Abstract
Background
Entomological monitoring of Aedes vectors has largely relied on surveillance of larvae, pupae and non-host-seeking adults, which have been poorly correlated with human disease incidence. Exposure to mosquito-borne diseases can be more directly estimated using human landing catches (HLC), although this method is not recommended for Aedes-borne arboviruses. We evaluated a new method previously tested with malaria vectors, the mosquito electrocuting trap (MET) as an exposure-free alternative for measuring landing rates of Aedes mosquitoes on people. Aims were to (i) compare the MET to the BG-sentinel (BGS) trap gold standard approach for sampling host-seeking Aedes vectors; and (ii) characterize the diel activity of Aedes vectors and their association with microclimatic conditions.
Methods
The study was conducted over 12 days in Quinindé (Ecuador) in May 2017. Mosquito sampling stations were set up in the peridomestic area of four houses. On each day of sampling, each house was allocated either a MET or a BGS trap, which were rotated amongst the four houses daily in a Latin square design. Mosquito abundance and microclimatic conditions were recorded hourly at each sampling station between 7:00–19:00 h to assess variation between vector abundance, trapping methods, and environmental conditions. All Aedes aegypti females were tested for the presence of Zika (ZIKV), dengue (DENV) and chikungunya (CHIKV) viruses.
Results
A higher number of Ae. aegypti females were found in MET than in BGS collections, although no statistically significant differences in mean Ae. aegypti abundance between trapping methods were found. Both trapping methods indicated female Ae. aegypti had bimodal patterns of host-seeking, being highest during early morning and late afternoon hours. Mean Ae. aegypti daily abundance was negatively associated with daily temperature. No infection by ZIKV, DENV or CHIKV was detected in any Aedes mosquitoes caught by either trapping method.
Conclusion
We conclude the MET performs at least as well as the BGS standard and offers the additional advantage of direct measurement of per capita human-biting rates. If detection of arboviruses can be confirmed in MET-collected Aedes in future studies, this surveillance method could provide a valuable tool for surveillance and prediction on human arboviral exposure risk.

Similar content being viewed by others
Background
Mosquito-borne viruses (arboviruses) are an important cause of diseases in humans and animals. In 2017, estimates suggested that mosquitoes were responsible for approximately 137 million human arboviral infections with dengue (DENV), chikungunya (CHIKV) and Zika virus (ZIKV) being the most important [1]. Arbovirus transmission to humans depends on multiple factors that involve spatial movement and immunity of human populations [2,3,4], socio-economic factors and access to basic services (especially water) [5, 6], and the ecology and distribution of the mosquito vectors that transmit them [7,8,9]. These factors combine to determine the distribution and intensity of arboviral transmission and generate often complex and highly heterogeneous patterns of exposure and infection [10, 11]. As safe and effective vaccines for DENV, CHIKV and ZIKV are not yet available [12,13,14], control of the Aedes mosquito vectors remains a primary strategy for reducing transmission [15,16,17].
Knowledge of where and when humans are at greatest risk of exposure to infected mosquito bites is vital for prediction of transmission intensity and effective deployment of vector control [18,19,20]. In the case of malaria, this information is used to estimate a time or site-specific “Entomological Inoculation Rate” (EIR); defined as the number of infected mosquito bites a person is expected to receive. This metric is usually derived from conducting human landing catches (HLCs); a method in which a participant collects and counts the number of mosquito vectors landing on them over a given sampling period, then the sample is tested for the presence of a pathogen [21]. By providing a direct estimate of human exposure, the HLC provides sensitive predictions of malaria transmission [19, 22,23,24]. However, this method raises ethical concerns due to the requirement for human participants to expose themselves to potentially infectious mosquito bites [25]. In the case of malaria, this risk can be minimized by providing participants with prophylaxis [26]. However, such remediation is not possible for arboviruses where often no prophylaxis is available, and therefore HLCs are not recommended for the surveillance of Aedes-borne arboviruses [27, 28].
Standard entomological monitoring for Aedes vectors is usually based on “exposure-free” surveillance of larvae or non-biting adults. This includes surveys of larvae or pupae in water containers [29, 30], and collection of adult mosquitoes resting inside and/or around houses to indirectly estimate human-vector contact rates [29, 31]. While such surveillance methods are useful for confirming vector abundance and distribution, they are poor predictors of epidemiological outcomes such as disease incidence and outbreak potential [32, 33]. Consequently, there is a need for vector sampling methods that can provide more reliable entomological indicators of arboviral transmission.
Human exposure to arboviral infection is likely best assessed by surveillance of “host-seeking” (human-biting) Aedes mosquitoes. Several methods have used to sample host-seeking Aedes including a variety of fan-operated traps that use visual attraction cues (e.g. Fay [34], the Fay-Prince trap [35], the black cylinder suction trap [36], duplex cone trap [37]) and lure-based traps. For the latter, artificial odours and attractants have been developed and tested for use in traps such as kairomone blends [38, 39], BG-Lure® cartridges [40, 41] and carbon dioxide (CO2) [42]. Additionally, other trapping methods have been developed that use live hosts as lures (e.g. animal-baited traps [43] and human-baited traps [44, 45]). Only a few studies have directly compared such alternative trapping methods against the HLC with most being outperformed by the latter [44, 45]. Out of all these methods, the BG-sentinel (BGS) trap has been demonstrated as one of the most effective and logistically feasible [46, 47], and thus often considered a gold standard for Aedes surveillance [48, 49]. In a range of trap evaluation studies, the BGS outperformed other methods for Aedes vectors except for HLC [50]. Despite these advantages of the BGS, its ability to accurately reflect the biting rates experienced by one person remains unclear. Consequently, there is still a need for a safe alternative for direct assessment of human biting rates.
Recently, a new mosquito electrocuting trap (MET) was developed as an exposure-free alternative to the HLC for sampling malaria vectors [51,52,53]. This trap was built on previous work using electrified nets and grids to trap tsetse flies [54, 55] and mosquitoes [56, 57] attracted to hosts or their odours. Similar to the HLC, this sampling method also uses human participants to lure mosquito vectors and trap them. However, the MET provides participants with full protection from mosquito bites so that no exposure is required. The MET consists of four squared-shaped electrocuting surfaces that are assembled around the legs of a host, with the rest of their body being protected by netting. Host-seeking mosquitoes are attracted towards the host by odour and heat cues as normal but are intercepted and killed before landing. In previous trials in Tanzania, the MET matched the performance of the HLC for sampling malaria vectors in rural and urban settings [51,52,53]. This trap has also been used to assess host preference by baiting with human and livestock hosts [53], although it has not yet been evaluated for sampling Aedes vectors. If successful in this context, the MET could significantly improve ability to monitor and predict arboviral transmission by facilitating an exposure-free direct estimation of EIR.
