Abstract
Background
Recent studies from different malaria-endemic regions including western Africa have now shown that Plasmodium vivax can infect red blood cells (RBCs) and cause clinical disease in Duffy-negative people, though the Duffy-negative phenotype was thought to confer complete refractoriness against blood invasion with P. vivax. The actual prevalence of P. vivax in local populations in Ghana is unknown and little information is available about the distribution of Duffy genotypes. The aim of this study was to assess the prevalence of P. vivax in both asymptomatic and symptomatic outpatients and the distribution of Duffy genotypes in Ghana.
Methods
DNA was extracted from dried blood spots (DBS) collected from 952 subjects (845 malaria patients and 107 asymptomatic persons) from nine locations in Ghana. Plasmodium species identification was carried out by nested polymerase chain reaction (PCR) amplification of the small-subunit (SSU) rRNA genes. For P. vivax detection, a second PCR of the central region of the Pvcsp gene was carried out. Duffy blood group genotyping was performed by allele-specific PCR to detect the presence of the FYES allele.
Results
No cases of P. vivax were detected in any of the samples by both PCR methods used. Majority of infections (542, 94.8%) in the malaria patient samples were due to P. falciparum with only 1 infection (0.0017%) due to Plasmodium malariae, and 2 infections (0.0034%) due to Plasmodium ovale. No case of mixed infection was identified. Of the samples tested for the FYES allele from all the sites, 90.5% (862/952) had the FYES allele. All positive samples were genotyped as FY*B-33/FY*B-33 (Duffy-negative homozygous) and therefore classified as Fy(a−b−).
Conclusions
No cases of P. vivax were detected by both PCRs and majority of the subjects tested carried the FYES allele. The lack of P. vivax infections observed can be attributed to the high frequency of the FYES allele that silences erythroid expression of the Duffy. These results provide insights on the host susceptibility for P. vivax infections that had not been investigated in Ghana before.
Similar content being viewed by others
Background
Malaria remains the most important parasitic infection in the world, with 228 million cases in 2018 (95% confidence interval [CI] 206–258 million) [1], caused by infection with one or more of the six species of Plasmodium parasites. Two species, Plasmodium falciparum and Plasmodium vivax, are responsible for most of the morbidity and mortality due to malaria globally [2, 3]. However, P. vivax malaria does not attract as much attention in almost every aspect as does the deadlier P. falciparum malaria because, traditionally, the infection was thought to be benign and self-limiting [4, 5]. Recent evidence is however challenging this long-held notion of the benign nature of P. vivax malaria, demonstrating that infection with P. vivax can also result in severe illness and death [6]. Globally, 53% of the P. vivax burden is in the WHO South-East Asia Region and P. vivax is the predominant parasite (75% of malaria cases) in the WHO Region of the Americas [1]. In 2018, an estimated 704,000 (95% CI 91,000–1,813,000) P. vivax malaria cases were reported in Africa [1].
An important biological difference between P. vivax and P. falciparum is that only P. vivax merozoites use the Duffy (Fy) antigen receptor for chemokines (DARC) to invade erythrocytes [7, 8]. The DARC-coding gene is polymorphic with multiple alleles as the codominant FY*A and FY*B, which encode for the two antigens—Fya and Fyb. Four genotypes are possible as a result of the combination of the major alleles, Fy(a+b+), Fy(a+b−), Fy(a−b+) and Fy(a−b−) [9]. The first three correspond to a Duffy-positive phenotype, mostly prevalent in Asian and in Caucasian populations and the last one corresponds to the Duffy-negative phenotype, mainly prevalent in African people, who are consequently deemed to be refractory to P. vivax infection. The Fy(a−b−) genotype results from a point mutation, − 33 T → C, in the promoter region of allele FY*B, in the GATA box region [10], preventing transcription and resulting in the null ‘erythrocyte silent’ (ES) phenotype.
