Abstract
Background
Malaria vectors vary in feeding preference depending on their innate behaviour, host availability and abundance. Host preference and human biting rate in malaria vectors are key factors in establishing zooprophylaxis and zoopotentiation. This study aimed at assessing the impact of non-human hosts in close proximity to humans on the human biting rate of primary and secondary malaria vectors, with varying host preferences.
Methods
The effect of the presence of non-human hosts in close proximity to the human host on the mean catches per person per night, as a proxy for mosquito biting rate, was measured using mosquito-electrocuting traps (METs), in Sagamaganga, Kilombero Valley, Tanzania. Two experiments were designed: (1) a human versus a calf, each enclosed in a MET, and (2) a human surrounded by three calves versus a human alone, with each human volunteer enclosed individually in a MET spaced 10 m apart. Each experiment was conducted on alternate days and lasted for 36 nights per experiment. During each experiment, the positions of hosts were exchanged daily (except the human in experiment 2). All anopheline mosquitoes caught were assayed for Plasmodium sporozoites using enzyme-linked immunosorbent assay.
Results
A total of 20,574 mosquitoes were captured and identified during the study, of which 3608 were anophelines (84.4% primary and 15.6% secondary malaria vectors) and 17,146 were culicines. In experiment 1, the primary malaria vector, Anopheles arabiensis, along with Culex spp. demonstrated a preference for cattle, while the primary vectors, Anopheles funestus, preferred humans. In experiment 2, both primary vectors, An. arabiensis and An. funestus, as well as the secondary vector Anopheles rivolurum, demonstrated behaviours amenable to zooprophylaxis, whereas Culex spp. increased their attraction to humans in the presence of nearby cattle. All anopheline mosquitoes tested negative for sporozoites.
Conclusions
The findings of this study provide support for the zooprophylaxis model for malaria vectors present in the Kilombero Valley, and for the zoopotentiation model, as it pertains to the Culex spp. in the region. However, the factors regulating zooprophylaxis and zoopotentiation are complex, with different species-dependent mechanisms regulating these behaviours, that need to be considered when designing integrated vector management programmes.
Similar content being viewed by others
Background
The host-feeding preference of malaria vectors is complex and may be modulated by the access and availability of preferred hosts, as well as the abundance of alternative hosts [1,2,3,4]. For example, using blood meal analysis, the highly anthropophilic Anopheles gambiae sensu stricto (s.s.) has been demonstrated to feed more frequently on non-human hosts in areas where human hosts are not readily accessible [3, 5,6,7]. In contrast, An. gambiae s.s. and Anopheles pharoensis exhibit greater anthropophily during direct side-by-side experimental comparison between human and non-human hosts [3, 4]. The demonstrated variability in host selection by vectors generally considered anthropophilic in the broader literature, may either increase or decrease the risk of transmission through changing human-vector contact, which is referred to as zoopotentiation (increased contact) or zooprophylaxis (decreased contact) [1, 8]. The usefulness, as well as the potential risks, associated with control strategies based on zooprophylaxis is fundamentally linked with the degree of host preference demonstrated by the local vector communities, and requires a standardized, ethically acceptable, direct measure of host preference in primary and secondary vectors at a local and regional level.
Host-feeding patterns determine both the frequency of blood feeding by vectors and the ability of the vector to transmit disease agents [9,10,11]. Changes in host preference in response to the use of control interventions and changes in host availability have been demonstrated for several primary malaria vectors, including Anopheles arabiensis, Anopheles funestus s.s. and An. gambiae s.s. [12, 13]. While many An. funestus s.s. and An. gambiae s.s. populations continue to demonstrate their ancestral anthropophagic, endophilic and endophagic behaviours [7, 14,15,16], these and other species, including An. arabiensis, currently the predominant malaria vector in sub-Saharan Africa [12, 17, 18], are reported to increasingly vary their patterns of blood feeding on hosts depending on host availability, particularly in the presence of cattle [2, 8, 19,20,21,22]. There is thus a need for regular surveillance of host-feeding preference in not only primary, but also secondary malaria vectors, to assess how these behavioural changes may affect the efficacy of current vector control tools, and the risk of disease transmission. As moving towards the malaria elimination target of 2030 set for Tanzania [23], the host feeding patterns of even the seemingly less important secondary vectors that are critical in sustaining transmission should be addressed.
