Abstract
The Cyclin-dependent kinase (CDK) class of serine/threonine kinases has crucial roles in the regulation of cell cycle transition and is mainly involved in the pathogenesis of cancers. The expression of CDKs is controlled by a complex regulatory network comprised of genetic and epigenetic mechanisms, which are dysregulated during the progression of cancer. The abnormal activation of CDKs results in uncontrolled cancer cell proliferation and the induction of cancer stem cell characteristics. The levels of CDKs can be utilized to predict the prognosis and treatment response of cancer patients, and further understanding of the function and underlying mechanisms of CDKs in human tumors would pave the way for future cancer therapies that effectively target CDKs. Defects in the regulation of cell cycle and mutations in the genes coding cell-cycle regulatory proteins lead to unrestrained proliferation of cells leading to formation of tumors. A number of treatment modalities have been designed to combat dysregulation of cell cycle through affecting expression or activity of CDKs. However, effective application of these methods in the clinical settings requires recognition of the role of CDKs in the progression of each type of cancer, their partners, their interactions with signaling pathways and the effects of suppression of these kinases on malignant features. Thus, we designed this literature search to summarize these findings at cellular level, as well as in vivo and clinical levels.
Similar content being viewed by others
Introduction
Cyclin-dependent kinases (CDKs) are a group of serine/threonine kinases with crucial roles in the regulation of cell cycle progression. The activity of these kinases is induced by cyclins. In fact, CDK/cyclin complexes control progression of the cell cycle in an orderly manner [1]. Emerging evidence suggest that CDKs and cyclins actively participate in the regulation of transcription, epigenetic mechanisms, metabolic processes and self-renewal capacity of stem cells [1]. Most notably, some of these functions are exerted in an independent manner from establishment of CDKs/cyclins complexes [1]. Another group of proteins, namely cyclin-dependent kinase inhibitors (CKIs) has been revealed to negatively regulate cyclin/CDKs. The main function of CDKIs is to obstruct cell cycle transition and suppress cell proliferation through inhibition of the enzymatic activity of CDKs. Inhibitor of CDK4 proteins and CDK-interacting protein/kinase inhibitory proteins belong to this group [2].
Defects in the regulation of cell cycle and mutations in the genes coding cell-cycle regulatory proteins result in unrestrained proliferation of cells leading to formation of tumors [3, 4]. Accordingly, modulation of activity of these proteins by therapeutic agents has been suggested as a promising strategy for treatment of cancers [5]. Successful introduction of these modalities into clinical settings needs proper recognition of the role of CDKs in the progression of each type of cancer, their interacting molecules and signaling pathways and the effects of suppression of these kinases on malignant features. Thus, we designed this literature search to summarize these findings at cellular level, as well as in vivo and clinical levels.
Cyclin-dependent kinase 1 (CDK1)
Cell line studies
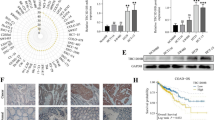
A recent study has demonstrated that vitro that centromere protein F (CENPF) through interaction with CDK1 can increase G2/M-phase transition, enhance cell proliferation and possibly activate the anti-tumor effects of p53 in a human adrenocortical carcinoma cell line. Moreover, assessment of GSEA has verified involvement of CENPF in the G2/M-phase cell cycle and p53 signaling [6].
Expression of CDK1 has also been found to be increased in bladder cancer cells, parallel with over-expression of the long non-coding RNA (lncRNA) PVT1. Notably, suppression of PVT1 has decreased activity, proliferative potential, colony formation, migratory capacity, and invasiveness of bladder cancer cells. miR-31 binding sites have been reported in both PVT1 and CDK1 transcripts. Taken together, PVT1-mediated reduction of miR-31 could increase expression of CDK1 in bladder cancer cells to enhance their proliferative potential, migration, and invasion [7]. Another study has shown the role of CDK1 in phosphorylation of TFCP2L1 at Thr177 in embryonic stem cells of mice as well as human bladder cancer cells. Notably, this type of phosphorylation has a crucial role in pluripotency and cell cycle progression of stem cells through modulation of expression of developmental genes. CDK1/TFCP2L1 axis is also involved in the induction of stemness characteristics and tumorigenic ability of bladder cancer cells [8]. Treatment of bladder cancer cells with the protein kinase D (PKD) inhibitor CRT0066101 has suppressed proliferation of these cells. CRT0066101 treatment or PKD2 silencing has induced cell cycle arrest at the G2/M phase, diminished expressions of cyclin B1, CDK1 and levels of CDK1 phosphorylated at Thr161, while increasing p27Kip1 and CDK1 phosphorylated at Thr14/Tyr15. This protein kinase inhibitor has also decreased expression of Cdc25C, which dephosphorylates and induces activity of CDK1, while enhancing function of Chk1, which suppresses CDK1 activity through phosphorylation and inactivation of Cdc25C. Moreover, CRT0066101 could elevate expression of a number of proteins that inhibit activity of the CDK1/cyclin B1 complex [9].
In breast cancer cells, the RNA binding protein KIAA1429 has been shown to interact with CDK1. Although this RNA binding protein is regarded as an N6-methyladenosine-associated regulatory protein, its oncogenic roles in breast cancer are exerted through regulation of CDK1 in an independent manner from its association with N6-methyladenosine (Fig. 1). Treatment of breast cancer cells with 5′-fluorouracil has efficiently reduced expressions of KIAA1429 and CDK1 [10]. Furthermore, siRNA-mediated silencing of CDK1 and CDC20 has significantly repressed cell migration and invasion of two breast cancer cell lines [11]. Another study has shown that knockdown of the ubiquitin-associated domain-containing gene UBAP2L in breast cancer cells suppresses their proliferation, impairs their colony formation aptitude and induces cell cycle arrest at G2/M phase. Most notably, this intervention has led to enhancement of p21 levels, while reducing levels of both CDK1 and Cyclin B1 [12].
A schematic diagram of CDK1 and the role of WTAP in modulating CDK2 in renal cell carcinoma. Mounting evidence has demonstrated the roles of N6-methyladenosine (m6A) in physiological processes and the progression of various human cancers such as cell cycle regulation that is mostly dependent on cyclins and CDKs. As a component in the m6A ‘writers’, WTAP is detected to be an RNA-binding protein and has a role in the m6A modification, mRNA splicing as well as processing. As an illustration, a recent study has detected that WTAP, an important component of the m6A writer complex, could have an oncogenic role in renal cell carcinoma tumorigenesis via physically binding to CDK2 transcript and promoting its transcript stability [68]
Cyclin B/CDK1 has been shown to phosphorylate inhibitor of apoptosis stimulating protein of P53 (iASPP), thus increasing nuclear localization of this protein and its inhibitory effects on p53. In Burkitt lymphoma cells, iASPP has been found to affect activity of transactivation domain p63 (TAp63). In fact, the interplay between CDK1 and iASPP can enhance the suppressive impact of iASPP on p53 and TAp63. Most notably, the tumor suppressor miR-129 has been shown to suppress expression of CDK1 and iASPP through binding with their transcripts. Moreover, CDK1 targeting by miR-129 can lead to inhibition of iASPP phosphorylation, therefore deterring nuclear localization of iASPP and its suppressive impact on p53 and TAp63 [13].