This study reports the first evaluation of METs for sampling host-seeking Aedes vectors in a hotspot of DENV and ZIKV transmission in coastal region of Ecuador. This region is endemic for such arboviral diseases and has accounted for most of the cases reported in Ecuador. For instance, during the CHIKV outbreak in 2015, a total of 33,625 cases were reported in Ecuador, from which 96.02% was reported in the coastal region [58]. A similar pattern occurred during the ZIKV outbreak in 2016 and 2017, where approximately 98.49% of the cases were reported in this region from a total of 5303 cases [59, 60]. DENV has been reported every year in high numbers and considering 2016 and 2017, 84.78% of cases came from the coastal region from a total of 25,537 cases [60, 61].
The objectives of this study were to: (i) evaluate the performance of the MET relative to the BGS trap for sampling host-seeking Ae. aegypti and other mosquitoes in the study area; and (ii) use the MET to characterize the biting time of Ae. aegypti and other relevant mosquito species and their association with microclimatic conditions.
In addition, we took the opportunity to test for the presence of arboviruses in the collected Aedes females by both trapping methods to investigate arboviral transmission in the local area.
Methods
Location and time of the study
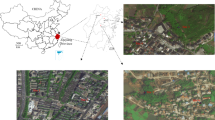
This study was conducted in the neighbourhood of “Los Higuerones” (0°19′34″N, 79°28′02″W, 78 meters above sea level), located in the city of Quinindé (Rosa Zárate) (Ecuador). This neighbourhood is located in an urban setting dominated by small, closely packed houses (Fig. 1c), bordering the eastern side with the Blanco River (Fig. 1d). Quinindé is located in the Province of Esmeraldas, the northernmost province in the coastal region of Ecuador. During the 2015 outbreak of CHIKV, this province accounted with the highest disease burden in the country, with a total of 10,477 cases [58]. While for DENV, during 2016, Quinindé alone accounted for 52% of the cases within Esmeraldas Province, with a total of 689 cases out of a total of 1319. In 2017, the number of DENV cases in Quinindé was much lower compared with 2016, where only 87 cases were reported out of 334 in the Province of Esmeraldas. Although there is a permanent incidence of arbovirus cases along the year, a higher incidence is usually reported during the first half of the year [6].
View of the urban area of the city of Quinindé. a Location of Ecuador in the Americas highlighted in red (taken from [96]). b Location of the city of Quinindé in the Pacific Coastal region, spotted by the red circle. c City of Quinindé showing Los Higuerones neighbourhood enclosed by the red line. d Enlarged view of Los Higuerones with the houses sampled spotted by the orange circles
The study was carried out across 12 days in May 2017 (4th–12th, and 16th–18th). On each day of the study, mosquito sampling was conducted over 12 h, from 7:00–19:00 h. Mosquito sampling was conducted within the peridomestic area (garden/yard) of four households (Fig. 1d). These houses were selected on the basis of being physically accessible, and having residents present and willing to participate during an initial tour of the area with a local guide. Houses were separated by approximately 90 m from one another.
Trapping methods
Over the study period, host-seeking mosquitoes were sampled by two different methods as described below.
BG-Sentinel trap (BGS)
The BG-Sentinel® trap (BioGents, Regensburg, Germany) is a white, cylinder-shaped trap made of plastic with a gauze cloth covering the top and a hollow black cylinder in the top centre of the trap (Fig. 2a). The trap operates with a 12 V battery that powers an internal fan that produces inwards artificial air currents. In this study, each trap was baited with two BG-Lure® cartridges and a 1.4 l cooler bottle filled with dry ice in order to maximize the attractiveness of traps to Aedes; as it is known that CO2 increases the catch efficiency of BGS traps [46, 47, 62]. Mosquitoes are attracted towards the baited traps and then sucked through the hollow black cylinder into an internal mesh bag that can be easily removed for subsequent processing.
Mosquito electrocuting trap (MET)
The METs used here consisted of four 30 × 30 cm panels which are assembled into a box around the lower legs of a seated person (Fig. 2b). Each panel is made up of stainless-steel electrified wires set within a PVC frame. The wires are positioned 5 mm apart, which is close enough so that mosquitoes could not pass through without making contact. Wires are vertically arranged in parallel, alternating positive with negative. When mosquitoes try to go through, contact is made and the voltage between wires kills them.
Mosquitoes attracted towards the volunteer were intercepted and killed on contact with these panels. The MET is powered by two 12 V batteries connected in series to a power source giving a power output of approximately 6 W (10 mA, 600 V). As an additional safety feature, a protective inner panel made from wide non-conductive plastic grid was fit into each frame preventing accidental contact between users and the electrified wires.
As an additional accessory to the MET, a retractable aluminium frame was built to cover the rest of the volunteer’s body with untreated mosquito-proof netting. Thus, volunteers were completely protected from mosquito bites during their participation in trapping. A plastic tarpaulin was erected over the MET station at a height of 2 m to protect users from direct rain and sunlight. Each MET was also set up on top of a white plastic sheet to isolate it from the ground and make it easier to see and collect shocked mosquitoes that fell onto the ground after touching the MET.
Experimental design
Every day of the study, four traps (two METs and two BGS traps) were set up in the peridomestic area of the four households (one trap per household) at the ground level under shade conditions. Traps were rotated among households each day, so that a different trapping method was used every consecutive day in each house. At the end of the study, this resulted in 6 days of trapping being conducted with each of the 2 methods at all houses.
MET collections were carried out by members of the research team, who were all adult men (30–50 years-old). During each hour of the collection period, one member sat within the MET for 45 min, with the trap being turned off for the remaining 15 min to allow volunteers to take a break. Members of the study team took turns sitting in the trap so that different collectors lured every hour. During the 15 min period when traps were turned off, mosquitoes were recovered from trap surfaces and the ground below using a pair of forceps, counted and placed in empty 15 ml falcon tubes; which were labelled with a unique code linked to the date, household ID, trap ID, hour period and collector ID. Tubes were stored in a cooler box of 45 l capacity filled with dry ice to kill, preserve and transport the specimens.
Each BGS was baited with two BG-Lure® cartridges on each day of sampling; with lures exchanged between the two BGS traps each day to minimize bias due to differential lure efficiency. BGS traps were further baited with carbon dioxide by adding one 1.2 l Coleman® polyethylene cooler bottle filled with dry ice. Dry ice containers were topped up every day. Like the MET, BGS sampling was conducted for 45 min of each sampling hour, with mosquito collection bags being checked and emptied during 15 min break periods. Mosquitoes from BGS collection bags were emptied into pre-labelled plastic bags and transferred into a cooler box with dry ice to kill and preserve the mosquitoes.