Malaria vectors in the Kilombero valley, and other parts of Tanzania, are exhibiting alterations in patterns of human feeding similar to that observed in other malaria endemic regions of Africa, regulated by the availability and abundance of alternative hosts, such as cattle [12,13,14, 24,25,26]. However, as in most malaria endemic areas, information on the extent of variation in anthropophagy is either mostly lacking, or nor regularly updated [27]. Major reasons for this include the lack of reliable and ethically acceptable tools for the direct assessment of mosquito biting rate, as well as a lack of funding to conduct studies in many localities for a more comprehensive conclusion on vector bionomics. To overcome the first challenge, recent studies have demonstrated that the mosquito-electrocuting trap (MET) is a viable, sensitive tool for directly assessing mosquito attraction to hosts, and a proxy for human biting rate [13, 28,29,30]. The current study employed the MET to assess the effect of non-human host (cattle) availability on the human attraction of primary and secondary malaria vectors within the Kilombero valley. How the availability of alternate hosts alters the anthropophagy of malaria vectors, and the implications for disease transmission risk and control, are discussed.
Methods
Study area
The study was conducted in Sagamaganga village (S 8° 3′ 50.352″ E 36° 47′ 46.254″), which is situated ca. 17 km from Ifakara town within the Kilombero River Valley, south-eastern Tanzania. The valley experiences an average annual rainfall ranging from 1200 to 1800 mm, and annual temperatures ranging between 20 and 32 °C [31, 32]. There are two main seasons: the wet season, between February to June, and the dry season, from July to January. Anopheles arabiensis is the predominant malaria vector in the area, followed by An. funestus s.s. and An. gambiae s.s. [13, 14, 33,34,35]. In the Kilombero Valley, the most commonly reported secondary vectors are Anopheles coustani and Anopheles squamosus [34, 35]. Most malaria cases are caused by Plasmodium falciparum, with the rate of prevalence decreasing from 14% in 2007–2011 [32, 36] to 0.4% in 2019 (Swai Kyeba, pers. commun.). Most of the residents in the area practice subsistence agriculture, particularly rice and maize cultivation, as well as livestock rearing. Cattle are the most common livestock species in the area, followed by sheep, goats, chickens and dogs [34].
Effect of non-human host availability on human mosquito biting rate
To assess the mean catches per person per night of primary and secondary malaria vectors, as a proxy for mosquito biting rate, and how this may be modulated by the presence of non-human hosts in near proximity, two experimental designs were used. In the first experiment, a single human and a single calf were each placed in METs (four panels placed in a square, each panel measuring 125 cm width × 122 cm height), which were set 20 m apart in an open field, 100 m away from human habitation, as per procedures described by Govella et al. and Githu et al. [28, 37]. The rationale for the short distance between the METs was to ensure competition between the hosts enclosed within each trap [37]. The traps were deployed so that neither trap was upwind of the other, in relation to the prevailing wind direction (Fig. 1). The treatments were exchanged between the two trap positions daily. Following the determination of host preference for each primary and secondary malaria vector caught in this location in experiment 1, a second experiment was conducted to assess the effect of non-human hosts in near proximity to a human host on the mosquito biting rate. In experiment 2, a single human in a MET was surrounded by three tethered calves, kept at 90° angle to one another and 10 m away from one of the MET-enclosed human volunteers (Fig. 1). A second human volunteer was enclosed in a MET and spaced 20 m apart from the other volunteer (Fig. 1). The rationale for the short distance between the human volunteers, and between the three calves and one of the human volunteers was to ensure interaction among the hosts, without influencing captures in the MET not surrounded by cattle (Fig. 1). The human volunteers were exchanged between the treatments daily. The two experiments were evaluated on alternate nights, for a total of 72 nights, from Dec 2019 to July 2021. For every instance of experiment 1, experiment 2 was conducted on subsequent nights to avoid any seasonal variation in the trap capture rates. The human volunteers weighed on average 70 ± 2 kg, while the calves weighed on average 68 ± 2 kg, in order to control for a similar release rate of host odour. In order to minimize distress, the calves were milk-fed immediately prior to the start of the experiments after returning with their mothers to the overnight enclosure, which delayed the onset of experiments by 1 h, compared to that which is deemed the standard onset time, 18h00, of similar field experiments in this region. Between 19h00 and 06h45, traps were active in 45 min bouts, and then the traps were switched off, the electrocuted mosquitoes collected using forceps and hand-held aspirators, and those which stuck to the surface of the panels were gently removed using a small brush prior to collection. Electrocuted mosquitoes were kept separately, by hour, in labelled paper cups. The voltage for the METs was checked regularly to ensure consistent power throughout the trapping period. Every morning, the collected mosquitoes were transported to the laboratory at Ifakara Health Institute, for morphological and molecular identification.
