Abstract
Background
Host preference is a critical determinant of human exposure to vector-borne infections and the impact of vector control interventions. Widespread use of long-lasting insecticide-treated nets (LLINs) and indoor residual spraying (IRS) across sub-Saharan Africa, which protect humans against mosquitoes, may select for altered host preference traits of malaria vectors over the long term. Here, the host preferences of Anopheles arabiensis and Anopheles gambiae sensu stricto (s.s.) were experimentally assessed in the field, using direct host-preference assays in two distinct ecological settings in Tanzania.
Methods
Eight Ifakara Tent Trap (ITT), four baited with humans and four with bovine calves, were simultaneously used to catch malaria vectors in open field sites in urban and rural Tanzania. The numbers of mosquitoes collected in human-baited traps versus calf-baited traps were used to estimate human feeding preference for each site's vector species.
Results
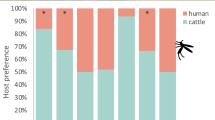
The estimated proportion [95% confidence interval (CI)] of mosquitoes attacking humans rather than cattle was 0.60 [0.40, 0.77] for An. arabiensis in the rural setting and 0.61 [0.32, 0.85] for An. gambiae s.s. in the urban setting, indicating no preference for either host in both cases (P = 0.32 and 0.46, respectively) and no difference in preference between the two (Odds Ratio (OR) [95%] = 0.95 [0.30, 3.01], P = 0.924). However, only a quarter of An. arabiensis in the urban setting attacked humans (0.25 [0.09, 0.53]), indicating a preference for cattle that approached significance (P = 0.08). Indeed, urban An. arabiensis were less likely to attack humans rather than cattle when compared to the same species in the rural setting (OR [95%] = 0.21 [0.05, 0.91], P = 0.037).
Conclusion
Urban An. arabiensis had a stronger preference for cattle than the rural population and urban An. gambiae s.s. showed no clear preference for either humans or cattle. In the urban setting, both species exhibited stronger tendencies to attack cattle than previous studies of the same species in rural contexts. Cattle keeping may, therefore, particularly limit the impact of human-targeted vector control interventions in Dar es Salaam and perhaps in other African towns and cities.
Similar content being viewed by others
Background
Apart from the distributions of bites between inside and outsides the houses and at different times of the night [1, 2], what mosquitoes feed upon critically determines the choice and impact of human-targeted vector control interventions [3,4,5,6,7,8]. For example, both historical and recent reports [9,10,11,12,13,14] show that the widespread use of long-lasting insecticide-treated nets (LLNs) or indoor residual spraying (IRS), which directly target humans or houses they live in, strongly suppressed or virtually eliminated the population of the main malaria vectors Anopheles gambiae sensu stricto (s.s.) and Anopheles funestus s.s. These two species preferentially feed upon human blood across sub-Saharan Africa (SSA) [10, 11, 15,16,17,18]. Beyond Africa, Anopheles darlingi was eliminated in British Guiana following three years of IRS with DDT [19]. This same species appears to have disappeared in Suriname in response to the scale-up of LLINs [20]. These vectors are highly vulnerable to insecticide-based interventions for protecting humans because these species rely heavily upon human blood for their survival [7, 19, 21,22,23].
While Anopheles arabiensis is commonly known to exhibit flexible host-feeding, switching biting between humans and domestic animals [24,25,26,27], recent evidence suggests that even the historically most inflexible human-feeding mosquito species in Africa, An. funestus s.s. can now attack non-human hosts, specifically cattle [24, 28]. This newly observed behavioural plasticity allows the mosquito to evade human-targeted insecticide-based interventions by allowing it to access safer alternative blood sources [29, 30]. This behaviour may help vector species sustain its population and contribute to residual malaria transmission by evading fatal contact with existing front-line interventions [6, 31, 32].
Inherent host preference is an innate behavioural trait of a mosquito population that is assessed in the field by allowing mosquitoes to freely select between two or more different host species experimentally presented in equal numbers simultaneously. Host choice, however, is a more complex function of both host preference and the availability of different host species that can be accessed locally and is assessed by surveying the sources of mosquito bloodmeals collected after they have fed [33, 34]. However, because the host choices exhibited by any given mosquito population can vary across spatial scales of only a few metres (e.g., in a cattle shed versus the house nearby), experimentally-controlled host preference measurements are a more reliable means of making direct comparisons between populations. Despite its critical importance as a metric to inform the selection of impact vector control interventions, there remains a paucity of data on vector host preference and its potential change over time.
