Abstract
Small extracellular vesicles (SEVs) are extracellular vesicles containing DNA, RNA, and proteins and are involved in intercellular communication and function, playing an essential role in the growth and metastasis of tumors. SEVs are present in various body fluids and can be isolated and extracted from blood, urine, and cerebrospinal fluid. Under both physiological and pathological conditions, SEVs can be released by some cells, such as immune, stem, and tumor cells, in a cytosolic manner. SEVs secreted by tumor cells are called tumor-derived exosomes (TEXs) because of their origin in the corresponding parent cells. Glioma is the most common intracranial tumor, accounting for approximately half of the primary intracranial tumors, and is characterized by insidious onset, high morbidity, and high mortality rate. Complete removal of tumor tissues by surgery is difficult. Chemotherapy can improve the survival quality of patients to a certain extent; however, gliomas are prone to chemoresistance, which seriously affects the prognosis of patients. In recent years, TEXs have played a vital role in the occurrence, development, associated immune response, chemotherapy resistance, radiation therapy resistance, and metastasis of glioma. This article reviews the role of TEXs in glioma progression, drug resistance, and clinical diagnosis.
Similar content being viewed by others
Introduction
Glioma is one of the most common primary malignant tumors in the adult central nervous system, accounting for approximately 70% of all primary intracranial tumors. More than half of them are the most malignant-glioblastoma multiforme (GBM) [1]. Although surgical treatment, radiotherapy, chemotherapy, and gene and immunotherapy have been significantly developed for gliomas in recent years, satisfactory results have not been achieved in clinical practice. Gliomas still have low cure rates, high recurrence rates, and poor prognosis [2,3,4]. The immunosuppressive tumor microenvironment that develops during glioma and infiltrative growth of glioma are the main reasons for the poor outcome of glioma treatment [5,6,7,8].
Small extracellular vesicles (SEVs) are a class of secretory vesicles with a single membrane structure in the form of cups or spheres, with a particle size of approximately 30–150 nm. They are rich in proteins, lipids, nucleic acids, and other biological information materials, and they can serve as a carrier of intercellular information transfer [9]. SEVs are present in almost all body fluids, such as blood and sweat, and almost all body cells can secrete SEVs [10, 11]. SEVs can be involved in various biological processes in a living organism, help in information transfer between cells, and play a vital role in the cellular microenvironment [12,13,14,15]. In the tumor microenvironment (TME), SEVs have an essential information transfer function, enabling the transmission of information between tumor cells. SEVs can effectively carry and deliver molecular signals to recipient cells, promoting various biological processes such as tumor growth, metastasis, invasion, tumor angiogenesis, tumor innervation, and chemotherapeutic drug resistance [16,17,18,19,20,21,22]. SEVs have been found in many types of cells, and tumor cell-derived exosomes (TEXs) are becoming a hot topic in oncology research due to their properties and functional characteristics [23,24,25]. TEXs can release their cytokines to promote tumorigenesis, development, proliferation, and migration and make tumors resistant to drugs, adversely affecting tumours’ treatment [26,27,28].
The TME is a local homeostatic environment composed of tumor cells, macrophages, fibroblasts, and extracellular matrix (ECM), which plays a significant role in cancer initiation, metastasis, recurrence, and chemotherapy resistance [29,30,31]. This review focuses on the biological properties of SEVs and the role and molecular mechanisms of TEXs in glioma development and progression and intends to provide novel insights into the developments in the clinical diagnosis and treatment of glioma.
Biogenesis, contents, and main biological function of SEVs
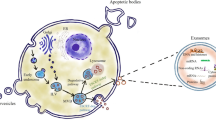
SEVs are regarded as a class of extracellular vesicles (EVs) formed by endocytosis at the nanometer scale (30–130 nm in diameter), which are formed in the endosomal network and released to fuse with the plasma membrane [32,33,34]. SEVs and micro-vesicles (MVs) are both EVs, but their origins are different. MVs are generally 100–1000 nm in diameter and are usually membranous vesicles formed by the shedding of cytoplasm from the cell membrane [35, 36]. In contrast, SEVs originate from the endosome, also known as the multivesicular endosome (MVE) or multivesicular body (MVB) [37, 38]. In the maturation process, the intraluminal vesicles (ILVs) are released from the membrane and fused with the cell membrane to form the ILV, the exosome [39,40,41]. Upon arrival at the recipient cell, SEVs release their contents in the specific cell by binding to receptors, endocytosis, and fusion with the plasma membrane, thereby altering the cell’s physiological status and biological function [36, 42, 43] (Fig. 1).
The main process of exosome biogenesis and release. Most cells in human body can release double-layer membrane-bound nanovesicles into the extracellular space. These membrane-derived vesicles can be divided into three types according to their size: small extracellular vesicles, microvesicles, and apoptotic bodies. Small extracellular vesicles originate from intracellular multivesicular bodies. Multivesicular bodies are vesicles with endocytosis. After fusion with the plasma membrane, a part is degraded by lysosomes, and the other part is released outside the cell to form small extracellular vesicles. Three main ways of information transmission exist between small extracellular vesicles and target cells. Exosome membrane proteins interact with target cell membrane proteins to activate intracellular signaling pathways. Small extracellular vesicles can transfer their own genetic material. The exosome membrane can be directly fused with the target cell membrane, and the genetic information carried in the exosome can be directly transferred to the recipient cell
SEVs primarily comprise nucleic acids, proteins, and lipids (Fig. 2). All SEVs commonly have proteins such as CD9, CD63, CD81, and CD82, which may serve as biomarkers and may also be related to the biological origin of SEVs [44, 45]. SEVs of different cellular origins express specific proteins, such as tumor susceptibility gene 101 protein (TSG101), ALG⁃2 interaction protein X (ALix), and heat shock protein 70 (HSP70), which are correlated to specific cellular functions [46, 47]. Additionally, SEVs contain a large number of nucleic acids, such as messenger RNA (mRNA), microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) [48,49,50]. These nucleic acids can fuse with target cells and act on the recipient cells to modulate gene expression and various signaling pathways in the recipient cells [51, 52]. SEVs contain lipids such as cholesterol, diglycerides, and phospholipids [53,54,55,56]. They are involved in the formation and maintenance of exosome morphology and serve as signaling molecules in the intercellular message communication process [57, 58]. These contents can be transported with body fluids to target cells to participate in angiogenesis, tumorigenesis, development, and metastasis [59,60,61].
The contents of small extracellular vesicles. Almost all types of cells can secrete small extracellular vesicles, and small extracellular vesicles widely contain nucleic acids such as microRNA (miRNA), lncRNA, circRNA, mRNA, proteins, and lipids. Their surface markers mainly include CD63, CD81, CD9, ALG-2 Interacting protein X (Alix), tumor susceptibility gene 101 (TSG101), and heat shock protein 70 (HSP70)
TEXs have been reported to play a vital role in several aspects of tumorigenesis and progression. Recently, Wang et al. reported that Linc01091 encapsulated in TEXs could activate the ELF4/CDX2 axis by binding to miR-128-3p and facilitate the malignant progression of gastric cancer [62]. Moreover, it has been reported that inhibition of PD-L1 exosome release transforming growth factor-β (TGF-β) could work synergistically to promote the release of granzyme and interferon-γ to relieve the burden of tumor and depicts the regeneration of depleted T cells. This study established the role of TGF-β as a promoter of exosomal PD-L1 in breast cancer [63]. Moreover, transferring exogenous miR-25-5p carried in TEXs to anoikis-resistant hepatocellular carcinoma cells could significantly enhance cell motility and promote tumor self-implantation [64].
The roles of TEXs in the initiation and development of glioma
TEXs were involved in glioma initiation and development by affecting TME, radiation therapy, chemoresistance, invasion and metastasis, angiogenesis, and tumor growth.
The roles of TEXs in TME
The TME consists of tumor cells, immune cells, mesenchymal stem cells, vascular endothelial cells and non-cellular components such as ECM, cytokines, and chemokines, which can affect reversible changes in tumor cell phenotype and promote tumor cell metastasis and proliferation (Fig. 3). Many factors within the TME influence the interaction between tumor cells and immune cells, including the negative regulation of immune regulation by cytokines, polarization response of macrophages, and negative regulation of metabolic activity of T cells, which may suppress the killing effect of immune cells on tumor cells and allow tumor cells to undergo immune escape [65, 66].
The role of SEVs in intercellular communication, particularly during tumor development, has been extensively studied. SEVs deliver biologically active molecules such as proteins, mRNA, and miRNA to cells in the TME and thus, play an immunomodulatory role [67,68,69]. The role of SEVs in tumor immunity is double-edged, with both immunostimulatory and immunosuppressive effects; it acts mainly through regulating relevant immune cells in TME. SEVs can exert their biological effects by interacting with receptor cells through various mechanisms, including activation of cell surface receptors through receptor–ligand interactions [8, 70]. In addition, SEVs can deliver their contents to receptor cells via membrane fusion [71, 72], and receptor cells internalize SEVs through cytosolic drinking, phagocytosis, and endocytosis [17, 73].