The effect of host availability on human preference. Diagrammatic representation of a two-choice assay with a human volunteer and a calf, each enclosed in mosquito-electrocuting traps (METs; red cubes), set 20 m apart (top panel); and b two human volunteers, each enclosed in METs, with one surrounded by three calves, each 10 m from the human volunteer (middle panel). a A human volunteer in the MET. b The collection of mosquitoes from the white sheets and electric grids around the calf every 45 min, throughout the night. c The set-up of the two-choice assay in an open field, 100 m away from human habitation
Mosquito identification and sporozoite detection
All mosquitoes collected were sorted, counted and morphologically identified in the laboratory with the aid of a dissection microscope [38, 39]. Morphologically, the mosquitoes were identified as members of the An. gambiae and An. funestus species complexes, as well as An. coustani, Anopheles ziemanni, Culex spp. and Aedes spp. Moreover, these mosquitoes were classified by sex. All mosquitoes identified as belonging to the An. gambiae (n = 2 532) and An. funestus (n = 577) species complexes were subsequently identified to species level using multiplexed polymerase chain reaction [40,41,42]. In addition, circumsporozoite enzyme linked immunosorbent assay (ELISA; IgG identifiers, KPL, Gaithersburg, US) was performed on all primary and secondary malaria vectors (n = 2 828) to detect the presence of malaria parasites as described by Burkot et al. and Wirtz et al. [43, 44]. To avoid false positives, the ELISA lysates were heated in a water bath at 100 °C for 10 min to inactivate heat-labile antigens other than the P. falciparum circumsporozoite proteins [45].
Statistical analysis
Data handling and analysis was done using R statistical software (version 4.0.2) and JMP® Pro (version 16.0.0, SAS Institute Inc., Cary, NC, 1989–2022). The total number of mosquitoes caught in the two experiments (Tables 1 and 2) were compared by species using nominal regression (JMP® Pro 16.0.0, SAS Institute Inc.). The proportions of primary and secondary malaria vectors captures were compared between traps in experiment 1, volunteer (METh) vs. calf (METc), and experiment 2, volunteer (METh0) vs. calf-surrounded volunteer (METhC), using a chi-squared test (JMP® Pro 16.0.0, SAS Institute Inc.). The mosquito biting rate, reported as mean catches per person per night, were calculated using the Rmisc package, and compared for each species using generalized linear mixed models (GLMMs) augmented with the matrix, lattice and lme4 packages [46] (R statistical software 4.0.2). Separate analyses was performed for experiment 1 and experiment 2, as well as for each mosquito species collected. Since the data were zero-inflated and over-dispersed (Shapiro test), a negative binomial distribution was employed [46]. The mosquito catches per person per night were treated as a dependent variable, with capturing method fitted as an independent fixed effect, and sampling night and positions of the traps as random effects. To quantify the likelihood associated with each comparison, the relative risks and their respective 95% confidence intervals are reported in Tables 3 and 4. All analyses used a significance level (alpha) of 0.05. Since all mosquitoes from both experiments 1 and 2 were sporozoite negative, no statistical analysis was performed on these datasets.
Results
Mosquito catches
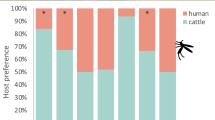
A total of 20754 mosquitoes were captured throughout the study, of which 3610 (3426 females, 184 males) were anophelines and 17146 (15209 females, 1937 males) were culicines. The collected female anophelines consisted of 2890 (84.4%) primary (Tables 1 and 2, asterisks) and 536 (15.6%) secondary malaria vector species (Tables 1 and 2). The presence of cattle surrounding one volunteer significantly affected the total numbers of mosquitoes caught in experiment 2 compared with experiment 1 in a species-specific manner (Fig. 2).
Experiment 1
To investigate mosquito host preference, the number of mosquitoes for each species caught in human- and calf-baited competing METs were analysed. The proportion of captured primary and secondary malaria vectors varied significantly between METc and METh (χ12 = 35.129, P < 0.001) in the two-choice experiment. For the most abundant primary malaria vector in the area, An. arabiensis, the mean number of mosquitoes caught in METh was significantly lower than that caught in METc (Table 3). In contrast, the mean number of An. funestus caught in METh was significantly higher than that caught in METc (Table 3). While no significant effect of host was found for the secondary malaria vectors, both An. coustani and An. rivolurum were caught in higher numbers in METc than that in METh (Table 3). Moreover, Culex spp. were captured in significantly higher numbers in METc than in METh (Table 3).