The oncogenic mutation HRASV12 has been found to induce activity of CDK1 and enhance protein O-GlcNAcylation, both of them having essential roles in induction of SOX2 expression and cancer stem cell properties in fibroblasts and cancer cell lines harboring RAS mutations. Most notably, the CDK inhibitor dinaciclib could reduce the quantities of cancer stem cells originated from these cells [14].
In colorectal cancer cells, knock-down of CDK1 has induced sensitivity to apoptosis. Moreover, CDK1 targeting with a MEK/ERK inhibitor has demonstrated effective impacts on proliferative abilities of these cells [15].
Notably, experiments in the vemurafenib-resistant colon cancer sublines have shown stable activation of CDK1, signifying the role of CDK1 activation in stimulation of resistance to vemurafenib. Adefovir dipivoxil that interrupts the interaction between CDK1 and KCTD12 and induces cell cycle arrest at G2 could inhibit colon cancer cells proliferation and induce sensitivity to vemurafenib [16]. Table 1 shows function of CDKs in cancer cell lines.
Animal studies
In vivo assessments have shown that down-regulation of miR-31 enhances expression of CDK1 at transcript and protein levels. Down-regulation of PVT1 (an lncRNA which increases expression of CDK1) has led to lessening of bladder tumor size, decrease in the proliferation rate of tumor cells and reduction of CDK1 and Ki-67-expressing cells as demonstrated by immunohistochemistry [7]. In animal models of breast cancer, up-regulation of RBM7 which induces activity of CDK1 has been shown to increase tumor growth [19]. In colorectal cancer, high levels of miR-378a-5p reduces tumor burden through decreasing expression of CDK1 [24]. Moreover, disruption of the interaction between CDK1 and KCTD12 using Adefovir dipivoxil has been shown to reduce in vivo tumorigenesis of colon cancer cells and induce vemurafenib sensitivity in xenografts [16].
Most notably, in animal models of hepatocellular carcinoma, administration of a CDK1 inhibitor along with sorafenib has enhanced the effectiveness of sorafenib [37]. Moreover, in animal models of pancreatic cancer, reduction of phosphorylation of CDK1, 2, 7, and 9 by AT7519 has been associated with reduction of tumor growth [63]. Studies in animal models of other cancers have also verified that decrease in activity of CDKs consistently reduces tumor burden and induces sensitivity to available therapies (Table 2).
Investigations in clinical samples
The CDK1-interacting protein CENPF has been found to be over-expressed in human adrenocortical carcinoma samples in correlation with tumor stage and poor overall survival (OS). Further assessment of immune cells infiltration has shown that over-expression of CENPF is associated with different pattern of infiltration of immune cells and high TMB/MSI score. Based on the results of gene-drug interaction assessments inhibitors of this protein, such as Cisplatin, Sunitinib, and Etoposide, can be putative therapeutic modalities for adrenocortical carcinoma [6]. In clinical samples of bladder cancer, activity of the CDK1/TFCP2L1 axis has been found to be associated with aggressive characteristics of tumors including advanced tumor grade, lymphovascular/muscularis-propria invasion, metastatic ability and poor clinical outcomes [8].
Assessment of expression profiles of three breast cancer datasets has led to identification of hub genes that indicate poor prognosis. Further analyses have indicated enrichment of four up-regulated genes, namely CDK1, CDC20, AURKA, and MCM4 in oocyte meiosis and cell cycle pathways. Taken together, bioinformatics methods and experimental validation have suggested these genes as reliable markers for breast cancer [11]. In breast cancer, up-regulation of CDK1 has been associated with short overall, relapse-free and progression-free survival times as well as advanced clinical stage [69]. In patients with cholangiocarcinoma, up-regulation of CDK1 or PSMC2 (which regulates CDK1) has been associated with lymph node metastasis and advanced clinical stage [22] and tumor grade [23], respectively. Table 3 shows the association between dysregulation of CDKs in clinical samples and clinical characteristics.
Cyclin-dependent kinase 2 (CDK2)
Cell line studies
Inactivation of CDK2 has been shown to effectively overcome the differentiation arrest of acute myeloid leukemia (AML) cells. Treatment of AML cells with CDK2-targeted proteolysis-targeting chimeras (PROTACs) has resulted in prompt and effective degradation of CDK2 in various cell lines without similar destruction of other targets. Moreover, this therapeutic agent has induced significant differentiation of AML cells as well as primary patient cells [92]. Another study in AML cells has shown that CDK2 is the only interphase CDK that is degraded through a ubiquitin-dependent proteasomal system. This mode of degradation of CDK2 is associated differentiation of AML cells. KLHL6 has been shown to be the specific E3 ubiquitin ligase which regulates CDK2 degradation. Notably, suppression of CDK2, but not CDK1/4/6, could induce granulocytic differentiation in AML cell lines. From a mechanistical point of view, CDK2 depletion results in reactivation of translation of differentiation pathway. Moreover, the effect of CDK2 in induction of differentiation blockade is exerted through preserving the activity of PRDX2 [93]. Moreover, CDK2 has been shown to down-regulate expression of C/EBPα through ubiquitin-dependent proteasomal degradation system resulting in differentiation blockade in AML. Mechanistically, CDK2-induced C/EBPα down-regulation is facilitated by SKP2. In fact, CDK2 enhances stability of SKP2 through Ser64 phosphorylation leading to C/EBPα ubiquitination. Suppression of CDK2 results in down-regulation of SKP2 and up-regulation of C/EBPα in myeloid cells. Cumulatively, CDK2-SKP2 axis has been identified as a therapeutic target for AML [94]. Another study has shown that GSK8612-mediated TBK1 inhibition and si-TBK1 can regulate CDK2 expression in AML cells through AKT pathway. Suppression of activity of AKT can enhance sensitivity of AML cells to daunorubicin, endorsing the interaction between TBK1 and the AKT/CDK2 axis [95].
Treatment of bladder cancer cells with propofol could inhibit their proliferation and enhance cell apoptosis through regulation of CDK2 expression. Mechanistically, propofol up-regulates expression of a CDK2-targeting miRNA, namely miR-340. Suppression of miR-340 has reversed the impacts of propofol on proliferation and apoptosis of bladder cancer cells. Moreover, suppression of CDK2 can partly reverse the impacts of miR-340 inhibition on proliferation and apoptosis of propofol-treated bladder cancer cells [96].