Here, the inherent host attack preferences of An. arabiensis and An. gambiae s.s. only was assessed in two distinct ecological settings (urban versus rural) in Tanzania. A competitive preference experimentally-controlled assay, baited with either a human or calf, was simultaneously presented to malaria vectors. This study focused only on these two vector species because they are both important primary malaria vectors across Tanzania and elsewhere in Africa. Other, mostly secondary, malaria vector species were caught in insufficient numbers to be reliably assessed.
Methods
Study sites
This study was conducted at two different Tanzania regions: the urban Dar es Salaam and the rural village within the Kilombero valley in the Morogoro region. Dar es Salaam is the largest City of Tanzania, situated at 6° 51′S, 39° 18′E along the Indian Ocean with an estimate of 5 million people according to the national census of 2012 [35]. A detailed description of the study area has been previously published elsewhere [36, 37]. The main malaria vectors are An. gambiae s.s. and An. arabiensis, but Anopheles merus and An. funestus s.s. are also available, though existing in very low numbers throughout the year [38]. Anopheles gambiae s.s., which is often regarded as the most anthropophagic vector (rely feeding heavily upon human blood), feeds predominantly in the middle of the night [36, 39]. In contrast, its sibling species, An. arabiensis, which is commonly referred to as zoophagic (prefers feeding on cattle) mosquito throughout SSA [22], starts actively feeding in the early evening and mainly outdoors, time which coincides with the period when most residents of this city are still outside [36, 39]. This overlaps overtime, and outdoor space between mosquito and human activity potentially increases the risk of human exposure to malaria transmission, which cannot be effectively addressed by using indoor-targeted interventions such as LLINs [39]. During this study, human Plasmodium falciparum malaria infection was around 10% among residents in all age groups [37], and with the strong reduction in malaria vectors densities of An. gambiae complex and An. funestus group [40]. This was achieved due to the scaling-up of larvicides [41] and LLINs [36, 37]. The scaling-up of larvicides and LLINs coincided spontaneously with the wide use of window screening across the city of Dar es Salaam [40]. The average annual rainfall ranging from 800 to 1300 mm with a 25ºC annual temperature [42].
The second study site was at Kilombero valley, Lupiro village (8°23′03.8″ S, 36°40′26.7″ E), which is located 40 km south of Ifakara town within the Kilombero Valley, south-eastern Tanzania [43]. The detail of an area can be found elsewhere [13, 43]. The area is located at 300 m above sea level on the floodplains of Kilombero valley. The average annual rainfall ranges between 1200 to 1800 mm between December to May, and the temperature is recorded at ranges from 20 to 32.6 °C. The most resident lives on subsistence farming of rice, fishing, and sparse livestock keeping. An. arabiensis and An. funestus group are the primary malaria vectors in the area, but the latter exist in relatively very low numbers throughout the year [12]. The historically-important malaria vector An. gambiae s.s. had been virtually eliminated, following the widespread use of LLINs [13].
Experimental design
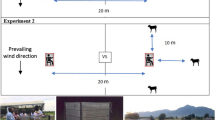
Eight Ifakara Tent Trap version C (ITT-C) [39] baited with either humans or calves were simultaneously used to catch wild malaria vectors in urban Dar es Salaam and rural Kilombero Valley. In each site, an open field ground measuring more than 500 m long was selected. Four (human versus calf) pairing catching stations, spaced about 50 m apart, were established within these field grounds. Within each pair, the host was spaced 5 m apart, allowing for a competitive host preference assay. A Latin square design involving the movement of trap-host combinations between positions was implemented to minimize possible biases associated with each position and natural variations in individual hosts' attractiveness to mosquitoes [44, 45]. Each pair was rotated after each experimental night through four stations. Four nights were required to make a complete round of experimentation (Fig. 1). After each round of four nights, the actual human volunteers and calves were replaced. The calf within each ITT-C was tethered to lure the mosquito entry inside the trap. Each morning, calves were taken out of the tent for daily grazing. There was no exchange of host between traps (calf-baited versus human-baited) because it was not acceptable to expect human participants to sleep in traps soiled by a calf. Trapping was conducted from 19:00 h to 06:00 h, and trapped mosquitoes were emptied from the trap every morning using a mouth aspirator. The details on how to empty mosquitoes inside the ITT-C can be found in the previous article [39]. In urban Dar es Salaam, 104 (60 nights between May to August 2009 and 44 nights between March and June 2010), experimental nights were conducted. In rural Kilombero Valley, only 16 nights (from August to September 2010) was conducted. It took longer in Urban Dar es Salaam due to the limited number of malaria vector densities.