Experiment 2
To evaluate the effect of non-human host presence in close proximity to a human host on mosquito biting rate, the number of mosquitoes for each species caught in human-baited METs, with and without surrounding calves, was analysed. The proportion of captured primary and secondary malaria vectors varied significantly between METh0 and METhC (χ12 = 9.9669, P = 0.002), in the dual-choice experiment. For An. arabiensis, the mean number of mosquitoes captured in METhC was significantly lower compared to that in METh0 (Table 4). In contrast, An. funestus, and An. rivolurum, were caught in significantly higher numbers in the METh0 compared to the METhC (Table 4). Culex spp. were captured in significantly higher numbers in METhC than in METh0 (Table 4).
Discussion
By limiting the number of factors associated with livestock-human interactions, the findings presented in this study demonstrate a species-dependent change in biting rate by primary and secondary vectors in the presence of cattle in close proximity. The availability of livestock, which act as dead-end hosts for malaria parasites, has the potential to change the interaction between vectors and humans, thereby modulating malaria transmission. While zooprophylaxis has been advocated as a vital part of integrated vector management, the underlying mechanism driving its effective design is still debated, with the characteristics of the local vectors and the location of the livestock in relation to human dwellings identified as key factors [1, 47]. The findings are discussed in relation to zoopotentiation and zooprophylaxis.
The host preference of mosquitoes exhibited in the two-choice experiment (experiment 1) reflected the anthropophilic response of An. funestus [14] and the generally zoophilic/opportunistic behaviour of An. arabiensis, An. rivulorum and Culex spp., as previously described in Kilombero Valley and beyond [2, 13, 47,48,49]. In such a scenario, the rationale for implementing zooprophylaxis as a malaria control measure appears valid [1, 8]. By increasing the number of cattle in relation to a single person, the zooprophylaxis model would suggest a reduction in the human biting rate, in favour of mosquitoes feeding on the surrounding cattle [50]. The findings from this study support the zooprophylaxis model for the An. arabiensis and An. rivolurum, as well as for the anthropophilic An. funestus s.s. In all three instances, fewer mosquitoes were caught by the human-baited MET surrounded by calves, compared to a similar MET without cattle positioned in close proximity. While a previous study suggested that the zooprophylactic effect of nearby cattle was dependent on the location of the human host and the malaria vectors present [51], this study demonstrated that this effect was present outdoors for all three species. A comparison between the overall number of mosquitoes caught in experiment 1 and 2, however, suggests that the mechanism underlying the zooprophylactic effect appears to be species dependent. The reduction by half of An. arabiensis caught by the human-baited METs in experiment 2 indicates that the calves are likely providing additional hosts for the mosquitoes, and thus reducing the biting rate. The doubling of the number of An. funestus s.s. caught during the same experiment, however, correlates with the doubling of its preferred human host, and suggests that the zooprophylaxis is related to the avoidance of cattle odour. The similar overall numbers of zoophilic An. rivolurum caught between the two experiments, together with the demonstrated reduction in mosquitoes caught in the human-baited trap surrounded by cattle, indicates that these mosquitoes may be avoiding the combination of human and cattle odour. While data from this study demonstrate that both primary and secondary vectors in the Kilombero Valley are amenable to zooprophylaxis, the mechanism by which this control measure may be effective differs according to vector species, rather than strictly host preference, as suggested by previous studies [1, 4, 12, 13, 52].
Zoopotentiation, an increase in the human biting rate in the presence of potential alternate hosts, appears to be species-dependent and can be a major detractor for the implementation of zooprophylaxis-based vector control strategies in multi-vector environments [4, 53,54,55,56,57,58,59]. The findings from this study demonstrated that more of the zoophilic Culex spp. [this study, 60–64] were caught in human-baited METs associated with cattle than those without, indicating that using a zooprophylaxis vector control strategy in areas with large numbers of Culex spp. will likely increase the human biting rate, and the disease transmission, associated with these species. These results call into question the previous reports that suggest a major role of host preference as a predictor of the potential efficacy of the zooprophylaxis model [1, 52].