The Cdk4/6 inhibitor palbociclib has been shown to exert antitumor effects against bladder cancer cells through modification of Cdk2. Palbociclib has been shown to induce apoptosis of bladder cancer cells rather than cell cycle arrest. Activation Cdk2 has an indispensable role in palbociclib-induced apoptosis, as depletion of Cdk2 has suppressed caspase-3 activation and apoptosis. Activation Cdk2 has been shown to induce p-Rad9 mitochondrial translocation and its interaction with Bcl-xl, resulting in Bak activation and induction of apoptosis [97].
In breast cancer cells, concurrent administartion of CDK2 and CDK4/6 inhibitiors could reverse palbociclib resistance through increasing cell senescence [98]. Another functional study has shown that CDK2-mediated phosphorylation of EZH2 induces and preserves proliferation of triple-negative breast cancer cells [99]. Table 4 summarizes function of CDK2 in different cancer cell lines. Figure 2 illustrates the interaction between STAT3 signaling pathway and CDK1 and CDK2 in lung cancer (Fig. 3).
A schematic illustration of the role of STAT3 signaling cascade in regulating CDK1 and CDK2 in lung cancer. Accumulating evidence has illustrated that CDK1/GP130/STAT3 signaling could promote lung cancer tumorigenesis. It has been reported that Iron-dependent CDK1 activity could phosphorylate 4E-BP1, which in turn elevates STAT3 signaling pathway via upregulation of GP130 [48]. Moreover, another research has revealed that PROS could downregulate VEGF induced proliferation, migration, and tube formation in non-small lung cancer cells and inhibits angiogenesis in chorioallantoic membrane assay through attenuating phosphorylation of VEGFR2, Src, and STAT3, thereby inducing sub G1 accumulation, S phase arrest [158]
Recent study has detected that upregulation of PTEN and Rb expression levels could lead to promoting sensitivity to CDK4/6 inhibitors, which could in turn result in reducing the expression of AKT and PI3K in ER-Positive Breast Cancer. Whereas, acquired loss of Rb and PTEN expression could induce resistance to CDK4/6 inhibitors in patients, and thereby promoting hyperactivation of CDK2 and CDK4 [172]. Moreover, other finding points out that IGF1R overexpression, as an escape mechanism, could elevate resistance to CDK4/6 inhibitors in Ewing sarcoma. Therefore, dual targeting of CDK4/6 and IGF1R could play an effective role in providing a candidate synergistic combination for clinical application in this disease and promoting inhibition of the cell cycle as well as PI3K/mTOR axis in tumor cells [173]. In addition, a recent clinical study has revealed that suppression of CDK4/6 phosphorylation and the complex with cyclin D as well as downregulating PI3K/AKT/mTOR signaling cascade could remarkably reduce cell viability, induce apoptosis, and promote the percentage of cells in G1 phase in hepatocellular carcinoma [174]. All the information regarding the role of these cascades involved in the regulation of CDK2 and CDK4/6 expression in various types of human cancers can be seen in Tables 4 and 10.
Animal studies
Depletion of CDK2 has led to blockade of AML cells growth in animal models and increased survival of xenograft mice models [93]. Another study in animal models of AML has shown that concomitant administration of chidamide and doxorubicin could inhibit HDAC3-AKT-P21-CDK2 signaling and reduce tumor growth [101].
Another experiment in an animal model of bladder cancer has shown the anticancer role of Cdk2 activation in palbociclib-treated animals, indicating that the anticancer effect of palbociclib is exerted via Cdk2 activation [97]. In xenogaft models of breast cancer, depletion of CDK2 and CDK4/6 has reduced tumor growth and palbociclib resistance [98]. Similar results have been reported in animal models of other types of cancers (Table 5).
Investigations in clinical samples
Up-regulation of CDK2 has been reported in diverse types of cancers. In AML, up-regulation of HDAC3-AKT-P21-CDK2 signaling has been associated with shorter event-free and overall survival (OS) times [101]. In bladder cancer, expression of CDK2 has been increased, while expression of a CDK2-targeting miRNA, namely miR-3619 has been decreased. These observations have been associated with advanced tumor stage and grade [105]. In breast cancer, up-regulation of MTHFD2, which contributes in the cell cycle through binding to CDK2, has been associated with shorter OS, tumor grade and stage [107]. Other studies have shown up-regulation of a number of CDK2-interactiong circRNAs such as hsa_circ_0000520 [128], circ_0084927 [129] and circZFR [130] in cervical cancer patients. Notably, up-regulation of circZFR has been associated with lymphatic metastasis in this type of cancer [130]. Several other studies have found association between dysregulation of CDK2 or its interacting partners and clinical data of patients (Table 6).
Cyclin-dependent kinase 3 (CDK3)
Cell line studies
CDK3 has been shown to participate in regulation of cell cycle transition at G0/G1 and G1/S phases. Up-regulation of CDK3 in breast cancer cells has suppressed their migration and invasion. Further experiments in these cells have identified miR-4469 as a CDK3-targeting miRNA. Consistent with this finding, miR-4469-induced enhancement of cell motility could be obliterated by CDK3 up-regulation. Assessments of RNA-seq data and western blot assay have indicated inhibition of Wnt pathway by CDK3 expression. Besides, Wnt3a treatment could abolish the inhibitory effect of CDK3 in cell motility, indicating the role of CDK3 as an upstream regulator of Wnt signaling in these cells [181].
CDK3 has also been reported to participate in ERα signaling and resistance to tamoxifen. The anti-cancer agent norcantharidin (NCTD) has been found to regulate miR-873/CDK3 axis. Treatment of breast cancer cells with NCTD has led to reduction of transcriptional activity of ERα but not ERβ via influencing activity of miR-873/CDK3 axis. Moreover, NCTD has been shown to inhibit proliferation of breast cancer cells and induce sensitivity to tamoxifen via this axis. Mechanistically, NCTD blocks tamoxifen induced transcriptional activity and ERα downstream gene expression. Moreover, it reestablishes tamoxifen induced recruitment of ERα co-repressors [182]. The CDK3 targeting miRNA, miR‐125a‐3p has also been revealed to inhibit transactivation of ERα and prevail tamoxifen resistance in ER + breast cancer cells [183]. Similarly, miR-873 has been found to regulate transcriptional activity of ERα and resistance to tamoxifen through influencing expression of CDK3 in breast cancer cells [184].
In colorectal cancer cells, Cdk3 has been shown to promote epithelial-mesenchymal transition (EMT) via enhancing activity of AP-1 [185]. Another study in esophageal squamous cell carcinoma cells has shown that the oncogenic circular RNA circRNA_141539 exerts its function through sponging miR-4469 and enhancing activity of CDK3 [186]. Table 7 shows the function of CDK3 based on cell line studies.
