Abstract
The use of nanomaterials in medicine offers multiple opportunities to address neurodegenerative disorders such as Alzheimer's and Parkinson's disease. These diseases are a significant burden for society and the health system, affecting millions of people worldwide without sensitive and selective diagnostic methodologies or effective treatments to stop their progression. In this sense, the use of gold nanoparticles is a promising tool due to their unique properties at the nanometric level. They can be functionalized with specific molecules to selectively target pathological proteins such as Tau and α-synuclein for Alzheimer’s and Parkinson’s disease, respectively. Additionally, these proteins are used as diagnostic biomarkers, wherein gold nanoparticles play a key role in enhancing their signal, even at the low concentrations present in biological samples such as blood or cerebrospinal fluid, thus enabling an early and accurate diagnosis. On the other hand, gold nanoparticles act as drug delivery platforms, bringing therapeutic agents directly into the brain, improving treatment efficiency and precision, and reducing side effects in healthy tissues. However, despite the exciting potential of gold nanoparticles, it is crucial to address the challenges and issues associated with their use in the medical field before they can be widely applied in clinical settings. It is critical to ensure the safety and biocompatibility of these nanomaterials in the context of the central nervous system. Therefore, rigorous preclinical and clinical studies are needed to assess the efficacy and feasibility of these strategies in patients. Since there is scarce and sometimes contradictory literature about their use in this context, the main aim of this review is to discuss and analyze the current state-of-the-art of gold nanoparticles in relation to delivery, diagnosis, and therapy for Alzheimer’s and Parkinson’s disease, as well as recent research about their use in preclinical, clinical, and emerging research areas.
Graphical Abstract
Similar content being viewed by others
Introduction
Neurodegenerative diseases have become emergent and prevalent illnesses with age, being Alzheimer’s disease (AD) and Parkinson’s disease (PD), two of the most extended disorders [1, 2]. Even though these neurodegenerative diseases have different cognitive disabilities, and clinical manifestations [3], both share a common molecular mechanism: misfolding proteins that underlie the progressive and chronic nature of each disease [4, 5]. Alzheimer´s disease (AD) is the most common kind of dementia [6,7,8], accompanied by memory loss and dependence on personal daily activities. It is characterized by the presence of two misfolding proteins: amyloid-β peptide (Aβ) and Tau protein, which form highly insoluble aggregates. In this regard, the Aβ peptide aggregates extracellularly, causing synaptic damage, activation of proinflammatory pathways, mitochondrial dysfunction, oxidative stress damage, and neuronal death, promoting the neurodegenerative process and cognitive impairment [9,10,11,12,13].
For over 30 years, Aβ has been considered the mainstream that underlies and dominate the AD research, with extensive literature and clinical trials at the expense of Tau protein [14, 15]. Recent studies have shown a preponderant role of Tau protein in the propagation of AD, playing a key interplay with Aβ in the neurodegenerative effect [16,17,18,19]. In fact, there are more evidence that show the key role of Tau as an active agent, refuting the Amyloid cascade as the main and unique actor of neurodegeneration[15, 20], and turning the focus on AD research field. In this sense, Tau protein is a soluble protein predominantly found in neuronal axons and associated with microtubules, playing a fundamental role in microtubule stability under physiological conditions, being relevant from a structural and axonal traffic point of view in neurons [21]. The loss of Tau protein function has been associated not only with AD, but also with various tauopathies: Pick disease, progressive supranuclear palsy, and corticobasal degeneration [22]. The human tau gene is localized in chromosome 17 and expresses six isoforms of 351–441 amino acid residues with a molecular weight of 45–65 kDa [23], being the called 4-R (4R) and 3-R (3R) isoforms mainly located in the axons of an adult brain. Under pathological conditions, 4R and 3R Tau are found to be hyperphosphorylated at threonine and serine residues [23], producing structural instability and disruptions in neuronal transport processes [24]. This hyperphosphorylation could also promote an aggregation process forming the neurofibrillary tangles (NFTs) [25]. Additionally, different intermediate and highly toxic oligomeric species are produced throughout this aggregation process, triggering inflammatory processes and oxidative stress, which ultimately lead to neuronal death [24, 26]. Thus, total Tau (T-tau), hyperphosphorylated Tau (P-Tau), and the oligomeric species have been used as key biomarkers for AD [27] even though they are found at low levels, making their detection difficult in biological fluids such as cerebrospinal fluid (CSF) (low 195 pg/mL or 4.7 pM) [28].
On the other hand, PD is characterized by motor impairments such as uncontrollable movements and difficulty with balance and coordination [5]. At the molecular level, there are intracellular lesions called Lewy bodies, which are composed of aggregates of α-synuclein (α-syn) protein [29]. This protein is part of the synuclein family which consists of three members: α-, β-, and γ-synuclein, that range from 127 to 140 residues [30]. However, only α-syn is present in the Lewy bodies and, therefore, possesses an attractive clinical perspective. There is no evidence that β- and γ-synuclein are present in the Lewy bodies because they have been demonstrated to fail to assemble into filaments, remaining in a random coil conformation [31]. α-syn is located in the neuronal presynaptic terminals. It is a small acidic protein of 14 kDa that is found as a monomer in the cytosol or as an α-helix structure in association with phospholipids under physiological conditions. The α-syn protein consists of three different parts: an amphiphilic N-terminal (residues 1–60), a hydrophobic region called NAC (non-amyloid-β component, residues 61–95), and an acidic C-terminal (residues 96–140) [32]. In this regard, the NAC region is highlighted because it plays an essential role in assembling monomeric α-syn into β-sheet-rich aggregates [33]. Under pathological conditions, monomers of α-syn exhibit a similar aggregation behavior as Tau, forming various species, including dimers, oligomers, protofibrils, and finally, fibrils, which eventually constitute the Lewy bodies [29]. Likewise, α-syn oligomers may drive the primary neurotoxicity, playing a critical role in neurodegeneration [34, 35]. Even though α-syn oligomers have emerged as potential biomarkers, the current methods have encountered challenges in accurately discriminating their morphology and size to detect them at appropriate concentrations, thus hindering their analysis and study [36, 37]. Finally, α-syn can also suffer genetic mutations and post-translational modifications such as phosphorylation, ubiquitination, and oxidation. These modifications have been observed to promote the misfolding process and correlate with disease progression [38] and, therefore, are being utilized as interesting biomarkers to follow the disease.
Even though the amino acid sequences of Tau and α-syn proteins are different, they both accumulate in pathological conditions, go through the same aggregation process, and generate toxic oligomers involved in the onset of AD and PD, respectively. In this sense, these oligomers possess a transient nature, low concentration in biological samples, and a wide range of different sizes and morphologies, making their analysis, quantification, and detection a remaining challenge in their use as promising biomarkers [37, 39]. Therefore, it is mandatory to develop a strategy that detects these species to generate future and potential therapies for AD and PD. Additionally, other species of Tau and α-syn may be used as targets to generate specific biomarkers to discriminate pathological concentrations in biological samples or develop therapies to modify the underlying aggregation processes [40,41,42]. Nevertheless, each of these approaches is hindered by factors that prevent the achievement of an effective treatment or diagnosis [43, 44], including side effects, the blood–brain barrier (BBB), and the challenges associated with the specificity and sensitivity of the new strategies [45, 46]. Overcoming these difficulties is crucial for developing new therapies or early diagnosis.
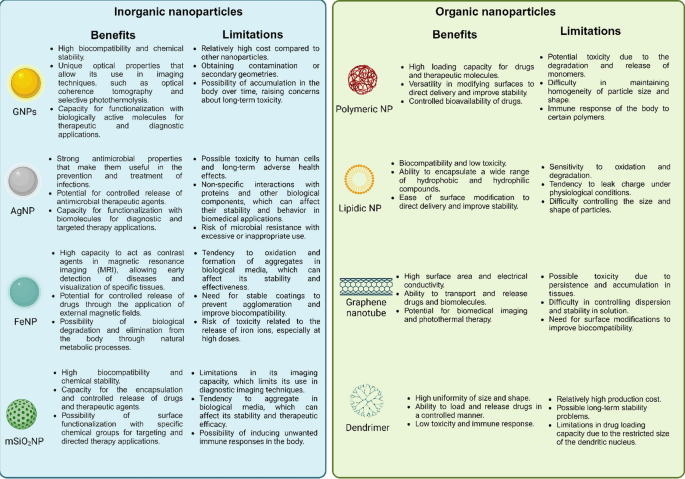
Nowadays, nanomaterials have acquired a high level of relevance in the nanobiotechnology field, and specifically in biomedicine, due to their several advantages, such as nanometer scale, relative low toxicity, and the capability of crossing biological membranes (including the BBB) [47]. Among these, gold nanoparticles (GNP) are the most studied and exhibit versatile features such as their high compatibility and stability in relevant biological mediums, tunable shape, and high surface area to be functionalized with different molecules over other kinds of nanomaterials [48,49,50]. In this regard, GNP are easily functionalized, becoming them drug carriers to target specific organs [51, 52] or specific molecules [53], enhancing their delivery capabilities compared to other materials (Fig. 1) [54]. Indeed, GNP possess unique properties to increase or amplify signals over other nanomaterials[55, 56], such as the surface plasmon resonance (SPR), surface enhanced Raman scattering (SERS), photothermal therapy, and even as a contrast enhancer in X-ray imaging; and thus, facilitating the recognition and sensing of biological molecules in complex samples compared to conventional methods [52, 53, 57,58,59]. Altogether, GNP have been demonstrated to have a multifunctional use as drug delivery, targeting, amplifiers, and even biomarkers being utilized in therapy, diagnoses, or the combination of both, the so-called theragnostic [49, 60]. Thus, GNP have become an interesting tool or complement to enhance conventional methods or techniques in order to increase their sensitivity, specificity, effectiveness, and safety. However, their use in preclinical studies and clinical trials for AD and PD is limited, and in the case of AD, they are mainly restricted to the Aβ peptide. Furthermore, the new therapeutic strategies for AD have shifted to include analyses that consider the Tau protein, and as with the α-syn protein, their relationship with GNP is scarce and has been poorly studied. Thus, both proteins have become an outstanding niche to develop research for new strategies and outlooks.
This review compiles and focuses on Tau and α-syn proteins as GNP targets, with a special attention to new functionalized nanosystems, how they can reach the central nervous system (CNS), and the detection at low concentrations of different Tau and α-syn species as biomarkers, including the current research in the oligomer field, to generate an early diagnosis. In addition, this review discusses not only in vitro and in vivo assays, state-of-art clinical studies, and FDA approvals, but also highlights the risks and concerns that underlie the uses and applications of GNP, as well as the latest advances and opportunities to generate and drive new methodologies to research the potential use of GNP with Tau and α-syn proteins for therapy, diagnosis, and theragnosis.
Obtention of GNP
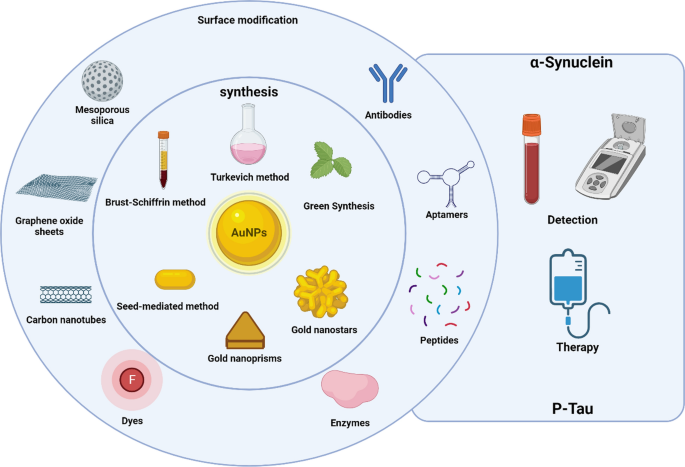
GNP have been widely used for electronic, optical, and biological applications including targeted drug delivery. GNP exhibit different geometries (Fig. 2), which can influence the interactions at the bio-nano interface, the functionalization degree, the molecular organization of these molecules as well as their chemical state, and even their biological uptake degree [61, 62]. Thus, the size and shape are fundamental for the chemical and surface properties, being a key part of the experimental design of a GNP [61, 63]. For these reasons, various methods have been developed to modulate these physical properties to obtain different geometries [64]. In this sense, Gold nanospheres (GNS) are one of the most studied shapes, possessing different synthesis methods such as Turkevich method [65], which involves citrate as a reducing and stabilizing agent to modulate their size [66,67,68]. Another strategy is the Brust-Schiffrin method, which allows to obtain small nanoparticles in the 1–3 nm range [69], but this synthesis generates a considerable amount of organic solvent used as a stabilizing agent.
GNP can be obtained by several methods that are shown in the central circle (Turkevich, Brust-Schiffrin, and green synthesis specifically for GNS), obtaining different shapes and sizes that depend on the methodology used. These GNP can also be functionalized with various types of molecules to target α-syn and Tau proteins, making them interesting tools to generate novel strategies to apply in the detection and therapy of PD and AD, respectively. Created with BioRender.com
Currently, the synthesis methods aim to develop a GNS synthesis more environmentally friendly and safe. Green synthesis involves the use of natural sources of chemicals to synthesize GNP [70, 71], avoiding the use of toxic chemicals and reducing waste products. These methods are mainly based on the presence of alkaloids and polyphenols, among other elements, from natural extracts, microorganisms, and molecules that allow the reduction of Au3+ to produce GNP. For example, polyphenols in the aqueous extract of Abutilon Indicum leaves [72] or alginate [73], a marine algae polysaccharide, have been used to synthesize GNS. However, these methods exhibit some limitations in polydispersity control because of the variety of molecules present in the extract or microorganism [74, 75]. To overcome these issues, the use of purified phytochemicals allows to obtain greater reproducibility, compared to the use of extracts, because there is an understating of which molecules participate as stabilizers and reducing agents [76]. Curcumin has been used to obtain GNS with promising effects in a human colorectal adenocarcinoma (HT29) cell line model [77], as well as their antioxidative and antimicrobial properties [78,79,80]. Gallic acid is another successful case used to synthesize GNP. This phytochemical is present in tea leaves, fruits, and nuts and has been used to synthesize GNS with a potential application as an antitumoral or anti-aging agent [81,82,83].
Anisotropic nanoparticles
Anisotropic nanoparticles are another area of research that has been developed because the shape drives their properties, and it also can be modulate with potential biomedical applications (Fig. 2). One of the most studied nanoparticles is gold nanorods (GNR) which can present their plasmon within the first and second biological windows, making them interesting for their applications in biomedicine [84,85,86]. The most common synthesis is the seed-mediated method using CTAB as a stabilizer agent [86], allowing to modulate the plasmon maximum, absorption, and scattering efficiency of the nanosystem [87, 88]. Gold nanoprisms (GNPr) also exhibit a plasmon that can be modulated in the biological window [89], but their synthesis is more friendly using sodium thiosulfate as a reducing and stabilizing agent [90]. Also, they scatter the light efficiently due to the greater number of hot spots, making them potential candidates as fluorescent probes in imaging [91]. Gold nanostars (GNSt) are another type of GNP that exhibit more hot spots than GNPr depending on the number of points and length of the arms presented, making them very interesting for their applications in plasmon-enhanced fluorescence (PEF) and surface-enhanced Raman spectroscopy (SERS) [91, 92]. Their synthesis is based on the use of HAuCl4 at basic pH and hydroxylamine as a stabilizer and reducing agent [93].
Hollow gold nanoparticles (HGNP) are another attractive type of nanoparticle because of their thickness and cavity size can be modified, allowing the maximum absorption to shift in different regions of the spectrum with potential application in photothermal therapy [94,95,96]. Also, their cavity can be used to encapsulate drugs, which makes it useful for drug delivery [97]. Their obtention is by templates over which Au is deposited from a HAuCl4 solution. Then, these templates are eliminated, and the size and shell thickness depend on the template used as well as the concentration of the added HAuCl4 solution [98]. Finally, even though anisotropic GNP exhibits great potential in many different areas, it is noteworthy that their obtention by green methods is still recent. Some issues related to their shape homogeneity and yield need to be improved and encouraged to develop an alternative to traditional chemical methods. For a complete revision see Table 1, and Ref. [99] and [100].
Because the control of morphology, size, and distribution is not trivial [48], these physical features may modify the biological effect at the nano-bio interface, resulting in different behavior of the nanosystems in in vitro approaches compared to in vivo analysis. Nowadays, efforts are focused on developing smart nanosystems with optimized properties to increase the responsiveness of biological systems [61]. However, some concerns emerge for their use in biological systems related to their stability and how GNP may interact or behave with the medium that surrounds them in physiological conditions. Also, different synthesis uses organic compounds that are incompatible with their application in biological assays, affecting their potential use in diagnosis and therapy [51, 101,102,103]. To overcome this issue, the surface of GNP can be functionalized easily with different molecules, adding new properties, giving more stability, increasing their biocompatibility and half-life (e.g., plasma), and enhancing their selective delivery to organs.
In this sense, polyethylene glycol (PEG) is the most common way of eliminating toxic stabilizer agents or residues from the surface of GNP by a coating process [104]. Also, PEG increases the half-life of GNP in the bloodstream due to their hydrophilic nature, conferring water solubility, physiological stability, and biocompatibility to the GNP [105,106,107]. Another widely used option is a silica shell, which has been reported to enhance GNP stability and biocompatibility for biomedical applications [51, 108, 109]. Silica coating is chemically inert and allows the encapsulation of different kinds of molecules, such as drugs, dyes, and antibodies, among others, as well as an effective drug release[108]. Another interesting approach to masking the GNP is the use of endogenous elements present in plasma is. This strategy consists of capping the GNP with serum albumin (SA), which is the most abundant protein in plasma. As reported, these nanosystems exhibit longer circulation times, biocompatibility, and low toxicity, making their use widely accepted in the pharmaceutical industry [110,111,112]. Finally, it is noteworthy that green synthesis has been reported to enable the synthesis of GNP as well as their coating, increasing their biocompatibility. Curcumin and Apigenin are highlighted due to their potential use in biomedical applications. Whereas GNP functionalized with curcumin increase their half-life in the bloodstream with potential applications in neurodegenerative diseases[113, 114], GNP coated with Apigenin exhibit anti-inflammatory and anti-cancer properties in a photothermal treatment [115].