Temperature and relative humidity data were collected every 10 min at each mosquito sampling point using TinyTag® Plus 2 TGP-4500 (Gemini Co., Chichester, UK) data loggers. Data loggers at the BGS sampling stations were tied and hung inside each of the traps, and loggers at MET sampling points were placed on top of the bottom border of the netting frame, next to the MET.
Morphological analysis
Mosquitoes collected in the field were transported to the Medical Entomology and Tropical Medicine Laboratory of the San Francisco de Quito University (LEMMT-USFQ) in cooler boxes filled with dry ice. At LEMMT-USFQ, mosquitoes were morphologically identified using taxonomic keys [63,64,65], counted and sorted into different cryo-vials according to date, household, trap type, hour of collection, species, sex and physiological status of females (blood-fed/gravid and non-blood-fed). All female Ae. aegypti specimens were retained for subsequent molecular analysis to test for the presence of ZIKV, DENV and CHIKV. These Ae. aegypti samples were grouped into pools of a maximum of 5 individuals.
Molecular detection of arboviruses
All pools of female Ae. aegypti specimens were screened for the presence of CHIKV, DENV and ZIKV. Details on the RNA extraction, reverse-transcription and PCR procedures are given in Additional file 1: Text S1, Table S1 and Table S2.
Data analysis
Statistical analyses were performed in R 3.5.0 and R Studio 1.1.419. Generalized linear mixed models (GLMM) were used to investigate variation in the abundance of host-seeking mosquitoes (per day and per hour) using the package lme4 in R [66]. As mosquito abundance data were overdispersed, all models were fitted with a negative binomial distribution. For all response variables of interest as described below, model selection was carried out through a process of backward stepwise elimination from a maximal model using likelihood ratio tests (LRT) [67].
Statistical analysis was performed for Ae. aegypti and Culex quinquefasciatus as the latter was the only other mosquito species found in high abundance in the study area. Culex quinquefasciatus is a nuisance biting mosquito and also a known vector of West Nile virus (WNV) [68].
The BGS traps functioned continuously across all days and sampling hours. However, the METs stopped running during some sampling hours; generally, under conditions of very high humidity due to rainfall which resulted in dampness on the traps and some temporary short circuiting (e.g. observed as plumes of smoke at the bottom junction with the frames). When these malfunctions occurred, the damaged traps were turned off and repaired. This resulted in variation in the total number of hours sampled with each trapping method (MET: 229 h; BGS: 270 h). This variation in sampling effort was accounted for in the statistical analysis. Days having less than 9 h were excluded from the analysis.
Four models were built to assess the variation in the abundance of each mosquito species and sex combination, respectively. For each of these four response variables, a maximal model was constructed that included the fixed explanatory variables of sampling effort (total number of hours of collection), trap type (MET or BGS), daily mean relative humidity (%RH), and daily mean temperature (°C). In addition, the interaction between daily mean temperature with relative humidity was also included. Sampling day (1 through 12), household ID, trap ID and attractant ID (BG-Lure cartridge ID or MET volunteers ID) were included as random effects.
Mosquito biting activity was assessed through analysis of variation in the mean number of females (Ae. aegypti and Cx. quinquefasciatus) caught per hour. Here, each mosquito species was analysed separately. Each model included the explanatory variables trap type (MET or BGS), sampling hour, mean temperature (°C) per hour, mean relative humidity (%RH) per hour, and the interaction between hourly temperature and relative humidity. Sampling hour was defined as a continuous variable recoding the first hour of trapping (7:00–8:00 h) into 1, and increasing “hour” by one digit for each subsequent hour until 12 h (17:00–18:00 h). Sampling hour was fit both as a linear and quadratic term, with the latter being used to test for peaks in biting time as have been previously reported for these mosquito species [69]. In addition, sampling day, trap ID, cluster ID, household ID (nested within cluster ID) and attractant ID (BG-Lure cartridge ID or MET volunteer ID) were fitted as random effects.
Results
Mosquito species and abundance
During the 12 day-experiment, a total of five mosquito species were collected by both trapping methods (Table 1). Culex quinquefasciatus was the most abundant species (78.6%) followed by Ae. aegypti (15.63%), and small numbers of Aedes angustivittatus (2.69%), Limatus durhami (2.33%) and Psorophora ferox (0.15%). A small proportion of mosquitoes could not be identified (0.51%, Table 1). Overall, more mosquitoes were collected with the BGS trap (60.77%) than with the MET (39.23%), but the numbers of Ae. aegypti were relatively similar (Table 1).
In the BGS traps, some non-target insects including house flies, butterflies, crane flies, and many fruit flies were caught. No insect taxa other than mosquitoes shown in Table 1 were caught in MET collections.
The mean daily abundance of Ae. aegypti was approximately 2 females and 3 males for the BGS trap, and 4 females and 4 males for the MET, but no significant differences between trapping methods were found (Table 2, Fig. 3a, b). The only significant predictor of daily abundance of females Ae. aegypti was temperature, which exhibited a negative association (Table 2, Fig. 4a). Similarly, the mean daily abundance of Cx. quinquefasciatus females did not significantly differ between trapping methods (Table 2, Fig. 3c, d); however, confidence intervals (especially for males) around estimates were very large, indicating that larger sample sizes may be required to robustly test if there were differences between trap types. The number of female Cx. quinquefasciatus per day varied between 16–207, with variation being even more pronounced for males where a high of 576 was caught on one day. The daily abundance of female Cx. quinquefasciatus was negatively associated with daily temperature (Table 2, Fig. 4b) and positively associated with the number of hours sampled in a day, while no significant differences were found in Cx. quinquefasciatus regarding any covariate (Table 2).
Mosquito biting activity
Hourly mosquito catches recorded for BGS and METs were used to characterize the biting activity of female Ae. aegypti and Cx. quinquefasciatus. Variation in the hourly biting activity of female Ae. aegypti was best explained by a quadratic association between hourly mosquito abundance and time (Table 3), with activity being highest in the early morning and late afternoon, and little activity during the middle of the day (Fig. 5a). After taking this hourly variation in biting rates into account, there was no additional impact of trapping method on the number of female Ae. aegypti collected per hour (Table 3, Fig. 6). Variation in the hourly biting activity of Ae. aegypti was also significantly associated with an interaction between temperature and relative humidity (Table 3). This interaction arose because the number of Ae. aegypti caught per hour was negatively associated with temperature under conditions of low relative humidity; but the strength of this association was lower as humidity increased (Table 3, Fig. 7), although temperature and humidity were strongly associated (Additional file 2: Figure S1).