The schematic illustration of a typical 4 × 4 Latin square experimental design with one complete round of experimentation through four mosquito-capturing stations in the field area. The dashed line indicates a screen bisecting the upper and lower part of the trap, which protects volunteers from being exposed to mosquito bites. The ring and the funnel shape on the side illustrate the mosquito entry point
Mosquito identification
Every morning, trapped adult mosquitoes from each trap were collected by mouth aspirator, placed in a respective paper cup prior labelled according to the host, and killed using chloroform. Morphological identification was conducted based on the keys of Gillies and Coetzee [46]. All collected An. gambiae sensu lato (s.l.) were stored individually in Eppendorf tubes (1.5 ml) with silica gel desiccant and cotton before transport for Polymerase chain reaction (PCR) assay for species identification. The field-collected data were recorded and linked with laboratory results using the designated forms adapted from Kiware et al. [47].
Statistical analysis
Statistical analyses were carried out using the R statistical software version 3.6.1, augmented with the matrix, lattice, and lme4 packages. To test the effect of species-specific on attacking human host, only PCR confirmed individuals from the An. gambiae complex (An. gambiae s.s. and An. arabiensis) were used. Because the response variable for each species is binary (that is, an individual mosquito can only attack a single host at a time and not both), a Generalized Linear Mixed Effect Models (GLMMs) [48], using binomial distribution and logit link function, was applied. The proportion of mosquitoes caught attacking humans was treated as the response variable, with a variable combination of PCR confirmed species and sites as a fixed effect. The experimental night and stations were fitted as a random effect. The model was run first without fitting an intercept so that the absolute proportion of mosquitoes attacking the human for each species and from each site can be estimated and compared. This was followed by fitting models that included intercept to obtain the contrast in human feeding preference between species with An. gambiae s.s. in urban Dar es Salaam treated as a reference species in the model. This detailed statistical analysis on the effect of species on the propensity of attacking upon human host species was restricted to An. arabiensis and An. gambiae s.s., partly because of their importance in driving malaria transmission in these settings, and their number captured was sufficient to detect the effect.
Results
Species composition
In urban Dar es Salaam, 197,155 mosquitoes were collected. 42,929 (21.8%) and 154,226 (78.2%) mosquitoes were collected from human and calf baited traps, respectively. The taxonomic group of mosquito collected included: An. gambiae s.l. (n = 97, 0.05%), Anopheles coustani (n = 2,144, 1.1%), Culex spp. (n = 192,836, 97.8%), Mansonia spp. (n = 1633, 0.8%) and Coquillettidia spp. (n = 460, 0.2%). All An. gambiae s.l. were subjected for PCR test, and 88 (88/97, 91%) specimens successfully amplified. Of which, 25 (28%) were An. gambiae s.s. and 63 (72%) An. arabiensis.
In rural Kilombero Valley, 41,876 mosquitoes were collected. 22,093 (53.0%) and 19,783 (47.2%) mosquitoes were collected from human and calf baited traps respectively. The taxonomic group of mosquito collected included: An. gambiae s.l. (n = 334, 0.8%), An. funestus group (n = 6, 0.01%), An. coustani (n = 185, 0.44%), Anopheles ziemanni (n = 31, 0.07%), Culex spp (n = 9539, 22.8%), Mansonia spp. (n = 31,749, 75.8%) and Coquillettidia spp. (n = 32, 0.08%). All An. gambiae s.l. were again subjected for PCR test, and all successful amplified specimens 313 (94%), confirmed to be An. arabiensis.
Based on the logistic model fitting to these data, the estimated proportion [95% confidence interval (CI)] of mosquitoes attacking humans rather than cattle was 0.60 [0.40, 0.77] for An. arabiensis in the rural setting and 0.61 [0.32, 0.85] for An. gambiae s.s. in the urban setting (Fig. 2), indicating no preference for either host in both cases (P = 0.32 and 0.46), respectively, with no evidence for any difference in preference between the two (Odds ratio (OR) [95%] = 0.95 [0.30, 3.01], P = 0.924)). However, only a quarter of An. arabiensis in the urban setting attacked humans (0.25 [0.09, 0.54]; Fig. 2), indicating a preference for cattle that approached significance (P = 0.081). Indeed, An. arabiensis in the urban setting were less likely to attack humans rather than cattle when compared to the same species in the rural setting (OR [95%] = 0.21 [0.05, 0.91], P = 0.037).