All the primary and secondary malaria vectors collected in this study were negative for malaria parasites, supporting the decreased malaria transmission in the region from 14% in the early 2000s to 0.4% in 2019 [33, 37, 65; Swai Kyeba, pers. commun.]. The lack of sporozoite-positive malaria vectors is consistent with reports from this region in recent studies [32]. The decline in malaria prevalence during the last two decades has been suggested to be due to urbanization, improved house construction and the use of LLINs in combination with a livestock-keeping lifestyle in the study area and beyond [32, 65]. The relatively low rate of malaria transmission described suggests that zooprophylaxis may be a useful control strategy in this region toward a further reduction in malaria [62], with the caveat that nuisance biting by Culex spp. is likely to remain a problem in the region, and may pose a risk of heightened transmission of diseases these mosquitoes may carry. It should be noted that this study was conducted on a single site thus limiting the generalization of the findings, while providing a clear workflow for the determination of the degree of host preference in local populations in a direct, ethically acceptable manner. While providing a proof-in-principle, this workflow is not cost-effective in the current state, and requires additional modification prior to implementation within established vector control programmes.
Conclusion
As previous studies indicate, the efficacy of zooprophylaxis as a control method is uncertain in ecosystems with several vectors and human disease agents. This study provides support for the zooprophylaxis model for malaria vectors present in the Kilombero Valley, but also for the zoopotentiation model as it pertains to the Culex spp. in the region. The role of host abundance in relation to human biting requires further investigation, particularly into which hosts regulate the observed effects. As a whole, this study emphasizes the complexity of factors regulating the efficacy of zooprophylaxis and highlights the danger of making assumptions concerning its use in controlling multi-vector systems based on previously determined host preference.
Availability of data and materials
Upon request, the data and materials will be made available under defined conditions expressed in writing through an exchange of letters between parties stipulating those conditions and any agreed limits thereof.
Abbreviations
- WHO:
-
World Health Organization
- MET:
-
Mosquito-electrocuting trap
- IRS:
-
Indoor residual spraying
- LLIN:
-
Long-lasting insecticidal nets
- CDC:
-
Centers for Disease Control and Prevention
- SSA:
-
Sub-Saharan Africa
References
Donnelly B, Berrang-Ford L, Ross NA, Michel P. A systematic, realist review of zooprophylaxis for malaria control. Malar J. 2015;14:313.
Mayagaya VS, Nkwengulila G, Lyimo IN, Kihonda J, Mtambala H. The impact of livestock on the abundance, resting behaviour and sporozoite rate of malaria vectors in southern Tanzania. Malar J. 2015;14:17.
Lefèvre T, Gouagna LC, Dabiré KR, Elguero E, Fontenille D, Renaud F, et al. Beyond nature and nurture: phenotypic plasticity in blood-feeding behavior of Anopheles gambiae s.s. when humans are not readily accessible. Am J Trop Med. 2009;81:1023–9.
Zeru MA, Shibru S, Massebo F. Exploring the impact of cattle on human exposure to malaria mosquitoes in the Arba Minch area district of southwest Ethiopia. Parasit Vectors. 2020;13:322.
Sousa CA, Pinto J, Almeida PG, Ferreira C, Do Rosário VE, Charlwood JD. Dogs as a favored host choice of Anopheles gambiae sensu stricto (Diptera: Culicidae) of Sao Tome, west Africa. J Med Entomol. 2001;38:122–5.
Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island. Equatorial Guinea Malar J. 2011;10:184.
Degefa T, Yewhalaw D, Zhou G, Lee MC, Atieli H, Githeko AK, et al. Indoor and outdoor malaria vector surveillance in western Kenya: implications for better understanding of residual transmission. Malar J. 2017;16:1–3.
Asale A, Duchateau L, Devleesschauwer B, Huisman G, Yewhalaw D. Zooprophylaxis as a control strategy for malaria caused by the vector Anopheles arabiensis (Diptera: Culicidae): a systematic review. Infect Dis Poverty. 2017;6:160.
Bashar K, Tuno N, Ahmed T, Howlader A. Blood-feeding patterns of Anopheles mosquitoes in a malaria-endemic area of Bangladesh. Parasit Vectors. 2012;5:39.
Pumpaibool T, Chakim I. The diversity of Anopheles blood feeding patterns suggests different malaria protection strategies in different localities. F1000Research. 2020;8:1217.
Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. 2013;58:433–53.
Mlacha YP, Chaki PP, Muhili A, Massue DJ, Tanner M, Majambere S, et al. Reduced human-biting preferences of the African malaria vectors Anopheles arabiensis and Anopheles gambiae in an urban context: controlled, competitive host-preference experiments in Tanzania. Malar J. 2020;19:418.