GNP delivery to the CNS
Currently, the engineering of GNP to deliver them comprises passive or active strategies for site-specific recognition [63]. The first strategy is based on nanoparticle accumulation into the brain depending on systemic circulation [47, 116, 117]. However, this mechanism is limited for the renal excretion, reticuloendothelial system, and immune system that eliminate the nanoparticles from bloodstream. Conversely, active targeting comprises the use of biological ligands functionalized onto GNP to recognize specific molecules on the cell surface (Fig. 3) [118, 119]. In fact, one of the current challenges is to assemble multiple ligands for delivery and targeting in one GNP. The best-case scenario is to design GNP in which the ligand merges both applications into one. Thus, the use of only one ligand is expected to yield a high selectivity for a particular body area, achieving better biocompatibility and ensuring the potential of the nanosystem for diagnosis and therapy.
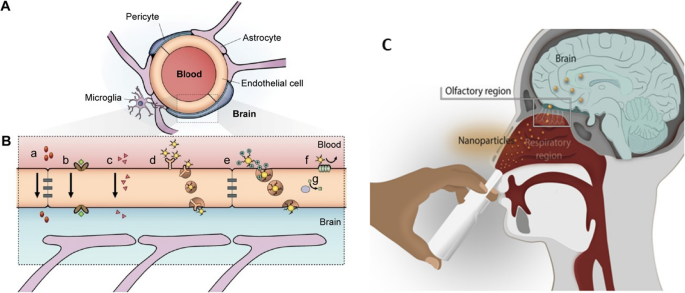
Copyright 2015, with permission from Dove Medical Press Limited. C Anatomical features of the intranasal route involved in nose-to-brain drug delivery. Adapted with permission from Ref. [133]. Copyright 2022, with permission from Elsevier
Principal pathways to overcome the BBB. A Scheme of the neurovascular unit of BBB constituted of pericytes, astrocytes, and endothelial cells. B The main mechanism of crossing through BBB: a) tight junctions restricting the pass of water-soluble compounds, b) carrier-mediated transport, [c] lipid-soluble agents, d) receptor-mediated endocytosis and transcytosis, e) adsorptive-mediated endocytosis and transcytosis, f) the efflux pump expulses the drugs from the endothelial cells to the blood, g) Cytochrome P450 enzymes. Adapted with permission from Ref. [126].
Regarding neurodegenerative diseases, the engineering of nanoparticles must consider another factor to achieve an effective therapy, which is that GNP must be able to arrive in the CNS by crossing the BBB [120, 121]. This biological barrier controls brain homeostasis through a tight and sophisticated mechanism where layers of endothelial cells limit the entrance of different foreign molecules into the CNS (Fig. 3a). Thus, the BBB has become a formidable barrier that must be surmounted in the generation of treatment options for neurodegenerative diseases [122,123,124]. Several approaches have been designed, but most of them imply a disruption of BBB cells, making them invasive methods that result in deleterious consequences for the cells [123, 125]. For this reason, the use of GNP has emerged as a novel alternative because they exhibit different mechanism to cross through the BBB. For example, their internalization can be mediated by endocytosis involving both pinocytosis and phagocytosis mechanisms, but this mechanism results in a widespread organ distribution including kidneys and liver [126, 127]. To overcome this issue GNPs can be capped or functionalize with targeting molecules to delivery into the brain [128,129,130], being adsorptive-mediated transcytosis (AMT) and receptor-mediated transcytosis (RMT), being RMT the most specific (Fig. 3b). Peptides Angiopep-2 and CLPFFD-THR functionalized onto GNP are successful examples of RMT [131, 132]. The present section describes and summarizes the advances in the field of delivery and targeting, considering the use of GNP with Tau and α-syn proteins for AD and PD, respectively.
A new opportunity: the nose-to-brain delivery
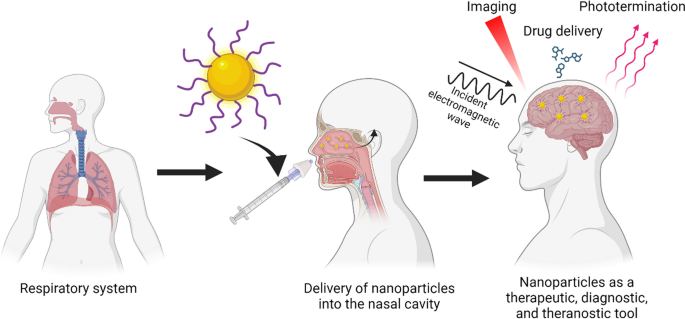
Even though RMT is an interesting strategy, some drawbacks may reduce its effectiveness. Low bioavailability, inadequate permeation, delay in onset of action, and limited presence or saturation of receptors in the BBB are the most common issues. To overcome these limitations, intranasal administration (IN) has emerged as a non-invasive strategy to bypass the BBB, reaching and delivering drugs and nanoparticles directly into the brain (Fig. 3c) [133]. Via IN therapy the nanosystems enter by two main pathways: the olfactory and the trigeminal nerves [134, 135]. Due to the unique anatomical organization of the olfactory nerves, compounds can circumvent the BBB through two main mechanisms: 1) the neuronal endocytosis, in which compounds are internalized by olfactory neurons of the olfactory epithelium and transported through the axons to reach the olfactory bulb, and then other brain areas; 2) paracellular diffusion, in which compounds can diffuse through intercellular gaps in the olfactory epithelium and channels created between the ensheathing cells surrounding olfactory axons, being externally transported along the neuronal axon and reaching the CSF and olfactory bulb [134,135,136]. Regarding GNP, different studies have shown their effective delivery enhances treatment efficacy [137,138,139,140]. GNP can reach the olfactory bulbs in minutes [141]. Gallardo-Toledo et al. [140] analyzed GNS and GNPr functionalized with PEG and the peptide D1 (qshyrhispaqv), as a potential targeting for Aβ and their delivery into the brain. Also, it compared the IN with the intravenous route, demonstrating the major effectiveness of the IN route and reinforcing the idea that IN administration is a promising pathway for the delivery of GNPs into CNS. However, IN delivery has presented some concerns about mucociliary clearance that can easily eliminate the drugs administrated; therefore, some special formulations may be required to decrease this effect. To overcome this issue, GNP surface can be coated to specifically target the olfactory region, exhibiting mucoadhesive properties that allow a major retention and avoiding the mucociliary clearance. In this regard, chitosan emerges as an attractive mucoadhesive polymer due to its cationic nature, biocompatibility, and suitability for high loading of hydrophilic drugs [142]. For example, Bhumkar et al. loaded insulin in GNP coated with chitosan. After transmucosal administration, they were able to reduce glucose levels on the blood of rats, noting a higher increase in the insulin level after nasal administration compared to oral administration [143].
GNP and diagnosis
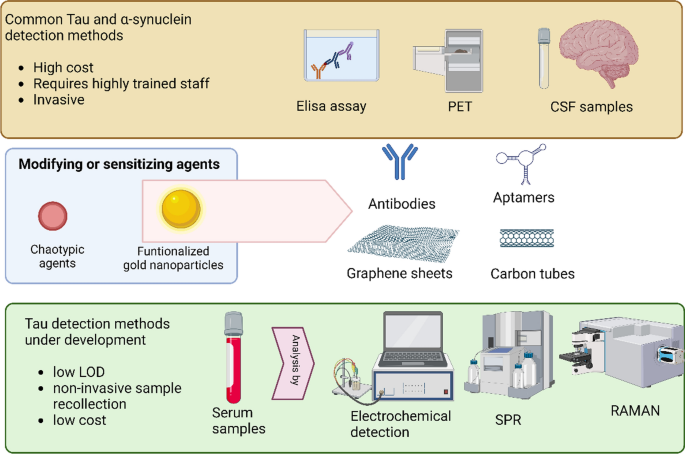
Tau protein is one of the main etiological agents of AD and other tauopathies [144]. Likewise, one of the key biofluid-based biomarkers for PD is the misfolded and aggregated α-syn protein [1, 145, 146], which is characteristic of Lewy bodies and hallmark of this disease [31, 147,148,149]. In this sense, both α-synuclein and Tau protein can be detected in CSF [144, 146], but this process involves the extraction of the sample by lumbar puncture, which is an invasive and painful procedure for the patient. Detection methods are currently limited to ELISA immunoassay and PET imaging (Fig. 4) [144]. The latter method is expensive, requiring access to a cyclotron, and uses radiotracers with a short half-life. In this context, research has been carried out to find new detection and diagnosis forms that are sensitive, economically accessible, and non-invasive [150,151,152,153]. Currently, researchers are investigating sensitive methodologies that allow the detection of Tau and α-syn in different biofluids. Numerous strategies based on GNP technology have emerged in the search for methodologies that allow the quantitative detection of both proteins (α-syn or Tau) with high sensitivity (for a complete review, see Table 2). Most of these biosensors are based on electrochemical methods, SERS, or localized surface plasmon resonance (LSPR) (Fig. 4) [154,155,156]. In general, these methods are based on immobilization on the surface of the GNP with a selective molecule for the target molecule (antibody, aptamer, etc.) and/or a reporter molecule. This strategy allows an improvement in both the limit of detection (LOD) and sensitivity (Fig. 4).
Conventional methods to detect Tau are often expensive, invasive, and require highly trained personnel. However, the use of nanoparticles is enabling the development of new detection methodologies that significantly improve the sensitivity and selectivity of the process. LOD: limit of detection. Created with BioRender.com
GNP designed for α-synuclein diagnosis
In this regard, Zhang et al. [155] showed that modifying a single-walled carbon nanotube with a gold-nanourchin-conjugated α-syn antibody significantly enhanced the LOD of the biosensor. Specifically, the biosensor displayed a 1000-fold increase in sensitivity, detecting concentrations as low as 1 fM compared to the surface coated solely with antibody, which detected concentrations as low as 1 pM (Fig. 5). Another notable immunosensor for α-syn is based on dual signal amplification (enzyme-assisted signal amplification and electrochemical measurement). The sensor is constructed with PAMAM-Au nanocomposites and an immunosensor surface using a horseradish peroxidase-secondary antibody (HRP-Ab2) linked to GNP. This sensor has high analytical performance, sensitivity (LOD: 14.6 pg mL−1), and stability [156]. The approach of using nanocomposites is very interesting since it allows for anchoring larger amounts of target protein with high stability and bioactivity. Furthermore, these detection analyses must exhibit both high sensitivity and high specificity for clinical examination using complex samples such as human plasma and CSF. In this context, there are some reports, such as that of Aminabad et al. [157], who developed a system based on GNP-modified graphene for bioconjugation with a biotinylated antibody (bioreceptor). Like the previous one, this nanosystem exhibits a synergistic effect between graphene and GNP, resulting in enhanced electrochemical activity. Consequently, it enables the development of a high-sensitivity electrochemical immunosensor (LOD: 4 ng/mL) for human plasma. In addition, there is a very interesting study in which the CSF is measured using a neuro-biosensor system that consists of an electrode with GNP-PGA-modified ITO, with a LOD of 0.135 pg/mL [158]. Essentially, a concentration of α-syn at the picograms per milliliter (pg/mL) level has been reported in the CSF of healthy individuals; however, it increases to nanograms per milliliter (ng/mL) in PD patients [159]. Thus, unlike the previous biosensor that detected α-syn in human plasma, this neurobiosensor system can identify and discriminate α-syn in the CSF of both healthy individuals and PD patients with high sensitivity, making it a promising tool for the diagnosis of PD.
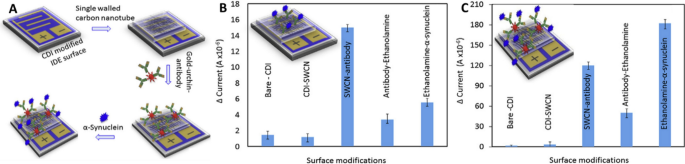
Adapted from Ref. [155]. Copyright 2020, with permission from Elsevier
The presence of gold nanourchin increases the sensitivity of detection for α-syn. A Schematic representation for detecting α-syn on a single-walled carbon nanotube (SWCN)-modified IDE surface, assisted by goldnanourchin-immobilized antibodies. Detection of 1 nM α-syn using the platform in the absence (B) or presence of gold nanourchin (C).
Early diagnosis is a key factor to evaluate in neurodegenerative diseases. Since the oligomer species are recognized as the most toxic species that appear in the first stages of the aggregation process, early detection of α-syn oligomer is necessary for PD or other neurodegenerative diseases [36, 160]. In recent research studies, aptamers have emerged in the search to identify the oligomers specifically. Aptamers are "artificial antibodies" selected from a randomized group of nucleic acids that are very specific and stable for the target molecule. For example, Wu et al. [161] successfully designed electrochemiluminescent systems based on GNP-cooper-metal–organic frameworks (NP-Cu-MOFs) with an aptamer as the recognition element for α-syn oligomer detection. In the search for an effective diagnosis of PD, the literature has described that a selective α-syn nitration is a modification associated with oxidative and nitrative damage of neurodegenerative synucleinopathies, and it has been found in biological fluids in patients with PD [162,163,164]. This has generated a new challenge for the design of diagnostic systems to detect the different conformations of α-syn. In this context, Zhang et al. [165] designed an electrochemical immunosensor as a sensitive sandwich assay that utilized supramolecule-mediated GNP composites (GNCs) with anti-α-syn-nitration magnetic nanoparticles (MNPs) as signal amplification tags (LOD: 310 pg mL−1). The significance of this work lies in the fact that it investigated the detection of α-syn nitration in diluted serum samples obtained from healthy donors and PD patients, indicating a significant difference between the two study groups and the potential use of this system for clinical applications.
GNP designed for Tau protein diagnosis
On the other hand, Tau protein is a key player in neurodegeneration that poses a diagnostic challenge. Some literature highlights such as Neely et al. [166], which synthesized GNP functionalized with an anti-Tau antibody (Tau-mab) that can detect up to 1 pg/ml of Tau protein, which is two orders of magnitude lower than the values found in CSF. A similar result was obtained using a monoclonal anti-Tau antibody-coated GNP based on a two-photon scattering assay, in which the sensitivity of the method increased about 16 times [166]. Kim et al. [167] investigated the use of guanidine chloride to enhance Tau detection in blood samples. In this case, the researchers demonstrated that the chaotropic agent guanidine chloride improved sensitivity and selectivity compared to traditional methods, preventing the interfering effects of other components in the biological fluid. This same effect was tested by LSPR using PEG-functionalized GNR and anti-Tau antibodies. Therefore, guanidine chloride allows differentiating samples from healthy individuals and patients with AD.
The development of sensitive, non-invasive diagnostic methods for neurodegenerative diseases, such as AD and PD, represents a crucial frontier in medical research. With advancements in systems based on GNP, it is possible to develop innovative approaches to detect biomarkers like Tau protein and α-syn with high sensitivity and specificity. Table 2 describes the main technologies currently developed as sensors to detect α-syn and Tau protein with potential diagnostic applications.
GNP designed for targeted therapies
The therapeutic potential of gold nanoparticles (GNP) in neurodegenerative diseases like Alzheimer's and Parkinson's is being explored. Researchers are leveraging the unique properties of GNPs to develop innovative approaches for more effective diagnosis and treatment, opening up new avenues for theragnosis.
Targeted therapies for α-synuclein
The accumulation of α-syn protein plays a central role in the pathogenesis of PD. Liu et al. [189] developed a nanosystem consisting of GNP-pDNA-Lipo-NGF-DHA to deliver plasmid DNA (pDNA) to inhibit α-syn expression. Liposomes (Lipo) are the carriers of the GNP-pDNA, and Nerve Growth Factor (NGF) and docosahexaenoic acid (DHA) are the targeting molecules in the nanosystem. NGF has an important role in the maintenance and growth of neurons, which have NGF receptors in their membranes, promoting the entrance of GNP into the neurons [190]. DHA is a polyunsaturated fatty acid that favors memory and cognitive functions, and it also promotes the movement of GNP across the BBB due to the presence of DHA receptors in the membrane of the BBB. This nanosystem shows effective GNP delivery into the CNS, reducing the overexpression of α-syn and improving motor dysfunction and exploration abilities in a PD mouse model. Hu et al. [191] developed a similar CTS@GNP-pDNA-NGF nanosystem. In this case, chitosan (CTS) works as a scaffold in which its cationic groups bind to pDNA, inhibiting of α-syn transcription and protecting from enzymatic digestion. This approach demonstrated effective delivery of GNPs to the central nervous system, significantly reducing α-syn overexpression and improving motor behavior in animal models of the disease.