Discussion
The findings indicate variation in the preference for feeding upon humans rather than cattle between two populations of An. arabiensis, in urban Dar es Salaam, and rural Kilombero. These observations become more interesting and seem to suggest an effect of urban environments on both An. arabiensis and An. gambiae s.s., compared with preceding studies that also measured host preference through carefully controlled experiments. The rural Tanzanian An. arabiensis population studied here had no strong preference for humans or cattle. Indeed these results compared particularly well with those of Meza et al. [24] (Fig. 3), which also used juvenile cattle with relatively low biomass, therefore, similar levels of attractiveness [49]. However, in urban Dar es Salaam, An. arabiensis appeared to exhibit a strong preference for cattle over humans and significantly different from the same species in rural Kilombero over approximately the same period (Figs. 2 and 3). Also unexpectedly, An. gambiae s.s. collected in Dar es Salaam, lacked its notoriously strong preference for humans compared with equivalent indices derived from a previous study of the same species in rural Tanzania [21]. It appears that both siblings species have a stronger preference for non-human hosts in this urban context than in previously reported studies of rural populations of the same species [21, 24, 26] (Fig. 3).
Previously estimated proportion of attacks on humans versus cattle (Ph)) when offered a direct choice between one of each host species (mean and 95% confidence intervals, for An. arabiensis in rural Tanzania (data extracted from Fig. 4 in [24], and rural Zimbabwe (data extracted from Fig. 7 in [26]), and the estimated proportion of attacks on humans for An. arabiensis and An. gambiae s.s. obtained from historical records in the rural coastal region of Tanzania [21] compared to those obtained by this study in Kilombero, rural southern Tanzania, and Dar es Salaam, urban coastal Tanzania. The estimated proportion of attacks on humans (Ph) from historical records were derived from modelling analysis of the relative availability of humans versus cattle (λ) models: Ph = 1/(1 + λ) [21]
The flexible feeding behaviour exhibited by the An. arabiensis in rural Kilombero is consistent with that reported by previous studies from the same setting [24] and beyond [26] that employed similarly direct, experimentally-controlled, host attack preference measurements but used different capture methods. It is also reassuring that fitting host preference and availability models to historical blood meal host choice data for the same species across entire villages [21, 50] yields similar indirect estimates, indicating only a slight preference for cattle (Fig. 3) even though such natural herds are dominated by larger adult cattle that may be reasonably expected to be more attractive [49]. Indeed the Torr et al. [26] direct host preference experiments using electric grids similar to Meza et al. [24], which also used adult cattle, yield almost identical estimates to these indirectly inferred from modelling analyses, confirming a slight preference of rural An. arabiensis for fully-grown cattle over humans. Such biologically and methodologically plausible triangulation of results from such different studies with such different methods suggests that the experimental approach applied here, including the first use of ITT-C [39] for experimental host preference studies, provides reliable and readily comparable indices of host preference. Therefore, it is reasonable to interpret the findings that An. arabiensis had a stronger preference for cattle in urban Dar es Salaam than in rural Kilombero or any previous studies population of the same species (Fig. 3) at face value.
It is also telling that a similar, and perhaps more surprising, the pattern was observed for the notoriously anthropophagic [6, 27, 34, 51] An. gambiae s.s. compared with a previous study of the same species in a rural Tanzanian context (Fig. 3). The lack of a clear preference for humans over cattle by An gambiae s.s. in this contemporary urban context contrasts starkly with historical records from Segera, only 258 km away from Dar es Salaam [21]. This unusually flexible feeding behaviour for An. gambiae s.s. in Dar es Salaam may also contribute to the persistence of this species in this settings, unlike other nearby ecological settings where it was virtually eliminated [11, 15], following widespread use of LLIN [11,12,13, 15]. The increasingly widespread use of LLINs [52], and high coverage of house window screening in urban Dar es Salaam [40], which limit safe access of mosquitoes to human blood, may have forced this species to develop a strategy which enables them to evade personal target protective interventions for humans by exploiting animal blood whenever they can find it.
Urban Dar es Salaam generally has fewer cattle than Kilombero, and probably in most other rural settings. It is, therefore, interesting that An. arabiensis now appears to have a stronger preference for feeding on cattle and perhaps on other non-human hosts that were not assessed here. It will be important to investigate whether the two populations are genetically distinct or not [53,54,55]. This may be especially important following the recent surge of interest in genetic manipulation approaches for malaria vector control [56]. Regardless of the underlying basis for this apparent trend towards greater zoophagy in both vector species in Dar es Salaam, on the one hand, it will limit the impacts of existing malaria vector control interventions like LLINs and mosquito-proofed window screening. On the other hand, it may provide opportunities for complementary approaches like veterinary insecticide treatments for livestock [6, 7, 57,58,59,60].