Meza FC, Kreppel KS, Maliti DF, Mlwale AT, Mirzai N, Killeen GF, et al. Mosquito electrocuting traps for directly measuring biting rates and host-preferences of Anopheles arabiensis and Anopheles funestus outdoors. Malar J. 2019;18:83.
Kaindoa EW, Matowo NS, Ngowo HS, Mkandawile G, Mmbando A, Finda M, et al. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south-eastern Tanzania. PLoS ONE. 2017;12: e0177807.
Mburu MM. Indoor and outdoor biting behaviour of malaria vectors and the potential risk factors that enhance malaria in southern Malawi [PhD thesis]. Wageningen University and Research, Wageningen 2019;198. https://edepot.wur.nl/471415. Accessed 9 May 2022.
Gibson G. Genetics, ecology and behaviour of anophelines. In: Ciba Foundation Symposium 200‐Olfaction in Mosquito‐Host Interactions. Chichester: Wiley. 2007:22–47.
Sherrard-Smith E, Skarp JE, Beale AD, Fornadel C, Norris LC, Moore SJ, et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc Natl Acad Sci USA. 2019;116:15086–95.
Cooke MK, Kahindi SC, Oriango RM, Owaga C, Ayoma E, Mabuka D, et al. “A bite before bed”: exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar J. 2015;14:259.
Ekoko WE, Awono-Ambene P, Bigoga J, Mandeng S, Piameu M, Nvondo N, et al. Patterns of anopheline feeding/resting behaviour and Plasmodium infections in North Cameroon, 2011–2014: implications for malaria control. Parasit Vectors. 2019;12:297.
Tirados I, Costantini C, Gibson G, Torr SJ. Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: implications for vector control. Med Vet Entomol. 2006;20:425–37.
Sougoufara S, Diédhiou SM, Doucouré S, Diagne N, Sembène PM, Harry M, et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malar J. 2014;13:125.
Killeen GF, McKenzie FE, Foy BD, Bøgh C, Beier JC. The availability of potential hosts as a determinant of feeding behaviours and malaria transmission by African mosquito populations. Trans R Soc Trop Med Hyg. 2001;95:469–76.
Finda MF, Christofides N, Lezaun J, Tarimo B, Chaki P, Kelly AH, et al. Opinions of key stakeholders on alternative interventions for malaria control and elimination in Tanzania. Malar J. 2020;19:164.
Lwetoijera DW, Harris C, Kiware SS, Dongus S, Devine GJ, McCall PJ, et al. Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley. Tanzania Malar J. 2014;13:331.
Adugna T, Yewhelew D, Getu E. Bloodmeal sources and feeding behavior of anopheline mosquitoes in Bure district, northwestern Ethiopia. Parasit Vectors. 2021;14:166.
Fornadel CM, Norris DE. Increased endophily by the malaria vector Anopheles arabiensis in southern Zambia and identification of digested blood meals. Am J Trop Med Hyg. 2008;79:876–80.
Stone C, Gross K. Evolution of host preference in anthropophilic mosquitoes. Malar J. 2018;17:257.
Govella NJ, Maliti DF, Mlwale AT, Masallu JP, Mirzai N, Johnson PCD, et al. An improved mosquito electrocuting trap that safely reproduces epidemiologically relevant metrics of mosquito human-feeding behaviours as determined by human landing catch. Malar J. 2016;15:465.
Ortega-López LD, Pondeville E, Kohl A, León R, Betancourth MP, Almire F, et al. The mosquito electrocuting trap as an exposure-free method for measuring human-biting rates by Aedes mosquito vectors. Parasit Vectors. 2020;13:31.
Sanou A, Moussa Guelbéogo W, Nelli L, Hyacinth Toé K, Zongo S, Ouédraogo P, et al. Evaluation of mosquito electrocuting traps as a safe alternative to the human landing catch for measuring human exposure to malaria vectors in Burkina Faso. Malar J. 2019;18:386.
Augustino S, Eriksen S, Makonda F, Amanzi N. Climate change adaptation strategies by local farmers in Kilombero District. Tanzania Ethiop J Environ Stud Manag. 2013;6:724–36.
Finda MF, Limwagu AJ, Ngowo HS, Matowo NS, Swai JK, Kaindoa E, et al. Dramatic decreases of malaria transmission intensities in Ifakara, south–eastern Tanzania since early 2000s. Malar J. 2018;17:362.