Until now, the Duffy-negative phenotype was viewed as giving almost total protection against infection with P. vivax because it prevents P. vivax from invading host erythrocytes and completing its complex life cycle [11]. Field observations indicate that the conclusion of the absolute dependence on the presence of Duffy on the red cell for P. vivax infection and development in the red cell no longer holds true because of a number of reports concerning findings of P. vivax in the blood of Duffy-negative persons in Brazil [12], Ethiopia [13, 14], Madagascar [15], Kenya [16], Equatorial Guinea and Angola [2] including West African countries, such as Mauritania [17], Cameroon [18, 19], Mali [20], and Benin [21]. Thus, contrary to expectation, there is evidence of P. vivax transmission even in areas mapped with highest Duffy-negativity frequencies [20, 22, 23].
The exact frequency of Duffy blood group is poorly documented across Africa, as indeed few populations have been surveyed and there are large gaps in the documentation on Duffy genotypes and phenotypes across Africa [24], with Ghana being no exception. Culleton et al. [25] have concluded that there are sufficient numbers of Duffy-positive individuals in some areas in Africa to maintain P. vivax transmission in areas where the majority of the population is Duffy-negative. The first objective of the present study was to evaluate the P. vivax circulation among both symptomatic and asymptomatic outpatients seeking medical care in various parts of Ghana. The second objective was to explore the Duffy antigen genotype frequency among the study population.
Methods
Study areas
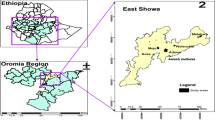
The study was conducted in nine sites across Ghana (Fig. 1): Accra, Bekwai, Cape Coast, Hohoe, Tarkwa, Sunyani, are urban sites, and Navrongo, Wa, and Yendi are rural sites. With the exception of Accra, the other eight are sentinel sites used as part of a surveillance programme for monitoring malaria drug resistance in Ghana. The description of the sentinel sites has already been published [26,27,28]. The sites are located in three ecological zones of Ghana.

(Adapted from Duah et al. [28])
A map of Ghana showing the 9 collection sites. U urban, R rural
The Accra metropolis is the capital of Ghana and has an estimated population of 2,052,341 in 2019 [29]. The city experiences generally low and erratic rainfall pattern. Annual rainfall in the metropolis is currently 1190 mm [30]. Most of the rainfall is recorded between March and July. Malaria transmission in the metropolis is perennial.
Sample collection and preparation
Sampling was both prospective and retrospective. Retrospective blood samples were collected by finger prick on filter paper (Whatman 3 MM filter paper) from subjects in the study areas. Informed consent was sought from all subjects. Prospective blood samples, all from Accra, were obtained from the blood banks at the Korle Bu Teaching Hospital and 37 Military Hospital to investigate the frequency of the Duffy allele. Samples were collected from 2013 to 2017.
DNA extraction
Dried blood spots (DBS) on filter paper were cut into small pieces with scissors and transferred into 1.5-ml microtubes. For lysis, a modified salting out DNA extraction protocol with several modifications based on [31] was used. Briefly, 200 µl TNES digestion buffer (10 mM Tris–HCl (pH 7.5), 400 mM NaCl, 100 mM EDTA, 0.60% SDS) was added to the filter papers, followed by the addition of 6 µl of proteinase K (10 mg/ml) to each tube and incubation at 55 °C overnight in a heat block (Thermo Block TDB-120, Warren, USA). Samples were retrieved, 200 µl 5 M NaCl was added to each tube and vortexed briefly. The contents were then spun at 15,000 rpm for 10 min. The supernatants were transferred into 1.5 ml Eppendorf tubes, and 800 µl of cold absolute ethanol added to each tube and rocked gently back and forth. Samples were stored in a − 18 °C freezer for 3 h. They were retrieved, allowed to thaw, and spun at 14,000 rpm for 30 min. Then absolute ethanol was carefully poured off the pellet, 500 µl 70% ethanol added and the tubes spun again at 14,000 rpm for 5 min. After the spun, the supernatant was poured off and the tubes were blotted on filter paper and air-dried. The formed pellets were finally re-suspended in 200 µl TE (10 mM Tris–HCl, pH 8.0; 1 mM EDTA, pH 8.0) and stored at − 21 °C.