While this study was quite limited in terms of scale and sample size, it does raise some important questions that merit consideration beyond Dar es Salaam and Tanzania. Urbanization is known to influence host preferences in other mosquito taxa [61], and similar effects to those reported here might also occur in other African settings where An. gambiae s.s. and An. arabiensis continue to mediate malaria transmission, despite widespread use of LLINs [54]. Indeed, it is notable that few experimentally controlled host preference studies could be found to populate Fig. 3, despite the vital role that this trait plays in malaria transmission and control. Therefore, this finding strongly encourages more widespread measurement of mosquito feeding preferences across a diversity of ecological settings through routine programmatic surveillance [62]. This may help inform the selection and evaluation of complementary vector control interventions, ideally in an ecologically stratified manner.
Conclusions
Urban An. arabiensis had a stronger preference for cattle than the rural population in this or previous studies. Furthermore, the urban An. gambiae s.s. assessed here had a weaker preference for humans over cattle than reported by a previous study of the same species in a nearby rural context. Cattle keeping may limit the impact of human-targeted vector control interventions in Dar es Salaam, and perhaps in other African towns and cities. Generalization of mosquito species host preferences across broad geographies or assuming that they may remain static traits may be misleading with respect to the selection of effective vector control interventions. Therefore, the characterization of vector feeding preferences across distinct ecological settings is recommended as a critical component of routine programmatic surveillance to inform the effective design, selection, implementation, and assessment of complementary new vector control interventions, ideally on an ecologically stratified basis.
Availability of data and materials
The Ifakara Health Institute, on behalf of the United Republic of Tanzania, owns all data. Data can be shared upon reasonable request in line with the Ifakara Health Institute’s data management and sharing policy.
Abbreviations
- ITT:
-
Ifakara Tent Trap (C type)
- LLINs:
-
Long-lasting insecticides treated nets
- IRS:
-
Indoor residual spray
- DDT:
-
Dichlorodiphenyltrichloroethane
- GLMM:
-
Generalized linear mixed effect model
- PCR:
-
Polymerase chain reaction
- CI:
-
Confidence interval
- SSA:
-
Sub-Saharan Africa
References
MF Finda IR Moshi A Monroe AJ Limwagu AP Nyoni JK Swai 2019 Linking human behaviours and malaria vector biting risk in south-eastern Tanzania PLoS One 14 0217414
A Monroe D Msaky S Kiware BB Tarimo S Moore K Haji 2020 Patterns of human exposure to malaria vectors in Zanzibar and implications for malaria elimination efforts Malar J 19 212
IN Lyimo HM Ferguson 2009 Ecological and evolutionary determinants of host species choice in mosquito vectors Trends Parasitol 25 189 196
I Tirados C Costantini G Gibson SJ Torr 2006 Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: implications for vector control Med Vet Entomol 20 425 437
GF Killeen 2014 Characterizing, controlling and eliminating residual malaria transmission Malar J 13 330
GF Killeen JM Marshall SS Kiware AB South LS Tusting PP Chaki 2017 Measuring, manipulating and exploiting behaviours of adult mosquitoes to optimise malaria vector control impact BMJ Glob Health 2 e000212
SS Kiware N Chitnis A Tatarsky S Wu HMS Castellanos R Gosling 2017 Attacking the mosquito on multiple fronts: Insights from the Vector Control Optimization Model (VCOM) for malaria elimination PLoS One 12 e0187680
E Sherrard-Smith JE Skarp AD Beale C Fornadel LC Norris SJ Moore 2019 Mosquito feeding behavior and how it influences residual malaria transmission across Africa Proc Natl Acad Sci USA 116 15086 15095
M Gillies A Smith 1960 The effect of a residual house-spraying campaign in East Africa on species balance in the Anopheles funestus group. The replacement of A. funestus Giles by A. rivulorum Leeson Bull Entomol Res. 51 243 52