Ngowo HS, Kaindoa EW, Matthiopoulos J, Ferguson HM, Okumu FO. Variations in household microclimate affect outdoor-biting behaviour of malaria vectors. Wellcome Open Res. 2017;2:102.
Katusi GC, Hermy MR, Makayula SM, Ignell R, Govella NJ, et al. Seasonal variation in abundance and blood meal sources of primary and secondary malaria vectors within Kilombero Valley. Southern Tanzania Parasit Vectors. 2022;15:479.
Kaindoa EW, Finda M, Kiplagat J, Mkandawile G, Nyoni A, Coetzee M, et al. Housing gaps, mosquitoes and public viewpoints: a mixed methods assessment of relationships between house characteristics, malaria vector biting risk and community perspectives in rural Tanzania. Malar J. 2018;17:298.
Harchut K, Standley C, Dobson A, Klaassen B, Rambaud-althaus C. Over-diagnosis of malaria by microscopy in the Kilombero Valley, Southern Tanzania: an evaluation of the utility and cost-effectiveness of rapid diagnostic tests. Malar J. 2013;12:159.
Githu V, Baravuga ME, Mbarawa A, Msuya HM, Mlacha YP, Chaki PP, et al. Comparative evaluation of different versions of exposure-free mosquito electrocuting traps and barrier screen trap for monitoring outdoor densities and biting time phenotypes by malaria and filariasis vectors in Tanzania. Parasit Vectors. 2022;15:420.
Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. Publ S Afr Inst Med Res. 1987;55:1–43.
Gillies M, Meillon D. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region). Johannesburg: S Afr Inst Med Res. 1968.
Cornel AJ, Porter CH, Collins FH. Polymerase chain reaction species diagnostic assay for Anopheles quadrimaculatus cryptic species (Diptera: Culicidae) based on ribosomal DNA ITS2 sequences. J Med Entomol. 1996;33:109–16.
Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;66:804–11.
Cohuet A, Simard F, Toto JC, Kengne P, Coetzee MA, Fontenille D. Species identification within the Anopheles funestus group of malaria vectors in Cameroon and evidence for a new species. Am J Trop Med. 2003;69:200–5.
Burkot TR, Graves PM, Cattan JA, Wirtz RA, Gibson FD. The efficiency of sporozoite transmission in the human malarias, Plasmodium falciparum and P. vivax. Bull World Health Organ. 1987;65:375–80.
Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot TR, Schneider I, et al. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65:39.
Durnez L, Van Bortel W, Denis L, Roelants P, Veracx A, Trung HD, et al. False positive circumsporozoite protein ELISA: A challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar J. 2011;10:195.
Team RC. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. 2016. https://www.R-project.org.
Mahande A, Mosha F, Mahande J, Kweka E. Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis. Malar J. 2007;6:100.
Massebo F, Balkew M, Gebre-Michael T, Lindtjørn B. Zoophagic behaviour of anopheline mosquitoes in southwest Ethiopia: opportunity for malaria vector control. Parasit Vectors. 2015;18:8.
Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2010;3:117.
Sota T, Mogi M. Effectiveness of zooprophylaxis in malaria control: a theoretical inquiry, with a model for mosquito populations with two bloodmeal hosts. Med Vet Entomol. 1989;3:337–45.
Tirados I, Gibson G, Young S, Torr SJ. Are herders protected by their herds? An experimental analysis of zooprophylaxis against the malaria vector Anopheles arabiensis. Malar J. 2011;10:68.
Saul A. Zooprophylaxis or zoopotentiation: the outcome of introducing animals on vector transmission is highly dependent on the mosquito mortality while searching. Malar J. 2003;2:32.
Temu EA, Coleman M, Abilio AP, Kleinschmidt I. High prevalence of malaria in Zambezia, Mozambique: the protective effect of IRS versus increased risks due to pig-keeping and house construction. PLoS ONE. 2012;7: e31409.
Bouma M, Rowland M. Failure of passive zooprophylaxis: cattle ownership in Pakistan is associated with a higher prevalence of malaria. Trans R Soc Trop Med Hyg. 1995;89:351–3.
Hasyim H, Dhimal M, Bauer J, Montag D, Groneberg DA, Kuch U, et al. Does livestock protect from malaria or facilitate malaria prevalence? A cross-sectional study in endemic rural areas of Indonesia. Malar J. 2018;17:302.
Chaccour C. Veterinary endectocides for malaria control and elimination: prospects and challenges. Philos Trans R Soc Lond B Biol Sci. 2021;376:20190810.