Animal studies
While a single study in breast cancer models has shown that up-regulation of CDK3 decreases metastatic abilities of breast cancer cells [181], other studies have shown that up-regulation of CDK3-targeting miRNAs miR-125a-3p [183] and miR-873 [184] leads to reduction of tumor growth. In xenograft models of colorectal cancer, up-regulation of CDK3 has been accompanied by enhancement of metastatic ability of cancer cells [185]. Table 8 summarizes function of CDK3 in animal models of cancer.
Investigations in clinical samples
Expression assays in breast cancer samples have shown that up-regulation of CDK3 is associated with chemoresistance [187]. In colorectal cancer samples, up-regulation of this member of CDK family has been associated with shorter progression-free survival and advanced TMN stage [186]. In clinical samples of nasopharyngeal carcinoma, up-regulation of CDK3 has been associated with tumor infiltration, lymph node metastasis and TNM staging [192]. Table 9 summarizes results of studies that reported association between up-regulation of CDK3 and clinical parameters.
Cyclin-dependent kinase 4/6 (CDK4/6)
Cell line studies
An in vitro study in AML has verified that suppression of CDK4/6 and autophagy enhances apoptosis in t(8; 21) AML cells in a synergic manner [194]. Similarly, CDK4/6 inhibition is a novel therapeutic modality for bladder cancer irrespective of RB1 status [195]. This treatment has reduced FOXM1 phosphorylation and exhibited synergy with cisplatin [195]. Another in vitro study in breast cancer cells has reported loss of the FAT1 as a mechanism for induction of resistance to CDK4/6 inhibitors. Mechanistically, FAT1 silencing has led to suppression of Hippo pathway in ER + cancer cells [196]. Single-cell assessment of CDK2 activity has confirmed difference in cell-cycle regulation between the luminal androgen receptor (LAR) subtype of triple negative breast cancer (TNBC) and basal-like cells. In fact, palbociclib-sensitive LAR cells leave mitotic cycle with low level of CDK2 activity, and enter a quiescent phase that needs activity of CDK4/6 for going back into cell-cycle. On the other hand, palbociclib-resistant basal-like cells leave mitosis and directly enter into a proliferative phase characterized by high level of CDK2 activity, circumventing the constraint point and the need for CDK4/6 activity. CDK4/6 inhibition has synergism with PI3 kinase inhibition in reduction of proliferation of PIK3CA-mutant TNBC cells, indicating that other subtypes of TNBC can be responsive to CDK4/6 inhibitors [197]. In breast and other solid tumors, CDK4/6 inhibitors could trigger anti-tumour immune responses [198]. Moreover, experiments in cervical cancer cells have shown that cyclin D-CDK4/6 inhibition enhances sensitivity of immune-refractory cancers through hindering the SCP3–NANOG axis [199]. Table 10 summarizes function of CDK4/6 based on cell line studies.
Animal studies
Experiments in animal models of AML have verified that CDK4/6 inhibition enhances autophagy. Moreover, concurrent administration CDK4/6 inhibitor and autophagy inhibitor has reduced tumor growth in these models [333]. Similarly, combination of cisplatin and CDK4/6 inhibitors has significantly reduced bladder cancer growth [195]. In xenograft models of breast cancer, CDK4/6 inhibitors could reduce proliferation, and enhance anti-tumor immune responses [198]. In addition, in this type of cancer, combined inhibition of CDK2 and CDK4/6 has enhaced sensitivity to palbociclib [98]. Besides, combination of CDK4/6 inhibitor, abemaciclib, with c-Met/Trk inhibitor, altiratinib has been shown to be effective against glioma-initiating cells [256]. Table 11 shows function of CDK4/6 in animal models of cancer.
Investigations in clinical samples
Investigations in breast cancer samples have shown up-regulation of CDK4/6 in different subtypes. For instance, CDK6 levels have been found to be higher in FAT1-deleted samples compared with those having wildtype FAT1 [196]. Another study has shown up-regulation of CDK4/6 and pRb levels in HER2 + breast cancer samples [334]. In ovarian cancer samples, up-regulation of CDK6 has been associated with shorted OS and immunosuppressive state [319]. Moreover, in this type of cancer, up-regulation of a functional counterpart of CDK4/6, i.e. COL6A3 has been associated with shorter OS and advanced clinical stage [330]. Table 12 shows dysregulation of CDK4/6 in clinical samples.
A number of clinical studies have evaluated the effects of CDK4/6 inhibition on survival of patients (Table 13). For instance, treatment of 22 breast cancer patients with a CDK4/6 inhibitor has resulted in complete response in one patient, partial response in 8 patients, and stable disease in 13 patients [336]. Another study in breast cancer patients has indicated better progression-free survival time in those treated with CDK4/6 inhibitors than those received PI3K inhibitors. Moreover, Combination of CDK4/6 inhibitors and endocrine therapy has yielded better OS than PI3K/mTOR inhibitors [337]. Promising results have also obtained from studies in other types of cancers.
Discussion
Expression and activity of CDKs have been assessed in animal models of cancers, cell lines and clinical samples of patients having different types of cancers. CDK1 and CDK2 are the most comprehensively assessed members of this family. Additionally, a number of studies have addressed involvement of CDKs 3, 4/6, 5, 7 and 9 in cancer cell lines. Other members of this protein family have not been thoroughly assessed.
The above-mentioned studies have revealed a number of CDKs-interacting molecules including mRNA coding genes as well as lncRNAs and miRNAs. PVT1, NCK1-AS1, FOXD2-AS1, SNHG4, SNORD52, TMPO-AS1, TONSL-AS1, DLEU1 and CASC11 are among lncRNAs that interact with CDKs. Meanwhile, miR-378a-5p, miR-34c-3p, miR-181a, miR-195-3p and miR-205 have been shown to regulate expression of certain CDKs through binding with the 3'UTR of their transcripts. Since miRNAs can efficiently reduce expression of CDKs, identification of additional CDKs-targeting miRNAs through in silico and experimental methods can facilitate design of novel treatment modalities for cancers. Moreover, available data indicate that expressions of CDKs are regulated through a complex regulatory network consisted of both genetic and epigenetic mechanisms which can be dysregulated during the course of cancer evolution. Application of various quantitative experimental and computational methods in a "system biology" approach is needed to unravel complicated aspects of the mentioned network and develop novel modalities to combat cancer-a prototype of disorders associated with dysregulation of CDKs.
Conclusion
Since activity of CDKs is associated with induction of stem cell properties, drugs targeting these proteins might be used for effective elimination of cancer stem cells and reduction of tumor metastases. This implicates that CDKs are involved in the pathogenesis of a high spectrum of cancers, including different types of carcinomas as well as non-epithelial malignancies. Coming from this point of view CDKs will come more and more in the focus as therapeutical targets.