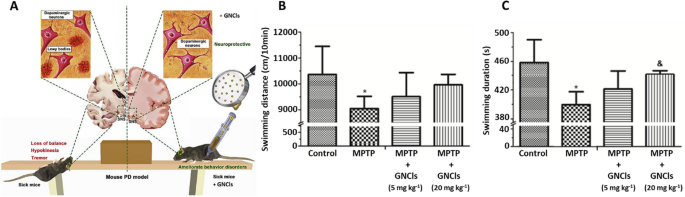
Additionally, Gao et al. [192] synthesized gold nanoclusters (GNCls) functionalized with N-isobutyryl-L-cysteine (L-NIBC) for the prevention of α-syn fibrillation in vitro. Furthermore, in vivo experiments using mouse PD models showed amelioration of behavior disorders with this nanosystem. The anti-PD effect of the L-NIBC-GNCLs system was evaluated using MPP + lesioned cells, and the in vivo models consisted of MPTP-induced mice, being MPTP a molecule commonly used to induce PD in animal models. The cell studies showed no obvious toxicity and a significant decrease in apoptosis compared to the MPP + control, demonstrating the neuroprotective effect of the GNCls. For the in vivo studies, the MPTP-induced mouse PD models were treated with different doses of the nanosystem administered via intraperitoneal injection. The results showed an improvement in locomotor activity in the mice in the presence of GNCls, significantly increasing the speed and distance traveled, thus, proving the effectiveness of the nanosystem and its delivery (Fig. 6).
Treatment of GNP in in vivo transgenic mouse models. A Experimental design of treatment with GNCls in a PD model. Effect of GNCls on motor coordination of mice in a mouse PD model using a swimming test. B Evaluation of swimming distance. C Evaluation of swimming duration. Adapted with permission from Ref. [192].
Targeted therapies for Tau Protein
Numerous therapeutic studies have focused on Tau protein, which is pivotal in neurodegeneration. Due to the limited studies conducted with GNP, we have included in the review studies involving other nanoparticles and approaches, which may serve as a guide for developing theragnostic using GNP.
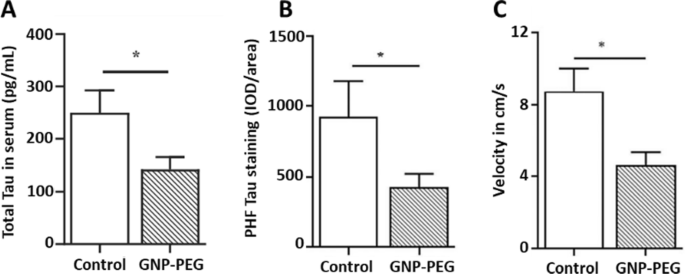
In this sense, Sonawane et al. developed two interesting types of protein-capped (PC) metal nanoparticles (PC-F3O4 and PC-CdS) for the inhibition of Tau aggregates, both obtained via biological synthesis with two fungal species (Funsarium oxysporum and Verticillium sp), showing that PC-CdS inhibited Tau aggregates by 63% and PC-F3O4 by 49% [193]. Gao et al. loaded Curcumin onto red blood cell membrane-coated PLGA nanoparticles bearing T807 molecules as an imaging agent for positron emission tomography. This nanosystem was able to cross the BBB, and curcumin reduced P-Tau levels and neuronal death both in vitro and in vivo. Vimal et al. [194] investigated the therapeutic potential of GNP functionalized with PEG in transgenic Tau P301L mutant mice and macaque monkey serum (Fig. 7). These nanostructures acted as pseudo-nanochaperones interfering with Tau aggregation and significantly improving neuronal health in transgenic models. Regarding the ex vivo experiment using monkey serum the authors observed that this nanosystem interfering with Tau aggregation. Additionally, Bhattacharyya et al. posited that GNP could act as nanochaperones [195]. GNP capped with citrate of approximately 5 nm were suggested to allow remodeling of the P301L mutant Tau protein in vitro, modifying its structure and recovering the transport function of the microtubules. Thus, these studies highlight the role of GNP in modulating Tau pathology and maintaining brain function.
A Quantification of total Tau concentration in serum samples of control and GNP-PEG-treated tau P301L mice using an ELISA assay after 30 days of treatment. B Quantification of integrated optical densities (IOD) values in control (n = 6) and treated groups (n = 6) for paired helical filament-positive cells using Image-Pro Plus analysis. C Novel object recognition test for the examination of learning and memory, in which the mice exhibited altered velocity. Adapted with permission from Ref. [194].
Opportunities in therapy
Even though the studies related to the targeting and delivery of Tau protein are scarce, some strategies may be applied or replicated from the research using the amyloid cascade theory, and GNP functionalized with different molecules. Liu et al. [6] functionalized GNR with the APH (ST0779) enzyme and the scFv (12B4) antibody, which binds to Aβ oligomers and fibrils and can cross the BBB. Javed et al. [196] also used GNP coated with β-casein, which binds to Aβ and can cross the BBB in zebrafish larvae and adult models via intracardial and cerebrovascular administration. In this sense, Martins et al. [197] functionalized GNP with Apolipoprotein E3 (ApoE3), which promotes both selective interaction with Aβ aggregates and can also cross the BBB. Altogether, if these approaches are used in a Tau protein context, they allow reaching the CNS.
Another potential strategy to replicate is the use of GNP functionalized with specific D-enantiomeric peptides, which are produced by mirror-image phage display to detect specific targets [198, 199]. These peptides exhibit several advantages for their use in biological systems, such as that they are not recognized by proteases, making them more resistant to degradation and increasing their half-life [200]. Interestingly, these D-peptides can cross the BBB and decrease both the amyloid load and neuroinflammation in transgenic models of AD [201,202,203,204,205,206,207,208]. Various studies have enhanced the inhibitory effect of these peptides on Aβ using GNP across multiple models (in vitro, in vivo, and even ex vivo) [84, 85, 209]. This progress highlights efforts to develop GNP-based systems for delivering targeted molecules, including these peptides, through the BBB for potential noninvasive CNS treatments. In this way, the use of enantiomers emerges as an interesting strategy to use with Tau protein. Recent reports have indicated that D-peptides can target Tau protein with a high inhibitory effect on the Tau aggregation process, and show valuable specificity, low toxicity, and high penetrance into neuron cell cultures [210,211,212,213,214], as was observed, for example, with the d-ttslqmrlyypp sequence [213]. Similarly, some studies have explored the use of D-peptides to disrupt the α-syn aggregation process. Shaltiel-Karyo et al. [215] found that β-syn exerts an inhibitory effect on α-syn. They designed a retro-inverso analogue of β-syn with high stability in mouse serum, which inhibited α-syn aggregation in in vitro and in vivo assays. Chemerovski-Glikman et al. [216] developed a self-assembled cyclic D,L-α-peptide which inhibited α-syn aggregation and even reduced its toxicity and intracellular accumulation. Horsley et al. [217] compared the effects of a D-peptide and an L-peptide to prevent the α-syn aggregation process, demonstrating that a D-peptide (d-gvlyvgs) was more effective in leading the α-syn monomers into a less aggregation-prone state, alleviating the cytotoxic effects of α-syn aggregates in cell models. Furthermore, these peptides have also exhibited the ability to cross the BBB and high stability in biological fluids, making them a promising strategy to develop new therapeutic agents. Thus, D-peptides open an interesting niche to functionalize them onto GNP as an effective future therapy. Thus, the studies with Aβ peptides can serve as models to follow for generating new treatments, considering the already described benefits as a guide.
Interestingly, since Tau protein is known for its close relationship with the microtubule network, it has also been the subject of interest as a target for treating diseases such as cancer. Ghalandari et al. [218] proposed the use of GNP or Fe3O4 nanoparticles coated with gold and functionalized with folic acid for photothermal treatment. The results of this study indicated that GNP interact with Tau and tubulin, preventing the formation of long polymers, and triggering apoptosis of malignant cells. Goto et al. [219] functionalized GNP with anti-Tau and anti-histone H1 antibodies to obtain high-resolution images that allowed the study of intracellular elements for protein localization [174]. Finally, Chia-Hsiung et al. [220] presented evidence that Plasmon-Activated Water (PAW) reduces β-amyloid and P-Tau burden in murine models of AD (APP/PS1), improving the behavioral parameters. In this article, the authors used resonantly illuminated GNP, reducing the hydrogen bonds present in the native structure of water and allowing PAW to be obtained. PAW could trap free radicals, which is one of the main mechanisms through which the damage produced in AD would be reduced in the model used. These results emerge as interesting approaches for a field that has not been sufficiently explored, along with their applications in neurodegenerative diseases, allowing the development of potential therapies.
Current clinical trials, GNP, and FDA
Nanomedicine research has expanded greatly in recent years, resulting in potential medical and pharmaceutical tools and systems that are either FDA-approved or in clinical trials. There are currently 537 clinical studies registered on Home-ClinicalTrials.gov referring to nanoparticles, including polymeric, metallic, and lipid nanoparticles, among others. Of these clinical studies, only 7 are clinical trials aimed to neurodegenerative diseases: 4 trials of GNP, 2 of lipid nanoparticles, and 1 of polymeric nanoparticles (Table 3). This data shows the huge difference between GNP and other types of nanoparticles, and the inconsistency between the preclinical and clinical studies. There is a large literature related to preclinical assays of GNP, but their clinical translation is scarce [221, 222]. In this sense, some concerns support this evidence. One of the reasons is that most research in this field is related to material science, where the main objective is to generate new materials with new properties instead of exploiting the already known materials. For example, the synthesis of GNP is commonly simple, easily scaled up, and tunable, and GNP can be functionalized with different kinds of molecules, drugs, etc., as was above mentioned. However, despite these promising features, clinical studies with these nanostructures are not common. One successful example is the AuroLase Therapy, which was the first FDA-approved inorganic material. AuroLase Therapy is based on gold nanoshells which accumulate preferentially in cancerous tissue and using a near-infrared laser the affected area is irradiated, destroying the cancer cells by photothermal therapy [223]. Contrarily, among the clinical studies conducted with GNP, only one focused on PD while there are no records of GNP-based treatments for AD (Table 3). Although this study is a great advance for nanomedicine in its clinical applications, the use of nanosystems based on GNP for the detection of α-syn or P-Tau in clinical settings has not been reported in spite of the fact that these biomarkers are relevant for PD and AD, respectively.
Another reason points to some concerns related to GNP toxicity and long-term accumulation [224]. However, GNP have been functionalized with different molecules to decrease their interaction with plasma proteins, increasing their targeting, and thus, showing a reduction in their side effects. For example, one clinical trial analyzed GNP functionalized with a siRNA for gliobastoma [225, 226]. This GNP-based therapy exhibited interesting results in patients treated for six months with tolerable side effects (which disappeared at the end of the treatment) being capable of crossing the BBB, promoting tumoral cell apoptosis, and tumor shrinkage. This example shows the potential use of GNP, their specificity, and in which their benefits outweigh the side effects.
In general, these sections collected and highlighted key literature contributing to the engineering of GNP and the development of a critical rational design to generate adequate analysis for targeting, delivery, diagnosis, therapy, or theragnosis. However, there is still another relevant point to address: the cytotoxic effect in elderly patients [227, 228]. It is known that their brain homeostatic capacity is reduced, and there is a lack of studies related to this age, requiring a further in-depth exploration of the toxic effect on this people. Thus, it is possible to generate a complete understanding of the GNP function in a physiological context. Strategies such as Nose-to-brain administration or the use of biodegradable nanosystems, such as those synthesized with poly-D-L-lactide-co-glycolide, polylactic acid, poly-e-caprolactone, or even natural materials such as chitosan [229] may help to minimize the possible toxic effects associated with bioaccumulation (Fig. 8) [140, 209, 230], improving their safety. Unfortunately, this research is still incipient and far from being analyzed in clinical trials. However, GNP are highly modifiable structures which their applications keep as potential and promising niches to explore and exploit to develop new therapies. In this sense, due to their tunable GNP nature, research that focuses on GNP behavior and their biological interactions can help to unravel and predict their potential effects and, therefore, raise the knowledge necessary for the rational design of gold nanosystems.
Impact of GNP on the aggregation process: relevant issues for a rational design
Interestingly, another function of GNP is acting as chaperones due to their modulation, inhibition, or promotion properties exhibited in the amyloid aggregation process [231]. As explained above, the aggregation process can produce several species, such as oligomers, which are considered the most toxic species. Targeting the aggregation process using GNP becomes an interesting and potential strategy, not only because there are few reports in the literature regarding α-syn or Tau protein, but also because it is a potential niche to develop different therapeutic strategies for rational design. This has been widely observed and demonstrated using other kinds of amyloid proteins, such as amyloid-β [232,233,234] or β-microglobulin [235,236,237]. Thus, understanding the molecular mechanisms of the GNP-protein interaction may guide to develop new and alternative strategy to gain control over the aggregation process.
In this regard, one strategy widely used is monitoring the fibrillation process with ThT [238,239,240]. In Álvarez et al. [241], the authors analyzed the effect of different diameters and concentrations of GNS on the kinetics of α-syn, observing all sizes accelerated α-syn kinetics, but only 22 nm GNS were found to be preferentially bound to the fibril surface (Fig. 9a–c). On the other hand, in presence of GNS functionalized with porphyrin, there was not formation of fibrils, monitored by ThT and AFM [242]. Indeed, when this sample was analyzed by circular dichroism, α-syn remained in its normal α-helical pattern without the characteristic β-sheet signal, making it a potential therapeutic strategy (Fig. 9d–f). Another recent study was carried out by Maity et al. [243] using GNS functionalized with naringenin, a polyphenolic compound obtained mainly from several citrus fruits, showing an inhibitory effect on α-syn kinetics and stabilizing α-syn in its helix-α structure [219]. Similar results were observed using GNS functionalized with β-boswellic acid, a plant-derived terpenoid, which inhibit the Tau aggregation process [244].
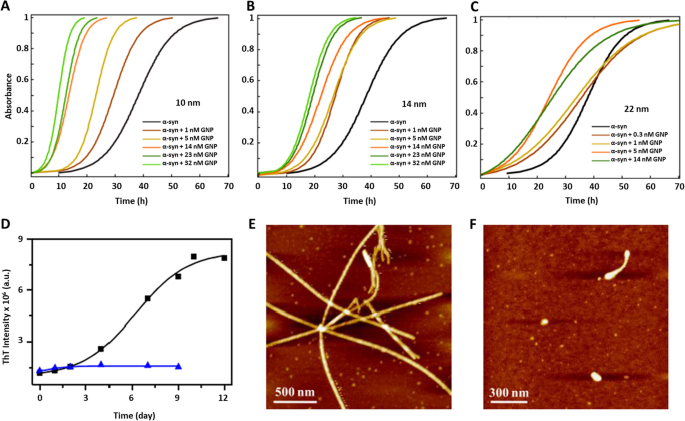
Copyright 2013, American Chemical Society. D Kinetics of α-syn in the absence (black trace) and in the presence of GNS functionalized with porphyrin (blue trace). AFM images of α-syn incubated alone E and in presence of GNS functionalized with porphyrin F after 7 days. Adapted with permission from Ref. [242]. Copyright 2020, American Chemical Society
Kinetic assays of α-syn. A–C Kinetics of α-syn in the presence of different sizes and concentrations of GNS. Adapted with permission from Ref. [241].
Another type of GNP analyzed by kinetics assays is GNCls. This GNP have diameters of 2 nm or less, allowing them an efficient urinary excretion compared to larger GNP diminishing their secondary problems [245]. GNCls also exhibit a strong fluorescence in the visible and near-infrared spectrum and therefore, they have emerged as interesting tools to use in fluorescent bioimaging [57, 246]. GNCls functionalized with N-isobutyryl-L-cysteine exhibited an inhibitory effect on the aggregation process of α-syn studied by ThT [192]. Mahapatra et al. [247] also studied the capping-charge effect by introducing either a negative charge or an apolar agent on the surface of GNCls. Although both nanosystems decreased the neurotoxic effect of α-syn aggregates, they worked in different ways. Whereas negative GNCls inhibited the aggregation process, producing nonfibrillar aggregates, apolar GNCls accelerated the aggregation process, producing ribbon-like aggregates. Thus, both classes of GNCls guided the toxic aggregates towards less toxic species. Finally, the authors only analyzed the apolar GNCls in in vivo studies, which exhibited an increase in brain delivery and blood–brain barrier (BBB) permeability in a mouse model.
However, although GNP exhibit interesting properties and uses, they have shown different roles in peptide aggregation that could lead to deleterious biological consequences [248]. Specifically, GNP-protein interactions can alter how proteins behave on the GNP surface, modifying the protein adsorption and orientation and modulating their activity. [249]. Thus, their potential use requires a detailed analysis to clarify the impact of GNP on biological systems for generating a rational design of GNP. Yang et al. [250] studied the effect of negatively and positively charged GNP on α-syn orientation. They demonstrated that protein orientation depends on the surface charge: negatively charged citrate-coated GNP interacted with the N-terminal whereas positively charged poly(allylamine hydrochloride)-coated GNP (PAH-GNP) interacted with the C-terminal. These results were accompanied by an increase in the β-sheet content, suggesting that PAH-GNP induce conformational changes resembling the aggregation state of α-syn. A similar result was observed by Lin et al. [251]. GNP coated with (16-mercaptohexadecyl) trimethylammonium bromide (MTAB), a cationic agent, also promoted N-terminus exposure similar to PAH. However, α-syn interacts with MTAB-coated or PAH-coated GNP in different ways because of their respective intermolecular interactions. While MTAB is unable to create hydrogen bonds with α-syn, PAH contains ammonium ions that facilitate the formation of hydrogen-bonding interactions.
Interestingly, McClain et al. [32] synthetized GNP coated with sodium dodecyl sulfate (SDS-GNP) to mimic the effect of biological membranes on α-syn conformation. This capping agent presents a negative charge that interacted with the N-terminus of α-syn, resembling the interaction of GNP with citrate. In fact, SDS-GNP were also found to expose the NAC region in a comparable manner to PAH-GNP. These results suggest that the effect of α-syn/GNP interaction could induce a higher α-syn propensity to aggregate depending on surface chemistry, modifying the orientation and adsorption of α-syn onto GNP. Therefore, determining this information becomes mandatory in order to predict and understand GNP behavior and their effects on biological systems and to apply this knowledge to develop future nanomaterials (Fig. 10).