Moreover, glioma cell-derived SEVs were reported to be involved in glioma development and progression by affecting TME (Table 1). CD133+ U87 glioblastoma cell-derived SEVs grown under hypoxic conditions are potent proliferation inducers of the tumor vascular system and glioma cell proliferation. CD133+ U87 glioblastoma cells may secrete exosome-derived miRNAs to promote angiogenic responses and glioma cell proliferation, which may be potential targetable drivers of hypoxia-dependent intercellular signaling upon tumorigenesis and progression [74]. Human astrocytoma U251 cell-derived SEVs induce tumorigenesis in human bone marrow mesenchymal stem cells (hBMSCs) by enhancing the cell proliferation, migration, and invasion; promoting the cell cycle; and activating glycolysis in hBMSCs [74]. Wang et al. reported that GBM could secrete multiple tumor-derived extracellular vesicles (TDEVs) with high immunosuppressive activity, thereby remotely suppressing the systemic immune system. The CD73+ TDEVs released by GBM cells could be taken up by T cells and inhibit cell cycle entry and clonal proliferation of T cells. Defects in exosome synthesis and CD73 expression remarkably repressed tumor growth in GBM-bearing mice and restored clonal proliferation of T cells in peripheral and central regions [75].
In addition, exosome-derived ncRNAs play a significant role in the malignant tension of gliomas. Normoxia-stimulated and hypoxia-stimulated glioma-derived SEVs (GDEs) were isolated. It was observed that hypoxia promotes the secretion of GDEs; myeloid-derived suppressor cells (MDSCs) can efficiently take up GDEs, and hypoxic glioma-derived SEVs (HGDEs) could significantly induce MDSCs compared with normoxic glioma-derived SEVs (N-GDEs) in vitro [76]. Hypoxia-induced expression of miR-10a and miR-21 in GDEs could mediate GDE-induced MDSC amplification and activation by targeting RORA and PTEN. Yang et al. reported that miR-214-5p expression was significantly higher in GBM and was strongly correlated to poorer clinical prognosis. Overexpression of miR-214-5p in cells markedly regulated cell proliferation and migration, which has been reported in various cancer cells [77, 78]. Mechanistic results demonstrated that GBM-derived exosomal miR-214-5p promotes inflammatory responses in primary microglia by targeting CXCR5, and thus, followed with lipopolysaccharide attack [79]. Qian et al. reported that HGDEs significantly induced M2 macrophage polarization compared with N-GDEs, thereby promoting glioma growth and metastasis. Furthermore, the next-generation sequencing results suggested that miR-1246 was enriched in the cerebrospinal fluid (CSF) of patients with GBM, and the expression was decreased after surgical resection. MiR-1246 could activate the STAT3 signaling pathway and repress NF-κB signaling-pathway-mediated H-GDE-induced M2-macrophage polarization via targeting TERF2IP and targeting miRNA-1246 might contribute to antitumor immunotherapy [80]. In addition, Xu et al. demonstrated that H-GDEs significantly promoted autophagy and M2-like macrophage polarization compared with N-GDEs, thereby promoting glioma growth and metastasis (possibly through an IL-6-pSTAT3-miR-155-3p-autophagy-pSTAT3 positive feedback loop), and in turn, its biological effects [81]. Pan et al. reported that circNEIL3 is packaged by hnRNPA2B1 into SEVs and delivered to infiltrating tumor-associated macrophages (TAM) via glioma cells, enabling them to acquire immunosuppressive properties via stabilizing IGF2BP3. This might, in turn, promote the malignant progression [82]. Mechanistically, circ-NEIL3 stabilizes the known oncogenic protein IGF2BP3 by blocking HECTD4-mediated ubiquitination.
The roles of TEXs in radiation therapy
Glioma-cell-derived SEVs have been reported to be involved in glioma development and progression by influencing radiation therapy (Table 2). The efficacy of radiation therapy as an important adjuvant therapy after surgical resection is well established for gliomas [1, 83] (Fig. 4). Radiation therapy can directly kill or inhibit the growth of residual tumors and prolong the survival time of patients. It has now become the standard treatment for high-grade glioma (HGG) [84].
Dai et al. reported that antisense transcript of hypoxia-inducible factor 1α (AHIF) expression was remarkably increased in GBM tissues and GBM cells with radiation tolerance. Overexpression of AHIF in GBM cells significantly increased cell viability and invasiveness and inhibited apoptosis. Furthermore, SEVs from AHIF-overexpressing GBM cells significantly promoted cell viability, invasion, and radioresistance, possibly through regulating migration and angiogenesis-related factors [85]. Yue et al. reported that Exo-miR-301a, specifically secreted by hypoxic GBM cells, could be transferred to the normoxic cultured cells and further enhance cellular radio-resistance. Hypoxic Exo-miR-301a could directly target the TCEAL7 gene and block the nuclear translocation of b-linked protein, thereby negatively regulating the Wnt/b-linked protein pathway [86]. Exo-miR-301a/TCEAL7 signaling axis may be a therapeutic target for radiation therapy resistance in patients with GBM.
The roles of TEXs in chemoresistance
Tumor resistance is the insensitivity or reduced sensitivity of tumor cells to antitumor drugs that would normally kill tumor cells. The development of tumor resistance is a major cause of chemotherapy failure. Chemotherapy is an important treatment strategy for glioma (Fig. 4). The main mechanisms for developing drug resistance in glioma are heterogeneity, hypermutation, immune evasion, and selective splicing of tumor activation. Procarbazine, lomustine, and vincristine combination regimens and combination regimens of cytotoxic chemotherapeutic agents such as teniposide, etoposide, isocyclophosphamide, cisplatin, or carboplatin have been used occasionally, but these regimens have limited efficacy and greater cytotoxic effects [1, 87]. Temozolomide (TMZ) is a novel alkylating agent with good CNS permeability and it can reach nearly 100% bioavailability when administered orally. Compared with traditional cytotoxic chemotherapeutic agents, TMZ has mild side effects, is well tolerated, and is easy to administer [87, 88]. Currently, TMZ-based chemotherapy regimens have been widely used in the adjuvant treatment of high-grade gliomas, in the salvage treatment of recurrent gliomas, and low-grade gliomas with poor prognostic factors [89, 90].
Additionally, glioma-cell-derived SEVs could be involved in glioma development and progression by affecting chemoresistance (Table 3). A study characterized the SEVs of GBM cells with and without PTPRZ1-MET fusion (ZM fusion) and assessed the role of ZM exosome-mediated intercellular communication in the GBM microenvironment. The results revealed that ZM-derived SEVs significantly promoted GBM cell migration and invasion, neurosphere growth, and angiogenesis and promoted temozolomide resistance in GBM cells [91]. Expression levels of exosome-linked protein 43 (Cx43) were significantly higher in TMZ-resistant GBM cells compared with TMZ-sensitive cells. Exosome-derived Cx43 from TMZ-resistant GBM cells significantly enhanced cell migration and invasion and conferred TMZ resistance to receptor-sensitive cells, suggesting that Cx43 is expected to be a future therapeutic target for glioblastoma [92]. EV secreted by Glioma stem cells (GSC-EV) could be involved in radiation resistance and malignant progression of glioma. GSC-EV was reported to be taken up by human glioblastoma cell line LN229 and U118 receptor cells. These receptor cells survived radiation exposure and could efficiently form colonies, significantly enhancing cell migration and invasion. Mechanistic findings indicated that GSC-EV and its specifically wrapped miRNAs might induce phenotypic changes in the recipient cells by activating the PTEN/Akt pathway [93]. In addition, it was also shown that recombinant SEVs (R-EXO) from homologous glioma cells could carry TMZ and dihydrotanshinone (DHT), can reverse drug resistance, and enhance focal targeted drug delivery, defined as R-EXO-TMZ/DHT (R-EXO-T/D). R-EXO-T/D was reported to have multiple advantages, including good blood–brain barrier (BBB) penetration with nanometer size, tumor homing aggregation with homologous effects, and enhanced antitumor activity by overcoming TMZ resistance and triggering immune responses [94]. Macrophage movement inhibitory factor (MIF) expression is increased in SEVs of TMZ-resistant cells and can be transferred from TMZ-resistant cells to sensitive cells. Mechanistic studies have reported that exosome-derived MIF can enhance the sensitivity of drug-resistant glioma cells to TMZ via repressing the expression of metalloproteinase inhibitor 3 (TIMP3) and subsequently activating the PI3K/AKT signaling pathway [95].
Recent studies have identified that exosome-derived non-coding RNAs play a significant role in chemoresistance in glioma. Yang et al. found that miR-221 expression was significantly increased in glioma tissues and SEVs, and inhibition of miR-221 expression in SHG-44 cells significantly inhibited cell proliferation, migration, and TMZ resistance; however, U87MG-derived SEVs produced protumorigenic effects [96]. u87MG-derived SEVs may contribute to glioma malignancy by regulating the RELA/. TMZ-resistant GBM cells may confer TMZ chemoresistance to the receptor by secreting SEVs into TMZ-sensitive cells and inhibiting the miR-151a/XRCC4/DNA repair signaling axis in the cells [97]. Furthermore, exosomal-miR-151a predicted chemotherapeutic response and is a potential therapeutic target for therapeutic GBM. In addition, miR-1238 expression levels were reported to be significantly higher in TMZ-resistant GBM cells and their SEVs than in TMZ-sensitive cells. TMZ-resistant GBM cells can promote their resistance by secreting miR-1238 into TMZ-sensitive GBM cells and directly targeting the CAV1/EGFR pathway [98]. SBF2-AS1 expression was upregulated in both TMZ-resistant GBM cells and tissues and overexpression of SBF2-AS1 in cells resulted in enhanced TMZ resistance. Mechanistic results revealed that TMZ-resistant GBM cells could reshape the TME and promote tumor chemoresistance by secreting exosome-derived SBF2-AS1 into TMZ-sensitive GBM cells and promoting XRCC4 expression through binding to miR-151a-3p [99]. Exosome-derived miR-25-3p was a TMZ-resistance-associated miRNA with remarkably higher expression in A172R cell SEVs and serum samples from patients with GBM treated with TMZ. Overexpression of miR-25-3p remarkably contributed to the proliferation and TMZ resistance of sensitive GBM cells. Mechanistic findings indicated that exosome-derived miR-25-3p might exert biological effects by promoting the expression of c-Myc and cyclin E through downregulation of FBXW7 [100].