Detection and identification of Plasmodium species
Detection of malaria infection and identification of Plasmodium species for all DBS samples were carried out using the nested-PCR amplification of the SSU rRNA genes as described by Snounou et al. [32]. For each PCR run, a negative control (sterile distilled water) and a positive control (P. falciparum 3D7 DNA) were used. PCR was performed using OneTaq® Quick-Load® 2X Master Mix with standard buffer from NEB (New England Biolabs Inc., Ipswich, MA, USA). PCR reactions were carried out in a SEEAMP™ SCE1000 thermal cycler (Seegene Inc., Seoul, Korea). Primers and PCR conditions are shown in Table 1.
Genotyping of Pvcsp genes
Further P. vivax parasite detection was carried out by analysis of the central region of the Pvcsp gene, following a slightly modified version of the protocol described by Alves et al. [33]. PCR was performed using OneTaq® Quick-Load® 2X Master Mix with standard buffer (NEB) and 0.4 µM of each primer. PCR reactions were carried out in a SEEAMP™ SCE1000 thermal cycler (Seegene Inc., Seoul, Korea). All PCR runs included a P. vivax positive control (courtesy of Michael Alifrangis). Primers and PCR conditions are shown in Table 1.
After the reaction 10 µl of the PCR product was run by electrophoresis at 120 V in 2% agarose gel (Biopioneer Co, USA) stained with 0.5 µg/ml ethidium bromide (Life Technologies Co, USA) in 1× Tris acetate EDTA (TAE) running buffer (Biopioneer Co, USA) using 2 µl of blue/orange DNA loading dye (6×) (Promega Co, USA). A 100-base pair DNA ladder (NEB) was run alongside the PCR products on the gel. The gel was photographed using UV-illumination (UVIsave gel documentation system, model GAS9200/1/2/3, version 12) and analysed.
Duffy blood group genotyping by allele-specific PCR
Duffy genotypes were determined for all blood samples by allele-specific PCR using the protocol described by Olsson et al. [34], with some modifications. Four PCRs were performed on each sample to genotype FY*A, FY*B, FY*AES and FY*BES alleles. The combination of GATAFY2 and FYAREV identified the FY*AES allele, GATAFY2 and FYBREV identified the FY*BES allele, FYAB2 and FYAREV primers the FY*A allele, and FYAB2 and FYBREV primers the FY*B allele. PCR was performed using OneTaq® Quick-Load® 2× Master Mix with standard buffer (NEB) and 0.4 µM of each primer. In addition, co-amplification of the human growth hormone gene (HGH) using 0.04 µM each of the HGH-F and HGH-R primers was run as an amplification control [35]. PCR reactions were carried out in a SEEAMP™ SCE1000 thermal cycler (Seegene Inc., Seoul, Korea). Primers and PCR conditions are shown in Table 1.
Results
Study population
A total of 952 subjects (845 malaria patients and 107 asymptomatic persons) from 9 locations in Ghana were used for the study.
Detection and identification of Plasmodium species
All the 107 asymptomatic persons were negative for Plasmodium species detection by nested PCR. Out of the 845 malaria patient samples, only 545 (64.5%) were found to be infected with malaria parasites following PCR diagnostic assays of the 18S rRNA gene (Table 2). As expected, majority of these infections (542, 94.8%) were due to P. falciparum with only 1 infection due to Plasmodium malariae (0.0017%) and 2 infections due to Plasmodium ovale (0.0034%). No mixed parasitic infections were detected. No cases of P. vivax were detected by PCR in the 845 patient samples tested from all the study sites.
Genotyping of Pvcsp genes
In order to confirm the results from the PCR analyses, a different gene of P. vivax (Pvcsp) was also PCR amplified but gave the same results in that the expected 1100 bp fragment was amplified in only the positive control DNA.
Duffy blood group genotyping by allele-specific PCR
All the 952 subjects were Duffy genotyped by allele-specific PCR. A negative reaction was defined as the presence of only the 434 bp amplification HGH control DNA fragment. A positive reaction was defined as the presence of a clearly visible 711-bp DNA fragment with or without the amplification control band (Fig. 2). Genotyping revealed the absence of FY*BES allele in 90.5% (862/952) of the samples which suggested the detection of Fy(a−b−) (Table 3).