Activity levels of CDKs can be used for prediction of cancer prognosis and response of patients to various therapeutic options. In fact, an appropriate approach for implementation of personalized medicine in the field of cancer therapy is measurement of activity of these proteins.
Cumulatively, CDKs represent ideal therapeutic targets for cancer. Thus, future studies should focus on assessment of their activities in different tumors and identification of their association with clinicopathological data. Moreover, the presence of putative genetic variants within CDK coding genes might affect their activity and susceptibility of persons to different cancers. This note should also be assessed in future studies.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
References
Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140(15):3079–93.
Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14(2):130–46.
Ding L, Cao J, Lin W, Chen H, Xiong X, Ao H, et al. The roles of cyclin-dependent kinases in cell-cycle progression and therapeutic strategies in human breast cancer. Int J Mol Sci. 2020;21(6):1960.
García-Reyes B, Kretz A-L, Ruff J-P, von Karstedt S, Hillenbrand A, Knippschild U, et al. The emerging role of cyclin-dependent kinases (CDKs) in pancreatic ductal adenocarcinoma. Int J Mol Sci. 2018;19(10):3219.
Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93–115.
Huang Y, Li D, Wang L, Su X, Tang X-B. CENPF/CDK1 signaling pathway enhances the progression of adrenocortical carcinoma by regulating the G2/M-phase cell cycle. J Transl Med. 2021;20(1):1–17.
Tian Z, Cao S, Li C, Xu M, Wei H, Yang H, et al. LncRNA PVT1 regulates growth, migration, and invasion of bladder cancer by miR-31/CDK1. J Cell Physiol. 2019;234(4):4799–811.
Heo J, Noh BJ, Lee S, Lee HY, Kim Y, Lim J, et al. Phosphorylation of TFCP2L1 by CDK1 is required for stem cell pluripotency and bladder carcinogenesis. EMBO Mol Med. 2020;12(1): e10880.
Li QQ, Hsu I, Sanford T, Railkar R, Balaji N, Sourbier C, et al. Protein kinase D inhibitor CRT0066101 suppresses bladder cancer growth in vitro and xenografts via blockade of the cell cycle at G2/M. Cell Mol Life Sci. 2018;75(5):939–63.
Qian J-Y, Gao J, Sun X, Cao M-D, Shi L, Xia T-S, et al. KIAA1429 acts as an oncogenic factor in breast cancer by regulating CDK1 in an N6-methyladenosine-independent manner. Oncogene. 2019;38(33):6123–41.
Wang N, Zhang H, Li D, Jiang C, Zhao H, Teng Y. Identification of novel biomarkers in breast cancer via integrated bioinformatics analysis and experimental validation. Bioengineered. 2021;12(2):12431–46.
He J, Chen Y, Cai L, Li Z, Guo X. UBAP2L silencing inhibits cell proliferation and G2/M phase transition in breast cancer. Breast Cancer. 2018;25(2):224–32.
Zou H, Zou R, Chen K, Zhu C, Tian X, You Y, et al. miR-129 targets CDK1 and iASPP to modulate Burkitt lymphoma cell proliferation in a TAp63-dependent manner. J Cell Biochem. 2018;119(11):9217–28.
Shimizu M, Shibuya H, Tanaka N. Enhanced O-GlcNAc modification induced by the RAS/MAPK/CDK1 pathway is required for SOX2 protein expression and generation of cancer stem cells. Sci Rep. 2022;12(1):1–13.
Zhang P, Kawakami H, Liu W, Zeng X, Strebhardt K, Tao K, et al. Targeting CDK1 and MEK/ERK overcomes apoptotic resistance in BRAF-mutant human colorectal cancer. Mol Cancer Res. 2018;16(3):378–89.
Yang J, Xu WW, Hong P, Ye F, Huang X-H, Hu H-F, et al. Adefovir dipivoxil sensitizes colon cancer cells to vemurafenib by disrupting the KCTD12-CDK1 interaction. Cancer Lett. 2019;451:79–91.
Xie D, Song H, Wu T, Li D, Hua K, Xu H, et al. MicroRNA-424 serves an anti-oncogenic role by targeting cyclin-dependent kinase 1 in breast cancer cells. Oncol Rep. 2018;40(6):3416–26.
Zhang X, Pan Y, Fu H, Zhang J. Nucleolar and spindle associated protein 1 (NUSAP1) inhibits cell proliferation and enhances susceptibility to epirubicin in invasive breast cancer cells by regulating cyclin D kinase (CDK1) and DLGAP5 expression. Med Sci Monit Int Med J Exp Clin Res. 2018;24:8553.
Xi P-W, Zhang X, Zhu L, Dai X-Y, Cheng L, Hu Y, et al. Oncogenic action of the exosome cofactor RBM7 by stabilization of CDK1 mRNA in breast cancer. NPJ Breast Cancer. 2020;6(1):1–10.
Tang J, Pan H, Wang W, Qi C, Gu C, Shang A, et al. MiR-495-3p and miR-143-3p co-target CDK1 to inhibit the development of cervical cancer. Clin Transl Oncol. 2021;23(11):2323–34.
Li H, Jia Y, Cheng J, Liu G, Song F. LncRNA NCK1-AS1 promotes proliferation and induces cell cycle progression by crosstalk NCK1-AS1/miR-6857/CDK1 pathway. Cell Death Dis. 2018;9(2):1–15.
Yamamura M, Sato Y, Takahashi K, Sasaki M, Harada K. The cyclin-dependent kinase pathway involving CDK1 is a potential therapeutic target for cholangiocarcinoma. Oncol Rep. 2020;43(1):306–17.
Duan X, Yang J, Jiang B, Duan W, Wei R, Zhang H, et al. Knockdown of PSMC2 contributes to suppression of cholangiocarcinoma development by regulating CDK1. Aging (Albany NY). 2021;13(17):21325.
Zhou N, Li S, Wu D, Zhang F, Tang F, Li Y. The lncRNA VPS9D1-AS1 promotes hepatocellular carcinoma cell cycle progression by regulating the HuR/CDK4 axis. DNA Cell Biol. 2021;40(10):1278–89.
Tong Y, Huang Y, Zhang Y, Zeng X, Yan M, Xia Z, et al. DPP3/CDK1 contributes to the progression of colorectal cancer through regulating cell proliferation, cell apoptosis, and cell migration. Cell Death Dis. 2021;12(6):1–12.
Zhou Z, Tan F, Pei Q, Li C, Zhou Y, Li Y, et al. lncRNA SNHG4 modulates colorectal cancer cell cycle and cell proliferation through regulating miR-590-3p/CDK1 axis. Aging (Albany NY). 2021;13(7):9838.