Long non-coding RNA Lnc-TALC can be encapsulated into SEVs and delivered by GBM cells to TAM, thereby promoting M2 polarization in microglia. Lnc-TALC can bind to ENO1 and promote phosphorylation of p38 MAPK, thereby promoting C5/C5a fractionation. C5 can significantly promote the repair of TMZ-induced DNA damage, leading to chemoresistance. In conclusion, exosome-derived lnc-TALC can remodel the GBM microenvironment and reduce tumor sensitivity to TMZ chemotherapy [101].
It has been reported that exosome-derived circRNAs play a vital role in TMZ resistance. circNFIX expression is significantly increased in serum SEVs of TMZ-resistant patients and is strongly associated with poor prognosis. TMZ-resistant GBM-cell-derived exosome-derived circNFIX can enhance TMZ resistance by binding to miR-132 and directly interacting with miR-132. Moreover, exosome-derived circ-HIPK3 can promote TMZ-resistant GBM cell growth and TMZ resistance by modulating the miR-421/ZIC5 axis and participating in intercellular communication between GBM cells [102]. Low-dose radiation stimulates GBM cells to secrete circ-METRN-rich SEVs, and circ-METRN enhances glioblastoma progression and radioresistance by regulating the miR-4709-3p/GRB14/PDGFRα pathway [103]. Circ-0072083 in TMZ-resistant GBM tissues and cells Expression was significantly increased in both TMZ-resistant GBM tissues and cells. It promoted drug resistance by promoting IC50, proliferation, migration, invasion, and xenograft tumor growth of TMZ and inhibiting apoptosis. Mechanistic studies reported that circ-007208 could enhance ALKBH5-mediated demethylation and thus promote NANOG expression by binding to miR-1252-5p [104]. Acetyl heparinase was upregulated in TMZ-resistant GBM cells, and overexpression of acetylheparinase significantly increased the resistance of U87 cells to TMZ. In addition, acetyl heparinase promoted exosome secretion by GBM cells and mediated the transfer of exosome-derived has_circ_0042003 from TMZ-resistant glioma cells to drug-sensitive cells [105].
The roles of TEXs in invasion and metastasis
Invasion and metastasis are the important reasons underlying poor tumor prognosis, and their occurrence involves several factors such as weakened adhesion between tumor cells and degradation of extracellular matrix, which can promote tumor metastasis [106, 107]. Extracellular matrix (ECM) acts as a natural barrier to tumor invasion and metastasis, and thus effectively prevents tumor metastasis [108, 109] (Fig. 5). During the establishment of the premetastatic niche, ECM changes through reorganization or a new ECM is deposited. Many factors contribute to this change, including solubility factors, immune cells, and exosomes, which is to create a permissive seeding and growth environment of circulating cancer cells [110]. Tumor-derived exosomes regulate ECM, and exosome-induced fibronectin deposition has been reported in both liver and lung premetastatic niches. In the liver, PaC exosomes carrying macrophage migration inhibitory factor (MIF) promote the secretion of TGF-b by Kupffer cells, thereby inducing stellate cells to produce fibronectin [111]. Exosomal small nuclear RNA enhances the expression of metalloproteinase-9 (MMP-9) and fibronectin in the lung premetastasis niche, thereby promoting the recruitment of neutrophils [112]. Moreover, non-small cell lung cancer (NSCLC) cells modulated the expression of podocalyxin in exosomes, which in turn impacted integrin trafficking in fibroblasts and created a supportive microenvironment for tumor cell migration and invasion by introducing tumor-promoting ECM components [113]. All these aforementioned studies highlighted the role of SEVs in modulating ECM.
The extracellular matrix in GBM tumorigenesis. The features of extracellular matrix in normal brain are low overall density and stiffness and absence of neurodevelopmental proteins. The features of extracellular matrix in GBM are increased overall density and stiffness, presence of oncofetal protein isoforms, and promotion of invasion and angiogenesis
Glioma-cell-derived SEVs were reported to be involved in glioma development and progression via influencing tumor invasion and metastasis (Table 4). MiR-148a levels in circulating SEVs were significantly higher in the serum of patients with GBM than in healthy volunteers. Inhibition of miR-148a expression in glioma cells significantly inhibited cancer cell proliferation and metastasis. The results of mechanistic experiments suggested that exosome-mediated miR-148a may promote cancer cell proliferation and metastasis by targeting cell adhesion molecule 1 (CADM1) to activate the STAT3 pathway [114]. Glioma-cell-derived SEVs can deliver lnc-ATB to astrocytes and activate astrocytes by suppressing miR-204-3p expression in an Ago2-dependent manner. Surprisingly, lnc-ATB-activated astrocytes could, in turn, promote glioma cell migration and invasion, indicating that lnc-ATB might contribute to regulating the glioma microenvironment through exosomal forms [115]. MiR-375 expression was significantly downregulated in gliomas; it could inhibit glioma growth by repressing the CTGF-epidermal growth factor receptor (EGFR) signaling pathway, thus inhibiting glioma proliferation, migration, and invasion. In addition, exosome-derived miR-375 was significantly downregulated in peripheral blood samples from patients with glioma and was strongly associated with patient prognosis. Exosome-derived miR-375 inhibits glioma cell proliferation and invasion through sustained inhibition of the CTGF-EGFR oncogenic pathway [116]. miR-1246 and miR-10b-5p are significantly upregulated in H-GDEs and can be delivered to normoxic glioma cells, promoting the migration and invasion of normoxia cells in vitro and in vivo. Mechanistic studies have reported that miR-1246 and miR-10b-5p can induce glioma migration and invasion by directly targeting FRK and TFAP2A [117]. Glioma cells secrete SEVs that significantly promote the malignant progression of gliomas, and exosome-derived circ-0001445 can be taken up by glioma cells. Circ-0001445 entering glioma cells can act as a sponge for miR-127-5p and upregulate the expression of sorting linker protein 5 (SNX5), which promotes glioma migration and invasion [118]. Exosome-derived circZNF652 is significantly up-day expressed in glioma cells and can be taken up by other glioma cells. The entry of circ-0001445 into glioma cells promotes glioma migration and invasion by regulating the miR-486-5p/SERPINE1 signaling axis and the epithelial–mesenchymal transition process [93].
The roles of TEXs in angiogenesis
Tumor angiogenesis plays a vital role in tumorigenesis and development. Angiogenesis refers to developing new blood vessels from existing capillaries or postcapillary veins [119, 120]. Generally, the whole process of angiogenesis occured in tissues is coordinated by angiogenic and vasopressor factors [121, 122]. Under external factors, internal genetic mutations, and tumorigenesis, the angiogenic factors are overactivated, while the vasopressor is suppressed. This kind of imbalance can activate the angiogenic system, resulting in excessive tissue angiogenesis [123, 124]. The rapid increase in blood vessels to meet the needs of tumor growth can lead to rapid tumor growth and increase the probability of tumor cell spread and metastasis (Fig. 6).
The features of tumor angiogenesis. Tumor angiogenesis is a complex process of interaction between tumor cells and endothelial cells. The main process is as follows: endothelial cells are stimulated by proangiogenic growth factors secreted by tumor cells. After degrading, endothelial cells proliferate under the action of various chemokines, form lumen with pericytes under the action of adhesion molecules, and generate new tumor blood vessels after maturation and stabilization. Tumor angiogenesis is an important basis for tumor growth, invasion, and metastasis. This process is regulated by various angiogenic growth-promoting or inhibitory factors and signaling pathways
Glioma-cell-derived SEVs were reported to be involved in glioma development and progression by affecting tumor angiogenesis (Table 5). A specific 120-kDa vascular endothelial growth factor (VEGF) isoform, namely, VEGF-C, was reported to be present in GBM-derived SEVs, which binds to VEGF receptor 2 (VEGFR2). Further, it was reported that VEGF-C in GBM-derived SEVs exhibited a strong stimulatory effect on tafazzin (TAZ) expression in endothelial cells via suppressing the Hippo signaling pathway, which ultimately stimulates endothelial cell viability, migration, and tubularization [125]. Linc-CCAT2 is overexpressed in glioma tissues and significantly leads to the malignant progression of gliomas. Further studies have reported that linc-CCAT2-rich glioma-cell-derived SEVs can be taken up by HUVEC cells and can increase the expression level of linc-CCAT2 in HUVEC cells in turn promoting HUVEC migration, proliferation, tubular formation in vitro, and small artery formation in vivo and inhibits hypoxia-induced HUVEC cell apoptosis. Mechanistic studies have reported that linc-CCAT2 can upregulate the expression of VEGFA and TGFβ, promote the expression of Bcl-2, and inhibit the expression of Bax and caspase-3 in HUVEC cells [126].