Example of an ethidium bromide-stained 1.0% agarose gel electrophoresis of allele specific PCR products for Duffy genotyping. Lanes 1 and 2 show PCR positive for both internal control and Duffy allele; Lane 3 shows a negative sample; Lane 4 shows PCR positive for only Duffy allele; Lanes 5 and 6 show PCR positive for only internal control. M = 100 bp ladder (NEB)
Discussion
Until relatively recently, P. vivax was rarely studied across most of sub-Saharan Africa and malaria diagnostics frequently remained limited to P. falciparum. Since 2010, however, evidence of the presence P. vivax in West Africa has emerged [21, 23, 36, 37] despite the high prevalence of Duffy-negative red blood cell phenotype. Nevertheless, no P. vivax infections were found in this molecular based study conducted in nine sites across Ghana.
Both symptomatic and asymptomatic outpatients were involved in this study. Malaria is hyperendemic in Ghana and 44% of outpatient visits at the various health facilities are attributed to malaria [38]. At the community level, fever or a history of fever is presumptively treated as malaria. However, according to [39], ≥ 75% of infections in malaria endemic areas are asymptomatic. This has been attributed to the development of protective immunity in adult populations against high parasitaemia and clinical disease due to the long-term continuous exposure to mosquito bites [40]. In Ghana, studies in both low and high transmission areas have found evidence of asymptomatic malaria in adult residents [41] as well as children [42] and pregnant women [43]. Asymptomatic malaria cases have been found to be higher than symptomatic cases in some studies [42, 44]. Most asymptomatic malaria infections are linked to submicroscopic parasite densities, and require the use molecular diagnostics methods [40, 45], since conventional microscopy and rapid diagnostic tests (RDTs) are of limited sensitivity.
Though P. vivax infections cannot be entirely ruled out in Ghana, it is important to note that an earlier study from China [46] reported a case of a 39-year-old Chinese man who had stayed in Ghana, for 6 months in 2012, for whom a microscopic examination of Giemsa-stained thin and thick blood smears initially indicated P. vivax infection. However, the results of a thrice conducted rapid diagnostic test were not in agreement with P. vivax and standard PCR analysis of the SSU rRNA gene, followed by gene sequencing, pointed to a variant P. ovale wallikeri. Microscopic identification of P. ovale and P. vivax due to their morphological similarities [47] may be unreliable since P. vivax can be misdiagnosed for P. ovale infections and conversely [48]. There is also potential for cross-reactivity between P. ovale- and P. vivax-specific antigens in serological screening [49].
In a 2019 case report also from China [50], a 49-year-old Chinese man was diagnosed by both microscopy and PCR as having uncomplicated P. vivax malaria on December 19, 2016. This was 39 days after he returned from Ghana after a stay of one and a half years. However, the Duffy genotype of the Chinese man was not given. The presence of the Fy(a−b−) phenotype outside the African continent and the Arabian Peninsula has been estimated to be at frequencies not exceeding 10% [22]. It is, therefore, highly likely that the Chinese man is Duffy-positive since the frequency of Fya among the Chinese has been estimated to exceed 97% [51].
Evidence relating to P. vivax transmission across Africa appears inconsistent [49]. In the West African countries were P. vivax infections have been recorded, Nigeria [52, 53], Mauritania [54], Mali [55], Cameroon [19], and Benin [21], the prevalence has been very low from these studies. These studies have varied in terms of sample size and diagnostic methods [56] and in some reports the Duffy antigen status of the patients was not determined [36, 52, 54]. As in this present study, extensive surveys using high-sensitivity molecular methods have repeatedly failed to diagnose P. vivax [25, 57].