Predicted abundance of biting mosquitoes between 7:00–19:00 h. a Ae. aegypti females. b Cx. quinquefasciatus females. Dots represent the observed values which correspond to the right Y-axes. The red line corresponds to the predicted mosquito abundance and the shaded area to the 95% confidence intervals (CI); both correspond to the left Y-axes
The biting activity of female Cx. quinquefasciatus also varied significantly across the sampling day. As with Ae. aegypti, this pattern was characterized as a quadratic relationship in which mosquito activity peaked during the early morning and late afternoon (Table 3, Fig. 5b). Accounting for this activity pattern, there was no difference in the number of Cx. quinquefasciatus caught per hour in different trapping methods (Table 3, Fig. 6b), and no association with temperature or humidity.
Molecular screening for ZIKV, DENV and CHIKV
Aedes aegypti females were tested for ZIKV, DENV 1-4 and CHIKV and none of the samples were found positive. For a detailed description on the molecular results, please see Additional file 1: Text S2 and Additional files 3, 4, 5, 6, 7, 8, 9, 10: Figures S2–S9. In Additional files 4, 5, 6, 7, 8, 9, 10: Figures S3–S9, asterisk indicates the samples that had a weak band at the corresponding expected size, and ^ indicates the samples that showed a size close to the expected one. The red dashed line is positioned at the corresponding expected size for each PCR run.
Discussion
Identifying an accurate method to predict the exposure of humans to infected mosquito vectors has been an enormous challenge for Aedes-borne pathogens [70, 71]. Here, we present the MET as a potential alternative for safe measurement of Aedes landing rates on humans. When tested in Ecuador, the MET provided similar estimates of Ae. aegypti abundance and biting activity as the current gold standard, the BGS sentinel method. While the BGS uses artificial odour baits and carbon dioxide (CO2) to lure mosquitoes into a standardized trap, the MET directly estimates the number of Aedes host-seeking within the immediate vicinity of a real host. The MET can also be used to measure biting rates on a range of different host species (e.g. [53]), which currently cannot be performed with the BGS and other methods. The standardization provided by the BGS makes it easy and effective to use in widescale surveillance [48, 50], although a limitation is that non-biogenic CO2 sources are not always available [72]. However, the degree to which BGS collections accurately reflect per capita human biting rates is unclear. For example, BGS trapping efficiency may vary with the type and number of lures used, rate of CO2 released (quantity per time), location and colour of the trap (e.g. BGS 1 and BGS 2) [38, 46, 73], making it difficult to infer how different variants translate into exposure experienced by one person in that environment. An advantage of the MET is that it is more directly analogous to the human landing catch in sampling mosquitoes in the process of host-seeking on a person and also estimate variability in attraction between individuals. This could also be seen in the total catches of the other mosquito species when compared to the total numbers trapped by the BGS. The MET could thus provide a useful supplementary surveillance method for estimation and validation of human-biting rates and the associated entomological inoculation rate (EIR).
By facilitating a safe and more direct estimation of the EIR for Aedes-borne viruses, the MET could provide robust and precise entomological indicators of transmission intensity [51,52,53]. Such indicators are much needed to understand heterogeneity in transmission [33, 74, 75] and evaluate the efficiency of vector control interventions. However, this relies on the assumption that the MET accurately reflects the true Aedes exposure of one person per unit of time. Estimates of human exposure to the malaria vector An. gambiae (s.l.) from the MET were similar to those of the human landing catch in some studies [53, 76], whereas in others mosquito abundance was underestimated by the MET compared to the HLC [52]. Here, it was not possible to directly compare the MET to the HLC because of ethical restrictions in using the latter in an area of high arboviral transmission. However, we speculate that one factor that could cause the MET to underestimate Aedes vectors biting rates is the area of the body protected. Whereas African Anopheles vectors generally prefer feeding on the lower legs and feet [77,78,79]; it is not clear if Aedes prefer to bite on specific parts of the body [80, 81]. As a next step in validating this approach, we recommend the MET to be directly compared to the HLC under controlled conditions with uninfected Aedes vectors (e.g. semi-field experiments), ideally using a defined Ae. aegypti strain and appropriate experimental design to act as a reference standard for future comparison.
Both the MET and BGS trap sampled a similar composition of mosquito species in the study period. However, estimates of the mean daily and hourly abundance of Ae. aegypti and Cx. quinquefasciatus were slightly but not statistically higher in MET than in BGS collections. The relatively short period of this (12 sampling days) may have limited power to detect for minor to moderate differences between trapping methods. We thus conclude the MET is at least as good as the BGS gold standard for sampling host-seeking Aedes vectors in this setting, but also recommend further longer-term comparisons over a wider range of seasons, sites and participants to evaluate whether the MET outperforms the BGS. If we assume that MET is equivalent to HLC, these results are also consistent to those shown by Kröckel et al. [50], who also observed that HLC captured more mosquitoes, although not statistically different from the BGS.
Mosquito collections conducted here were also used to test for associations between Aedes host-seeking activity and microclimatic conditions. The impact of temperature and humidity on the life history, physiology, behaviour and ecology of Ae. aegypti has been extensively investigated under laboratory conditions [82,83,84,85]. However, relatively little is known about how microclimate impacts the diel host-seeking behaviour of wild Aedes. In general, the host-seeking activity Ae. aegypti and Cx. quinquefasciatus was higher on days when mean temperatures were lower (across the range of 25–30 °C). Additionally, the hourly biting rates of Aedes were negatively associated with temperature but only under conditions of low humidity. As mean hourly temperatures were strongly negatively correlated with relative humidity (Additional file 2: Figure S1), these results indicate that Ae. aegypti biting activity is highest during relatively cool and humid hours of the day. These microclimatic associations may account for the observed biting activity of Ae. aegypti and Cx. quinquefasciatus. A comprehensive review [69] of Ae. aegypti biting behaviour indicates that bimodal and trimodal activity patterns are often reported, with evidence of specific adaptations to other ecological features (e.g. artificial light availability) [69]. Such variability seems to be common and related to optimal humidity and temperature conditions available during such hours [86, 87].
A key feature of any method for estimating EIR is its ability to estimate human-biting rates and infection rates in mosquitoes. While the results here presented indicate that the MET could be used to estimate the human-biting rates, the infection rates could not be measured as none of the Aedes mosquitoes collected with either trapping method were positive for arboviruses. Reported rates of arboviruses in Aedes vectors are generally very low (0.1–10%) even in high transmission areas (e.g. [88,89,90,91,92,93,94,95]). Thus, failure to detect arboviruses within the relatively small sample size of vectors tested here (e.g. 207 individuals tested in 122 pools) is not unexpected.