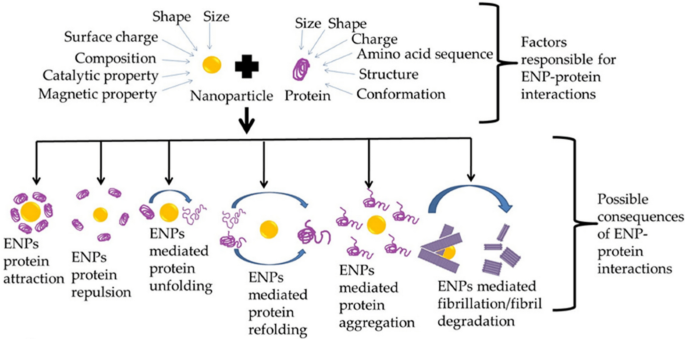
Different functions or consequences of engineering nanoparticles (ENP) interacting with proteins. As a product of this interaction, nanoparticles are capable of unfolding, refolding, promoting protein aggregation, or mediating the fibrillation or defibrillation process. Adapted with permission from Ref. [231]. 2022, by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/)
Challenges and potential risks of GNP use: neurotoxicity
The explosive development of GNP has raised interesting concerns related to their uses and applications. The surface and geometric design of GNP can modify or alter the biological responses both in in vitro and in vivo studies and in fact, the use of GNP on biological models is not free of limitations, risky factors, or side effects [252, 253]. In this regard, current research has been conducted on cytotoxicity analysis, and especially related to neurotoxicity [103, 254]. These studies have become relevant because any issue as a result of the use of GNP in the CNS has a major impact due to the low rate of regeneration of the CNS, and considering that the brain is a sensitive organ to the oxidative stress generated by GNP [130, 255]. Thus, the neurotoxicity of GNP has become a key parameter to improve and optimize nanostructures in order to design safer and non-toxic GNP for potential therapeutic applications in neurodegenerative diseases [256].
In fact, GNP have exhibited significant cytotoxic effects, such as generation of oxidative stress, disruption of the lipid bilayer, necrosis/apoptosis, mitochondrial dysfunction, and DNA damage [103]. These harmful effects are controversial because there is a wide spectrum of studies and analyses using different sizes and shapes of GNP, and there are also differences in the physicochemical nature of GNP coating and the doses or administration routes of GNP [103, 130, 253]. Thus, this wide variety of studies makes it difficult to generalize and point out the effect or behavior of GNP on biological systems, rendering this analysis nontrivial. In this sense, there have been numerous in vitro studies published in the literature describing the neurotoxic effects of GNP, but only are few in vivo studies have been reported [103]. Because the use and application of GNP have sparked an explosive growing interest in biomedicine, especially in CSN disorders, it is mandatory to disclose the major spectrum of neurotoxic effects that GNP can exhibit. Therefore, this review will concentrate on considering and collecting in vivo, in vitro, and also ex vivo studies that have documented the neurotoxic effects of GNP in order to display the current state-of-art research related to this key parameter.
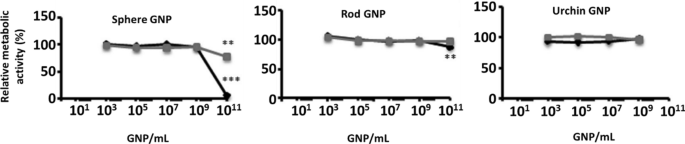
Nowadays, the most common and widely studied GNP shape is the sphere. Different sizes, treatments, administration routes, coatings, and also the type of animal, their sex, and their age, are all parameters that make it difficult to assemble the results in order to surmise the effect of a particular GNP. Bare GNS with different sizes have been analyzed in in vivo studies, yielding somewhat conflicting findings. GNS with a diameter of 10 or 30 nm have shown neurotoxic effects [257], while GNS with a diameter of 12.5 or 50 nm have been reported to be non-neurotoxic [258]. Both studies were carried out with different GNP concentrations, types of animals, and treatments. Similar neurotoxic effects, learning impairment, biochemical changes, and a slight apoptosis and inflammatory response were exhibited in the case of GNS [259,260,261] administered either intraperitoneally or by a unilateral stereotactic injection into the cerebral cortex [262, 263]. Of note GNS with a diameter of 50 nm injected intravenously showed a non-neurotoxic effect [264]. Another kind of analysis reported is the ex vivo study using the patch-clamp method on CA1 and CA3 neurons. In this case, stars and spheres were analyzed, but both types of GNP provoked disturbances in neuronal functions similar to those found under pathological conditions [265, 266].
Interestingly, GNP can diminish these toxic effects with an appropriate coating [130, 252]. Although GNS and GNCls with different coatings have commonly exhibited a non-neurotoxic effect [267,268,269], some coatings have exhibited a slight decrease in cell viability in in vitro assays [270]. Regarding in vivo assays, GNS functionalized with imidazole exhibited a neurotoxic effect, whereas bare GNS did not impact cell viability [271]. Another example of the apparent contradictory effect of GNS is coating with PEG, which is the most common molecule used to increase GNP stability [272]. GNS-PEG showed a toxic effect in mice that was reverted by a second functionalization with Trolox, an antioxidant molecule [273]. However, GNS-PEG exhibited a non-neurotoxic effect compared to the bare GNS in rats [274]. This non-neurotoxic effect was also observed using different shapes of GNP functionalized with PEG in ex vivo assays [275, 276]. Another interesting analysis was carried out using GNS or GNCls with different coatings in a PD-induced model. These studies demonstrated that GNP exhibited a neuroprotective effect, reversing the deleterious damage observed in this disease [191, 192, 277], and showed an anti-neuroinflammatory effect compared to the controls [277]. This neuroprotective effect was also observed in a transgenic Tau mouse model of AD, in which the presence of GNP improved the cognitive functions of the mice (Fig. 12) [194].
Commonly, the neurotoxic effect of GNP has been evaluated only on neurons from different models through in vitro and in vivo assays, as described above. However, the CNS not only contains neurons but also glial cells, which are closely related to the normal functioning of the brain. There are different kinds of glia with specific functions, but in general, all of them participate in providing a key support system to the neurons [278, 279]. Thereby, the evaluation of GNP cytotoxicity both in neuronal models and in glia models allows for a general idea of the impact of GNP on the CNS. Only a few reports of the GNP effect on glia have been reported so far. Leite et al. [270] analyzed the effect of GNS-PEG on a 3D neural model to precisely determine the cytotoxicity that mimics the in vivo physiological conditions. This novel approach demonstrated that brainspheres were not affected by the different GNP used. Similar results were observed using GNS-PEG in either a human astrocyte cell line or primary cultures [275, 280]. This kind of information is key because astrocytes participate in maintaining adequate levels of some neurotransmitters, support the synaptic connection, and consolidate the learning and memory cognitive process [281]. Another relevant type of cell are microglia, which are the immune cells of the brain. Their activation or death could lead to the generation of neurodegenerative diseases or the loss of homeostasis. GNS with different coatings exhibited a non-cytotoxic effect in different microglial cell lines or primary cultures (Fig. 11) [267, 275, 277, 282, 283] as well as in in vivo assays analyzing either astrocytes or microglia [273, 284]. A similar non-cytotoxic effect was observed in GNCls with different coatings in in vitro assays for astrocytes or microglia [268, 269, 285,286,287]. Thus, the cytotoxic effect exhibited by GNP highly depends on the coating used, and modulating this parameter allows decreasing or avoiding these side effects in order to design and obtain safer GNP for potential therapies.
Cytotoxicity effect of GNP coated with either CTAB or PEG in a microglial (N9) cell line using different concentrations of GNP. Metabolic activity was evaluated using the MTT assay. Adapted with permission from Ref. [283].
Perspectives on strategies to enhance the analysis of GNP effect: 3D culture and the microfluidic model
GNP are potential tools to carry drugs and treat brain disorders. It is already known that GNP can be evaluated using in vitro cells in 2D cell cultures, which do not resemble the complexity of the in vivo nanoparticle-cell interaction. In this sense, dynamic methodologies highlight as interesting tools to introduce different variables that normally are not considered in static methods, mainly because a dynamic environment produces shear stress, changing the composition and structure of the biomolecules when they interact with nanoparticles [288,289,290,291]. In this sense, microfluidic (MF) has several advantages over conventional methods by incorporating fluid flow and mechanical forces, bringing a step closer to mimicking the in vivo microenvironment, being an in vivo-like method for studying nano-bio interfaces [292,293,294,295], for example, emulating the physiological conditions when administering nanoparticles into the bloodstream to reach different targets. In fact, MF corresponds to one of the pioneering techniques to analyze and study hydrodynamic regimes at small scales. This technique utilizes microscale channels to manipulate fluids at the nanoliter scale and suspend objects in a controlled manner [296], and it has rapidly progressed over the past decade, improving from basic devices to large-scale two-dimensional integration of components, 3D architectures, and nonlinear autoregulatory systems [297].
Currently, one of the future challenges is to obtain 3D in vitro nervous system models because the 2D cell cultures of neurite networks and in vitro brain cell architecture is limited and does not fully represent the function and complexity of the brain, especially during development and neuronal plasticity. The primary challenge in investigating drug delivery or any kind of molecule in 3D brain models lies in the complex and non-repetitive 3D structure of the brain, as well as its complex integrated circuits. In contrast, the current 2D cell culture models lack the ability to accurately replicate the complexity of the brain. At he moment, neuronal networks arranged in sophisticated 3D matrices or optimum combinations of different cells represent the inception of a groundbreaking generation that has the potential to revolutionize future results [298, 299]. Now, the field of bioengineering is striving to emulate the physiological systems with utmost accuracy to study the administration of nanoparticles. Accordingly, 3D in vitro models are the latest and most advanced method in this field. They provide the ability to replicate the physiological conditions seen in vivo [298, 300], resulting in improved cell survival, differentiation, and more accurate reproduction of electrical activity [270]. Lab-on-a-chip (LOC) has been used for neurobiology applications due to the ability of microfluidic channels to approximate the size and flow conditions found in in vivo capillaries, the so called in vivo-like flow. Therefore, studying the delivery of nanoparticles or their impact on specific biological systems is challenging due to dramatic variations in their efficacy across 2D and 3D culture systems [301].
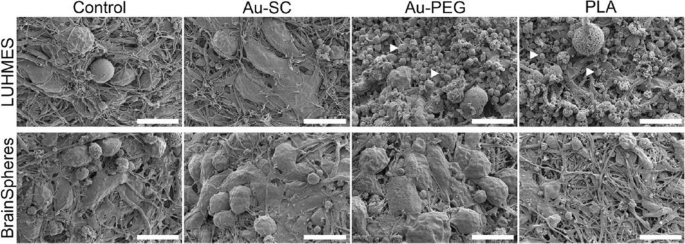
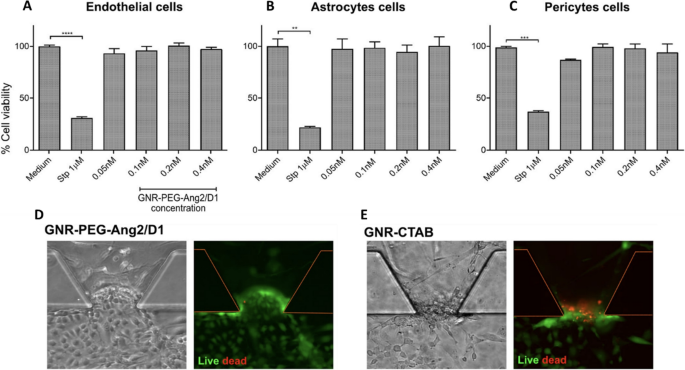
In fact, there are microfluidic devices that simulate a complex functional and anatomical structure composed of endothelial cells and their BBB, forming tight junctions and constructing a 3D brain spheroid model to study the effect of GNP. Nevertheless, there are few publications describing these models to study the use of GNP [293, 302]. For example, Smirnova et al. [270] studied the effect of GNP functionalized with citrate and PEG using two different human 3D CNS in vitro models. In this work, they fabricated a 3D Lund human mesencephalic spheroid and a human iPSC-derived brain spheroid, called BrainSphere. The BrainSpheres is a multicellular 3D brain model composed of neurons, astrocytes, and oligodendrocytes, forming a complex multicellular model (Fig. 12). The comparison between single 3D models and a complex multicellular 3D model was relevant in the study of nanoparticle uptake, morphological and molecular alterations, and cytotoxicity, among others. The results indicated that GNP and their application in the 3D brain spheroid models are well suited to characterize the neurotoxicity of nanoparticles, and that the mixed populations of neural cells within the model allow the presence of glial cells (which participate in brain support). Therefore, GNP neurotoxicity decreased in comparison with the single 3D model, enhancing the comprehensiveness of this study in capturing physiological conditions. In this sense, a similar result was obtained by Palma-Florez et al. [292]. The authors mimicked the BBB with human astrocytes, pericytes and endothelial cells in a microfluidic device and analyzed a gold nanosystem, GNR-PEG-D1/Ang2, determining its non-cytotoxic effect on the tri-culture and the enhancement of nanosystem permeability (Fig. 13).
Morphology of 3D LUHMES and BrainSpheres exposed to Au-SC (6 μg/mL), Au-PEG (20 μg/mL) and PLA-NP (20 μg/mL) for 72 h. The control represents untreated spheroids. White arrowheads indicate cell debris. Scale bars: 10 μm. Adapted with permission from Ref. [270].
Cytotoxic effect of GNR-PEG-Ang2/D1 on cells that constitute the BBB at three different concentrations. A-C, cytotoxic effect of the nanosystem on each cell line, evaluated separately. D and E, GNR-PEG-Ang2/D1 analyzed on the endothelial barrier in the BBB-on-a-chip. GNR-CTAB was used as a control. Adapted with permission from Ref. [292]. Copyright, 2023 Springer Nature
Likewise, it is important to mention that one of the key aspects of most protein folding or aggregation studies, as well as studies performed in the presence of inhibitory agents or GNP, are performed in bulk assays. This implies that the degree of freedom possessed by proteins or peptides is greater, so it is uncertain whether these studies truly reflect the behavior of a protein in living organisms [303]. Indeed, various studies have shown that the use of confined environments alters the kinetics and thermodynamics of protein–protein interactions [304], even promoting the appearance of β-secondary structures [305]. Additionally, since in vivo protein folding occurs mainly under rigid confinement characteristics, studies with models in confined spaces should be included in in vitro studies, constituting an interesting and challenging approach to replicate a typical scenario of the in vivo aggregation process [306,307,308]. In this sense, obtaining this information is mandatory because, bulk studies have indicated that the modification of the stirring speeds completely alters the aggregation process and morphology of the Aβ42 aggregates. In the same way, studies using microfluidics have shown that modifying the flow inside the microchannels together through a spatial confinement alters the morphology of amyloid aggregates [309, 310]. Furthermore, the confinement added to the low retention times achieved in the microchannels takes advantage of the initial stages of the amyloid aggregation process [311, 312], which are key steps directly related to the generation of neurotoxic oligomeric species of amyloid aggregates. Thus, the use of microfluidics emerges as a powerful methodology to study the aggregation process of amyloids in the presence of GNP, as well as their effect on the generation of toxic oligomeric species of amyloids. To date, no studies have used microfluidics to investigate the effect of functionalized GNP on the amyloid aggregation process in a confined space using, Tau or α-syn amyloid protein in a dynamic regime. Therefore, for the purpose of future studies, MF research has taken a keen interest in investigating this particular field of study due to the limited exploration of the aggregation process within microfluidic devices.
Conclusions
Plasmonic GNP have gained interest in the biomedical field due to their high versatility and optical and electronic properties. This review highlighted their application in the development of diagnostic tools for PD and AD and described how the selectivity and sensitivity of current methods have been improved. In this sense, their use as delivery therapeutic agents or in vivo imaging has been proposed for the therapy and diagnosis of neurodegenerative diseases, taking advantage of the plasmonic properties of these nanomaterials. However, the research on GNP and their risk factors, neurotoxicity, targeting, selectivity, and sensitivity in the early stages of these diseases in preclinical models is incipient and remains a challenge. Furthermore, the investigation of the interactions between nanoparticles and biological barriers, as well as cellular responses, plays a critical role in developing preclinical and clinical applications of nanomaterials. For these reasons, cutting-edge methodologies such as 3D cultures and microfluidics have emerged as significant innovations by mimicking in vivo biological conditions, thus overcoming the limitations of bulk assays, and providing valuable and representative data for characterizing nanomaterials in relevant biological contexts. Furthermore, using microfluidics and "organ-on-a-chip" platforms highlights the emulation of physiological conditions to reduce the need for animal experimentation and contribute to understanding how GNP can interact with cells and cross the BBB. These advanced approaches are an essential step towards a deeper understanding of nanomaterial-cell interactions and the accurate assessment of their impact on the biological environment. In summary, the use of GNP in the diagnosis, targeting, therapy, and monitoring of neurodegenerative diseases represents an exciting and promising field in biomedical research. Although there are challenges ahead, such as safety, potential intranasal administration, the capping with biodegradable materials, or GNP biodistribution, the positive impact of these nanotools on the quality of patients’ lives and the advance in understanding these complex disorders is undeniable. It is crucial to maintain the collaboration between researchers and experts in various disciplines to make progress in this exciting field and potentially revolutionize the treatment of neurological diseases.
Availability of data and materials
Not applicable.