CC Draper JL Lelijveld YG Matola GB White 1972 Malaria in the Pare area of Tanzania. IV. Malaria in the human population 11 years after the suspension of residual insecticide spraying, with special reference to the serological findings Trans R Soc Trop Med Hyg. 66 905 12
MN Bayoh DK Mathias MR Odiere FM Mutuku L Kamau JE Gimnig 2010 Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province Kenya Malar J 9 62
DW Lwetoijera C Harris SS Kiware S Dongus GJ Devine PJ McCall 2014 Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley Tanzania Malar J 13 331
EW Kaindoa NS Matowo HS Ngowo G Mkandawile A Mmbando M Finda 2017 Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south–eastern Tanzania PLoS One 12 e0177807
BL Sharp D Sueur le 1996 Malaria in South Africa: the past, the present and selected implications for the future S Afr Med J 86 83 89
YA Derua M Alifrangis KM Hosea DW Meyrowitsch SM Magesa EM Pedersen 2012 Change in composition of the Anopheles gambiae complex and its possible implications for the transmission of malaria and lymphatic filariasis in north-eastern Tanzania Malar J 11 188
J Kitau RM Oxborough PK Tungu J Matowo RC Malima SM Magesa 2012 Species shifts in the Anopheles gambiae complex: do LLINs successfully control Anopheles arabiensis? PLoS One 7 e31481
GG Padonou G Gbedjissi A Yadouleton R Azondekon O Razack O Oussou 2012 Decreased proportions of indoor feeding and endophily in Anopheles gambiae s.l. populations following the indoor residual spraying and insecticide-treated net interventions in Benin (West Africa) Parasit Vectors. 5 262
A Smith 1962 Studies on Domestic Habits of A. gambiae that affect its Vulnerability to Insecticides East Afr Med J. 39 15 24
G Giglioli 1951 Nation-wide malaria eradication projects in the America. III. Eradication of Anopheles darlingi from the inhabited areas of British Guiana by DDT residual spraying J Natl Malar Soc. 10 142 61
H Hiwat S Mitro A Samjhawan P Sardjoe T Soekhoe W Takken 2012 Collapse of Anopheles darlingi populations in Suriname after introduction of insecticide-treated nets (ITNs); malaria down to near elimination level Am J Trop Med Hyg 86 649 655
GF Killeen FE McKenzie BD Foy C Bøgh JC Beier 2001 The availability of potential hosts as a determinant of feeding behaviours and malaria transmission by African mosquito populations Trans R Soc Trop Med Hyg 95 469 476
A Kiszewski A Mellinger A Spielman P Malaney SE Sachs J Sachs 2004 A global index representing the stability of malaria transmission Trans R Soc Trop Med Hyg 70 486 498
SS Kiware N Chitnis GJ Devine SJ Moore S Majambere GF Killeen 2012 Biologically meaningful coverage indicators for eliminating malaria transmission Biol Lett 8 874 877
FC Meza KS Kreppel DF Maliti AT Mlwale N Mirzai GF Killeen 2019 Mosquito electrocuting traps for directly measuring biting rates and host-preferences of Anopheles arabiensis and Anopheles funestus outdoors Malar J 18 83
F Massebo M Balkew T Gebre-Michael B Lindtjorn 2015 Zoophagic behaviour of anopheline mosquitoes in southwest Ethiopia: opportunity for malaria vector control Parasit Vectors 8 645
SJ Torr A Della Torre M Calzetta C Costantini GA Vale 2008 Towards a fuller understanding of mosquito behaviour: use of electrocuting grids to compare the odour-orientated responses of Anopheles arabiensis and An. quadriannulatus in the field Med Vet Entomol. 22 93 108
GB White 1974 Anopheles gambiae complex and disease transmission in Africa Trans R Soc Trop Med Hyg 68 278 298
NF Lobo B St Laurent CH Sikaala B Hamainza J Chanda D Chinula 2015 Unexpected diversity of Anopheles species in Eastern Zambia: implications for evaluating vector behavior and interventions using molecular tools Sci Rep 5 17952
B Pluess FC Tanser C Lengeler BL Sharp 2010 Indoor residual spraying for preventing malaria Cochrane Database Syst Rev. 4 CD006657
J Pryce M Richardson C Lengeler 2018 Insecticide-treated nets for preventing malaria Cochrane Database Syst Rev. 11 CD000363