Bury M, Le Calve B, Lessard F, Dal Maso T, Saliba J, Michiels C, et al. NFE2L3 controls colon cancer cell growth through regulation of DUX4, a CDK1 inhibitor. Cell Rep. 2019;29(6):1469-81.e9.
Zeng Q, Lei F, Chang Y, Gao Z, Wang Y, Gao Q, et al. An oncogenic gene, SNRPA1, regulates PIK3R1, VEGFC, MKI67, CDK1 and other genes in colorectal cancer. Biomed Pharmacother. 2019;117: 109076.
Li L, Qu Y, Li Y. Over-expression of miR-1271 inhibits endometrial cancer cells proliferation and induces cell apoptosis by targeting CDK1. Eur Rev Med Pharmacol Sci. 2017;21(12):2816–22.
Bi L, Wang H, Tian Y. Silencing FAM135B enhances radiosensitivity of esophageal carcinoma cell. Gene. 2021;772: 145358.
Zhu Y, Xing Y, Chi F, Sun W, Zhang Z, Piao D. Long noncoding RNA SNHG6 promotes the progression of colorectal cancer through sponging miR-760 and activation of FOXC1. Onco Targets Ther. 2018;11:5743–52.
Li F-N, Zhang Q-Y, Li O, Liu S-L, Yang Z-Y, Pan L-J, et al. ESRRA promotes gastric cancer development by regulating the CDC25C/CDK1/CyclinB1 pathway via DSN1. Int J Biol Sci. 2021;17(8):1909.
Huang Z, Zhang S, Du J, Zhang X, Zhang W, Huang Z, et al. Cyclin-Dependent kinase 1 (CDK1) is co-expressed with CDCA5: their functions in gastric cancer cell line MGC-803. Med Sci Monit Int Med J Exp Clin Res. 2020;26:e923664–71.
Shi Q, Ni X, Lei M, Xia Q, Dong Y, Zhang Q, et al. Phosphorylation of islet-1 serine 269 by CDK1 increases its transcriptional activity and promotes cell proliferation in gastric cancer. Mol Med. 2021;27(1):1–11.
Voce DJ, Bernal GM, Cahill KE, Wu L, Mansour N, Crawley CD, et al. CDK1 is up-regulated by temozolomide in an NF-κB dependent manner in glioblastoma. Sci Rep. 2021;11(1):1–10.
Wang J, Li B, Wang C, Luo Y, Zhao M, Chen P. Long noncoding RNA FOXD2-AS1 promotes glioma cell cycle progression and proliferation through the FOXD2-AS1/miR-31/CDK1 pathway. J Cell Biochem. 2019;120(12):19784–95.
Wu CX, Wang XQ, Chok SH, Man K, Tsang SHY, Chan ACY, et al. Blocking CDK1/PDK1/β-Catenin signaling by CDK1 inhibitor RO3306 increased the efficacy of sorafenib treatment by targeting cancer stem cells in a preclinical model of hepatocellular carcinoma. Theranostics. 2018;8(14):3737.
Dang X-W, Pan Q, Lin Z-H, Wang H-H, Li L-H, Li L, et al. Overexpressed DEPDC1B contributes to the progression of hepatocellular carcinoma by CDK1. Aging (Albany NY). 2021;13(16):20094.
Liu H-M, Tan H-Y, Lin Y, Xu B-N, Zhao W-H, Xie Y-A. MicroRNA-1271-5p inhibits cell proliferation and enhances radiosensitivity by targeting CDK1 in hepatocellular carcinoma. J Biochem. 2020;167(5):513–24.
Li L, Huang K, Zhao H, Chen B, Ye Q, Yue J. CDK1-PLK1/SGOL2/ANLN pathway mediating abnormal cell division in cell cycle may be a critical process in hepatocellular carcinoma. Cell Cycle. 2020;19(10):1236–52.
Li C, Wu L, Liu P, Li K, Zhang Z, He Y, et al. The C/D box small nucleolar RNA SNORD52 regulated by Upf1 facilitates Hepatocarcinogenesis by stabilizing CDK1. Theranostics. 2020;10(20):9348.
Wang C, Li M-Y, Shen X-H, Wang S-J, Wang W-Q, Liu Y-F. Effect of CDK1 interferes with the regulation of PLK1, Aurora B and TRF1 on the proliferation of leukemia cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29(4):1129–35.
Huang Z, Shen G, Gao J. CDK1 promotes the stemness of lung cancer cells through interacting with Sox2. Clin Transl Oncol. 2021;23(9):1743–51.
Tong W, Han T, Wang W, Zhao J. LncRNA CASC11 promotes the development of lung cancer through targeting microRNA-302/CDK1 axis. Eur Rev Med Pharmacol Sci. 2019;23(15):6539–47.
Palma F, Affinito A, Nuzzo S, Roscigno G, Scognamiglio I, Ingenito F, et al. miR-34c-3p targets CDK1 a synthetic lethality partner of KRAS in non-small cell lung cancer. Cancer Gene Ther. 2021;28(5):413–26.
Zha L, Zhang L, Pan H, Ma H. Upregulation of lncRNA NCK1-AS1 predicts poor prognosis and contributes to non-small cell lung cancer proliferation by regulating CDK1. Eur Rev Med Pharmacol Sci. 2021;25(3):1351–7.
Li L, Zhang Z, Yang Q, Ning M. Lycorine inhibited the cell growth of non-small cell lung cancer by modulating the miR-186/CDK1 axis. Life Sci. 2019;231: 116528.
Kuang Y, Guo W, Ling J, Xu D, Liao Y, Zhao H, et al. Iron-dependent CDK1 activity promotes lung carcinogenesis via activation of the GP130/STAT3 signaling pathway. Cell Death Dis. 2019;10(4):1–12.
Li Q, Bian Y, Li Q. Down-regulation of TMPO-AS1 induces apoptosis in lung carcinoma cells by regulating miR-143-3p/CDK1 axis. Technol Cancer Res Treat. 2021;20:1533033820948880.
Shi Q, Zhou Z, Ye N, Chen Q, Zheng X, Fang M. MiR-181a inhibits non-small cell lung cancer cell proliferation by targeting CDK1. Cancer Biomark. 2017;20(4):539–46.
Hossian A, Mackenzie GG, Mattheolabakis G. Combination of miR-143 and miR-506 reduces lung and pancreatic cancer cell growth through the downregulation of cyclin-dependent kinases. Oncol Rep. 2021;45(4):1.
Hossian A, Sajib M, Tullar PE, Mikelis CM, Mattheolabakis G. Multipronged activity of combinatorial miR-143 and miR-506 inhibits lung cancer cell cycle progression and angiogenesis in vitro. Sci Rep. 2018;8(1):1–14.