Although promising, the MET has a number of limitations relative to the BGS for sampling host-seeking Aedes. First, although both trapping methods require a power supply, the current version of the MET requires two 12 V batteries compared to the one required by the BGS), requires human participants and the trap itself is heavier, which is more labour-intensive than using BGS. Also, as the METs used here are still research prototypes produced on a bespoke basis without a licensed manufacturer, their production cost is currently more expensive than BGS traps (approximately £650 vs £170 per trap, respectively). In addition, some technical problems were experienced including a tendency to short circuit under conditions of high air humidity. These limitations are expected to be improved if manufactured at scale as manufacturing costs would fall and technical improvements should make the MET suitable for humid environments. The primary advantage of the MET is, therefore, its potential ability to directly estimate the EIR for arboviral infections. This advantage could be leveraged to calibrate other existing trapping methods that are less labour intensive and more feasible to be deployed at large scale. Additionally, the MET could be used in combination with other trapping methods to identify hotspots of transmission before large scale deployment with other traps is carried out.
Conclusions
Here, we evaluated the MET as a tool for estimating human biting rates of the arboviral vector Ae. aegypti in a high transmission setting in coastal Ecuador. The MET performed at least as well as the current BG-Sentinel trap gold standard for estimating the mean abundance per hour of host-seeking Aedes and provided a realistic representation of hourly activity patterns. We conclude that MET is a promising tool for Ae. aegypti and other mosquito species surveillance, which could uniquely enable a relatively direct estimate of the arboviral entomological inoculation rate experienced by communities.
Availability of data and materials
Data supporting the conclusions of this article are included within the article and its additional files. The dataset generated and analysed during this study is publicly available in the Open Science Framework repository at https://osf.io/zwbs8.
Abbreviations
- HLC:
-
human landing catches
- EIR:
-
entomological inoculation rate
- MET:
-
mosquito electrocuting trap
- BGS:
-
BG-sentinel trap
- ZIKV:
-
Zika virus
- DENV:
-
dengue virus
- CHIKV:
-
chikungunya virus
- WNV:
-
West Nile virus
- GLMM:
-
generalized linear mixed models
- LRT:
-
likelihood ratio test
- PCR:
-
polymerase chain reaction
References
WHO. Global burden of major vector-borne diseases. Global vector control response 2017–2030 Geneva: World Health Organization; 2017. p. 2. https://www.who.int/vector-control/publications/global-control-response/en/. Accessed 4 Aug 2019.
Wesolowski A, Qureshi T, Boni MF, Sundsøy PR, Johansson MA, Rasheed SB, et al. Impact of human mobility on the emergence of dengue epidemics in Pakistan. Proc Natl Acad Sci USA. 2015;112:11887–92.
Sutherland LJ, Cash AA, Huang YJS, Sang RC, Malhotra I, Moormann AM, et al. Serologic evidence of arboviral infections among humans in Kenya. Am J Trop Med Hyg. 2011;85:158–61.
Carver S, Bestall A, Jardine A, Ostfeld RS. Influence of hosts on the ecology of arboviral transmission: potential mechanisms influencing dengue, Murray Valley encephalitis, and Ross River virus in Australia. Vector Borne Zoonotic Dis. 2008;9:51–64.
Setbon M, Raude J. Population response to the risk of vector-borne diseases: lessons learned from socio-behavioural research during large-scale outbreaks. Emerg Health Threats J. 2012;2:7083.
Stewart Ibarra AM, Ryan SJ, Beltrán E, Mejía R, Silva M, Muñoz Á. Dengue vector dynamics (Aedes aegypti) influenced by climate and social factors in Ecuador: implications for targeted control. PLoS One. 2013;8:639–47.
Kraemer M, Reiner R, Brady O, Messina J, Gilbert M, Pigott D, et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4:854–63.
Kamal M, Kenawy MA, Rady MH, Khaled AS, Samy AM. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS One. 2018;13:021012.
Dickens BL, Sun H, Jit M, Cook AR, Carrasco LR. Determining environmental and anthropogenic factors which explain the global distribution of Aedes aegypti and Ae. albopictus. BMJ Glob Health. 2018;3:e000801.
Eder M, Cortes F, de Teixeira Siqueira Filha N, de Araújo França GV, Degroote S, Braga C, et al. Scoping review on vector-borne diseases in urban areas: transmission dynamics, vectorial capacity and co-infection. Infect Dis Poverty. 2018;7:1–24.
Kuno G, Chang GJ. Biological transmission of arboviruses: reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin Microbiol Rev. 2005;18:608–37.
Abbink P, Stephenson KE, Barouch DH. Zika virus vaccines. Nat Rev Microbiol. 2018;16:594–600.
Rezza G, Weaver SC. Chikungunya as a paradigm for emerging viral diseases: evaluating disease impact and hurdles to vaccine development. PLoS Negl Trop Dis. 2019;13:e0006919.
World Health Organization. Dengue vaccine: WHO position paper, September 2018—recommendations. Vaccine. 2018;37:4848–9.
Yakob L, Walker T. Zika virus outbreak in the Americas: the need for novel mosquito control methods. Lancet Glob Health. 2016;4:e148–9.
WHO. Dengue: guidelines for diagnosis, treatment, prevention, and control. Geneva: World Health Organization; 2009. http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf. Accessed 16 Dec 2016.
Gubler DJ. Emerging vector-borne flavivirus diseases: are vaccines the solution? Expert Rev Vaccines. 2011;10:563–5.
Tusting LS, Bousema T, Smith DL, Drakeley C. Measuring changes in Plasmodium falciparum transmission: precision, accuracy and costs of metrics. Adv Parasitol. 2014;84:151–208.
MacDonald G. The epidemiology and control of malaria. London: Oxford University Press; 1957.
Beier JC, Killeen GF, Githure JI. Short report: entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am J Trop Med Hyg. 2017;61:109–13.
Beier JC. Vector incrimination and entomological inoculation rates. In: Doolan DL, editor. Malaria methods and protocols. Methods in molecular medicine™, vol. 72. New Jersey: Humana Press Inc.; 2002. p. 3–11.
Kelly-Hope LA, McKenzie FE. The multiplicity of malaria transmission: a review of entomological inoculation rate measurements and methods across sub-Saharan Africa. Malar J. 2009;8:1–16.
WHO. Malaria entomology and vector control (Guide for participants). Geneva: World Health Organization; 2013. https://apps.who.int/iris/bitstream/handle/10665/85890/9789241505819_eng.pdf?sequence=1. Accessed 14 Mar 2019.
Service M. A critical review of procedures for sampling populations of adult mosquitoes. Bull Entomol Res. 1977;67:343–82.
Ndebele P, Musesengwa R. View point: ethical dilemmas in malaria vector research in Africa: making the difficult choice between mosquito, science and humans. Malawi Med J. 2012;24:65–8.
Gimnig JE, Walker ED, Otieno P, Kosgei J, Olang G, Ombok M, et al. Incidence of malaria among mosquito collectors conducting human landing catches in western Kenya. Am J Trop Med Hyg. 2013;88:301–8.
WHO. Ethical issues associated with vector-borne diseases. Geneva: World Health Organization; 2017. https://apps.who.int/iris/bitstream/handle/10665/259687/WHO-HTM-NTD-VEM-2017.07-eng.pdf. Accessed 18 Mar 2019.