Studies have reported that glioma stem cells may contribute to the prognosis of glioblastoma by mediating cellular communication, mainly through the secretion of exosome-derived miRNAs. miR-21 expression is increased in glioblastoma, and it upregulates VEGF expression. Mechanistic experiments revealed that GSC-EXs could promote the angiogenic capacity of endothelial cells (ECs) through miR-21/VEGF signaling [127]. Proangiogenic propermeability factor (VEGF-A) is enriched in SEVs from the cells originating from patients with glioma and contributes to the increased permeability and angiogenic potential of human brain endothelial cells in vitro. Inhibition of VEGF-A expression significantly inhibited the increase in extracellular vesicle-mediated permeability and angiogenesis in vitro. Therefore, targeting EVs released from gliomas may be a therapeutic tool to inhibit tumor-induced angiogenesis and vascular permeability in GBM [128]. miR-26a is enriched in GSCs SEVs and promotes proliferation, migration, tube formation, and angiogenesis in HBMEC via targeting PTEN to activate PI3K/Akt signaling pathway [129]. Circulating miR-182-5p levels are elevated in serum and cerebrospinal fluid samples from patients with glioma, and its expression level is negatively correlated with prognosis. Under hypoxic conditions, miR-182-5p expression was significantly increased in the SEVs of GBM cells, which directly inhibited KLF2 and KLF4 and led to the accumulation of VEGFR, thereby promoting HUVEC cell proliferation and tumor angiogenesis. In addition, exosome-mediated miR-182-5p inhibits tight junction-related proteins (such as ZO-1, occludin, and claudin-5), thereby enhancing vascular permeability and transendothelial migration of tumors [130]. miR-148a-3p is enriched in glioma-cell-derived SEVs and can be transferred to HUVEC cells in an exosome-mediated form and promotes its proliferation. HUVEC and promote their proliferation and angiogenesis. Mechanistic findings suggested that miR-148a-3p activates the epidermal growth factor receptor (EGFR)/mitogen-activated protein kinase (MAPK) signaling pathway by inhibiting ERRFI1 expression [131]. It was reported that miR-944 levels were significantly lower in high-grade gliomas (HGGs) than in low-grade gliomas (LGGs), and overall survival was significantly lower in patients with glioma with low miR-944 expression than in patients with glioma with high miR-944 expression. In addition, GSC-derived exosome-derived miR-944 was delivered to HUVEC cells and significantly reduced cell proliferation, migration, and test tube formation in vitro. Mechanistic findings revealed that miR-944 significantly reduced VEGFC levels and inhibited activation of the AKT/ERK signaling pathway [132]. circGLIS3 expression was significantly increased in HGGs, and it promoted migration and invasion of glioma cells, which exhibited an aggressive profile in hormonal mice. Mechanistic findings suggested that circGLIS3 promotes the phosphorylation level of Ezrin T567. In addition, gliomas can secrete circGLIS3 into endothelial cells via SEVs and induce endothelial cell angiogenesis, thereby promoting glioma invasion and angiogenesis[133].
The roles of TEXs in tumor growth
Glioma-cell-derived SEVs have been reported to be involved in glioma development and progression by affecting tumor growth (Table 6). It was reported that treating non-GSC glioma cells with GSC SEVs significantly enhanced cell proliferation, neurosphere formation, invasiveness, and tumorigenicity. Further studies revealed that the Notch1 signaling pathway was activated in GSC and highly enriched in GSC SEVs. GSC could deliver SEVs to nonGSC glioma cells and increase Notch1 expression, which in turn mediated the dedifferentiation of nonGSC glioma cells into GSC and enhanced the stemness and tumorigenicity of nonGSC glioma cells [134]. Linc01060 was upregulated in gliomas and significantly correlated with tumor grade and poor clinical prognosis. Linc01060 expression was significantly increased in hypoxic GSC (H-GSC), which promoted malignant proliferation of cells by transferring SEVs into glioma cells and leading to significantly higher Linc01060 expression in cells. Mechanistic findings suggested that Linc01060 promotes nuclear translocation of MZF1 and facilitates MZF1-mediated c-Myc transcriptional activity, whereas c-Myc enhances the accumulation of hypoxia-inducible factor-1α (HIF1a) at post-transcriptional levels. HIF1a binds to the hormone-responsive element of the Linc01060 promoter and upregulates Linc01060 gene transcription. Overall, inhibiting Linc01060-containing SEVs or targeting the Linc01060/MZF1/c-Myc/HIF1a axis may be an effective therapeutic strategy for glioma [135]. ROR1-AS1 is upregulated in glioma tissues, and high expression of ROR1-AS1 predicts poor prognosis in patients with glioma. ROR1-AS1 can be packaged into the SEVs of glioma cells and can significantly promote cell growth and metastasis. Mechanistic findings suggested that ROR1-AS1 acts as a sponge for miR-4686 and inhibits its expression. Tumor-cell-derived Exo-ROR1-AS1 may be a target for clinical treatment of glioma [136].
TEXs serve as diagnostic and prognostic biomarkers in glioma
The search for a specific and nontoxic tumor marker has become increasingly urgent because of the late detection of gliomas, which are often not very effectively cured. In recent years, it has been reported that SEVs promise new tumor markers because they are widely distributed in eukaryotic cells, and the contents of SEVs secreted by various types of tumor cells can be helpful in the diagnosis of various tumors.
Patients with recurrent GBM with higher serum exosomal SBF2-AS1 levels had a worse prognosis, forecasting a poor response to TMZ treatment [99]. Exosomal circNFIX is biologically important in the early diagnosis of patients with recurrent GBM and prognostic assessment [137]. Detection of circ-METRN expression levels in serum SEVs of patients with glioma early in radiation therapy not only helps to predict radioresistance and prognosis but also assists in the early detection of glioblastoma recurrence by MRI [103]. circ_0072083 expression levels were significantly elevated in serum SEVs of drug-resistant patients with glioma and predicted a lower overall survival of patients [104]. Glioma can be diagnosed early by measuring the expression level of circulating exosomal miR-148a [114]. The prognosis of patients with glioma can be assessed by measuring the expression level of circulating exosomal miR-375 [116].
Future prospections and conclusions
TEXs are rich in protein, miRNA, mRNA, DNA, and lipid contents and play an essential role in the early diagnosis, development, and treatment of tumors [138,139,140]. However, because of the differences in experimental materials and research methods, the detection of single exosome contents is prone to false-positive results. Therefore, there is still a long way to go for the clinical application and promotion of TEXs. To improve the accuracy of exosome content detection technology, we can focus on developing multiple exosome content assays in the future so that exosome-related developments can be better applied for the early diagnosis, treatment, and prognosis of tumors [141, 142].
Tumor-cell-derived SEVs are released into the extracellular space to bind to receptors on target cells through the humoral circulation, and transport characteristic proteins and nucleic acids. They are essential in promoting tumor invasion and metastasis [101, 143, 144]. Therefore, clarifying the function of SEVs is vital in understanding their role in the TME. With the in-depth study of SEVs, we have gained a deep understanding of the interactions and mechanisms between tumor cells, which provides a new theoretical basis for diagnosing and treating tumors. For a long time, studies on SEVs have focused on developing specific targeted antitumor vaccines. Their intrinsic mechanisms and their immune-enhancing or tolerogenic nature should be explored. Because SEVs are widely distributed in body fluids and have a long half-life, they may be suitable drug carriers. However, the feasibility of SEVs as drug carriers needs to be investigated. In conclusion, with the deepening of the understanding of SEVs and the clarification of tumor detection, diagnosis, and treatment methods based on exosome research, new antitumor drugs and clinical tumor interventions are expected to be developed.
Under both physiological and pathological conditions, SEVs mediate the exchange of information between cells and their surroundings [145,146,147,148,149]. TEXs have emerged as a major communication mechanism between tumor cells and TMEs and have a vital role in tumor progression and metastasis [11, 150,151,152,153,154]. The application of TEXs in the diagnosis and treatment of glioma is still in the nascent stages. Follow-up studies should focus on the biogenesis and secretion of TEXs, their interaction with target cells, and the role of exosomal components of TEXs, which are expected to improve the application of drug therapy and increase the survival rate of patients with glioma. However, still, some issues are to be resolved. For example, the sensitivity and specificity of TEXs in the early diagnosis and prognostic assessment of glioma still need to be improved. The acquisition of high-purity SEVs remains a challenge due to technical limitations and high costs, and the presence of impurity proteins in the extracted SEVs may affect the efficiency of the assay. The quantification, purification, and preservation of SEVs have not been standardized SEVs as tumor micro. The specific mechanism of SEVs as an important component of the TME in glioma evolution is still unclear. The adverse effects of SEVs in targeted therapy cannot be fully determined. These problems limit the application of TEXs in treating glioma.
This article reviewed the multifaceted nature of TEXs and their biological role in glioma genesis and development. In the future, the clinical application of TEXs will provide a new path for the treatment of glioma.
Data availability
The data in the current study are available from the corresponding authors on reasonable request.