Abbreviations
- AD:
-
Alzheimer’s disease
- BBB:
-
Blood–brain barrier
- CNS:
-
Central nervous system
- GNP:
-
Gold nanoparticles
- NFTs:
-
Neurofibrillary tangles
- PD:
-
Parkinson’s disease
- P-Tau:
-
Hyperphosphorylated Tau
- T-Tau:
-
Total Tau
- α-syn:
-
α-Synuclein
- GNS:
-
Gold nanospheres
- GNR:
-
Gold nanorods
- GNPr:
-
Gold nanoprisms
- GNSts:
-
Gold nanostars
- GNCls:
-
Gold nanoclusters
- PEG:
-
Polyethylene glycol
- GNCs:
-
Gold nanocomposites
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- ThT:
-
Thioflavin-based kinetic assay
- MNPs:
-
Magnetic nanoparticles
- LOD:
-
Limit od detection
- NGF:
-
Nerve growth factor
- MF:
-
Microfluidics
References
Emamzadeh FN, Surguchov A. Parkinson’s disease: biomarkers, treatment, and risk factors. Front Neurosci. 2018. https://doi.org/10.3389/fnins.2018.00612.
Du X, Wang X, Geng M. Alzheimer’s disease hypothesis and related therapies. Transl Neurodegener. 2018;7:2.
Irvine GB, El-Agnaf OM, Shankar GM, Walsh DM. Protein aggregation in the brain: the molecular basis for Alzheimer’s and Parkinson’s diseases. Mol Med. 2008. https://doi.org/10.2119/2007-00100.Irvine.
Tan SH, Karri V, Tay NWR, Chang KH, Ah HY, Ng PQ, et al. Emerging pathways to neurodegeneration: dissecting the critical molecular mechanisms in Alzheimer’s disease Parkinson’s disease. Biomed Pharmacother. 2019. https://doi.org/10.1016/j.biopha.2018.12.101.
Xie A, Gao J, Xu L, Meng D. Shared mechanisms of neurodegeneration in alzheimer’s disease and parkinson’s disease. Biomed Res Int. 2014. https://doi.org/10.1155/2014/648740.
Liu D, Li W, Jiang X, Bai S, Liu J, Liu X, et al. Using near-infrared enhanced thermozyme and scFv dual-conjugated Au nanorods for detection and targeted photothermal treatment of Alzheimer’s disease. Theranostics. 2019. https://doi.org/10.7150/thno.30649.
Liu W, Dong X, Liu Y, Sun Y. Photoresponsive materials for intensified modulation of Alzheimer’s amyloid-β protein aggregation: a review. Acta Biomater. 2021. https://doi.org/10.1016/j.actbio.2021.01.018.
Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M. Improving healthcare for people living with dementia. Coverage, Quality and costs now and in the future. World Alzheimer Report 2016. 2016
Karapetyan G, Fereshetyan K, Harutyunyan H, Yenkoyan K. The synergy of β amyloid 1–42 and oxidative stress in the development of Alzheimer’s disease-like neurodegeneration of hippocampal cells. Sci Rep. 2022. https://doi.org/10.1038/s41598-022-22761-5.
Yana MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med. 2013. https://doi.org/10.1016/j.freeradbiomed.2012.11.014.
Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, et al. Structural conversion of neurotoxic amyloid-beta(1–42) oligomers to fibrils. Nat Struct Mol Biol. 2010;17:561–7.
Zheng H, Koo EH. The amyloid precursor protein: beyond amyloid. Mol Neurodegener. 2006;1:5.
LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509.
Liu P-P, Xie Y, Meng X-Y, Kang J-S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct Target Ther. 2019;4:29. https://doi.org/10.1038/s41392-019-0063-8.
Silvestro S, Valeri A, Mazzon E. Aducanumab and its effects on tau pathology: is this the turning point of amyloid hypothesis? Int J Mol Sci. 2022;23:2011.
Kametani F, Hasegawa M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front Neurosci. 2018. https://doi.org/10.3389/fnins.2018.00025.
Marcatti M, Fracassi A, Montalbano M, Natarajan C, Krishnan B, Kayed R, et al. Aβ/tau oligomer interplay at human synapses supports shifting therapeutic targets for Alzheimer’s disease. Cell Mol Life Sci. 2022;79:222. https://doi.org/10.1007/s00018-022-04255-9.
Tripathi T, Khan H. Direct interaction between the β-amyloid core and tau facilitates cross-seeding: a novel target for therapeutic intervention. Biochemistry. 2020;59:341–2. https://doi.org/10.1021/acs.biochem.9b01087.
Weglinski C, Jeans A. Amyloid-β in Alzheimer’s disease—front and centre after all? Neuronal Signal. 2023;7:NS20220086. https://doi.org/10.1042/NS20220086.
Gulisano W, Maugeri D, Baltrons MA, Fà M, Amato A, Palmeri A, et al. Role of amyloid-β and tau proteins in Alzheimer’s disease: confuting the amyloid cascade. J Alzheimer’s Dis. 2018. https://doi.org/10.3233/JAD-179935.
Avila J, Lucas JJ, Pérez M, Hernández F. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004. https://doi.org/10.1152/physrev.00024.2003.
Kovacs GG. Chapter 25 Tauopathies. Handb Clin Neurol. 2017;145:355–68.
Pîrşcoveanu DFV, Pirici I, Tudorică V, Bălşeanu TA, Albu VC, Bondari S, et al. Tau protein in neurodegenerative diseases—a review. Roman J Morphol Embryol. 2017;58(4):1141–50.
Ameri M, Shabaninejad Z, Movahedpour A, Sahebkar A, Mohammadi S, Hosseindoost S, et al. Biosensors for detection of Tau protein as an Alzheimer’s disease marker. Int J Biol Macromol. 2020. https://doi.org/10.1016/j.ijbiomac.2020.06.239.
Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci. 2016;17:5–21.
Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Rev. 2000. https://doi.org/10.1016/B978-012351830-9/50023-8.
Molinuevo JL, Ayton S, Batrla R, Bednar MM, Bittner T, Cummings J, et al. Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol. 2018. https://doi.org/10.1007/s00401-018-1932-x.
Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, et al. Decreased β-amyloid1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003. https://doi.org/10.1001/jama.289.16.2094.
Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. α-synuclein in Lewy bodies [8]. Nature. 1997. https://doi.org/10.1038/42166.
George JM. The synucleins. Genome Biol. 2002;3:1–6.
Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001. https://doi.org/10.1038/35081564.
McClain SM, Ojoawo AM, Lin W, Rienstra CM, Murphy CJ. Interaction of alpha-synuclein and its mutants with rigid lipid vesicle mimics of varying surface curvature. ACS Nano. 2020. https://doi.org/10.1021/acsnano.0c03420.
Giasson BI, Murray IVJ, Trojanowski JQ, Lee VMY. A hydrophobic stretch of 12 amino acid residues in the middle of α-synuclein is essential for filament assembly. J Biol Chem. 2001. https://doi.org/10.1074/jbc.M008919200.
Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, et al. In vivo demonstration that α-synuclein oligomers are toxic. Proc Natl Acad Sci U S A. 2011. https://doi.org/10.1073/pnas.1100976108.
Du XY, Xie XX, Liu RT. The role of α-synuclein oligomers in parkinson’s disease. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21228645.
Bengoa-Vergniory N, Roberts RF, Wade-Martins R, Alegre-Abarrategui J. Alpha-synuclein oligomers: a new hope. Acta Neuropathol. 2017. https://doi.org/10.1007/s00401-017-1755-1.
Kulenkampff K, Wolf Perez AM, Sormanni P, Habchi J, Vendruscolo M. Quantifying misfolded protein oligomers as drug targets and biomarkers in Alzheimer and Parkinson diseases. Nat Rev Chem. 2021. https://doi.org/10.1038/s41570-021-00254-9.
Schmid AW, Fauvet B, Moniatte M, Lashuel HA. Alpha-Synuclein post-Translational modifications as potential biomarkers for parkinson disease and other synucleinopathies. Mol Cell Proteomics. 2013. https://doi.org/10.1074/mcp.R113.032730.
Schuster J, Funke SA. Methods for the specific detection and quantitation of amyloid-β oligomers in cerebrospinal fluid. JAD. 2016. https://doi.org/10.3233/JAD-151029.
Ntetsika T, Papathoma PE, Markaki I. Novel targeted therapies for Parkinson’s disease. Mol Med. 2021. https://doi.org/10.1186/s10020-021-00279-2.
Polanco JC, Li C, Bodea LG, Martinez-Marmol R, Meunier FA, Götz J. Amyloid-β and tau complexity—towards improved biomarkers and targeted therapies. Nat Rev Neurol. 2018. https://doi.org/10.1038/nrneurol.2017.162.
BulckVan M, Sierra-Magro A, Alarcon-Gil J, Perez-Castillo A, Morales-Garcia JA. Novel approaches for the treatment of alzheimer’s and parkinson’s disease. Int J Mol Sci. 2019;20:719.
Linazasoro G. Recent failures of new potential symptomatic treatments for Parkinson’s disease: Causes and solutions. Movement Disord. 2004. https://doi.org/10.1002/mds.20120.
Yiannopoulou KG, Anastasiou AI, Zachariou V, Pelidou SH. Reasons for failed trials of disease-modifying treatments for alzheimer disease and their contribution in recent research. Biomedicines. 2019;7:97.
Baldacci F, Mazzucchi S, Della Vecchia A, Giampietri L, Giannini N, Koronyo-Hamaoui M, et al. The path to biomarker-based diagnostic criteria for the spectrum of neurodegenerative diseases. Expert Rev Mol Diagn. 2020. https://doi.org/10.1080/14737159.2020.1731306.
Obrocki P, Khatun A, Ness D, Senkevich K, Hanrieder J, Capraro F, et al. Perspectives in fluid biomarkers in neurodegeneration from the 2019 biomarkers in neurodegenerative diseases course—a joint PhD student course at University College London and University of Gothenburg. Alz Res Ther. 2020. https://doi.org/10.1186/s13195-020-00586-.
Sela H, Cohen H, Elia P, Zach R, Karpas Z, Zeiri Y. Spontaneous penetration of gold nanoparticles through the blood brain barrier (BBB). J Nanobiotechnology. 2015;13:71.
Hammami I, Alabdallah NM, Jomaa A, Kamoun M. Gold nanoparticles: Synthesis properties and applications. J King Saud Univ Sci. 2021. https://doi.org/10.1016/j.jksus.2021.101560.
Nejati K, Dadashpour M, Gharibi T, Mellatyar H, Akbarzadeh A. Biomedical applications of functionalized gold nanoparticles: a review. J Clust Sci. 2022. https://doi.org/10.1007/s10876-020-01955-9.
Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012;41:2740–79.
Siddique S, Chow JCL. Gold nanoparticles for drug delivery and cancer therapy. Appl Sci. 2020. https://doi.org/10.3390/app10113824.
Zhao J, Xu N, Yang X, Ling G, Zhang P. The roles of gold nanoparticles in the detection of amyloid-β peptide for Alzheimer’s disease. Colloids Interface Sci Commun. 2022. https://doi.org/10.1016/j.colcom.2021.100579.
Sperling RA, Rivera Gil P, Zhang F, Zanella M, Parak WJ. Biological applications of gold nanoparticles. Chem Soc Rev. 2008;37:1896–908.
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021. https://doi.org/10.1038/s41573-020-0090-8.
Huang X, El-Sayed MA, GolHuang X, El-Sayed MA. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J Adv Res. 2010. https://doi.org/10.1016/j.jare.2010.02.002d.
Huang X, El-Sayed MA. Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J Adv Res. 2010. https://doi.org/10.1016/j.jare.2010.02.002.
Zhang G. Functional gold nanoparticles for sensing applications. Nanotechnol Rev. 2013. https://doi.org/10.1515/ntrev-2012-0088.
Oyarzún MP, Tapia-Arellano A, Cabrera P, Jara-Guajardo P, Kogan MJ. Plasmonic nanoparticles as optical sensing probes for the detection of alzheimer’s disease. Sensors. 2021. https://doi.org/10.3390/s21062067.
Saha K, Agasti SS, Kim C, Li X, Rotello VM. Gold nanoparticles in chemical and biological sensing. Chem Rev. 2012. https://doi.org/10.1021/cr2001178.
Bagheri S, Yasemi M, Safaie-Qamsari E, Rashidiani J, Abkar M, Hassani M, et al. Using gold nanoparticles in diagnosis and treatment of melanoma cancer. Artif Cells Nanomed Biotechnol. 2018. https://doi.org/10.1080/21691401.2018.1430585.
Gonzalez Solveyra E, Szleifer I. What is the role of curvature on the properties of nanomaterials for biomedical applications? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:334–54.
Xie X, Liao J, Shao X, Li Q, Lin Y. The effect of shape on cellular uptake of gold nanoparticles in the forms of stars, rods, and triangles. Sci Rep. 2017;7:3827. https://doi.org/10.1038/s41598-017-04229-z.
Petros RA, Desimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010. https://doi.org/10.1038/nrd2591.
Zare I, Yaraki MT, Speranza G, Najafabadi AH, Shourangiz-Haghighi A, Nik AB, et al. Gold nanostructures: synthesis, properties, and neurological applications. Chem Soc Rev. 2022. https://doi.org/10.1039/D1CS01111A.
Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951. https://doi.org/10.1039/df9511100055.
Afrapoli ZB, Majidi RF, Negahdari B, Tavoosidana G. ‘Inversed Turkevich’ method for tuning the size of Gold nanoparticles: evaluation the effect of concentration and temperature. Nanomed Res J. 2018;3(4):190–6.
Tran M, DePenning R, Turner M, Padalkar S. Effect of citrate ratio and temperature on gold nanoparticle size and morphology. Mater Res Express. 2016. https://doi.org/10.1088/2053-1591/3/10/105027.
Yazdani S, Daneshkhah A, Diwate A, Patel H, Smith J, Reul O, et al. Model for gold nanoparticle synthesis: effect of pH and reaction time. ACS Omega. 2021. https://doi.org/10.1021/acsomega.1c01418.
Slepička P, Kasálková NS, Siegel J, Kolská Z, Švorčík V. Methods of gold and silver nanoparticles preparation. Materials. 2020. https://doi.org/10.3390/ma13010001.
Santhosh PB, Genova J, Chamati H. Review green synthesis of gold nanoparticles: an eco-friendly approach. Chemistry. 2022. https://doi.org/10.3390/chemistry4020026.
Rónavári A, Igaz N, Adamecz DI, Szerencsés B, Molnar C, Kónya Z, et al. Green silver and gold nanoparticles: biological synthesis approaches and potentials for biomedical applications. Molecules. 2021;26:844.
Mata R, Nakkala JR, Sadras SR. Polyphenol stabilized colloidal gold nanoparticles from Abutilon indicum leaf extract induce apoptosis in HT-29 colon cancer cells. Colloids Surf B Biointerfaces. 2016. https://doi.org/10.1016/j.colsurfb.2016.03.069.
Zhao X, Li Z, Deng Y, Zhao Z, Li X, Xia Y. Facile synthesis of gold nanoparticles with alginate and its catalytic activity for reduction of 4-nitrophenol and H2O2 detection. Materials. 2017. https://doi.org/10.3390/ma10050557.
Cai F, Li S, Huang H, Iqbal J, Wang C, Jiang X. Green synthesis of gold nanoparticles for immune response regulation: mechanisms, applications, and perspectives. J Biomed Mater Res A. 2022;110(2):424–42.
Dhanasekar NN, Rahul GR, Narayanan KB, Raman G, Sakthivel N. Green chemistry approach for the synthesis of gold nanoparticles using the fungus Alternaria sp. J Microbiol Biotechnol. 2015. https://doi.org/10.4014/jmb.1410.10036.
Zuhrotun A, Oktaviani DJ, Hasanah AN. Biosynthesis of gold and silver nanoparticles using phytochemical compounds. Molecules. 2023. https://doi.org/10.3390/molecules28073240.
Akbari A, Eshkiki ZS, Mayahi S, Amini SM. In-vitro investigation of curcumin coated gold nanoparticles effect on human colorectal adenocarcinoma cell line. Nanomedicine Res J. 2022;7:66–72.
Amini SM, Hadighi R, Najm M, Alipour M, Hasanpour H, Vosoogh M, et al. The therapeutic effects of curcumin-coated gold nanoparticle against leishmania major causative agent of zoonotic cutaneous leishmaniasis (ZCL): an in vitro and in vivo study. Curr Microbiol. 2023. https://doi.org/10.1007/s00284-022-03172-1.
Nambiar S, Osei E, Fleck A, Darko J, Mutsaers AJ, Wettig S. Synthesis of curcumin-functionalized gold nanoparticles and cytotoxicity studies in human prostate cancer cell line. Appl Nanosci. 2018. https://doi.org/10.1007/s13204-018-0728-6.
Muniyappan N, Pandeeswaran M, Amalraj A. Green synthesis of gold nanoparticles using Curcuma pseudomontana isolated curcumin: Its characterization, antimicrobial, antioxidant and anti- inflammatory activities. Environ Chem Ecotoxicol. 2021;3:117.
Fathy MM, Elfiky AA, Bashandy YS, Hamdy MM, Elgharib AM, Ibrahim IM, et al. An insight into synthesis and antitumor activity of citrate and gallate stabilizing gold nanospheres. Sci Rep. 2023. https://doi.org/10.1038/s41598-023-29821-4.
Wu YZ, Tsai YY, Chang LS, Chen YJ. Evaluation of gallic acid-coated gold nanoparticles as an anti-aging ingredient. Pharmaceuticals. 2021;14:1071.