Menon DR, Luo Y, Arcaroli JJ, Liu S, KrishnanKutty LN, Osborne DG, et al. CDK1 interacts with Sox2 and promotes tumor initiation in human melanoma. Can Res. 2018;78(23):6561–74.
Sun W, Zhao F, Xu Y, Huang K, Guo X, Zheng B, et al. Chondroitin polymerizing factor (CHPF) promotes development of malignant melanoma through regulation of CDK1. Cell Death Dis. 2020;11(7):1–13.
Yang Y, Dai Y, Yang X, Wu S, Wang Y. DNMT3A mutation-induced CDK1 overexpression promotes leukemogenesis by modulating the interaction between EZH2 and DNMT3A. Biomolecules. 2021;11(6):781.
Hu L, Pan X, Hu J, Zeng H, Liu X, Jiang M, et al. Proteasome inhibitors decrease paclitaxel-induced cell death in nasopharyngeal carcinoma with the accumulation of CDK1/cyclin B1. Int J Mol Med. 2021;48(4):1–11.
Wang J, Chang L, Lai X, Li X, Wang Z, Huang Z, et al. Tetrandrine enhances radiosensitivity through the CDC25C/CDK1/cyclin B1 pathway in nasopharyngeal carcinoma cells. Cell Cycle. 2018;17(6):671–80.
Xie F, Xiao W, Tian Y, Lan Y, Zhang C, Bai L. MicroRNA-195-3p inhibits cyclin dependent kinase 1 to induce radiosensitivity in nasopharyngeal carcinoma. Bioengineered. 2021;12(1):7325–34.
Li J, Zhi X, Shen X, Chen C, Yuan L, Dong X, et al. Depletion of UBE2C reduces ovarian cancer malignancy and reverses cisplatin resistance via downregulating CDK1. Biochem Biophys Res Commun. 2020;523(2):434–40.
Zhang R, Shi H, Ren F, Zhang M, Ji P, Wang W, et al. The aberrant upstream pathway regulations of CDK1 protein were implicated in the proliferation and apoptosis of ovarian cancer cells. J Ovarian Res. 2017;10(1):1–11.
Zhou L, Liu R, Liang X, Zhang S, Bi W, Yang M, et al. lncRNA RP11–624L4. 1 is associated with unfavorable prognosis and promotes proliferation via the CDK4/6-cyclin D1-Rb-E2F1 pathway in NPC. Mol Ther Nucleic Acids. 2020;22:1025–39.
Wang LL, Sun KX, Wu DD, Xiu YL, Chen X, Chen S, et al. DLEU 1 contributes to ovarian carcinoma tumourigenesis and development by interacting with miR-490-3p and altering CDK 1 expression. J Cell Mol Med. 2017;21(11):3055–65.
Kazi A, Chen L, Xiang S, Vangipurapu R, Yang H, Beato F, et al. Global phosphoproteomics reveal CDK suppression as a vulnerability to KRas addiction in pancreatic cancer. Clin Cancer Res. 2021;27(14):4012–24.
Pecoraro C, Parrino B, Cascioferro S, Puerta A, Avan A, Peters GJ, et al. A new oxadiazole-based topsentin derivative modulates cyclin-dependent kinase 1 expression and exerts cytotoxic effects on pancreatic cancer cells. Molecules. 2021;27(1):19.
Huang J, Chen P, Liu K, Liu J, Zhou B, Wu R, et al. CDK1/2/5 inhibition overcomes IFNG-mediated adaptive immune resistance in pancreatic cancer. Gut. 2021;70(5):890–9.
Zhang B, Zhang M, Li Q, Yang Y, Shang Z, Luo J. TPX2 mediates prostate cancer epithelial-mesenchymal transition through CDK1 regulated phosphorylation of ERK/GSK3β/SNAIL pathway. Biochem Biophys Res Commun. 2021;546:1–6.
Ji G, He S, Huang C, Gong Y, Li X, Zhou L. Upregulation of ATP binding cassette subfamily C member 5 facilitates prostate cancer progression and enzalutamide resistance via the CDK1-mediated AR Ser81 phosphorylation pathway. Int J Biol Sci. 2021;17(7):1613.
Tang J, Wang F, Cheng G, Si S, Sun X, Han J, et al. Wilms’ tumor 1-associating protein promotes renal cell carcinoma proliferation by regulating CDK2 mRNA stability. J Exp Clin Cancer Res. 2018;37(1):1–12.
Xing Z, Wang X, Liu J, Zhang M, Feng K, Wang X. Expression and prognostic value of CDK1, CCNA2, and CCNB1 gene clusters in human breast cancer. J Int Med Res. 2021;49(4):0300060520980647.
Peng X, Wang J, Li D, Chen X, Liu K, Zhang C, et al. Identification of grade-related genes and construction of a robust genomic-clinicopathologic nomogram for predicting recurrence of bladder cancer. Medicine. 2020;99(47):e23179.
Liu Z, Liang G, Tan L, Su A, Jiang W, Gong C. High-efficient screening method for identification of key genes in breast cancer through microarray and bioinformatics. Anticancer Res. 2017;37(8):4329–35.
Li J, Wang Y, Wang X, Yang Q. CDK1 and CDC20 overexpression in patients with colorectal cancer are associated with poor prognosis: evidence from integrated bioinformatics analysis. World J Surg Oncol. 2020;18(1):1–11.
Yun Z-J, Wang H-J, Yu Y-X, Sun Z-Y, Yao S-K. Screening of differentially expressed genes for colorectal cancer and prediction of potential traditional Chinese medicine: based on bioinformatics. Zhongguo Zhong yao za zhi Zhongguo Zhongyao Zazhi China J Chin Mater Med. 2022;47(6):1666–76.
Zhang HJ, Chen G, Chen SW, Fu ZW, Zhou HF, Feng ZB, et al. Overexpression of cyclin-dependent kinase 1 in esophageal squamous cell carcinoma and its clinical significance. FEBS Open Bio. 2021;11(11):3126–41.
Zhang L, Kang W, Lu X, Ma S, Dong L, Zou B. LncRNA CASC11 promoted gastric cancer cell proliferation, migration and invasion in vitro by regulating cell cycle pathway. Cell Cycle. 2018;17(15):1886–900.
Zou Y, Ruan S, Jin L, Chen Z, Han H, Zhang Y, et al. CDK1, CCNB1, and CCNB2 are prognostic biomarkers and correlated with immune infiltration in hepatocellular carcinoma. Med Sci Monit Int Med J Exp Clin Res. 2020;26:e925289–91.
Zhou Z, Li Y, Hao H, Wang Y, Zhou Z, Wang Z, et al. Screening hub genes as prognostic biomarkers of hepatocellular carcinoma by bioinformatics analysis. Cell Transplant. 2019;28(1_suppl):76S-86S.