Achee N, Youngblood L, Bangs M, Lavery J, James S. Considerations for the use of human participants in vector biology research: a tool for investigators and regulators. Vector-Borne Zoonotic Dis. 2015;15:89–102.
Focks D. A review of entomological sampling methods and indicators for dengue vectors. Geneva: World Health Otganisation; 2003. https://apps.who.int/iris/bitstream/handle/10665/68575/TDR_IDE_DEN_03.1.pdf?sequence=1&isAllowed=y. Accessed 18 Mar 2019.
Barrera R, Amador M, Clark GG. Use of the pupal survey technique for measuring Aedes aegypti (Diptera: Culicidae) productivity in Puerto Rico. Am J Trop Med Hyg. 2006;74:290–302.
Vazquez-Prokopec GM, Galvin WA, Kelly R, Kitron U. A new, cost-effective, battery-powered aspirator for adult mosquito collection. J Med Entomol. 2009;46:1256–9.
Cromwell EA, Stoddard ST, Barker CM, Van Rie A, Messer WB, Meshnick SR, et al. The relationship between entomological indicators of Aedes aegypti abundance and dengue virus infection. PLoS Negl Trop Dis. 2017;11:e0005429.
Bowman LR, Runge-Ranzinger S, McCall PJ. Assessing the relationship between vector indices and dengue transmission: a systematic review of the evidence. PLoS Negl Trop Dis. 2014;8:e2848.
Fay RW. A trap based on visual responses of adult mosquitoes. Mosq News. 1968;28:1–7.
Fay R, Prince W. A modified visual trap for Aedes aegypti. Mosq News. 1970;30:20–3.
Wilton DP, Kloter KO. Preliminary evaluation of a black cylinder suction trap for Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). J Med Entomol. 1985;22:113–4.
Freier JE, Francy DB. A duplex cone trap for the collection of adult Aedes albopictus. J Am Mosq Control Assoc. 1991;7:73–9.
Williams CR, Bergbauer R, Geier M, Kline DL, Bernier UR, Russell RC, et al. Laboratory and field assessment of some kairomone blends for host-seeking Aedes aegypti. J Am Mosq Control Assoc. 2006;22:641–7.
Ong S-Q, Jaal Z. Assessment of potential kairomones and combination with the presence of insecticides in attracting Aedes aegypti (L.). Serangga. 2017;22:179–92.
Akaratovic KI, Kiser JP, Gordon S, Abadam CF. Evaluation of the trapping performance of four biogents AG traps and two lures for the surveillance of Aedes albopictus and other host-seeking mosquitoes. J Am Mosq Control Assoc. 2017;33:108–15.
Arimoto H, Harwood JF, Nunn PJ, Richardson AG, Gordon S, Obenauer PJ. Comparison of trapping performance between the original BG-Sentinel® trap and BG-Sentinel 2® trap. J Am Mosq Control Assoc. 2015;31:384–7.
Sukumaran D. A review on use of attractants and traps for host seeking Aedes aegypti mosquitoes. Indian J Nat Prod Resour. 2016;7:207–14.
Sallam MF, Pereira RM, Batich C, Koehler P. Factors affecting short-range host-seeking for the yellow fever mosquito (Diptera: Culicidae). J Med Entomol. 2019;56:609–16.
Gao Q, Wang F, Lv X, Cao H, Zhou J, Su F, et al. Comparison of the human-baited double net trap with the human landing catch for Aedes albopictus monitoring in Shanghai, China. Parasit Vectors. 2018;11:483.
Krajacich B, Slade J, Mulligan R, La Brecque B, Kobylinski K, Gray M, et al. Design and testing of a novel, protective human-baited tent trap for the collection of anthropophilic disease vectors. J Med Entomol. 2014;51:253–63.
Wilke ABB, Carvajal A, Medina J, Anderson M, Nieves VJ, Ramirez M, et al. Assessment of the effectiveness of BG-Sentinel traps baited with CO2 and BG-Lure for the surveillance of vector mosquitoes in Miami-Dade County, Florida. PLoS One. 2019;14:e0212688.
Meeraus WH, Armistead JS, Arias JR. Field comparison of novel and gold standard traps for collecting Aedes albopictus in northern Virginia. J Am Mosq Control Assoc. 2008;24:244–8.
WHO. Efficacy-testing of traps for control of Aedes spp. mosquito vectors. Geneva: World Health Organization; 2018. http://eprints.gla.ac.uk/173660/1/173660.pdf. Accessed 25 July 2019.
Centers for Disease Control and Prevention. Surveillance and control of Aedes aegypti and Aedes albopictus in the United States. 2017. https://www.cdc.gov/chikungunya/pdfs/Surveillance-and-Control-of-Aedes-aegypti-and-Aedes-albopictus-US.pdf. Accessed 25 July 2019.
Kröckel U, Rose A, Eiras ÁE, Geier M. New tools for surveillance of adult yellow fever mosquitoes: comparison of trap catches with human landing rates in an urban environment. J Am Mosq Control Assoc. 2006;22:229–38.
Govella NJ, Maliti DF, Mlwale AT, Masallu JP, Mirzai N, Johnson PCD, et al. An improved mosquito electrocuting trap that safely reproduces epidemiologically relevant metrics of mosquito human-feeding behaviours as determined by human landing catch. Malar J. 2016;15:465.
Maliti DV, Govella NJ, Killeen GF, Mirzai N, Johnson PCD, Kreppel K, et al. Development and evaluation of mosquito-electrocuting traps as alternatives to the human landing catch technique for sampling host-seeking malaria vectors. Malar J. 2015;14:502.
Meza FC, Kreppel KS, Maliti DF, Mlwale AT, Mirzai N, Killeen GF, et al. Mosquito electrocuting traps for directly measuring biting rates and host-preferences of Anopheles arabiensis and Anopheles funestus outdoors. Malar J. 2019;10:1–11.
Vale GA, Hargrove JW, Cullis NA, Chamisa A, Torr SJ. Efficacy of electrocuting devices to catch tsetse flies (Glossinidae) and other Diptera. PLoS Negl Trop Dis. 2015;9:e0004169.
Vale GA. Attractants for controlling and surveying tsetse populations. Trans R Soc Trop Med Hyg. 1974;68:11.
Torr SJ, Della Torre A, Calzetta M, Costantini C, Vale GA. Towards a fuller understanding of mosquito behaviour: use of electrocuting grids to compare the odour-orientated responses of Anopheles arabiensis and An. quadriannulatus in the field. Med Vet Entomol. 2008;22:93–108.
Knols BGJ, Mboera LEG, Takken W. Electric nets for studying odour-mediated host-seeking behaviour of mosquitoes. Med Vet Entomol. 1998;12:116–20.