Hewitt S, Rowland M. Control of zoophilic malaria vectors by applying pyrethroid insecticides to cattle. Trop Med Int Health. 1999;4:481–6.
Schultz GW. Animal influence on man-biting rates at a malarious site in Palawan, Philippines. Southeast Asian J Trop Med Public Health. 1989;20:49–53.
Waite JL, Swain S, Lynch PA, Sharma SK, Haque MA, Montgomery J, Thomas MB. Increasing the potential for malaria elimination by targeting zoophilic vectors. Sci Rep. 2017;7:40551.
Boyer S, Durand B, Yean S, Brengues C, Maquart PO, Fontenille D, et al. Host-feeding preference and diel activity of mosquito vectors of the Japanese encephalitis virus in rural Cambodia. Pathogens. 2021;10:376.
Musa AA, Muturi MW, Musyoki AM, Ouso DO, Oundo JW, Makhulu EE, et al. Arboviruses and blood meal sources in zoophilic mosquitoes at human-wildlife interfaces in Kenya. Vector Borne Zoonotic Dis. 2020;20:444–53.
Richards SL, Anderson SL, Yost SA. Effects of blood meal source on the reproduction of Culex pipiens quinquefasciatus (Diptera: Culicidae). J Vector Ecol. 2012;37:1–7.
Muturi EJ, Muriu S, Shililu J, Mwangangi JM, Jacob BG, Mbogo C, Githure J, et al. Blood-feeding patterns of Culex quinquefasciatus and other culicines and implications for disease transmission in Mwea rice scheme. Kenya Parasitol Res. 2008;102:1329–35.
Finda MF, Moshi IR, Monroe A, Limwagu AJ, Nyoni AP, Swai JK, et al. Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PLoS ONE. 2019;14: e0217414.
Bulterys PL, Mharakurwa S, Thuma PE. Cattle, other domestic animal ownership, and distance between dwelling structures are associated with reduced risk of recurrent Plasmodium falciparum infection in southern Zambia. Trop Med Int Health. 2009;14:522–8.
Acknowledgements
We would like to express our deep appreciation to all volunteers, community leaders and residents of Sagamaganga village for their cooperation throughout the study. We are also indebted to the residents who kindly provided us with experimental calves. We also thank Sambo Maganga, Nicolaus Mwakalinga, Mseti William, Anold Mmbando, Halfan Ngowo and Emmanuel Kaindoa for great technical support. Also, all volunteers who participated in the collection of mosquitoes, namely Shinje Giduka, Ngunda Elia, Kulwa Gwala, and Giloti Gwala, are warmly thanked. The authors wish to especially thank the staff from Ifakara Health Institute and NIMR Amani centre, and retired Mr. J Myamba for their support during the project.
Funding
Open access funding provided by Swedish University of Agricultural Sciences. This work was supported by the Swedish Research council by a grant awarded to SRH (nr. 2017–05536). Salary support for NJG during the writing of the manuscript, is supported by the African Research Leaders Award (Grant Ref: MR/T008873/1), jointly funded by the UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO) under the MRC/FCDO Concordat agreement, which is part of the EDCTP2 programme supported by the European Union.
Author information
Authors and Affiliations
Contributions
GCK, NJG, SRH, RI and LLM designed the study and prepared the detailed protocol. GCK and SMM conducted the experiments. GCK performed the data analysis, interpreted the results and drafted the manuscript. NJG, SRH, RI, LLM and MRGH contributed to the data analysis, interpretation of results and conducted a series of revisions on the manuscript. All authors have read and approved the final manuscript submitted for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the study was obtained from the Ifakara Health Institute Institutional Review Board (IHI/IRB/EXT/No: 23–2019) and the Medical Research Coordination Committee of the National Institute for Medical Research (Certificate No. NIMR/HQ/R.8a/Vol.IX/3085). Before the commencement of data collection, we organised meetings with the study communities to explain the purpose and data collection procedures. A signed informed consent was obtained from the human volunteers and the heads of households, which provided us with experimental calves. The informed consent was translated to Kiswahili, since all the participants understood this language.
Consent for publication
Permission to publish this work was also obtained from NIMR (Ref: NIMR/HQ/P.12 VOL XXXIV/68).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Katusi, G.C., Hermy, M.R.G., Makayula, S.M. et al. Effect of non-human hosts on the human biting rate of primary and secondary malaria vectors in Tanzania. Malar J 22, 340 (2023). https://doi.org/10.1186/s12936-023-04778-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-023-04778-x