The low prevalence of P. vivax infection in West Africa has been attributed to the high frequency of the Duffy-negative phenotype in this region [7, 22, 58]. In this study, 90.5% (862/952) of the malaria patients had the FYES allele and were classified as Fy(a−b−) in agreement with the report by Howes et al. [49]. It is clearly obvious that Duffy-negativity provides significant protection against P. vivax blood-stage infection, particularly in symptomatic patients presenting for treatment, though this protection is not absolute. This is in agreement with long-prevailing thinking that for P. vivax invasions to occur an interaction between the parasites and antigens of the Duffy blood group system is necessary [59, 60]. However, several other host cell receptors have recently been identified as being involved in the parasite invasion pathway of RBCs. Gruszczyk et al. [61] identified host transferrin receptor 1 (TfR1 or CD71) as an alternative receptor, critical for P. vivax entry into reticulocytes. CD98 has also been shown to be involved in entrance of the parasite into the host cell [62]. Lack of the Duffy antigen thus seems to places a certain restriction on the invasion mechanism, but not completely. A better understanding of the mechanisms that allow interaction between P. vivax and the Fya and Fyb host antigens may allow more specific assessments of the risks of P. vivax infection and clinical disease across the Duffy-negative populations previously considered fully protected, as well as identifying potential vaccine targets.
Conclusions
No P. vivax infections were confirmed by both PCRs and the high FYES allele frequency could explain the sparse evidence of P. vivax infections in the samples studied across the country. Despite the fact that P. vivax infections cannot be entirely ruled out in Ghana, P. vivax malaria at present does not pose a public health risk in the country.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DBS:
-
Dried blood spots
- DNA:
-
Deoxyribonucleic acid
- ES:
-
Erythrocyte silent
- PCR:
-
Polymerase chain reaction
- rRNA:
-
Ribosomal RNA
References
WHO. World malaria report 2019. Geneva: World Health Organization; 2019.
Mendes C, Dias F, Figueiredo J, Mora VG, Cano J, de Sousa B, et al. Duffy negative antigen is no longer a barrier to Plasmodium vivax—molecular evidences from the African West Coast (Angola and Equatorial Guinea). PLoS Negl Trop Dis. 2011;5:e1192.
WHO. World malaria report, 2016. Geneva: World Health Organization; 2016.
Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–66.
Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77(Suppl 6):79–87.
Alexandre MA, Ferreira CO, Siqueira AM, Magalhaes BL, Mourao MP, Lacerda MV, et al. Severe Plasmodium vivax malaria, Brazilian Amazon. Emerg Infect Dis. 2010;16:1611–4.
Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med. 1976;295:302–3044.
Miller LH, Mason SJ, Dvorak JA, McGinniss MH, Rothman IK. Erythrocyte receptors for Plasmodium knowlesi malaria: Duffy blood group determinants. Science. 1975;189:561–3.
Parasol N, Reid M, Rios M, Castilho L, Harari I, Kosower NS. A novel mutation in the coding sequence of the FY*B allele of the Duffy chemokine receptor gene is associated with an altered erythrocyte phenotype. Blood. 1998;92:2237–43.
Tournamille C, Colin Y, Cartron JP, Le Van KC. Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat Genet. 1995;10:224–8.
Miller LH, Aikawa M, Johnson JG, Shiroishi T. Interaction between cytochalasin B-treated malarial parasites and erythrocytes. Attachment and junction formation. J Exp Med. 1979;149:172–84.
Carvalho TA, Queiroz MG, Cardoso GL, Diniz IG, Silva AN, Pinto AY, et al. Plasmodium vivax infection in Anajas, State of Para: no differential resistance profile among Duffy-negative and Duffy-positive individuals. Malar J. 2012;11:430.
Lo E, Yewhalaw D, Zhong D, Zemene E, Degefa T, Tushune K, et al. Molecular epidemiology of Plasmodium vivax and Plasmodium falciparum malaria among Duffy-positive and Duffy-negative populations in Ethiopia. Malar J. 2015;14:84.
Woldearegai TG, Kremsner PG, Kun JF, Mordmuller B. Plasmodium vivax malaria in Duffy-negative individuals from Ethiopia. Trans R Soc Trop Med Hyg. 2013;107:328–31.