Neshastehriz A, Amini SM, Mohammadi A, Mahdavi SR, Mahabadi VP, Akbari A. In-vitro investigation of green synthesized gold nanoparticle’s role in combined photodynamic and radiation therapy of cancerous cells. Adv Nat Sci. 2020;11:045006.
Morales-Zavala F, Arriagada H, Hassan N, Velasco C, Riveros A, Álvarez AR, et al. Peptide multifunctionalized gold nanorods decrease toxicity of β-amyloid peptide in a Caenorhabditis elegans model of Alzheimer’s disease. Nanomedicine. 2017. https://doi.org/10.1016/j.nano.2017.06.013.
Jara-Guajardo P, Cabrera P, Celis F, Soler M, Berlanga I, Parra-Muñoz N, et al. Gold nanoparticles mediate improved detection of β-amyloid aggregates by fluorescence. Nanomaterials. 2020;10:1–16.
Cabrera P, Jara-Guajardo P, Oyarzún MP, Parra-Muñoz N, Campos A, Soler M, et al. Surface enhanced fluorescence effect improves the in vivo detection of amyloid aggregates. Nanomedicine. 2022;44:102569.
Park K, Drummy LF, Wadams RC, Koerner H, Nepal D, Fabris L, et al. Growth mechanism of gold nanorods. Chem Mater. 2013. https://doi.org/10.1021/cm303659q.
Chhatre A, Thaokar R, Mehra A. Formation of gold nanorods by seeded growth: mechanisms and modeling. Cryst Growth Des. 2018;18(6):3269–82.
Yu X, Wang Z, Cui H, Wu X, Chai W, Wei J, et al. A review on gold nanotriangles: synthesis, self-assembly and their applications. Molecules. 2022. https://doi.org/10.3390/molecules27248766.
Tapia-Arellano A, Gallardo-Toledo E, Ortiz C, Henríquez J, Feijóo CG, Araya E, et al. Functionalization with PEG/Angiopep-2 peptide to improve the delivery of gold nanoprisms to central nervous system: in vitro and in vivo studies. Mater Sci Eng C. 2021;121:111785.
Gao Y, Wang J, Wang W, Zhao T, Cui Y, Liu P, et al. More symmetrical “hot spots” ensure stronger plasmon-enhanced fluorescence: from Au nanorods to nanostars. Anal Chem. 2021;93:2480–9.
Becerril-Castro IB, Calderon I, Pazos-Perez N, Guerrini L, Schulz F, Feliu N, et al. Gold nanostars: synthesis, optical and SERS analytical properties. Anal Sens. 2022;2:e202200005.
Donoso-González O, Lodeiro L, Aliaga ÁE, Laguna-Bercero MA, Bollo S, Kogan MJ, et al. Functionalization of gold nanostars with cationic β-cyclodextrin-based polymer for drug co-loading and sers monitoring. Pharmaceutics. 2021;13(2):261.
Oldenburg SJ, Averitt RD, Westcott SL, Halas NJ. Nanoengineering of optical resonances. Chem Phys Lett. 1998;288:243–7.
Jaque D, Martínez Maestro L, Del Rosal B, Haro-Gonzalez P, Benayas A, Plaza JL, et al. Nanoparticles for photothermal therapies. Nanoscale. 2014;6:9494–530.
Day ES, Morton JG, West JL. Nanoparticles for thermal cancer therapy. J Biomech Eng. 2009. https://doi.org/10.1115/1.3156800.
Li Y, He D, Tu J, Wang R, Zu C, Chen Y, et al. The comparative effect of wrapping solid gold nanoparticles and hollow gold nanoparticles with doxorubicin-loaded thermosensitive liposomes for cancer thermo-chemotherapy. Nanoscale. 2018;10:8628–41.
Abdollahi SN, Naderi M, Amoabediny G. Synthesis and characterization of hollow gold nanoparticles using silica spheres as templates. Colloids Surf A Physicochem Eng Asp. 2013;436:1069–75.
Ortiz-Castillo JE, Gallo-Villanueva RC, Madou MJ, Perez-Gonzalez VH. Anisotropic gold nanoparticles: a survey of recent synthetic methodologies. Coord Chem Rev. 2020;425: 213489.
Hassan H, Sharma P, Hasan MR, Singh S, Thakur D, Narang J. Gold nanomaterials—the golden approach from synthesis to applications. Mater Sci Energy Technol. 2022;5:375–90.
Zhang XD, Wu D, Shen X, Liu PX, Yang N, Zhao B, et al. Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int J Nanomedicine. 2011. https://doi.org/10.2147/IJN.S21657.
Jia YP, Shi K, Liao JF, Peng JR, Hao Y, Qu Y, et al. Effects of cetyltrimethylammonium bromide on the toxicity of gold nanorods both in vitro and in vivo: molecular origin of cytotoxicity and inflammation. Small Methods. 2020. https://doi.org/10.1002/smtd.201900799.
Sani A, Cao C, Cui D. Toxicity of gold nanoparticles (AuNPs): a review. Biochem Biophys Rep. 2021. https://doi.org/10.1016/j.bbrep.2021.100991.
Howard MD, Jay M, Dziubla TD, Lu X. PEGylation of nanocarrier drug delivery systems: state of the art. J Biomed Nanotechnol. 2008. https://doi.org/10.1166/jbn.2008.021.
Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990. https://doi.org/10.1016/0014-5793(90)81016-H.
Spinelli A, Girelli M, Arosio D, Polito L, Podini P, Martino G, et al. Intracisternal delivery of PEG-coated gold nanoparticles results in high brain penetrance and long-lasting stability. J Nanobiotechnology. 2019;17:1–3.
Navarro JRG, Manchon D, Lerouge F, Blanchard NP, Marotte S, Leverrier Y, et al. Synthesis of PEGylated gold nanostars and bipyramids for intracellular uptake. Nanotechnology. 2012;23: 465602.
Moreira AF, Rodrigues CF, Reis CA, Costa EC, Correia IJ. Gold-core silica shell nanoparticles application in imaging and therapy: a review. Microporous Mesoporous Materials. 2018;270:168–79.
Mamaeva V, Sahlgren C, Lindén M. Mesoporous silica nanoparticles in medicine-recent advances. Adv Drug Deliv Rev. 2013;65(5):689–702.
Bolaños K, Kogan MJ, Araya E. Capping gold nanoparticles with albumin to improve their biomedical properties. Int J Nanomedicine. 2019;14:6387–406.
Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Controlled Release. 2012. https://doi.org/10.1016/j.jconrel.2011.07.031.
Matei I, Buta CM, Turcu IM, Culita D, Munteanu C, Ionita G. Formation and stabilization of gold nanoparticles in bovine serum albumin solution. Molecules. 2019;24:3395.
Amini SM, Emami T, Rashidi M, Zrrinnahad H. Curcumin-gold nanoformulation: synthesis, characterizations and biomedical application. Food Biosci. 2024;57:103446.
Mohammadi A, Colagar AH, Khorshidian A, Amini SM. The functional roles of curcumin on astrocytes in neurodegenerative diseases. NeuroImmunoModulation. 2022. https://doi.org/10.1159/000517901.
Amini SM, Mohammadi E, Askarian-amiri S, Azizi Y, Shakeri-zadeh A, Neshastehriz A. Investigating the in vitro photothermal effect of green synthesized apigenin-coated gold nanoparticle on colorectal carcinoma. IET Nanobiotechnol. 2021. https://doi.org/10.1049/nbt2.12016.
Hersh AM, Alomari S, Tyler BM. Crossing the blood-brain barrier: advances in nanoparticle technology for drug delivery in neuro-oncology. Int J Mol Sci. 2022;23(8):4153.
Wu D, Chen Q, Chen X, Han F, Chen Z, Wang Y. The blood–brain barrier: structure, regulation, and drug delivery. Sig Transduct Target Ther. 2023. https://doi.org/10.1038/s41392-023-01481-w.
De Bem SG, Muller AP, Machado-De-Ávila RA, Silveira PCL. Advance in the use of gold nanoparticles in the treatment of neurodegenerative diseases: new perspectives. Neural Regen Res. 2021. https://doi.org/10.4103/1673-5374.313040.
Shukla R, Srivastava V, Sethi A, Ruwali M. Nanomaterial-based drug delivery systems as tools for targeted therapy of neurodegenerative diseases. Nanomed Drug Delivery Neurodegenerative Dis. 2022. https://doi.org/10.1016/B978-0-323-85544-0.00003-4.
Lockman PR, Mumper RJ, Khan MA, Allen DD. Nanoparticle technology for drug delivery across the blood-brain barrier. Drug Dev Ind Pharm. 2002;28:1–13.
Kumar A, Chaudhary RK, Singh R, Singh SP, Wang SY, Hoe ZY, et al. Nanotheranostic applications for detection and targeting neurodegenerative diseases. Front Neurosci. 2020. https://doi.org/10.3389/fnins.2020.00305.
Asha Spandana KM, Bhaskaran M, Karri VV, Natarajan J. A comprehensive review of nano drug delivery system in the treatment of CNS disorders. J Drug Deliv Sci Technol. 2020;57:101628.
Rai M, Yadav A. Nanobiotechnology in neurodegenerative diseases. Nanobiotechnol Neurodegenerative Dis. 2019. https://doi.org/10.1007/978-3-030-30930-5.
Re F, Gregori M, Masserini M. Nanotechnology for neurodegenerative disorders. Maturitas. 2012. https://doi.org/10.1016/j.nano.2012.05.007.
Meng J, Agrahari V, Youm I. Advances in targeted drug delivery approaches for the central nervous system tumors: the inspiration of nanobiotechnology. J Neuroimmune Pharmacol. 2017. https://doi.org/10.1007/s11481-016-9698-1.
Velasco-Aguirre C, Morales F, Gallardo-Toledo E, Guerrero S, Giralt E, Araya E, et al. Peptides and proteins used to enhance gold nanoparticle delivery to the brain: preclinical approaches. Int J Nanomedicine. 2015;10:4919–36.
Cheng G, Liu Y, Ma R, Cheng G, Guan Y, Chen X, et al. Anti-parkinsonian therapy: strategies for crossing the blood-brain barrier and nano-biological effects of nanomaterials. Nanomicro Lett. 2022;14:105.
Khongkow M, Yata T, Boonrungsiman S, Ruktanonchai UR, Graham D, Namdee K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood–brain barrier penetration. Sci Rep. 2019;9:8278.
Feng X, Chen A, Zhang Y, Wang J, Shao L, Wei L. Central nervous system toxicity of metallic nanoparticles. Int J Nanomedicine. 2015;2015:4321–40.
Flora SJS. The applications, neurotoxicity, and related mechanism of gold nanoparticles. Neurotoxicity of Nanomaterials and Nanomedicine. 2017.
Prades R, Guerrero S, Araya E, Molina C, Salas E, Zurita E, et al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials. 2012;33:7194–205.
Habib S, Singh M. Angiopep-2-modified nanoparticles for brain-directed delivery of therapeutics: a review. Polymers (Basel). 2022;14:712.
Formica ML, Real DA, Picchio ML, Catlin E, Donnelly RF, Paredes AJ. On a highway to the brain: a review on nose-to-brain drug delivery using nanoparticles. Appl Mater Today. 2022;29: 101631.
Agrawal M, Saraf S, Saraf S, Antimisiaris SG, Chougule MB, Shoyele SA, et al. Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J Controlled Release. 2018;281:139–77.
Crowe TP, Greenlee MHW, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52.
Sintov AC, Velasco-Aguirre C, Gallardo-Toledo E, Araya E, Kogan MJ. Metal nanoparticles as targeted carriers circumventing the blood-brain barrier. Int Rev Neurobiol. 2016;130:199–227.
Wang L, Tang S, Yu Y, Lv Y, Wang A, Yan X, et al. Intranasal delivery of temozolomide-conjugated gold nanoparticles functionalized with anti-EphA3 for glioblastoma targeting. Mol Pharm. 2021;18(3):915–27.
Raliya R, Saha D, Chadha TS, Raman B, Biswas P. Non-invasive aerosol delivery and transport of gold nanoparticles to the brain. Sci Rep. 2017;7(1):44718.
Ul Islam S, Shehzad A, Bilal Ahmed M, Lee YS. Intranasal delivery of nanoformulations: a potential way of treatment for neurological disorders. Molecules. 2020;7(1):44718.
Gallardo-Toledo E, Tapia-Arellano A, Celis F, Sinai T, Campos M, Kogan MJ, et al. Intranasal administration of gold nanoparticles designed to target the central nervous system: fabrication and comparison between nanospheres and nanoprisms. Int J Pharm. 2020;590:119957.
Han S, Wang JTW, Yavuz E, Zam A, Rouatbi N, Utami RN, et al. Spatiotemporal tracking of gold nanorods after intranasal administration for brain targeting. J Controlled Release. 2023. https://doi.org/10.1016/j.jconrel.2023.04.022.
Casettari L, Illum L. Chitosan in nasal delivery systems for therapeutic drugs. Jof Controlled Release. 2014;190:189–200.
Bhumkar DR, Joshi HM, Sastry M, Pokharkar VB. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm Res. 2007;24:1415–26.
Pietrzak K, Czarnecka K, Mikiciuk-Olasik E, Szymanski P. New perspectives of Alzheimer disease diagnosis—the most popular and future methods. Med Chem (Los Angeles). 2018;14:34–43.
Fayyad M, Salim S, Majbour N, Erskine D, Stoops E, Mollenhauer B, et al. Parkinson’s disease biomarkers based on α-synuclein. J Neurochem. 2019. https://doi.org/10.1111/jnc.14809.
Li T, Le W. Biomarkers for Parkinson’s disease: how good are they? Neurosci Bull. 2020;36(2):183–94.
Lee HJ, Bae EJ, Lee SJ. Extracellular α-synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol. 2014;10:92–8.
Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48.
Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: Mechanism and therapeutic strategies. Nat Med. 2017;23(2):1–3.
Leuzy A, Chiotis K, Lemoine L, Gillberg PG, Almkvist O, Rodriguez-Vieitez E, et al. Tau PET imaging in neurodegenerative tauopathies—still a challenge. Mol Psychiatry. 2019;24(8):1112–34.
Cassinelli Petersen G, Roytman M, Chiang GC, Li Y, Gordon ML, Franceschi AM. Overview of tau PET molecular imaging. Curr Opin Neurol. 2022;35(2):230–9.
Paciotti S, Bellomo G, Gatticchi L, Parnetti L. Are we ready for detecting α-synuclein prone to aggregation in patients? The case of “Protein-Misfolding Cyclic Amplification” and “Real-Time Quaking-Induced Conversion” as diagnostic tools. Front Neurol. 2018;9:376477.
Shahnawaz M, Tokuda T, Waraga M, Mendez N, Ishii R, Trenkwalder C, et al. Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol. 2017;74(2):163–72.
An Y, Tang L, Jiang X, Chen H, Yang M, Jin L, et al. A photoelectrochemical immunosensor based on au-doped TiO2 nanotube arrays for the detection of α-synuclein. Chemistry. 2010;16:14439–46.
Zhang R, Wang S, Huang X, Yang Y, Fan H, Yang F, et al. Gold-nanourchin seeded single-walled carbon nanotube on voltammetry sensor for diagnosing neurogenerative Parkinson’s disease. Anal Chim Acta. 2020;1094:142–50.
An Y, Jiang X, Bi W, Chen H, Jin L, Zhang S, et al. Sensitive electrochemical immunosensor for α-synuclein based on dual signal amplification using PAMAM dendrimer-encapsulated Au and enhanced gold nanoparticle labels. Biosens Bioelectron. 2012;32:224–30.
Aminabad ED, Mobed A, Hasanzadeh M, Hosseinpour Feizi MA, Safaralizadeh R, Seidi F. Sensitive immunosensing of α-synuclein protein in human plasma samples using gold nanoparticles conjugated with graphene: an innovative immuno-platform towards early stage identification of Parkinson’s disease using point of care (POC) analysis. RSC Adv. 2022;12:4346–57. https://doi.org/10.1039/D1RA06437A.
Sonuç Karaboğa MN, Sezgintürk MK. Cerebrospinal fluid levels of alpha-synuclein measured using a poly-glutamic acid-modified gold nanoparticle-doped disposable neuro-biosensor system. Analyst. 2019;144:611–21. https://doi.org/10.1039/C8AN01279B.
Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–18.
Zoey FLG, Palanivel M, Padmanabhan P, Gulyás B. Parkinson’s disease: a nanotheranostic approach targeting alpha-synuclein aggregation. Front Cell Dev Biol. 2021. https://doi.org/10.3389/fcell.2021.707441.
Wu Q, Tan R, Mi X, Tu Y. Electrochemiluminescent aptamer-sensor for alpha synuclein oligomer based on a metal–organic framework. Analyst. 2020;145:2159–67. https://doi.org/10.1039/D0AN00169D.
Giasson BI, Duda JE, Murray IVJ, Chen Q, Souza JM, Hurtig HI, et al. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–9. https://doi.org/10.1126/science.290.5493.985.
Yu Z, Xu X, Xiang Z, Zhou J, Zhang Z, Hu C, et al. Nitrated α-synuclein induces the loss of dopaminergic neurons in the substantia nigra of rats. PLoS ONE. 2010;5:e9956. https://doi.org/10.1371/journal.pone.0009956.
Kovacs GG, Botond G, Budka H. Protein coding of neurodegenerative dementias: The neuropathological basis of biomarker diagnostics. Acta Neuropathol. 2010;119:389–408.
Zhang ZH, Hu J, Chen Q, Chen J, Hu X, Koh K, et al. The magnetic-nanoparticle-assisted sensitive detection of nitrated α-syn in blood based on a sensitizing electrochemical layer. Nanoscale. 2021;13:8107–17.
