cancer Res Treat 161, 259–267 (2017). https://doi.org/10.1007/s10549-016-4064-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-4064-9