Menard D, Barnadas C, Bouchier C, Henry-Halldin C, Gray LR, Ratsimbasoa A, et al. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci USA. 2010;107:5967–71.
Ryan JR, Stoute JA, Amon J, Dunton RF, Mtalib R, Koros J, et al. Evidence for transmission of Plasmodium vivax among a duffy antigen negative population in Western Kenya. Am J Trop Med Hyg. 2006;75:575–81.
Wurtz N, Mint Lekweiry K, Bogreau H, Pradines B, Rogier C, Ould Mohamed Salem Boukhary A, et al. Vivax malaria in Mauritania includes infection of a Duffy-negative individual. Malar J. 2011;10:336.
Fru-Cho J, Bumah VV, Safeukui I, Nkuo-Akenji T, Titanji VP, Haldar K. Molecular typing reveals substantial Plasmodium vivax infection in asymptomatic adults in a rural area of Cameroon. Malar J. 2014;13:170.
Ngassa Mbenda HG, Das A. Molecular evidence of Plasmodium vivax mono and mixed malaria parasite infections in Duffy-negative native Cameroonians. PLoS ONE. 2014;9:e103262.
Bernabeu M, Gomez-Perez GP, Sissoko S, Niambele MB, Haibala AA, Sanz A, et al. Plasmodium vivax malaria in Mali: a study from three different regions. Malar J. 2012;11:405.
Poirier P, Doderer-Lang C, Atchade PS, Lemoine JP, de l’Isle MC, Abou-Bacar A, et al. The hide and seek of Plasmodium vivax in West Africa: report from a large-scale study in Beninese asymptomatic subjects. Malar J. 2016;15:570.
Howes RE, Patil AP, Piel FB, Nyangiri OA, Kabaria CW, Gething PW, et al. The global distribution of the Duffy blood group. Nat Commun. 2011;2:266.
Russo G, Faggioni G, Paganotti GM, Djeunang Dongho GB, Pomponi A, De Santis R, et al. Molecular evidence of Plasmodium vivax infection in Duffy negative symptomatic individuals from Dschang, West Cameroon. Malar J. 2017;16:74.
Mercereau-Puijalon O, Ménard D. Plasmodium vivax and the Duffy antigen: a paradigm revisited. Transfus Clin Biol. 2010;17:176–83.
Culleton RL, Mita T, Ndounga M, Unger H, Cravo PV, Paganotti GM, et al. Failure to detect Plasmodium vivax in West and Central Africa by PCR species typing. Malar J. 2008;7:174.
Abuaku B, Duah N, Quaye L, Quashie N, Koram K. Therapeutic efficacy of artemether-lumefantrine combination in the treatment of uncomplicated malaria among children under five years of age in three ecological zones in Ghana. Malar J. 2012;11:388.
Duah NO, Quashie NB, Abuaku BK, Sebeny PJ, Kronmann KC, Koram KA. Surveillance of molecular markers of Plasmodium falciparum resistance to sulphadoxine-pyrimethamine 5 years after the change of malaria treatment policy in Ghana. Am J Trop Med Hyg. 2012;87:966–1003.
Duah NO, Wilson M, Ghansah A, Abuaku B, Edoh D, Quashie N, et al. Mutations in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance genes, and treatment outcomes in Ghanaian children with uncomplicated malaria. J Trop Pediatr. 2007;53:27–31.
Ghana Statistical Services. Demography: population projection 2020. (updated 2019) https://statsghana.gov.gh/nationalaccount_macros.php?Stats=MTA1NTY1NjgxLjUwNg==/webstats/s679n2sn87.
Accra Metropolitan Assembly & C40 Cities. Accra Climate Action Plan: first five-year plan (2020–2025). Accra, 2020.
Asadzaheh N, Javanmard A, Nassiry MR. Comparison of rapid DNA extraction techniques for conventional PCR-RFLP analysis from mammalian whole blood cells. J Mol Genet. 2010;2:32–5.
Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–20.