NJ Govella H Ferguson 2012 Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination Front Physiol 3 199
JL Waite S Swain PA Lynch SK Sharma MA Haque J Montgomery 2017 Increasing the potential for malaria elimination by targeting zoophilic vectors Sci Rep 7 40551
T Lefèvre L-C Gouagna KR Dabiré E Elguero D Fontenille F Renaud 2009 Beyond nature and nurture: phenotypic plasticity in blood-feeding behavior of Anopheles gambiae s.s. when humans are not readily accessible Am J Trop Med Hyg 81 1023 1029
W Takken NO Verhulst 2013 Host preferences of blood-feeding mosquitoes Annu Rev Entomol 58 433 453
National Bureau of Statistics (NBS), Office of Chief Government Statistician (OCGS): Population and housing census 2012 Report: Population Distribution by Administrative Areas United Republic of Tanzania Dar es Salaam 2013
Y Geissbühler P Chaki B Emidi NJ Govella R Shirima V Mayagaya 2007 Interdependence of domestic malaria prevention measures and mosquito-human interactions in urban Dar es Salaam, Tanzania Malar J 6 126
D Msellemu HI Namango VM Mwakalinga AJ Ntamatungiro Y Mlacha ZJ Mtema 2016 The epidemiology of residual Plasmodium falciparum malaria transmission and infection burden in an African city with high coverage of multiple vector control measures Malar J 15 288
Y Geissbühler K Kannady PP Chaki B Emidi NJ Govella V Mayagaya 2009 Microbial larvicide application by a large-scale, community-based program reduces malaria infection prevalence in urban Dar es Salaam, Tanzania PloS One 4 e5107
NJ Govella JD Moore GF Killeen 2010 An exposure-free tool for monitoring adult malaria mosquito populations Am J Trop Med Hyg 83 596 600
Killeen GF, Govella NJ, Mlacha YP, Chaki PP. Suppression of malaria vector densities and human infection prevalence associated with scale-up of mosquito-proofed housing in Dar es Salaam, Tanzania: re-analysis of an observational series of parasitological and entomological surveys. Lancet Planet Health. 2019;3: e132.
M Maheu-Giroux MC Castro 2013 Impact of community-based larviciding on the prevalence of malaria infection in Dar es Salaam Tanzania PLoS One 8 e71638
Dar es Salaam City Council. City Profile for Dar Es Salaam, United Republic of Tanzania; 2004.
G Killeen A Tami J Kihonda F Okumu M Kotas H Grundmann 2007 Cost-sharing strategies combining targeted public subsidies with private-sector delivery achieve high bednet coverage and reduced malaria transmission in Kilombero Valley, southern Tanzania BMC Infect Dis 7 121
WR Mukabana W Takken R Coe BG Knols 2002 Host-specific cues cause differential attractiveness of Kenyan men to the African malaria vector Anopheles gambiae Malar J 1 17
SW Lindsay JH Adiamah JE Miller RJ Pleass JRM Armstrong 1993 Variation in attractiveness of human-subjects to malaria mosquitos (Diptera, Culicidae) in the Gambia J Med Entomol 30 368 373
MT Gillies TJ Wilkes 1965 A study of the age-composition of populations of Anopheles gambiae Giles and A. funestus Giles in North-Eastern Tanzania Bull Entomol Res. 56 237 62
SS Kiware TL Russell ZJ Mtema AD Malishee P Chaki D Lwetoijera 2016 A generic schema and data collection forms applicable to diverse entomological studies of mosquitoes Source Code Biol Med 11 4
AO Franco MGM Gomes M Rowland PG Coleman CR Davies 2014 Controlling malaria using livestock-based interventions: a one health approach PLoS One 9 e101699
G Port P Boreham JH Bryan 1980 The relationship of host size to feeding by mosquitoes of the Anopheles gambiae Giles complex (Diptera: Culicidae) Bull Entomol Res 70 133 144
G White SA Magayuka P Boreham 1972 Comparative studies on sibling species of the Anopheles gambiae Giles complex (Dipt., Culicidae): bionomics and vectorial activity of species A and species B at Segera Tanzania. Bull Entomol Res. 62 295 317
M Coetzee M Craig D Sueur le 2000 Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex Parasitol Today 16 74 77
National Bureau of Statistics (NBS/Tanzania), ICF Macro. Tanzania Demographic and Health Survey 2010. Dar es Salaam,Tanzania, NBS/Tanzania and ICF Macro. 2011. https://dhsprogram.com/publications/publication-fr243-dhs-final-reports.cfm.