Abbreviations
- TEXs:
-
Tumor-derived exosomes
- GBM:
-
Glioblastoma multiforme
- EVs:
-
Extracellular vesicles
- MVs:
-
Micro-vesicles
- MVE:
-
Multivesicular endosome
- MVB:
-
Multivesicular body
- ILV:
-
Intraluminal vesicle
- TSG101:
-
Tumor susceptibility gene 101 protein
- Alix:
-
ALG-2 interaction protein X
- HSP70:
-
Heat shock protein 70
- mRNA:
-
Messenger RNA
- miRNA:
-
MicroRNA
- lncRNAs:
-
Long non-coding RNA
- circRNA:
-
Circular RNA
- TME:
-
Tumor microenvironment
- hBMSCs:
-
Human bone marrow mesenchymal stem cells
- TDEVs:
-
Tumor-derived extracellular vesicles
- GDEs:
-
Glioma-derived exosomes
- HGDEs:
-
Hypoxic glioma-derived exosomes
- N-GDEs:
-
Normoxic glioma-derived exosomes
- CSF:
-
Cerebrospinal fluid
- TAM:
-
Tumor-associated macrophages
- AHIF:
-
Hypoxia inducible factor 1α
- TMZ:
-
Temozolomide
- Cx43:
-
Exosome-linked protein 43
- BBB:
-
Blood–brain barrier
- MIF:
-
Macrophage movement inhibitory factor
- TIMP3:
-
Metalloproteinase inhibitor 3
- ECM:
-
Extracellular matrix
- CADM1:
-
Cell adhesion molecule 1
- Ago2:
-
Argonaute 2
- EGFR:
-
Epidermal growth factor receptor
- SNX5:
-
Sorting linker protein 5
- VEGF:
-
Vascular endothelial growth factor
- ECs:
-
Endothelial cells
- HGGs:
-
High-grade gliomas
- LGGs:
-
Low-grade gliomas
References
Yang K, Wu Z, Zhang H, Zhang N, Wu W, Wang Z, Dai Z, Zhang X, Zhang L, Peng Y, Ye W, Zeng W, Liu Z, Cheng Q. Glioma targeted therapy: insight into future of molecular approaches. Mol Cancer. 2022;21:39.
Zhou Q, Xue C, Ke X, Zhou J. Treatment response and prognosis evaluation in high-grade glioma: an imaging review based on MRI. J Magn Reson Imaging. 2022;56:325–40.
Ho RLY, Ho IAW. Recent advances in glioma therapy: combining vascular normalization and immune checkpoint blockade. Cancers (Basel). 2021;13:3686.
Takacs GP, Flores-Toro JA, Harrison JK. Modulation of the chemokine/chemokine receptor axis as a novel approach for glioma therapy. Pharmacol Ther. 2021;222:107790.
Tamai S, Ichinose T, Tsutsui T, Tanaka S, Garaeva F, Sabit H, Nakada M. Tumor microenvironment in glioma invasion. Brain Sci. 2022;12:505.
Li X, Geng X, Chen Z, Yuan Z. Recent advances in glioma microenvironment-response nanoplatforms for phototherapy and sonotherapy. Pharmacol Res. 2022;179:106218.
Kim PL. Targeting gene fusions in glioma. Curr Opin Neurol. 2021;34:840–7.
Barthel L, Hadamitzky M, Dammann P, Schedlowski M, Sure U, Thakur BK, Hetze S. Glioma: molecular signature and crossroads with tumor microenvironment. Cancer Metastasis Rev. 2022;41:53–75.
Xia X, Wang Y, Qin Y, Zhao S, Zheng JC. Exosome: a novel neurotransmission modulator or non-canonical neurotransmitter? Ageing Res Rev. 2022;74:101558.
Yu W, Hurley J, Roberts D, Chakrabortty SK, Enderle D, Noerholm M, Breakefield XO, Skog JK. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32:466–77.
Li MY, Liu LZ, Dong M. Progress on pivotal role and application of exosome in lung cancer carcinogenesis, diagnosis, therapy and prognosis. Mol Cancer. 2021;20:22.
Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49:347–60.
Ranjbaran A, Latifi Z, Nejabati HR, Abroon S, Mihanfar A, Sadigh AR, Fattahi A, Nouri M, Raffel N. Exosome-based intercellular communication in female reproductive microenvironments. J Cell Physiol. 2019;234:19212–22.
Wan Z, Gao X, Dong Y, Zhao Y, Chen X, Yang G, Liu L. Exosome-mediated cell-cell communication in tumor progression. Am J Cancer Res. 2018;8:1661–73.
Sun Z, Yang S, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Xu J, Xia K, Chang Y, Liu J, Yuan W. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol Cancer. 2018;17:82.
Kim H, Jang H, Cho H, Choi J, Hwang KY, Choi Y, Kim SH, Yang Y. Recent advances in exosome-based drug delivery for cancer therapy. Cancers (Basel). 2021;13:4435.
Pathania AS, Prathipati P, Challagundla KB. New insights into exosome mediated tumor-immune escape: clinical perspectives and therapeutic strategies. Biochim Biophys Acta Rev Cancer. 2021;1876:188624.
Santos P, Almeida F. Exosome-based vaccines: history, current state, and clinical trials. Front Immunol. 2021;12:711565.
Noonin C, Thongboonkerd V. Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics. 2021;11:4436–51.
Han C, Zhang C, Wang H, Zhao L. Exosome-mediated communication between tumor cells and tumor-associated macrophages: implications for tumor microenvironment. Oncoimmunology. 2021;10:1887552.
Duan L, Xu L, Xu X, Qin Z, Zhou X, Xiao Y, Liang Y, Xia J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2021;13:1387–97.
Yousafzai NA, Wang H, Wang Z, Zhu Y, Zhu L, Jin H, Wang X. Exosome mediated multidrug resistance in cancer. Am J Cancer Res. 2018;8:2210–26.
Wang B, Tan Z, Guan F. Tumor-derived exosomes mediate the instability of cadherins and promote tumor progression. Int J Mol Sci. 2019;20:3652.
Wan M, Ning B, Spiegel S, Lyon CJ, Hu TY. Tumor-derived exosomes (TDEs): how to avoid the sting in the tail. Med Res Rev. 2020;40:385–412.
Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8:307.
Shenoy GN, Bhatta M, Bankert RB. Tumor-associated exosomes: a potential therapeutic target for restoring anti-tumor T cell responses in human tumor microenvironments. Cells. 2021;10:3155.
Whiteside TL, Diergaarde B, Hong CS. Tumor-derived exosomes (TEX) and their role in immuno-oncology. Int J Mol Sci. 2021;22:6234.
McAndrews KM, Kalluri R. Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer. 2019;18:52.
Yang E, Wang X, Gong Z, Yu M, Wu H, Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther. 2020;5:242.
Wu Q, Zhou L, Lv D, Zhu X, Tang H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J Hematol Oncol. 2019;12:53.
Liu J, Wu F, Zhou H. Macrophage-derived exosomes in cancers: biogenesis, functions and therapeutic applications. Immunol Lett. 2020;227:102–8.
Fraga de Andrade I, Mehta C, Bresnick EH. Post-transcriptional control of cellular differentiation by the RNA exosome complex. Nucleic Acids Res. 2020;48:11913–28.
Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomed. 2020;15:6917–34.
Agarwal S, Agarwal V, Agarwal M, Singh M. Exosomes: structure biogenesis, types and application in diagnosis and gene and drug delivery. Curr Gene Ther. 2020;20:195–206.
Shan X, Zhang C, Mai C, Hu X, Cheng N, Chen W, Peng D, Wang L, Ji Z, Xie Y. The biogenesis biological functions, and applications of macrophage-derived exosomes. Front Mol Biosci. 2021;8:715461.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau977.
Yue B, Yang H, Wang J, Ru W, Wu J, Huang Y, Lan X, Lei C, Chen H. Exosome biogenesis, secretion and function of exosomal miRNAs in skeletal muscle myogenesis. Cell Prolif. 2020;53:e12857.
Kumar A, Deep G. Hypoxia in tumor microenvironment regulates exosome biogenesis: molecular mechanisms and translational opportunities. Cancer Lett. 2020;479:23–30.
Boriachek K, Islam MN, Möller A, Salomon C, Nguyen NT, Hossain MSA, Yamauchi Y, Shiddiky MJA. Biological functions and current advances in isolation and detection strategies for exosome nanovesicles. Small. 2018;14:1702153.
Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193–208.
He G, Peng X, Wei S, Yang S, Li X, Huang M, Tang S, Jin H, Liu J, Zhang S, Zheng H, Fan Q, Liu J, Yang L, Li H. Exosomes in the hypoxic TME: from release, uptake and biofunctions to clinical applications. Mol Cancer. 2022;21:19.
Farooqi AA, Desai NN, Qureshi MZ, Librelotto DRN, Gasparri ML, Bishayee A, Nabavi SM, Curti V, Daglia M. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol Adv. 2018;36:328–34.
Syn N, Wang L, Sethi G, Thiery JP, Goh BC. Exosome-mediated metastasis: from epithelial-mesenchymal transition to escape from immunosurveillance. Trends Pharmacol Sci. 2016;37:606–17.
Xie S, Zhang Q, Jiang L. Current knowledge on exosome biogenesis, cargo-sorting mechanism and therapeutic implications. Membranes (Basel). 2022;12:498.
Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19:47.