Alves RT, Póvoa MM, Goldman IF, Cavasini CE, Rossit AR, Machado RL. A new polymerase chain reaction/restriction fragment length polymorphism protocol for Plasmodium vivax circumsporozoite protein genotype (VK210, VK247, and P. vivax-like) determination. Diagn Microbiol Infect Dis. 2007;59:415–9.
Olsson ML, Hansson C, Avent ND, Akesson IE, Green CA, Daniels GL. A clinically applicable method for determining the three major alleles at the Duffy (FY) blood group locus using polymerase chain reaction with allele-specific primers. Transfusion. 1998;38:168–73.
Crottet SL, Henny C, Meyer S, Still F, Stolz M, Gottschalk J, et al. Implementation of a mandatory donor RHD screening in Switzerland. Transfus Apher Sci. 2014;50:169–74.
Ba H, Duffy CW, Ahouidi AD, Deh YB, Diallo MY, Tandia A, et al. Widespread distribution of Plasmodium vivax malaria in Mauritania on the interface of the Maghreb and West Africa. Malar J. 2016;15:80.
Rogier E, Moss DM, Chard AN, Trinies V, Doumbia S, Freeman MC, et al. Evaluation of immunoglobulin G Responses to Plasmodium falciparum and Plasmodium vivax in Malian school children using multiplex bead assay. Am J Trop Med Hyg. 2017;96:312–8.
Abdul-Aziz AR, Harris E, Munyakazi L. Risk factors in malaria mortality among children in Northern Ghana: a case study at the Tamale Teaching Hospital. Int J Bus Soc Res. 2012;2:35–45.
Greenwood BM. Asymptomatic malaria infections—do they matter? Parasitol Today. 1987;3:206–14.
Okell LC, Bousema T, Griffin JT, Ouédraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun. 2012;3:1237.
Heinemann M, Phillips RO, Vinnemeier CD, Rolling CC, Tannich E, Rolling T. High prevalence of asymptomatic malaria infections in adults, Ashanti Region, Ghana, 2018. Malar J. 2020;19:366.
Owusu ED, Buabeng V, Dadzie S, Brown CA, Grobusch MP, Mens P. Characteristics of asymptomatic Plasmodium spp. parasitaemia in Kwahu-Mpraeso, a malaria endemic mountainous district in Ghana, West Africa. Malar J. 2016;15:38.
Lamptey H, Ofori MF, Kusi KA, Adu B, Owusu-Yeboa E, Kyei-Baafour E, et al. The prevalence of submicroscopic Plasmodium falciparum gametocyte carriage and multiplicity of infection in children, pregnant women and adults in a low malaria transmission area in Southern Ghana. Malar J. 2018;17:331.
Diallo N, Akweongo P, Maya E, Aikins M, Sarfo B. Burden of malaria in mobile populations in the Greater Accra region, Ghana: a cross-sectional study. Malar J. 2017;16:109.
Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12:833–40.
Li Y, Wang G, Sun D, Meng F, Lin S, Hu X, et al. A case of Plasmodium ovale wallikeri infection in a Chinese worker returning from West Africa. Korean J Parasitol. 2013;51:557–62.
Collins WE, Jeffery GM. Plasmodium ovale: parasite and disease. Clin Microbiol Rev. 2005;18:570–81.
Rosenberg R. Plasmodium vivax in Africa: hidden in plain sight? Trends Parasitol. 2007;23:193–6.
Howes RE, Reiner RC Jr, Battle KE, Longbottom J, Mappin B, Ordanovich D, et al. Plasmodium vivax transmission in Africa. PLoS Negl Trop Dis. 2015;9:e0004222.
He X, Pan M, Zeng W, Zou C, Pi L, Qin Y, et al. Multiple relapses of Plasmodium vivax malaria acquired from West Africa and association with poor metabolizer CYP2D6 variant: a case report. BMC Infect Dis. 2019;19:704.
Yan L, Fu Q, Jin L, Li L. Duffy blood group phenotypes and genotypes in Chinese. Transfusion. 2001;41:970.
Ayorinde AF, Oyeyiga AM, Nosegbe NO, Folarin OA. A survey of malaria and some arboviral infections among suspected febrile patients visiting a health centre in Simawa, Ogun State, Nigeria. J Infect Public Health. 2016;9:52–9.