M Gillies B Mellion De 1968 The Anophelinae of Africa South of the Sahara (Ethiopian zoogeographical region) Publ S Afr Inst Med Res 54 1 343
MR Reddy HJ Overgaard S Abaga VP Reddy A Caccone AE Kiszewski 2011 Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island Equatorial Guinea Malar J 10 184
BJ Main Y Lee HM Ferguson KS Kreppel A Kihonda NJ Govella 2016 The genetic basis of host preference and resting behavior in the major African malaria vector Anopheles arabiensis PLoS Genet 12 e1006303
VM Gantz N Jasinskiene O Tatarenkova A Fazekas VM Macias E Bier 2015 Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi Proc Natl Acad Sci USA 112 E6736 E6743
C Chaccour GF Killeen 2016 Mind the gap: residual malaria transmission, veterinary endectocides and livestock as targets for malaria vector control Malar J 15 24
SS Imbahale JM Lopez J Brew K Paaijmansz C Rist C Chaccour 2019 Mapping the potential use of endectocide-treated cattle to reduce malaria transmission Sci Rep 9 5826
The Ivermectin Roadmappers 2020 A roadmap for the development of ivermectin as a complementary malaria vector control tool Am J Trop Med Hyg 102 3 24
CJ Chaccour K Ngha'bi G Abizanda AI Barrio A Aldaz F Okumu 2018 Targeting cattle for malaria elimination: marked reduction of Anopheles arabiensis survival for over six months using a slow-release ivermectin implant formulation Parasit Vectors 11 287
NH Rose M Sylla A Badolo J Lutomiah D Ayala OB Aribodor 2020 Climate and urbanization drive mosquito preference for humans Curr Biol 30 3570 3579
Killeen GF, Chaki PP, Reed TE, Moyes CL, Govella NJ. Entomological surveillance as a cornerstone of malaria elimination: a critical appraisal. In: Towards malaria elimination - a leap forward. Manguin S, Vas D, Eds. IntechOpen; 2018: 403–29
Acknowledgments
We are enormously grateful for the support provided by all the study participants both in urban Dar es Salaam and in Kilombero. We are thankful for the cattle owners from these two communities to trust and allow us to use their calves during the study. Thanks to Halfan Ngowo and Josephine Malinga for their inputs during statistical analysis. We are grateful to our Drivers, Jonael Msangi and Eldadi Govella, for calves availability coordination, picks up, and drop off volunteers timely and facilitation of spot-check over the nights.
Funding
Data collection for this study was financially supported by the Bill & Melinda Gates Foundation through the Malaria Transmission Consortium [Award number 45114] and Wellcome Trust Masters Fellowship Awarded to Dennis J. Massue [Grant Number 089326/z/09/z]. UK-Medical Research Council under the African Research Leaders Award (Grant Ref: MR/T008873/1) awarded to Nicodem Govella, and an AXA Research Chair award to Gerry Killeen supported analysis and writing of the manuscript. YPM is a recipient of a Swiss Government Excellence Scholarship via the Federal Commission for Scholarships for Foreign Students FCS (ESKAS)(Ref Number: 2017.0786).
Author information
Authors and Affiliations
Contributions
GFK, SM, PPC, and NJG conceived the study and designed experiments. YPM, AM, and DJM contributed to the study design, trained the mosquito collectors, implemented and supervised the field activities. YPM, NJG, and GFK performed the analysis. YPM and NJG drafted and revised the manuscript. SM, PC, DJM, and MT reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The permission to carry out this study was approved by the Ethical Committee of the National Institute of Medical Research [NIMR/HQ/R.8a/Vol.IX/279 and 324] and from the Institutional Review Board of the Ifakara Health Institute [IHI-IRB-A.50]. The volunteer was provided with informed consent after a verbal explanation about study design in the local language. Before participation and after the experimentation days, participants were screened by a trained clinician for malaria parasite using a rapid diagnostic test (mRDT), and none was found positive. In case they were found positive, they would be treated free of charge following the national guideline of malaria treatment and withdrawn from the study. Information sheets of the informed consent included assurance of confidentiality, voluntary participation, potential risks, and benefit associated with the study. They also had the right to withdraw from the study at any time without justification.
Consent for publication
As part of the consenting procedure and information sheet’s content, they also included a request that results obtained will be disseminated through scientific articles to reach broad audiences, including academics. The permission to publish was thereafter obtained from the National Institute of Medical Research (NIMR), Tanzania that has the legal mandate to approve publications of results from public health research data.
Competing interests
The authors declare that no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mlacha, Y.P., Chaki, P.P., Muhili, A. et al. Reduced human-biting preferences of the African malaria vectors Anopheles arabiensis and Anopheles gambiae in an urban context: controlled, competitive host-preference experiments in Tanzania. Malar J 19, 418 (2020). https://doi.org/10.1186/s12936-020-03495-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-020-03495-z