Nicolini A, Ferrari P, Biava PM. Exosomes and cell communication: from tumour-derived exosomes and their role in tumour progression to the use of exosomal cargo for cancer treatment. Cancers (Basel). 2021;13:822.
Daßler-Plenker J, Küttner V, Egeblad M. Communication in tiny packages: exosomes as means of tumor-stroma communication. Biochim Biophys Acta Rev Cancer. 2020;1873:188340.
Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19.
Kahroba H, Hejazi MS, Samadi N. Exosomes: from carcinogenesis and metastasis to diagnosis and treatment of gastric cancer. Cell Mol Life Sci. 2019;76:1747–58.
Fanale D, Taverna S, Russo A, Bazan V. Circular RNA in exosomes. Adv Exp Med Biol. 2018;1087:109–17.
Colletti M, Ceglie D, Di Giannatale A, Nazio F. Autophagy and exosomes relationship in cancer: friends or foes? Front Cell Dev Biol. 2020;8:614178.
Sinha D, Roy S, Saha P, Chatterjee N, Bishayee A. Trends in research on exosomes in cancer progression and anticancer therapy. Cancers (Basel). 2021;13:326.
Romano E, Netti PA, Torino E. Exosomes in gliomas: biogenesis, isolation, and preliminary applications in nanomedicine. Pharmaceuticals (Basel). 2020;13:319.
Tang Z, Li D, Hou S, Zhu X. The cancer exosomes: clinical implications, applications and challenges. Int J Cancer. 2020;146:2946–59.
Jabbari N, Karimipour M, Khaksar M, Akbariazar E, Heidarzadeh M, Mojarad B, Aftab H, Rahbarghazi R, Rezaie J. Tumor-derived extracellular vesicles: insights into bystander effects of exosomes after irradiation. Lasers Med Sci. 2020;35:531–45.
Stahl PD, Raposo G. Exosomes and extracellular vesicles: the path forward. Essays Biochem. 2018;62:119–24.
Huang T, Deng CX. Current progresses of exosomes as cancer diagnostic and prognostic biomarkers. Int J Biol Sci. 2019;15:1–11.
Gao D, Jiang L. Exosomes in cancer therapy: a novel experimental strategy. Am J Cancer Res. 2018;8:2165–75.
Mousavi S, Moallem R, Hassanian SM, Sadeghzade M, Mardani R, Ferns GA, Khazaei M, Avan A. Tumor-derived exosomes: potential biomarkers and therapeutic target in the treatment of colorectal cancer. J Cell Physiol. 2019;234:12422–32.
Jella KK, Nasti TH, Li Z, Malla SR, Buchwald ZS, Khan MK. Exosomes, their biogenesis and role in inter-cellular communication, tumor microenvironment and cancer immunotherapy. Vaccines (Basel). 2018;6:69.
Samanta S, Rajasingh S, Drosos N, Zhou Z, Dawn B, Rajasingh J. Exosomes: new molecular targets of diseases. Acta Pharmacol Sin. 2018;39:501–13.
Wang Q, Zhang C, Cao S, Zhao H, Jiang R, Li Y. Tumor-derived exosomes orchestrate the microRNA-128–3p/ELF4/CDX2 axis to facilitate the growth and metastasis of gastric cancer via delivery of LINC01091. Cell Biol Toxicol. 2022. https://doi.org/10.1007/s10565-022-09728-y.
Chatterjee S, Chatterjee A, Jana S, Dey S, Roy H, Das MK, Alam J, Adhikary A, Chowdhury A, Biswas A, Manna D, Bhattacharyya A. Transforming growth factor beta orchestrates PD-L1 enrichment in tumor-derived exosomes and mediates CD8 T-cell dysfunction regulating early phosphorylation of TCR signalome in breast cancer. Carcinogenesis. 2021;42:38–47.
Liu H, Chen W, Zhi X, Chen EJ, Wei T, Zhang J, Shen J, Hu LQ, Zhao B, Feng XH, Bai XL, Liang TB. Tumor-derived exosomes promote tumor self-seeding in hepatocellular carcinoma by transferring miRNA-25-5p to enhance cell motility. Oncogene. 2018;37:4964–78.
Jia Z, Jia J, Yao L, Li Z. Crosstalk of exosomal non-coding RNAs in the tumor microenvironment: novel frontiers. Front Immunol. 2022;13:900155.
Jing X, Xie M, Ding K, Xu T, Fang Y, Ma P, Shu Y. Exosome-transmitted miR-769-5p confers cisplatin resistance and progression in gastric cancer by targeting CASP9 and promoting the ubiquitination degradation of p53. Clin Transl Med. 2022;12:e780.
Li Y, Zhao W, Wang Y, Wang H, Liu S. Extracellular vesicle-mediated crosstalk between pancreatic cancer and stromal cells in the tumor microenvironment. J Nanobiotechnol. 2022;20:208.
Thakur A, Johnson A, Jacobs E, Zhang K, Chen J, Wei Z, Lian Q, Chen HJ. Energy sources for exosome communication in a cancer microenvironment. Cancers (Basel). 2022;14:1698.
Xu Y, Feng K, Zhao H, Di L, Wang L, Wang R. Tumor-derived extracellular vesicles as messengers of natural products in cancer treatment. Theranostics. 2022;12:1683–714.
Attaran S, Bissell MJ. The role of tumor microenvironment and exosomes in dormancy and relapse. Semin Cancer Biol. 2022;78:35–44.
Bondhopadhyay B, Sisodiya S, Alzahrani FA, Bakhrebah MA, Chikara A, Kasherwal V, Khan A, Rani J, Dar SA, Akhter N, Tanwar P, Agrawal U, Hussain S. Exosomes: a forthcoming era of breast cancer therapeutics. Cancers (Basel). 2021;13:4672.
Vautrot V, Bentayeb H, Causse S, Garrido C, Gobbo J. Tumor-derived exosomes: hidden players in PD-1/PD-L1 resistance. Cancers (Basel). 2021;13:4537.
Jiang C, Zhang N, Hu X, Wang H. Tumor-associated exosomes promote lung cancer metastasis through multiple mechanisms. Mol Cancer. 2021;20:117.
Zhang G, Zhang Y, Cheng S, Wu Z, Liu F, Zhang J. CD133 positive U87 glioblastoma cells-derived exosomal microRNAs in hypoxia- versus normoxia-microenviroment. J Neurooncol. 2017;135:37–46.
Wang M, Jia J, Cui Y, Peng Y, Jiang Y. CD73-positive extracellular vesicles promote glioblastoma immunosuppression by inhibiting T-cell clonal expansion. Cell Death Dis. 2021;12:1065.
Guo X, Qiu W, Liu Q, Qian M, Wang S, Zhang Z, Gao X, Chen Z, Xue H, Li G. Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten Pathways. Oncogene. 2018;37:4239–59.
Cao TH, Ling X, Chen C, Tang W, Hu DM, Yin GJ. Role of miR-214-5p in the migration and invasion of pancreatic cancer cells. Eur Rev Med Pharmacol Sci. 2018;22(21):7214–21. https://doi.org/10.26355/eurrev_201811_16255.
Gong F, Chai W, Wang J, Cheng H, Shi Y, Cui L, Jia G. miR-214-5p suppresses the proliferation, migration and invasion of trophoblast cells in pre-eclampsia by targeting jagged 1 to inhibit the notch signaling pathway. Acta Histochem. 2020;122(3):151527. https://doi.org/10.1016/j.acthis.2020.151527.
Yang JK, Liu HJ, Wang Y, Li C, Yang JP, Yang L, Qi XJ, Zhao YL, Shi XF, Li JC, Sun GZ, Jiao BH. Exosomal miR-214-5p released from glioblastoma cells modulates inflammatory response of microglia after lipopolysaccharide stimulation through targeting CXCR5. CNS Neurol Disord Drug Targets. 2019;18:78–87.
Qian M, Wang S, Guo X, Wang J, Zhang Z, Qiu W, Gao X, Chen Z, Xu J, Zhao R, Xue H, Li G. Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-κB pathways. Oncogene. 2020;39:428–42.
Xu J, Zhang J, Zhang Z, Gao Z, Qi Y, Qiu W, Pan Z, Guo Q, Li B, Zhao S, Guo X, Qian M, Chen Z, Wang S, Gao X, Zhang S, Wang H, Guo X, Zhang P, Zhao R, Xue H, Li G. Hypoxic glioma-derived exosomes promote M2-like macrophage polarization by enhancing autophagy induction. Cell Death Dis. 2021;12:373.
Pan Z, Zhao R, Li B, Qi Y, Qiu W, Guo Q, Zhang S, Zhao S, Xu H, Li M, Gao Z, Fan Y, Xu J, Wang H, Wang S, Qiu J, Wang Q, Guo X, Deng L, Zhang P, Xue H, Li G. EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3. Mol Cancer. 2022;21:16.
Vilar JB, Christmann M, Tomicic MT. Alterations in molecular profiles affecting glioblastoma resistance to radiochemotherapy: where does the good go? Cancers (Basel). 2022;14:2416.
Janjua TI, Rewatkar P, Ahmed-Cox A, Saeed I, Mansfeld FM, Kulshreshtha R, Kumeria T, Ziegler DS, Kavallaris M, Mazzieri R, Popat A. Frontiers in the treatment of glioblastoma: past, present and emerging. Adv Drug Deliv Rev. 2021;171:108–38.