Dirección Nacional de Vigilancia Epidemiológica, Ministerio de Salud Pública, Ecuador. Gaceta 2–2016. Virus chikungunya; 2016. https://www.salud.gob.ec/gacetas-vectoriales/. Accessed 25 Oct 2016.
Dirección Nacional de Vigilancia Epidemiológica, Ministerio de Salud Pública, Ecuador. Gaceta 52–2016. Virus Zika; 2016. https://www.salud.gob.ec/gacetas-vectoriales/. Accessed 4 Jan 2017.
Dirección Nacional de Vigilancia Epidemiológica, Ministerio de Salud Pública, Ecuador. Gaceta 52–2017. Enfermedades vectoriales; 2017. https://www.salud.gob.ec/gacetas-vectoriales/. Accessed 18 June 2018.
Dirección Nacional de Vigilancia Epidemiológica, Ministerio de Salud Pública, Ecuador. Gaceta 52–2016. Virus Dengue; 2016. https://www.salud.gob.ec/gacetas-vectoriales/. Accessed 20 Mar 2017.
Irish SR, Chandre F, N’Guessan R. Comparison of octenol- and BG Lure®-baited Biogents sentinel traps and an encephalitis virus surveillance trap in Portland. OR. J Am Mosq Control Assoc. 2008;24:393–7.
Lane J. Neotropical Culicidae, vol. 1. Sao Paulo, Brazil: University of Sao Paulo; 1953.
Lane J. Neotropical Culicidae, vol. 2. Sao Paulo: University of Sao Paulo; 1953.
Consoli R, de Ricardo Lourenco O. Principais mosquitos de importância sanitária no Brasil. Río de Janeiro: SciELO-Editora Fiocruz; 1994.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48.
Chambers JM, Hastie TJ. Statistical models in S. London: Wadsworth & Brooks/Cole; 1992.
Farajollahi A, Fonseca DM, Kramer LD, Marm Kilpatrick A. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect Genet Evol. 2011;11:1577–85.
Lima-Camara TN. Activity patterns of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) under natural and artificial conditions. Oecologia Aust. 2010;14:737–44.
Reiter P. Surveillance and control of urban dengue vectors. In: Gubler DJ, Ooi EE, Vasudevan S, Farrar J, editors. Dengue and dengue hemorrhagic fever. 2nd ed. Wallingford: CAB International; 2014. p. 481–518.
Sivagnaname N, Gunasekaran K. Need for an efficient adult trap for the surveillance of dengue vectors. Indian J Med Res. 2012;136:739–49.
Harwood JF, Arimoto H, Nunn P, Richardson AG, Obenauer PJ. Assessing carbon dioxide and synthetic lure-baited traps for dengue and chikungunya vector surveillance. J Am Mosq Control Assoc. 2015;31:242–7.
Roiz D, Duperier S, Roussel M, Boussès P, Fontenille D, Simard F, et al. Trapping the tiger: efficacy of the novel BG-Sentinel 2 with several attractants and carbon dioxide for collecting Aedes albopictus (Diptera: Culicidae) in southern France. J Med Entomol. 2016;53:460–5.
Reiner RC, Stoddard ST, Scott TW. Socially structured human movement shapes dengue transmission despite the diffusive effect of mosquito dispersal. Epidemics. 2014;6:30–6.
Stoddard ST, Kochel TJ, Halsey ES, Scott TW, Kitron U, Astete H, et al. House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci USA. 2013;110:994–9.
Govella NJ, Chaki PP, Mpangile JM, Killeen GF. Monitoring mosquitoes in urban Dar es Salaam: evaluation of resting boxes, window exit traps, CDC light traps, Ifakara tent traps and human landing catches. Parasit Vectors. 2011;4:40.
Dekker T, Takken W, Knols B, Bouman E, Van Der Laak S, de Bever A, et al. Selection of biting sites on a human host by Anopheles gambiae sensu stricto, An. arabiensis and An. quadriannulatus. Entomol Exp Appl. 1998;87:295–300.
De Jong R, Knols BGJ. Selection of biting sites on man by two malaria mosquito species. Experientia. 1995;51:80–4.
Braack L, Hunt R, Koekemoer LL, Gericke A, Munhenga G, Haddow AD, et al. Biting behaviour of African malaria vectors: 1. Where do the main vector species bite on the human body? Parasit Vectors. 2015;8:76.
Derek Charlwood J, Tomás EVE, Kelly-Hope L, Briët OJT. Evidence of an “invitation” effect in feeding sylvatic Stegomyia albopicta from Cambodia. Parasit Vectors. 2014;7:324.
Shirai Y, Funada H, Kamimura K, Seki T, Morohashi M. Landing sites on the human body preferred by Aedes albopictus. J Am Mosq Control Assoc. 2002;18:97–9.
Rivas GBS, Teles-de-Freitas R, Pavan MG, Lima JBP, Peixoto AA, Bruno RV. Effects of light and temperature on daily activity and clock gene expression in two mosquito disease vectors. J Biol Rhythms. 2018;33:272–88.
Beserra EB, Fernandes CRM, de Silva SAO, de Silva LA, dos Santos JW. Efeitos da temperatura no ciclo de vida, exigências térmicas e estimativas do número de gerações anuais de Aedes aegypti (Diptera, Culicidae). Iheringia Série Zool. 2009;99:142–8.
Lumsden WHR. Observations on the effect of microclimate on biting by Aedes aegypti (L.) (Dipt.; Culicidae). J Exp Biol. 1947;24:361–73.
Reinhold JM, Lazzari CR, Lahondère C. Effects of the environmental temperature on Aedes aegypti and Aedes albopictus mosquitoes: a review. Insects. 2018;9:158.
Atmosoedjono S, Van Peenen PFD, See R, Saroso JS. Man-biting activity of Aedes aegypti in Djakarta, Indonesia. Mosq News. 1972;32:467–9.
Chadee DD, Martinez R. Landing periodicity of Aedes aegypti with implications for dengue transmission in Trinidad, West Indies. J Vector Ecol. 2000;25:158–63.
de Ferreira-Brito A, Ribeiro IP, de Moraes Miranda R, Surubi Fernandes R, Silva Campos S, da Barbosa Silva KA, et al. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Mem Inst Oswaldo Cruz. 2016;111:655–8.
Barrera R, Amador M, Acevedo V, Beltran M, Muñoz JL. A comparison of mosquito densities, weather and infection rates of Aedes aegypti during the first epidemics of Chikungunya (2014) and Zika (2016) in areas with and without vector control in Puerto Rico. Med Vet Entomol. 2019;33:68–77.
Diallo D, Sall AA, Diagne CT, Faye O, Faye O, Ba Y, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One. 2014;9:e109442.