Oboh MA, Badiane AS, Ntadom G, Ndiaye YD, Diongue K, Diallo MA, et al. Molecular identification of Plasmodium species responsible for malaria reveals Plasmodium vivax isolates in Duffy negative individuals from southwestern Nigeria. Malar J. 2018;17:439.
Ba H, Ahouidi AD, Duffy CW, Deh YB, Diedhiou C, Tandia A, et al. Evaluation of malaria rapid diagnostic test Optimal-IT(R) pLDH along the Plasmodium falciparum distribution limit in Mauritania. Bull Soc Pathol Exot. 2017;110:31–7 (in French).
Niangaly A, Karthigayan G, Amed O, Coulibaly D, Sa JM, Adams M, et al. Plasmodium vivax infections over 3 years in Duffy blood group negative Malians in Bandiagara, Mali. Am J Trop Med Hyg. 2017;97:744–52.
Twohig KA, Pfeffer DA, Baird JK, Price RN, Zimmerman PA, Hay SI, et al. Growing evidence of Plasmodium vivax across malaria-endemic Africa. PLoS Negl Trop Dis. 2019;13:e0007140.
Sundararaman SA, Liu W, Keele BF, Learn GH, Bittinger K, Mouacha F, et al. Plasmodium falciparum-like parasites infecting wild apes in southern Cameroon do not represent a recurrent source of human malaria. Proc Natl Acad Sci USA. 2013;110:7020–5.
Livingstone FB. The Duffy blood groups, vivax malaria, and malaria selection in human populations: a review. Hum Biol. 1984;56:413–25.
Gunalan K, Lo E, Hostetler JB, Yewhalaw D, Mu J, Neafsey DE, et al. Role of Plasmodium vivax Duffy-binding protein 1 in invasion of Duffy-null Africans. Proc Nat Acad Sci USA. 2016;113:6271–6.
de Carvalho GB, de Carvalho GB. Duffy Blood Group System and the malaria adaptation process in humans. Rev Bras Hematol Hemoter. 2011;33:55–64.
Gruszczyk J, Kanjee U, Chan LJ, Menant S, Malleret B, Lim NTY, et al. Transferrin receptor 1 is a reticulocyte-specific receptor for Plasmodium vivax. Science. 2018;359:48–55.
Malleret B, El Sahili A, Howland SW, Suwanarusk R, Ong ASM, Kosaisavee V, et al. CD98 is a Plasmodium vivax receptor for human reticulocytes. In: Proceedings of international conference on Plasmodium vivax, Campinas, Galoá; 2017.
Acknowledgements
The authors thank Michael Alifrangis for the P. vivax positive control samples. We are also grateful to the Developing Excellence in Leadership and Genetic Training for Malaria Elimination (DELGEME) for the funding and support given to EAA as a Masters Fellow to enable her to complete this study. The co-author AG, is currently supported through the DELTAS Africa Initiative an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust [DELGEME Grant #107740/Z/15/Z] and the UK government. AG worked on this project as part of her DELGEME aspiring leadership fellowship. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust, H3Africa or the UK government.
Funding
Financial support for this study was provided by the University of Ghana Office of Research Innovation and Development (ORID) and the Wellcome Trust [DELGEME Grant #107740/Z/15/Z].
Author information
Authors and Affiliations
Contributions
The study was conceived and designed by CAB, KK, and EA. CAB, PAP, ND, AG, and HA contributed to data collection and analysis. All authors contributed to data interpretation. The article was drafted by CAB with critique and revision from PAP, ND, KK and AG. All authors contributed to the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study was granted by the Noguchi Memorial Institute for Medical Research Institutional Review Board (NMIMR IRB), University of Ghana.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Brown, C.A., Pappoe-Ashong, P.J., Duah, N. et al. High frequency of the Duffy-negative genotype and absence of Plasmodium vivax infections in Ghana. Malar J 20, 99 (2021). https://doi.org/10.1186/s12936-021-03618-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-021-03618-0