Dai X, Liao K, Zhuang Z, Chen B, Zhou Z, Zhou S, Lin G, Zhang F, Lin Y, Miao Y, Li Z, Huang R, Qiu Y, Lin R. AHIF promotes glioblastoma progression and radioresistance via exosomes. Int J Oncol. 2019;54:261–70.
Yue X, Lan F, Xia T. Hypoxic glioma cell-secreted exosomal miR-301a activates Wnt/β-catenin signaling and promotes radiation resistance by targeting TCEAL7. Mol Ther. 2019;27:1939–49.
Kannan S, Murugan AK, Balasubramanian S, Munirajan AK, Alzahrani AS. Gliomas: genetic alterations, mechanisms of metastasis, recurrence, drug resistance, and recent trends in molecular therapeutic options. Biochem Pharmacol. 2022;201:115090.
Qiao L, Yang H, Shao XX, Yin Q, Fu XJ, Wei Q. Research progress on nanoplatforms and nanotherapeutic strategies in treating glioma. Mol Pharm. 2022;19:1927–51.
Aldoghachi AF, Aldoghachi AF, Breyne K, Ling KH, Cheah PS. Recent advances in the therapeutic strategies of glioblastoma multiforme. Neuroscience. 2022;491:240–70.
van Solinge TS, Nieland L, Chiocca EA, Broekman MLD. Advances in local therapy for glioblastoma—taking the fight to the tumour. Nat Rev Neurol. 2022;18:221–36.
Zeng AL, Yan W, Liu YW, Wang Z, Hu Q, Nie E, Zhou X, Li R, Wang XF, Jiang T, You YP. Tumour exosomes from cells harbouring PTPRZ1-MET fusion contribute to a malignant phenotype and temozolomide chemoresistance in glioblastoma. Oncogene. 2017;36:5369–81.
Yang ZJ, Zhang LL, Bi QC, Gan LJ, Wei MJ, Hong T, Tan RJ, Lan XM, Liu LH, Han XJ, Jiang LP. Exosomal connexin 43 regulates the resistance of glioma cells to temozolomide. Oncol Rep. 2021;45:44.
Ma C, Nguyen HPT, Jones JJ, Stylli SS, Whitehead CA, Paradiso L, Luwor RB, Areeb Z, Hanssen E, Cho E, Putz U, Kaye AH, Morokoff AP. Extracellular vesicles secreted by glioma stem cells are involved in radiation resistance and glioma progression. Int J Mol Sci. 2022;23:2770.
Wang R, Liang Q, Zhang X, Di Z, Wang X, Di L. Tumor-derived exosomes reversing TMZ resistance by synergistic drug delivery for glioma-targeting treatment. Colloids Surf B Biointerfaces. 2022;215:112505.
Wei QT, Liu BY, Ji HY, Lan YF, Tang WH, Zhou J, Zhong XY, Lian CL, Huang QZ, Wang CY, Xu YM, Guo HB. Exosome-mediated transfer of MIF confers temozolomide resistance by regulating TIMP3/PI3K/AKT axis in gliomas. Mol Ther Oncolytics. 2021;22:114–28.
Yang JK, Yang JP, Tong J, Jing SY, Fan B, Wang F, Sun GZ, Jiao BH. Exosomal miR-221 targets DNM3 to induce tumor progression and temozolomide resistance in glioma. J Neurooncol. 2017;131:255–65.
Zeng A, Wei Z, Yan W, Yin J, Huang X, Zhou X, Li R, Shen F, Wu W, Wang X, You Y. Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma. Cancer Lett. 2018;436:10–21.
He L, Li H, Wu A, Peng Y, Shu G, Yin G. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. 2019;18:176.
Zhang Z, Yin J, Lu C, Wei Y, Zeng A, You Y. Exosomal transfer of long non-coding RNA SBF2-AS1 enhances chemoresistance to temozolomide in glioblastoma. J Exp Clin Cancer Res. 2019;38:166.
Wang J, Li T, Wang B. Exosomal transfer of miR-25-3p promotes the proliferation and temozolomide resistance of glioblastoma cells by targeting FBXW7. Int J Oncol. 2021;59:64.
Li Z, Meng X, Wu P, Zha C, Han B, Li L, Sun N, Qi T, Qin J, Zhang Y, Tian K, Li S, Yang C, Ren L, Ming J, Wang P, Song Y, Jiang C, Cai J. Glioblastoma cell-derived lncRNA-containing exosomes induce microglia to produce complement C5, promoting chemotherapy resistance. Cancer Immunol Res. 2021;9:1383–99.
Han C, Wang S, Wang H, Zhang J. Exosomal circ-HIPK3 facilitates tumor progression and temozolomide resistance by regulating miR-421/ZIC5 axis in glioma. Cancer Biother Radiopharm. 2021;36:537–48.
Wang X, Cao Q, Shi Y, Wu X, Mi Y, Liu K, Kan Q, Fan R, Liu Z, Zhang M. Identification of low-dose radiation-induced exosomal circ-METRN and miR-4709-3p/GRB14/PDGFRα pathway as a key regulatory mechanism in Glioblastoma progression and radioresistance: functional validation and clinical theranostic significance. Int J Biol Sci. 2021;17:1061–78.
Ding C, Yi X, Chen X, Wu Z, You H, Chen X, Zhang G, Sun Y, Bu X, Wu X, Lin Z, Gu J, Lin Y, Kang D. Warburg effect-promoted exosomal circ_0072083 releasing up-regulates NANGO expression through multiple pathways and enhances temozolomide resistance in glioma. J Exp Clin Cancer Res. 2021;40:164.
Si J, Li W, Li X, Cao L, Chen Z, Jiang Z. Heparanase confers temozolomide resistance by regulation of exosome secretion and circular RNA composition in glioma. Cancer Sci. 2021;112:3491–506.
Jerabkova-Roda K, Dupas A, Osmani N, Hyenne V, Goetz JG. Circulating extracellular vesicles and tumor cells: sticky partners in metastasis. Trends Cancer. 2022. https://doi.org/10.1016/j.trecan.2022.05.002.
Rehman AU, Khan P, Maurya SK, Siddiqui JA, Santamaria-Barria JA, Batra SK, Nasser MW. Liquid biopsies to occult brain metastasis. Mol Cancer. 2022;21:113.
Tyagi A, Wu SY, Watabe K. Metabolism in the progression and metastasis of brain tumors. Cancer Lett. 2022;539:215713.
Rogiers A, Lobon I, Spain L, Turajlic S. The genetic evolution of metastasis. Cancer Res. 2022;82:1849–57.
Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, MorenoBueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar CM, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–91.
Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–26.
Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 2016;30:668–81.
Novo D, Heath N, Mitchell L, Caligiuri G, MacFarlane A, Reijmer D, Charlton L, Knight J, Calka M, McGhee E, et al. Mutant p53s generate pro-invasive niches by influencing exosome podocalyxin levels. Nat Commun. 2018;9:5069.
Cai Q, Zhu A, Gong L. Exosomes of glioma cells deliver miR-148a to promote proliferation and metastasis of glioblastoma via targeting CADM1. Bull Cancer. 2018;105:643–51.
Bian EB, Chen EF, Xu YD, Yang ZH, Tang F, Ma CC, Wang HL, Zhao B. Exosomal lncRNA-ATB activates astrocytes that promote glioma cell invasion. Int J Oncol. 2019;54:713–21.
Xu X, Liu Y, Li Y, Chen H, Zhang Y, Liu J, Deng S, Zheng Y, Sun X, Wang J, Chen T, Huang M, Ke Y. Selective exosome exclusion of miR-375 by glioma cells promotes glioma progression by activating the CTGF-EGFR pathway. J Exp Clin Cancer Res. 2021;40:16.
Qian M, Chen Z, Guo X, Wang S, Zhang Z, Qiu W, Qi Y, Zhang S, Xu J, Zhao R, Xue H, Li G. Exosomes derived from hypoxic glioma deliver miR-1246 and miR-10b-5p to normoxic glioma cells to promote migration and invasion. Lab Invest. 2021;101:612–24.
Han Y, Liu Y, Zhang B, Yin G. Exosomal circRNA 0001445 promotes glioma progression through miRNA-127-5p/SNX5 pathway. Aging (Albany NY). 2021;13:13287–99.
Geindreau M, Bruchard M, Vegran F. Role of cytokines and chemokines in angiogenesis in a tumor context. Cancers (Basel). 2022;14:2446.
Liu J, Zhang Q, Yang D, Xie F, Wang Z. The role of long non-coding RNAs in angiogenesis and anti-angiogenic therapy resistance in cancer. Mol Ther Nucleic Acids. 2022;28:397–407.
Kumar VBS, Anjali K. Tumour generated exosomal miRNAs: a major player in tumour angiogenesis. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166383.
Kasherman L, Liu SL, Karakasis K, Lheureux S. Angiogenesis: a pivotal therapeutic target in the drug development of gynecologic cancers. Cancers (Basel). 2022;14:1122.
Poto R, Cristinziano L, Modestino L, de Paulis A, Marone G, Loffredo S, Galdiero MR, Varricchi G. Neutrophil extracellular trap,s angiogenesis and cancer. Biomedicines. 2022;10:431.
Huang M, Lei Y, Zhong Y, Chung C, Wang M, Hu M, Deng L. New insights into the regulatory roles of extracellular vesicles in tumor angiogenesis and their clinical implications. Front Cell Dev Biol. 2021;9:791882.