Guerbois M, Fernandez-Salas I, Azar SR, Danis-Lozano R, Alpuche-Aranda CM, Leal G, et al. Outbreak of Zika virus infection, Chiapas state, Mexico, 2015, and first confirmed transmission by Aedes aegypti mosquitoes in the Americas. J Infect Dis. 2016;214:1349–56.
Dzul-Manzanilla F, Martínez NE, Cruz-Nolasco M, Gutiérrez-Castro C, López-Damián L, Ibarra-López J, et al. Arbovirus surveillance and first report of chikungunya virus in wild populations of Aedes aegypti from Guerrero, Mexico. J Am Mosq Control Assoc. 2015;31:275–7.
Sang RC, Ahmed O, Faye O, Kelly CLH, Yahaya AA, Mmadi I, et al. Entomologic investigations of a chikungunya virus epidemic in the Union of the Comoros, 2005. Am J Trop Med Hyg. 2008;78:77–82.
Díaz-González EE, Kautz TF, Dorantes-Delgado A, Malo-García IR, Laguna-Aguilar M, Langsjoen RM, et al. First report of Aedes aegypti transmission of chikungunya virus in the Americas. Am J Trop Med Hyg. 2015;93:1325–9.
Urdaneta L, Herrera F, Pernalete M, Zoghbi N, Rubio-Palis Y, Barrios R, et al. Detection of dengue viruses in field-caught Aedes aegypti (Diptera: Culicidae) in Maracay, Aragua state, Venezuela by type-specific polymerase chain reaction. Infect Genet Evol. 2005;5:177–84.
User:Rei-artur. A large blank world map with oceans marked in blue.svg. Wikimedia Commons. Licence: CC BY-SA 3.0; 2006.
Acknowledgements
We thank all the residents from the local communities for granting access to their properties every day of the study. We also want to thank Jakub Czyzewski and the rest of the Bioelectronics Unit team of the University of Glasgow for building the mosquito electrocuting traps (METs) and providing with their technical assistance before and during the experiments. We also want to specially thank Miguel Ortega-López and Teresa López-Cuesta for designing and building the retractable aluminium frames and the mosquito netting for the METs. We finally thank Ana Espinoza from Fibios Science Communication (https://www.fibios.org/scicomm) for the design of the graphical abstract of this article.
Funding
Funding for LDOL to develop this research as part of his PhD studies was provided by the Government of Ecuador through SENESCYT (AR2Q-9554) under the programme “Primera Convocatoria Abierta 2013”. Funding for this research work was provided by the Medical Research Council from the UK under the Grant Number MC_PC_15081 (to AK, HMF, LDOL and RL) and MC_UU_12014/8 (to EP and AK).
Author information
Authors and Affiliations
Contributions
LDOL, HMF, RL and AK conceptualized the project. NM developed and built the mosquito electrocuting traps (METs). LDOL, MPB, ST and SS conducted the field work of mosquito collections under the supervision of NM, RL and HMF. MPB and LDOL carried out the morphological analyses. LDOL and FA carried out the molecular analyses under permanent supervision of EP and AK. LDOL carried out the statistical analyses and wrote the manuscript under the guidance of HMF. AK, EP, HMF, MPB, NM and RL edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this research was granted by the MVLS College Ethics Committee of the University of Glasgow (Project No.: 200150175), and by the Ethics Committee of Research on Human Beings of the San Francisco de Quito University (2016-146M). Prior to the study, the objectives of this research and risks and benefits for taking part in this were explained to the participants in MET collections and their written informed consent was obtained. Oral informed consent was also obtained from the heads of households where mosquito collections were performed. The purpose and objectives of the study was explained to householders before requesting permission for the study team to collect mosquitoes within their properties.
The Government of Ecuador, through the Ministry of Environment (MAE), granted the permits to carry out the present study under the Framework Agreement on Access to Genetic Resources No. MAE-DNB-CM-2016-0052. Transportation of samples from the study site in Quinindé (Rosa Zárate) to the LEMMT-USFQ in Quito was authorized by MAE through the document No. MAE-DPAE-2017-1163-O. Transfer of samples from the LEMMT-USFQ in Ecuador to the MRC-University of Glasgow CVR in the UK, was firstly established by a Material Transfer Agreement signed between Universidad San Francisco de Quito and the University of Glasgow on the 1st of September of 2017. The exportation of biological samples from Ecuador was authorized by MAE under the document 076-17-EXP-IC-FAU-DNB/MA. Finally, a certification of no-need of importation permit to Scotland was issued through a letter from the Animal Health and Welfare Division of the Agriculture and Rural Economy Directorate of the Scottish Government on the 14th of September of 2017.
Consent for publication
Individuals pictured in the photographs of this article granted their oral consent of the photographs in which they appear to be published.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Text 1:
Additional Methods. Table S1. Primers used for detection of arboviruses by RT-PCR. Table S2. Positive control DNA sequences used as PCR positive controls. Text 2. Additional Results.
Additional file 2: Figure S1.
Relationship between observed temperature (°C) and relative humidity (%). Red dots represent individual observations per hour recorded.
Additional file 3: Figure S2.
Visualization of the PCR products of S7 gene on agarose gels. All samples were positive, except 920-1.
Additional file 4: Figure S3.
Visualization of the first PCR products of ZIKV on agarose gels. Expected size of positive fragments: 76 bp. ZIKV+: positive control.
Additional file 5: Figure S4.
Visualization of the first PCR products of DENV 1–3 on agarose gels. Expected size of positive fragments: 63 bp. DENV1+: positive control.
Additional file 6: Figure S5.
Visualization of the first PCR products of DENV 4 on agarose gels. Expected size of positive fragments: 63 bp. DENV4+: positive control.
Additional file 7: Figure S6.
Visualization of the second PCR products of ZIKV on agarose gels. Expected size of positive fragments: 76 bp. ZIKV+: positive control.
Additional file 8: Figure S7.
Visualization of the second PCR products of DENV 4 on agarose gels. All samples were negative. Expected size of positive fragments: 63 bp. DENV4+: positive control.
Additional file 9: Figure S8.
Visualization of the second PCR products of DENV 1-3 on agarose gels. Expected size of positive fragments: 63 bp. DENV1+: positive control.
Additional file 10: Figure S9.
Visualization of the individual PCR products of DENV1, DENV2 and DENV3. Samples from Figure S8 are shown for individual runs for each of the three DENV isotypes. Expected size for DENV1 run was 71 bp, 199 bp for the DENV2 run, and 167 bp for the DENV3 run. DENV1+, DENV2+ and DENV3+: positive controls.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ortega-López, L.D., Pondeville, E., Kohl, A. et al. The mosquito electrocuting trap as an exposure-free method for measuring human-biting rates by Aedes mosquito vectors. Parasites Vectors 13, 31 (2020). https://doi.org/10.1186/s13071-020-3887-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-020-3887-8