Neely A, Perry C, Varisli B, Singh AK, Arbneshi T, Senapati D, et al. Ultrasensitive and highly selective detection of alzheimer’s disease biomarker using two-photon rayleigh scattering properties of gold nanoparticle. ACS Nano. 2009. https://doi.org/10.1021/nn900813b.
Kim H, Lee JU, Kim S, Song S, Sim SJ. A nanoplasmonic biosensor for ultrasensitive detection of Alzheimer’s disease biomarker using a chaotropic agent. ACS Sens. 2019;4:595–602.
Khatri A, Punjabi N, Ghosh D, Maji SK, Mukherji S. Detection and differentiation of α-Synuclein monomer and fibril by chitosan film coated nanogold array on optical sensor platform. Sens Actuators B Chem. 2018;255:692–700.
Kumar J, Eraña H, López-Martínez E, Claes N, Martín VF, Solís DM, et al. Detection of amyloid fibrils in Parkinson’s disease using plasmonic chirality. Proc Natl Acad Sci. 2018;115:3225–30.
You X, Gopinath SCB, Lakshmipriya T, Li D. High-affinity detection of alpha-synuclein by aptamer-gold conjugates on an amine-modified dielectric surface Buccolieri A, editor. J Anal Methods Chem. 2019;2019:6526850.
Yao T, Wang R, Meng Y, Hun X. Photoelectrochemical sensing of α-synuclein based on a AuNPs/graphdiyne-modified electrode coupled with a nanoprobe. ACS Appl Mater Interfaces. 2021;13:26515–21.
Hun X, Kong X. An enzyme linked aptamer photoelectrochemical biosensor for Tau-381 protein using AuNPs/MoSe2 as sensing material. J Pharm Biomed Anal. 2021;192: 113666.
Razzino CA, Serafín V, Gamella M, Pedrero M, Montero-Calle A, Barderas R, et al. An electrochemical immunosensor using gold nanoparticles-PAMAM-nanostructured screen-printed carbon electrodes for tau protein determination in plasma and brain tissues from Alzheimer patients. Biosens Bioelectron. 2020;163:112238.
Sonuç Karaboga MN, Sezgintürk MK. Analysis of Tau-441 protein in clinical samples using rGO/AuNP nanocomposite-supported disposable impedimetric neuro-biosensing platform: towards Alzheimer’s disease detection. Talanta. 2020;219:121257.
Stegurová L, Dráberová E, Bartos A, Dráber P, Řípová D, Dráber P. Gold nanoparticle-based immuno-PCR for detection of tau protein in cerebrospinal fluid. J Immunol Methods. 2014;406:137–42.
Yang SJ, Lee JU, Jeon MJ, Sim SJ. Highly sensitive surface-enhanced Raman scattering-based immunosensor incorporating half antibody-fragment for quantitative detection of Alzheimer’s disease biomarker in blood. Anal Chim Acta. 2022;1195: 339445.
Zhang X, Liu S, Song X, Wang H, Wang J, Wang Y, et al. Robust and universal sers sensing platform for multiplexed detection of Alzheimer’s disease core biomarkers using PAapt-AuNPs conjugates. ACS Sens. 2019;4:2140–9.
Serafín V, Razzino CA, Gamella M, Pedrero M, Povedano E, Montero-Calle A, et al. Disposable immunoplatforms for the simultaneous determination of biomarkers for neurodegenerative disorders using poly(amidoamine) dendrimer/gold nanoparticle nanocomposite. Anal Bioanal Chem. 2021;413:799–811.
Kim H, Lee JU, Song S, Kim S, Sim SJ. A shape-code nanoplasmonic biosensor for multiplex detection of Alzheimer’s disease biomarkers. Biosens Bioelectron. 2018;101:96–102.
Vestergaard M, Kerman K, Kim DK, Hiep HM, Tamiya E. Detection of Alzheimer’s tau protein using localised surface plasmon resonance-based immunochip. Talanta. 2008;74(4):1038–42.
Tao D, Shui B, Gu Y, Cheng J, Zhang W, Jaffrezic-Renault N, et al. Development of a label-free electrochemical aptasensor for the detection of Tau381 and its preliminary application in AD and non-AD patients’ sera. Biosensors (Basel). 2019;9:84.
Zengin A, Tamer U, Caykara T. A SERS-based sandwich assay for ultrasensitive and selective detection of Alzheimer’s tau protein. Biomacromol. 2013;14:3001–9.
Sinha SS, Jones S, Pramanik A, Ray PC. Nanoarchitecture based SERS for biomolecular fingerprinting and label-free disease markers diagnosis. Acc Chem Res. 2016;49:2725–35.
Maurer V, Frank C, Porsiel JC, Zellmer S, Garnweitner G, Stosch R. Step-by-step monitoring of a magnetic and SERS-active immunosensor assembly for purification and detection of tau protein. J Biophotonics. 2020;13:1–10.
Shui B, Tao D, Cheng J, Mei Y, Jaffrezic-Renault N, Guo Z. A novel electrochemical aptamer-antibody sandwich assay for the detection of tau-381 in human serum. Analyst. 2018;143:3549–54.
Li X, Jiang M, Cheng J, Ye M, Zhang W, Jaffrezic-Renault N, et al. Signal multi-amplified electrochemical biosensor for voltammetric determination of tau-441 protein in biological samples using carbon nanomaterials and gold nanoparticles to hint dementia. Microchim Acta. 2020;187:1–8.
Lu X, Hou X, Tang H, Yi X, Wang J. A high-quality CdSe/CdS/ZnS quantum-dot-based FRET aptasensor for the simultaneous detection of two different alzheimer’s disease core biomarkers. Nanomaterials. 2022;12:4031.
Zhang L, Cao K, Su Y, Hu S, Liang X, Luo Q, et al. Colorimetric and surface-enhanced Raman scattering dual-mode magnetic immunosensor for ultrasensitive detection of blood phosphorylated tau in Alzheimer’s disease. Biosens Bioelectron. 2023;222: 114935.
Liu L, Li M, Xu M, Wang Z, Zeng Z, Li Y, et al. Actively targeted gold nanoparticle composites improve behavior and cognitive impairment in Parkinson’s disease mice. Mater Sci Eng C. 2020;114:111028.
Iulita MF, Bistué Millón MB, Pentz R, Aguilar LF, Do Carmo S, Allard S, et al. Differential deregulation of NGF and BDNF neurotrophins in a transgenic rat model of Alzheimer’s disease. Neurobiol Dis. 2017;108:307–23.
Hu K, Chen X, Chen W, Zhang L, Li J, Ye J, et al. Neuroprotective effect of gold nanoparticles composites in Parkinson’s disease model. Nanomedicine. 2018;14:1123–36.
Gao G, Chen R, He M, Li J, Wang L, Sun T. Gold nanoclusters for Parkinson’s disease treatment. Biomaterials. 2019;194:36–46.
Sonawane SK, Ahmad A, Chinnathambi S. Protein-capped metal nanoparticles inhibit tau aggregation in Alzheimer’s disease. ACS Omega. 2019;4:12833–40.
Vimal SK, Zuo H, Wang Z, Wang H, Long Z, Bhattacharyya S. Self-therapeutic nanoparticle that alters tau protein and ameliorates tauopathy toward a functional nanomedicine to tackle Alzheimer’s. Small. 2020;16(16):1906861.
Bhattacharyya S, Kim K, Teizer W. Remodeling tau and prion proteins using nanochaperons. Adv Biosyst. 2017;1:1700108.
Javed I, Peng G, Xing Y, Yu T, Zhao M, Kakinen A, et al. Inhibition of amyloid beta toxicity in zebrafish with a chaperone-gold nanoparticle dual strategy. Nat Commun. 2019;10:3780.
Martins PAT, Alsaiari S, Julfakyan K, Nie Z, Khashab NM. Self-assembled lipoprotein based gold nanoparticles for detection and photothermal disaggregation of β-amyloid aggregates. Chem Commun. 2017;53(13):2102–5.
Wiesehan K, Willbold D. Mirror-image phage display: aiming at the mirror. ChemBioChem. 2003;4:811–5.
Wiesehan K, Buder K, Linke RP, Patt S, Stoldt M, Unger E, et al. Selection of D-amino-acid peptides that bind to Alzheimer’s disease amyloid peptide Aβ1–42 by mirror image phage display. ChemBioChem. 2003;4:748–53.
Wiesehan K, Stöhr J, Nagel-Steger L, Van Groen T, Riesner D, Willbold D. Inhibition of cytotoxicity and amyloid fibril formation by a D-amino acid peptide that specifically binds to Alzheimer’s disease amyloid peptide. Protein Eng Design Select. 2008;21:241–6.
Van Groen T, Kadish I, Wiesehan K, Funke SA, Willbold D. In vitro and in vivo staining characteristics of small, fluorescent, Aβ42-binding D-enantiomeric peptides in transgenic AD mouse models. ChemMedChem. 2009;4:276–82.
Van Groen T, Wiesehan K, Funke SA, Kadish I, Nagel-Steger L, Willbold D. Reduction of Alzheimer’s disease amyloid plaque load in transgenic mice by D3, a D-enantiomeric peptide identified by mirror image phage display. ChemMedChem. 2008;3:1848–52.
Liu H, Funke SA, Willbold D. Transport of alzheimer disease amyloid-β-binding d-amino acid peptides across an in vitro blood-brain barrier model. Rejuvenation Res. 2010;13:210–3.
Van Groen T, Kadish I, Funke A, Bartnik D, Willbold D. Treatment with Aβ42 binding d-amino acid peptides reduce amyloid deposition and inflammation in APP/PS1 double transgenic mice. Adv Protein Chem Struct Biol. 2012;88:133–52.
Van Groen T, Kadish I, Funke SA, Bartnik D, Willbold D. Treatment with D3 removes amyloid deposits, reduces inflammation, and improves cognition in aged AβPP/PS1 double transgenic mice. JAD. 2013;34:609–20.
Jiang N, Leithold LHE, Post J, Ziehm T, Mauler J, Gremer L, et al. Preclinical pharmacokinetic studies of the tritium labelled D-enantiomeric peptide D3 developed for the treatment of Alzheimer’s disease. PLoS ONE. 2015;10:e0128553.
Bartnik D, Funke SA, Andrei-Selmer LC, Bacher M, Dodel R, Willbold D. Differently selected d-enantiomeric peptides Act on different aβ species. Rejuvenation Res. 2010;13:202–5.
Aileen Funke S, Van Groen T, Kadish I, Bartnik D, Nagel-Steger L, Brener O, et al. Oral treatment with the d-enantiomeric peptide D3 improves the pathology and behavior of alzheimer’s disease transgenic mice. ACS Chem Neurosci. 2010;1:639–48.
Morales-Zavala F, Jara-Guajardo P, Chamorro D, Riveros AL, Chandia-Cristi A, Salgado N, et al. In vivo micro computed tomography detection and decrease in amyloid load by using multifunctionalized gold nanorods: a neurotheranostic platform for Alzheimer’s disease. Biomater Sci. 2021;9:4178–90.
Altendorf T, Gering I, Santiago-Schübel B, Aghabashlou Saisan S, Tamgüney G, Tusche M, et al. Stabilization of monomeric tau protein by All D-enantiomeric peptide ligands as therapeutic strategy for alzheimer’s disease and other tauopathies. Int J Mol Sci. 2023;24(3):2161.
Aillaud I, Funke SA. Tau aggregation inhibiting peptides as potential therapeutics for Alzheimer disease. Cell Mol Neurobiol. 2023;43(3):951–61.
Aillaud I, Kaniyappan S, Chandupatla RR, Ramirez LM, Alkhashrom S, Eichler J, et al. A novel D-amino acid peptide with therapeutic potential (ISAD1) inhibits aggregation of neurotoxic disease-relevant mutant Tau and prevents Tau toxicity in vitro. Alzheimers Res Ther. 2022;14(1):15.
Dammers C, Yolcu D, Kukuk L, Willbold D, Pickhardt M, Mandelkow E, et al. Selection and characterization of tau binding D-enantiomeric peptides with potential for therapy of Alzheimer disease. PLoS ONE. 2016;11:e0167432.
Malhis M, Kaniyappan S, Aillaud I, Chandupatla RR, Ramirez LM, Zweckstetter M, et al. Potent tau aggregation inhibitor D-peptides selected against tau-repeat 2 using mirror image phage display. ChemBioChem. 2021;22(21):3049–59.
Shaltiel-Karyo R, Frenkel-Pinter M, Egoz-Matia N, Frydman-Marom A, Shalev DE, Segal D, et al. Inhibiting α-synuclein oligomerization by stable cell-penetrating β-synuclein fragments recovers phenotype of parkinson’s disease model flies. PLoS ONE. 2010;5(11):e13863.
Chemerovski-Glikman M, Rozentur-Shkop E, Richman M, Grupi A, Getler A, Cohen HY, et al. Self-assembled cyclic d, l-α-peptides as generic conformational inhibitors of the α-synuclein aggregation and toxicity: in vitro and mechanistic studies. Chem A Eur J. 2016. https://doi.org/10.1002/chem.201601830.
Horsley JR, Jovcevski B, Pukala TL, Abell AD. Designer D-peptides targeting the N-terminal region of α-synuclein to prevent parkinsonian-associated fibrilization and cytotoxicity. Biochim Biophys Acta Proteins Proteom. 2022;1870:140826.
Ghalandari B, Asadollahi K, Shakerizadeh A, Komeili A, Riazi G, Kamrava SK, et al. Microtubule network as a potential candidate for targeting by gold nanoparticle-assisted photothermal therapy. J Photochem Photobiol B. 2019. https://doi.org/10.1016/j.jphotobiol.2019.01.012.
Goto T, Kasai N, Filip R, Sumitomo K, Nakashima H. Observation of intracellular protein localization area in a single neuron using gold nanoparticles with a scanning electron microscope. Micron. 2019;126:102740.
Cheng CH, Lin KJ, Hong CT, Wu D, Chang HM, Liu CH, et al. Plasmon-activated water reduces amyloid burden and improves memory in animals with Alzheimer’s disease. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-49731-8.
Yao L, Bojic D, Liu M. Applications and safety of gold nanoparticles as therapeutic devices in clinical trials. J Pharm Anal. 2023.
Zhang R, Kiessling F, Lammers T, Pallares RM. Clinical translation of gold nanoparticles. Drug Deliv Transl Res. 2023;13(2):378–85.
Sung D, Sanchez A, Tward JD. Successful salvage brachytherapy after infusion of gold auroshell nanoshells for localized prostate cancer in a human patient. Adv Radiat Oncol. 2023;8(4):101202.
Balfourier A, Kolosnjaj-Tabi J, Luciani N, Carn F, Gazeau F, Murphy CJ. Gold-based therapy: from past to present. Proc Natl Acad Sci U S A. 2020;117(37):22639–48.
Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med. 2013;5(209):209ra152.
Kumthekar P, Ko CH, Paunesku T, Dixit K, Sonabend AM, Bloch O, et al. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci Transl Med. 2021. https://doi.org/10.1126/scitranslmed.abb3945.
Duan L, Li X, Ji R, Hao Z, Kong M, Wen X, et al. Nanoparticle-based drug delivery systems: an inspiring therapeutic strategy for neurodegenerative diseases. Polymers (Basel). 2023. https://doi.org/10.3390/polym15092196.
Olaru DG, Olaru A, Kassem GH, Popescu-Drigă MV, Pinoşanu LR, Dumitraşcu DI, et al. Toxicity and health impact of nanoparticles. Basic biology and clinical perspective. Roman J Morphol Embryol. 2019;60:787–92.
Su S, Kang PM. Systemic review of biodegradable nanomaterials in nanomedicine. Nanomaterials. 2020;10:656.
P. Rajput A, Kulkarni M, Pingale PL, Tekade M, Shakya AK, Tekade RK. Understanding the bioaccumulation of pharmaceuticals and personal care products. Essentials of Pharmatoxicology in Drug Research: Toxicity and Toxicodynamics: Volume 1. 2023. https://doi.org/10.1016/B978-0-443-15840-7.00024-5
Jaiswal S, Manhas A, Pandey AK, Priya S, Sharma SK. Engineered nanoparticle-protein interactions influence protein structural integrity and biological significance. Nanomaterials. 2022;12(7):1214.
Kim Y, Park JH, Lee H, Nam JM. How do the size, charge and shape of nanoparticles affect amyloid β aggregation on brain lipid bilayer? Sci Rep. 2016;6:19548.
Sanati M, Khodagholi F, Aminyavari S, Ghasemi F, Gholami M, Kebriaeezadeh A, et al. Impact of gold nanoparticles on amyloid β-induced alzheimer’s disease in a rat animal model: involvement of STIM proteins. ACS Chem Neurosci. 2019;10:2299–309.
Tapia-Arellano A, Gallardo-Toledo E, Celis F, Rivera R, Moglia I, Campos M, et al. The curvature of gold nanoparticles influences the exposure of amyloid-β and modulates its aggregation process. Mater Sci Eng C. 2021;128: 112269.
Brancolini G, Toroz D, Corni S. Can small hydrophobic gold nanoparticles inhibit β2- microglobulin fibrillation? Nanoscale. 2014;6(14):7903–11.
Cantarutti C, Raimondi S, Brancolini G, Corazza A, Giorgetti S, Ballico M, et al. Citrate-stabilized gold nanoparticles hinder fibrillogenesis of a pathological variant of β2-microglobulin. Nanoscale. 2017;9(11):3941–51.
Cantarutti C, Raj G, Fogolari F, Giorgetti S, Corazza A, Bellotti V, et al. Interference of citrate-stabilized gold nanoparticles with β2-microglobulin oligomeric association. Chem Commun. 2018;54(43):5422–5.