Wang Z, Yuan Y, Ji X, Xiao X, Li Z, Yi X, Zhu Y, Guo T, Wang Y, Chen L, Liu Y. The Hippo-TAZ axis mediates vascular endothelial growth factor C in glioblastoma-derived exosomes to promote angiogenesis. Cancer Lett. 2021;513:1–13.
Lang HL, Hu GW, Zhang B, Kuang W, Chen Y, Wu L, Xu GH. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol Rep. 2017;38:785–98.
Sun X, Ma X, Wang J, Zhao Y, Wang Y, Bihl JC, Chen Y, Jiang C. Glioma stem cells-derived exosomes promote the angiogenic ability of endothelial cells through miR-21/VEGF signal. Oncotarget. 2017;8:36137–48.
Treps L, Perret R, Edmond S, Ricard D, Gavard J. Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles. J Extracell Vesicles. 2017;6:1359479.
Wang ZF, Liao F, Wu H, Dai J. Glioma stem cells-derived exosomal miR-26a promotes angiogenesis of microvessel endothelial cells in glioma. J Exp Clin Cancer Res. 2019;38:201.
Li J, Yuan H, Xu H, Zhao H, Xiong N. Hypoxic cancer-secreted exosomal miR-182-5p promotes glioblastoma angiogenesis by targeting Kruppel-like Factor 2 and 4. Mol Cancer Res. 2020;18:1218–31.
Wang M, Zhao Y, Yu ZY, Zhang RD, Li SA, Zhang P, Shan TK, Liu XY, Wang ZM, Zhao PC, Sun HW. Glioma exosomal microRNA-148a-3p promotes tumor angiogenesis through activating the EGFR/MAPK signaling pathway via inhibiting ERRFI1. Cancer Cell Int. 2020;20:518.
Jiang J, Lu J, Wang X, Sun B, Liu X, Ding Y, Gao G. Glioma stem cell-derived exosomal miR-944 reduces glioma growth and angiogenesis by inhibiting AKT/ERK signaling. Aging (Albany NY). 2021;13:19243–59.
Li Y, Chen J, Chen Z, Xu X, Weng J, Zhang Y, Mo Y, Liu Y, Wang J, Ke Y. CircGLIS3 promotes high-grade glioma invasion via modulating ezrin phosphorylation. Front Cell Dev Biol. 2021;9:663207.
Sun Z, Wang L, Zhou Y, Dong L, Ma W, Lv L, Zhang J, Wang X. Glioblastoma stem cell-derived exosomes enhance stemness and tumorigenicity of glioma cells by transferring notch1 protein. Cell Mol Neurobiol. 2020;40:767–84.
Li J, Liao T, Liu H, Yuan H, Ouyang T, Wang J, Chai S, Li J, Chen J, Li X, Zhao H, Xiong N. Hypoxic glioma stem cell-derived exosomes containing Linc01060 promote progression of glioma by regulating the MZF1/c-Myc/HIF1α axis. Cancer Res. 2021;81:114–28.
Chai Y, Wu HT, Liang CD, You CY, Xie MX, Xiao SW. Exosomal lncRNA ROR1-AS1 derived from tumor cells promotes glioma progression via regulating miR-4686. Int J Nanomed. 2020;15:8863–72.
Ding C, Yi X, Wu X, Bu X, Wang D, Wu Z, Zhang G, Gu J, Kang D. Exosome-mediated transfer of circRNA CircNFIX enhances temozolomide resistance in glioma. Cancer Lett. 2020;479:1–12.
Li X, Li X, Zhang B, He B. The role of cancer stem cell-derived exosomes in cancer progression. Stem Cells Int. 2022;2022:9133658.
Jin Y, Du N, Huang Y, Shen W, Tan Y, Chen YZ, Dou WT, He XP, Yang Z, Xu N, Tan C. Fluorescence analysis of circulating exosomes for breast cancer diagnosis using a sensor array and deep learning. ACS Sens. 2022;7:1524–32.
Du S, Qian J, Tan S, Li W, Liu P, Zhao J, Zeng Y, Xu L, Wang Z, Cai J. Tumor cell-derived exosomes deliver TIE2 protein to macrophages to promote angiogenesis in cervical cancer. Cancer Lett. 2022;529:168–79.
Oey O, Ghaffari M, Li JJ, Hosseini-Beheshti E. Application of extracellular vesicles in the diagnosis and treatment of prostate cancer: Implications for clinical practice. Crit Rev Oncol Hematol. 2021;167:103495.
Liu J, Ren L, Li S, Li W, Zheng X, Yang Y, Fu W, Yi J, Wang J, Du G. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021;11:2783–97.
Chen SW, Zhu SQ, Pei X, Qiu BQ, Xiong D, Long X, Lin K, Lu F, Xu JJ, Wu YB. Cancer cell-derived exosomal circUSP7 induces CD8(+) T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Mol Cancer. 2021;20:144.
Lin Y, Zheng ZH, Wang JX, Zhao Z, Peng TY. Tumor cell-derived exosomal Circ-0072088 suppresses migration and invasion of hepatic carcinoma cells through regulating MMP-16. Front Cell Dev Biol. 2021;9:726323.
Wang C, Huang CH, Gao Z, Shen J, He J, MacLachlan A, Ma C, Chang Y, Yang W, Cai Y, Lou Y, Dai S, Chen W, Li F, Chen P. Nanoplasmonic sandwich immunoassay for tumor-derived exosome detection and exosomal PD-L1 profiling. ACS Sens. 2021;6:3308–19.
Yu Z, Lin S, Xia F, Liu Y, Zhang D, Wang F, Wang Y, Li Q, Niu J, Cao C, Cui D, Sheng N, Ren J, Wang Z, Chen D. ExoSD chips for high-purity immunomagnetic separation and high-sensitivity detection of gastric cancer cell-derived exosomes. Biosens Bioelectron. 2021;194:113594.
He R, Wang Z, Shi W, Yu L, Xia H, Huang Z, Liu S, Zhao X, Xu Y, Yam JWP, Cui Y. Exosomes in hepatocellular carcinoma microenvironment and their potential clinical application value. Biomed Pharmacother. 2021;138:111529.
Yuan X, Qian N, Ling S, Li Y, Sun W, Li J, Du R, Zhong G, Liu C, Yu G, Cao D, Liu Z, Wang Y, Qi Z, Yao Y, Wang F, Liu J, Hao S, Jin X, Zhao Y, Xue J, Zhao D, Gao X, Liang S, Li Y, Song J, Yu S, Li Y. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics. 2021;11:1429–45.
Lin B, Tian T, Lu Y, Liu D, Huang M, Zhu L, Zhu Z, Song Y, Yang C. Tracing Tumor-derived exosomal PD-L1 by dual-aptamer activated proximity-induced droplet digital PCR. Angew Chem Int Ed Engl. 2021;60:7582–6.
Qiu Y, Yang Y, Yang R, Liu C, Hsu JM, Jiang Z, Sun L, Wei Y, Li CW, Yu D, Zhang J, Hung MC. Activated T cell-derived exosomal PD-1 attenuates PD-L1-induced immune dysfunction in triple-negative breast cancer. Oncogene. 2021;40:4992–5001.
Xiong H, Huang Z, Yang Z, Lin Q, Yang B, Fang X, Liu B, Chen H, Kong J. Recent progress in detection and profiling of cancer cell-derived exosomes. Small. 2021;17:e2007971.
Lin L, Wang H, Guo W, He E, Huang K, Zhao Q. Osteosarcoma-derived exosomal miR-501-3p promotes osteoclastogenesis and aggravates bone loss. Cell Signal. 2021;82:109935.
Chen F, Xu B, Li J, Yang X, Gu J, Yao X, Sun X. Hypoxic tumour cell-derived exosomal miR-340-5p promotes radioresistance of oesophageal squamous cell carcinoma via KLF10. J Exp Clin Cancer Res. 2021;40:38.
Jing B, Gai Y, Qian R, Liu Z, Zhu Z, Gao Y, Lan X, An R. Hydrophobic insertion-based engineering of tumor cell-derived exosomes for SPECT/NIRF imaging of colon cancer. J Nanobiotechnol. 2021;19:7.
Ma Z, Cui X, Lu L, Chen G, Yang Y, Hu Y, Lu Y, Cao Z, Wang Y, Wang X. Exosomes from glioma cells induce a tumor-like phenotype in mesenchymal stem cells by activating glycolysis. Stem Cell Res Ther. 2019;10:60.
Yin J, Zeng A, Zhang Z, Shi Z, Yan W, You Y. Exosomal transfer of miR-1238 contributes to temozolomide-resistance in glioblastoma. EBioMedicine. 2019;42:238–51.
Acknowledgements
We thank the generous support of Liaoning Cancer Hospital & Institute (Shenyang) and Dalian University of Technology (Dalian).
Funding
This work is supported by the Fundamental Research Funds for the Central University (2021-YGJC-17).
Author information
Authors and Affiliations
Contributions
Original draft preparation, allocation, revision: XG and RS. Supplement and edition: HP. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, X., Sui, R. & Piao, H. Tumor-derived small extracellular vesicles: potential roles and mechanism in glioma. J Nanobiotechnol 20, 383 (2022). https://doi.org/10.1186/s12951-022-01584-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-022-01584-6