Arosio P, Vendruscolo M, Dobson CM, Knowles TP. Chemical kinetics for drug discovery to combat protein aggregation diseases. Trends Pharmacol Sci. 2014;35:127–35.
Arosio P, Knowles TP, Linse S. On the lag phase in amyloid fibril formation. Phys Chem Chem Phys. 2015;17:7606–18.
Arosio P, Michaels TC, Linse S, Månsson C, Emanuelsson C, Presto J, et al. Kinetic analysis reveals the diversity of microscopic mechanisms through which molecular chaperones suppress amyloid formation. Nat Commun. 2016;7:10948.
Álvarez YD, Fauerbach JA, Pellegrotti JV, Jovin TM, Jares-Erijman EA, Stefani FD. Influence of gold nanoparticles on the kinetics of α-synuclein aggregation. Nano Lett. 2013;13:6156–63.
Bera K, Mondal A, Pal U, Maiti NC. Porphyrin-armored gold nanospheres modulate the secondary structure of α-synuclein and arrest its fibrillation. J Phys Chem C. 2020;124:6418–34.
Maity A, Mondal A, Kundu S, Shome G, Misra R, Singh A, et al. Naringenin-functionalized gold nanoparticles and their role in α-synuclein stabilization. Langmuir. 2023;39:7231–48. https://doi.org/10.1021/acs.langmuir.2c03259.
Gharb M, Nouralishahi A, Riazi A, Riazi G. Inhibition of tau protein aggregation by a chaperone-like β-boswellic acid conjugated to gold nanoparticles. ACS Omega. 2022;7:30347–58.
Yang G, Phua SZF, Bindra AK, Zhao Y. Degradability and clearance of inorganic nanoparticles for biomedical applications. Adv Mater. 2019;31(10):1805730.
van de Looij SM, Hebels ER, Viola M, Hembury M, Oliveira S, Vermonden T. Gold nanoclusters: imaging, therapy, and theranostic roles in biomedical applications. Bioconjug Chem. 2022;33(1):4–23.
Mahapatra A, Sarkar S, Biswas SC, Chattopadhyay K. Modulation of α-synuclein fibrillation by ultrasmall and biocompatible gold nanoclusters. ACS Chem Neurosci. 2020;11(20):3442–54.
Sivanesan S, Rajeshkumar S. Gold nanoparticles in diagnosis and treatment of Alzheimer’s disease. Nanobiotechnol Neurodegenerative Dis. 2019.
Yang JA, Johnson BJ, Wu S, Woods WS, George JM, Murphy CJ. Study of wild-type α-synuclein binding and orientation on gold nanoparticles. Langmuir. 2013;29:4603–15.
Yang JA, Lin W, Woods WS, George JM, Murphy CJ. α-Synuclein’s adsorption, conformation, and orientation on cationic gold nanoparticle surfaces seeds global conformation change. J Phys Chem B. 2014;118:3559–71.
Lin W, Insley T, Tuttle MD, Zhu L, Berthold DA, Král P, et al. Control of protein orientation on gold nanoparticles. J Phys Chem C. 2015;119:21035–43.
Vinod C, Jena S. Nano-neurotheranostics: impact of nanoparticles on neural dysfunctions and strategies to reduce toxicity for improved efficacy. Front Pharmacol. 2021;12:612692.
Báez DF, Gallardo-Toledo E, Oyarzún MP, Araya E, Kogan MJ. The influence of size and chemical composition of silver and gold nanoparticles on in vivo toxicity with potential applications to central nervous system diseases. Int J Nanomedicine. 2021;16:2187–201.
Sun H, Jia J, Jiang C, Zhai S. Gold nanoparticle-induced cell death and potential applications in nanomedicine. Int J Mol Sci. 2018;19(3):754.
Bencsik A, Lestaevel P, Guseva CI. Nano- and neurotoxicology: an emerging discipline. Prog Neurobiol. 2018;160:45–63.
Teleanu DM, Chircov C, Grumezescu AM, Volceanov A, Teleanu RI. Impact of nanoparticles on brain health: an up to date overview. J Clin Med. 2018;7:490.
Cardoso E, Rezin GT, Zanoni ET, de Souza NF, Leffa DD, Damiani AP, et al. Acute and chronic administration of gold nanoparticles cause DNA damage in the cerebral cortex of adult rats. Mutat Res. 2014;766:25–30.
Lasagna-Reeves C, Gonzalez-Romero D, Barria MA, Olmedo I, Clos A, Sadagopa Ramanujam VM, et al. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem Biophys Res Commun. 2010;393:649–55.
Chen YS, Hung YC, Lin LW, Liau I, Hong MY, Huang GS. Size-dependent impairment of cognition in mice caused by the injection of gold nanoparticles. Nanotechnology. 2010;21:485102.
Noor NA, Fahmy HM, Mourad IM. Evaluation of the potential neurotoxicity of gold nanoparticles in the different rat brain regions. IJSBAR. 2016;30:114–29.
Siddiqi NJ, Abdelhalim MAK, El-Ansary AK, Alhomida AS, Ong WY. Identification of potential biomarkers of gold nanoparticle toxicity in rat brains. J Neuroinflammation. 2012. https://doi.org/10.1186/1742-2094-9-123.
Lee U, Yoo CJ, Kim YJ, Yoo YM. Cytotoxicity of gold nanoparticles in human neural precursor cells and rat cerebral cortex. J Biosci Bioeng. 2016. https://doi.org/10.1016/j.jbiosc.2015.07.004.
Yoo C-J, Lee U, Kim Y-J, Park J, Yoo Y-M. Dose-dependent cytotoxicity of gold nanoparticles on human neural progenitor cells and rat brain. J Nanosci Nanotechnol. 2019. https://doi.org/10.1166/jnn.2019.16547.
Pannerselvam B, Devanathadesikan V, Alagumuthu TS, Kanth SV, Thangavelu KP. Assessment of in-vivo biocompatibility evaluation of phytogenic gold nanoparticles on Wistar albino male rats. IET Nanobiotechnol. 2020. https://doi.org/10.1049/iet-nbt.2019.0116.
Jung S, Bang M, Kim BS, Lee S, Kotov NA, Kim B, et al. Intracellular gold nanoparticles increase neuronal excitability and aggravate seizure activity in the mouse brain. PLoS ONE. 2014;9:e91360.
Salinas K, Kereselidze Z, DeLuna F, Peralta XG, Santamaria F. Transient extracellular application of gold nanostars increases hippocampal neuronal activity. J Nanobiotechnology. 2014;12:1–7.
Stojiljković A, Kuehni-Boghenbor K, Gaschen V, Schüpbach G, Mevissen M, Kinnear C, et al. High-content analysis of factors affecting gold nanoparticle uptake by neuronal and microglial cells in culture. Nanoscale. 2016;8:16650–61.
Xiao L, Wei F, Zhou Y, Anderson GJ, Frazer DM, Lim YC, et al. Dihydrolipoic acid-gold nanoclusters regulate microglial polarization and have the potential to alter neurogenesis. Nano Lett. 2020;20(1):478–95.
Yuan Q, Yao Y, Zhang X, Yuan J, Sun B, Gao X. The gold nanocluster protects neurons directly or via inhibiting cytotoxic secretions of microglia cell. J Nanosci Nanotechnol. 2018;19(4):1986–95.
Leite PEC, Pereira MR, Harris G, Pamies D, Dos Santos LMG, Granjeiro JM, et al. Suitability of 3D human brain spheroid models to distinguish toxic effects of gold and poly-lactic acid nanoparticles to assess biocompatibility for brain drug delivery. Part Fibre Toxicol. 2019;16:1–20.
Imperatore R, Carotenuto G, Di Grazia MA, Ferrandino I, Palomba L, Mariotti R, et al. Imidazole-stabilized gold nanoparticles induce neuronal apoptosis: an in vitro and in vivo study. J Biomed Mater Res A. 2015;103(4):1436–46.
Manson J, Kumar D, Meenan BJ, Dixon D. Polyethylene glycol functionalized gold nanoparticles: the influence of capping density on stability in various media. Gold Bull. 2011;44:99–105. https://doi.org/10.1007/s13404-011-0015-8.
Zhang X, Guo X, Kang X, Yang H, Guo W, Guan L, et al. Surface functionalization of pegylated gold nanoparticles with antioxidants suppresses nanoparticle-induced oxidative stress and neurotoxicity. Chem Res Toxicol. 2020;33(5):1195–205.
Pereira MC, Adewale OB, Roux S, Cairncross L, Davids H. Biochemical assessment of the neurotoxicity of gold nanoparticles functionalized with colorectal cancer-targeting peptides in a rat model. Hum Exp Toxicol. 2021;40(11):1962–73.
Ji J, Moquin A, Bertorelle F, Chang P, Antoine R, Luo J, et al. Organotypic and primary neural cultures as models to assess effects of different gold nanostructures on glia and neurons. Nanotoxicology. 2019;13(3):285–304.
Tuna BG, Yesilay G, Yavuz Y, Yilmaz B, Culha M, Maharramov A, et al. Electrophysiological effects of polyethylene glycol modified gold nanoparticles on mouse hippocampal neurons. Heliyon. 2020;6:e05824.
Xue J, Liu T, Liu Y, Jiang Y, Seshadri VDD, Mohan SK, et al. Neuroprotective effect of biosynthesised gold nanoparticles synthesised from root extract of Paeonia moutan against Parkinson disease—in vitro & in vivo model. J Photochem Photobiol B. 2019;200:111635.
Sancho L, Contreras M, Allen NJ. Glia as sculptors of synaptic plasticity. Neurosci Res. 2021;167:17–29.
Allen NJ, Lyons DA. Glia as architects of central nervous system formation and function. Science. 2018;362(6411):181–5.
Mytych J, Lewinska A, Zebrowski J, Wnuk M. Gold nanoparticles promote oxidant-mediated activation of NF-κB and 53BP1 recruitment-based adaptive response in human astrocytes. Biomed Res Int. 2015. https://doi.org/10.1155/2015/304575.
Kofuji P, Araque A. Astrocytes and behavior. Annu Rev Neurosci. 2021;44:49–67.
Park SY, Yi EH, Kim Y, Park G. Anti-neuroinflammatory effects of ephedra sinica stapf extract-capped gold nanoparticles in microglia. Int J Nanomedicine. 2019;14:2861–77.
Hutter E, Boridy S, Labrecque S, Lalancette-Hébert M, Kriz J, Winnik FM, et al. Microglial response to gold nanoparticles. ACS Nano. 2010;4:2595–606.
Lira-Diaz E, Gonzalez-Pedroza MG, Vasquez C, Morales-Luckie RA, Gonzalez-Perez O. Gold nanoparticles produce transient reactive gliosis in the adult brain. Neurosci Res. 2021;170:76–86.
Gran ER, Bertorelle F, Fakhouri H, Antoine R, Perić Bakulić M, Sanader Maršić Ž, et al. Size and ligand effects of gold nanoclusters in alteration of organellar state and translocation of transcription factors in human primary astrocytes. Nanoscale. 2021;13:3173–83.
Maysinger D, Sanader Maršić Ž, Gran ER, Shobo A, MacAiran JR, Zhang I, et al. Insights into the impact of gold nanoclusters Au10SG10on human microglia. ACS Chem Neurosci. 2022;13:464–76.
Sobska J, Waszkielewicz M, Podleśny-Drabiniok A, Olesiak-Banska J, Krężel W, Matczyszyn K. Gold nanoclusters display low immunogenic effect in microglia cells. Nanomaterials. 2021;11:1066.
Weiss ACG, Kempe K, Förster S, Caruso F. Microfluidic examination of the “hard” biomolecular corona formed on engineered particles in different biological milieu. Biomacromol. 2018;19:2580–94. https://doi.org/10.1021/acs.biomac.8b00196.
Palchetti S, Pozzi D, Capriotti AL, BarberaLa G, Chiozzi RZ, Digiacomo L, et al. Influence of dynamic flow environment on nanoparticle-protein corona: from protein patterns to uptake in cancer cells. Colloids Surf B Biointerfaces. 2017;153:263–71.
Braun NJ, DeBrosse MC, Hussain SM, Comfort KK. Modification of the protein corona–nanoparticle complex by physiological factors. Mater Sci Eng C. 2016;64:34–42.
Palchetti S, Colapicchioni V, Digiacomo L, Caracciolo G, Pozzi D, Capriotti AL, et al. The protein corona of circulating PEGylated liposomes. Biochimica et Biophysica Acta (BBA) Biomembranes. 2016;1858:189–96.
Palma-Florez S, López-Canosa A, Moralez-Zavala F, Castaño O, Kogan MJ, Samitier J, et al. BBB-on-a-chip with integrated micro-TEER for permeability evaluation of multi-functionalized gold nanorods against Alzheimer’s disease. J Nanobiotechnology. 2023;21:115.
Fan Y, Xu C, Deng N, Gao Z, Jiang Z, Li X, et al. Understanding drug nanocarrier and blood-brain barrier interaction based on a microfluidic microphysiological model. Lab Chip. 2023;23:1935–44.
Ziółkowska K, Kwapiszewski R, Brzózka Z. Microfluidic devices as tools for mimicking the in vivo environment. New J Chem. 2011;35(5):979–90.
Valencia PM, Farokhzad OC, Karnik R, Langer R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat Nanotechnol. 2012;7:93–112.
Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–73.
Shepherd SJ, Issadore D, Mitchell MJ. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials. 2021;274: 120826.
Alépée N, Bahinski A, Daneshian M, De Wever B, Fritsche E, Goldberg A, et al. State-of-the-art of 3D cultures (organs-on-a-chip) in safety testing and pathophysiology. Altex. 2014. https://doi.org/10.14573/altex.1406111.
Prasanna P, Rathee S, Rahul V, Mandal D, Goud MSC, Yadav P, et al. Microfluidic platforms to unravel mysteries of Alzheimer’s disease: How far have we come? Life. 2021;11:1022.
Pamies D, Hartung T, Hogberg HT. Biological and medical applications of a brain-on-a-chip. Exp Biol Med. 2014;239:1096–107.
Hartung T. 3D—a new dimension of in vitro research. Adv Drug Deliv Rev. 2014;69:vi.
Sokolova V, Nzou G, van der Meer SB, Ruks T, Heggen M, Loza K, et al. Ultrasmall gold nanoparticles (2 nm) can penetrate and enter cell nuclei in an in vitro 3D brain spheroid model. Acta Biomater. 2020;111:349–62.
Simpson LW, Good TA, Leach JB. Protein folding and assembly in confined environments: Implications for protein aggregation in hydrogels and tissues. Biotechnol Adv. 2020;42:107573.
Ellis RJ. Protein folding: importance of the anfinsen cage. Curr Biol. 2003. https://doi.org/10.1016/j.cub.2003.10.051.
Javidpour L, Sahimi M. Confinement in nanopores can destabilize α-helix folding proteins and stabilize the β structures. J Chem Phys. 2011;135:125101.
Gospodarczyk W, Kozak M. Microchip circulation drastically accelerates amyloid aggregation of 1–42 β-amyloid peptide from felis catus. ACS Chem Neurosci. 2017;8:2558–67.
Park J, Lee BK, Jeong GS, Hyun JK, Lee CJ, Lee SH. Three-dimensional brain-on-a-chip with an interstitial level of flow and its application as an in vitro model of Alzheimer’s disease. Lab Chip. 2015;15:141–50.
Gospodarczyk W, Kozak M. The severe impact of in vivo-like microfluidic flow and the influence of gemini surfactants on amyloid aggregation of hen egg white lysozyme. RSC Adv. 2017;7:10973–84.
Foderá V, Pagliara S, Otto O, Keyser UF, Donald AM. Microfluidics reveals a flow-induced large-scale polymorphism of protein aggregates. J Phys Chem Lett. 2012;3:2803–7.
Lee JS, Um E, Park JK, Park CB. Microfluidic self-assembly of insulin monomers into amyloid fibrils on a solid surface. Langmuir. 2008;24:7068–71.
Shammas SL, Garcia GA, Kumar S, Kjaergaard M, Horrocks MH, Shivji N, et al. A mechanistic model of tau amyloid aggregation based on direct observation of oligomers. Nat Commun. 2015. https://doi.org/10.1038/ncomms8025.
Horrocks MH, Tosatto L, Dear AJ, Garcia GA, Iljina M, Cremades N, et al. Fast flow microfluidics and single-molecule fluorescence for the rapid characterization of α-synuclein oligomers. Anal Chem. 2015. https://doi.org/10.1021/acs.analchem.5b01811.
Acknowledgements
The authors would like to acknowledge The National Research and Development Agency of Chile (ANID), depending on the Ministry of Science, Technology, Knowledge, and Innovation.
Funding
This work was funded by Fondecyt Postdoctoral Fellowship 3220583, ANID doctoral scholarships 202110617 and 21200403, Millenium Nucleus in NanobioPhysics (N2BP) N°NCN2021_021, Fondecyt 1230830 and 1211482, and Fondap 15130011.
Author information
Authors and Affiliations
Contributions
ATA, NH and MJK conceived the topic and drew up the outline. ATA, PC, ECA, AR and NH wrote and revised the original draft. ATA and MJK reviewed and edited the draft. ATA, NH and MJK provided funding for the project. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors gave their consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tapia-Arellano, A., Cabrera, P., Cortés-Adasme, E. et al. Tau- and α-synuclein-targeted gold nanoparticles: applications, opportunities, and future outlooks in the diagnosis and therapy of neurodegenerative diseases. J Nanobiotechnol 22, 248 (2024). https://doi.org/10.1186/s12951-024-02526-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-024-02526-0