Li Y, Wu D, Wei C, Yang X, Zhou S. CDK1, CCNB1 and NDC80 are associated with prognosis and progression of hepatitis B virus-associated hepatocellular carcinoma: a bioinformatic analysis. Nan fang yi ke da xue xue bao J South Med Univ. 2021;41(10):1509–18.
Liu J, Han F, Ding J, Liang X, Liu J, Huang D, et al. Identification of multiple hub genes and pathways in hepatocellular carcinoma: a bioinformatics analysis. BioMed Res Int. 2021;2021.
Ni W, Zhang S, Jiang B, Ni R, Xiao M, Lu C, et al. Identification of cancer-related gene network in hepatocellular carcinoma by combined bioinformatic approach and experimental validation. Pathol Res Pract. 2019;215(6): 152428.
Lei X, Zhang M, Guan B, Chen Q, Dong Z, Wang C. Identification of hub genes associated with prognosis, diagnosis, immune infiltration and therapeutic drug in liver cancer by integrated analysis. Hum Genom. 2021;15(1):1–21.
Li M, He F, Zhang Z, Xiang Z, Hu D. CDK1 serves as a potential prognostic biomarker and target for lung cancer. J Int Med Res. 2020;48(2):0300060519897508.
Li S, Li H, Cao Y, Geng H, Ren F, Li K, et al. Integrated bioinformatics analysis reveals CDK1 and PLK1 as potential therapeutic targets of lung adenocarcinoma. Medicine. 2021;100(32):e26474.
Liu W-T, Wang Y, Zhang J, Ye F, Huang X-H, Li B, et al. A novel strategy of integrated microarray analysis identifies CENPA, CDK1 and CDC20 as a cluster of diagnostic biomarkers in lung adenocarcinoma. Cancer Lett. 2018;425:43–53.
Qin W, Yuan Q, Liu Y, Zeng Y, Dai X, Shuai Y, et al. Identification of key molecular markers in epithelial ovarian cancer by integrated bioinformatics analysis. Taiwan J Obstet Gynecol. 2021;60(6):983–94.
Piao J, Zhu L, Sun J, Li N, Dong B, Yang Y, et al. High expression of CDK1 and BUB1 predicts poor prognosis of pancreatic ductal adenocarcinoma. Gene. 2019;701:15–22.
Dong S, Huang F, Zhang H, Chen Q. Overexpression of BUB1B, CCNA2, CDC20, and CDK1 in tumor tissues predicts poor survival in pancreatic ductal adenocarcinoma. Biosci Rep. 2019;39(2).
Sun Y, Li S-H, Cheng J-W, Chen G, Huang Z-G, Gu Y-Y, et al. Downregulation of miRNA-205 expression and biological mechanism in prostate cancer tumorigenesis and bone metastasis. BioMed Res Int 2020; 2020.
Li Q, Zhang L, Jiang J, Zhang Y, Wang X, Zhang Q, et al. CDK1 and CCNB1 as potential diagnostic markers of rhabdomyosarcoma: validation following bioinformatics analysis. BMC Med Genom. 2019;12(1):1–13.
Yunoki T, Hirano T, Tabuchi Y, Furusawa Y, Torigoe M, Nakajima T, et al. CDKN2A, CDK1, and CCNE1 overexpression in sebaceous gland carcinoma of eyelid. Int Ophthalmol. 2020;40(2):343–50.
Zheng H-P, Huang Z-G, He R-Q, Lu H-P, Dang Y-W, Lin P, et al. Integrated assessment of CDK1 upregulation in thyroid cancer. Am J Transl Res. 2019;11(12):7233.
Wang L, Shao X, Zhong T, Wu Y, Xu A, Sun X, et al. Discovery of a first-in-class CDK2 selective degrader for AML differentiation therapy. Nat Chem Biol. 2021;17(5):567–75.
Ying M, Shao X, Jing H, Liu Y, Qi X, Cao J, et al. Ubiquitin-dependent degradation of CDK2 drives the therapeutic differentiation of AML by targeting PRDX2. Blood J Am Soc Hematol. 2018;131(24):2698–711.
Thacker G, Mishra M, Sharma A, Singh AK, Sanyal S, Trivedi AK. CDK2-instigates C/EBPα degradation through SKP2 in Acute myeloid leukemia. Med Oncol. 2021;38(6):1–10.
Chen S, Ni M, Hu T, Gu Y, Feng C, Pan C, et al. TANK-binding kinase 1 inhibitor GSK8612 enhances daunorubicin sensitivity in acute myeloid leukemia cells via the AKT-CDK2 pathway. Am J Transl Res. 2021;13(12):13640.
Tan S-H, Ding H-J, Mei X-P, Liu J-T, Tang Y-X, Li Y. Propofol suppressed cell proliferation and enhanced apoptosis of bladder cancer cells by regulating the miR-340/CDK2 signal axis. Acta Histochem. 2021;123(5): 151728.
Bai Y, Zhang G, Chu H, Li P, Li J. The positive feedback loop of lncRNA DANCR/miR-138/Sox4 facilitates malignancy in non-small cell lung cancer. Am J Cancer Res. 2019;9(2):270.
Pandey K, Park N, Park K-S, Hur J, Cho YB, Kang M, et al. Combined CDK2 and CDK4/6 inhibition overcomes palbociclib resistance in breast cancer by enhancing senescence. Cancers. 2020;12(12):3566.
Nie L, Wei Y, Zhang F, Hsu Y-H, Chan L-C, Xia W, et al. CDK2-mediated site-specific phosphorylation of EZH2 drives and maintains triple-negative breast cancer. Nat Commun. 2019;10(1):1–15.
Thacker G, Mishra M, Sharma A, Singh AK, Sanyal S, Trivedi AK. CDK2 destabilizes tumor suppressor C/EBPα expression through ubiquitin-mediated proteasome degradation in acute myeloid leukemia. J Cell Biochem. 2020;121(4):2839–50.
Wang H, Liu Y-C, Zhu C-Y, Yan F, Wang M-Z, Chen X-S, et al. Chidamide increases the sensitivity of refractory or relapsed acute myeloid leukemia cells to anthracyclines via regulation of the HDAC3-AKT-P21-CDK2 signaling pathway. J Exp Clin Cancer Res. 2020;39(1):1–19.
Shao X, Xiang S, Fu H, Chen Y, Xu A, Liu Y, et al. CDK2 suppression synergizes with all-trans-retinoic acid to overcome the myeloid differentiation blockade of AML cells. Pharmacol Res. 2020;151: 104545.
Rashid A, Duan X, Gao F, Yang M, Yen A. Roscovitine enhances All-trans retinoic acid (ATRA)-induced leukemia cell differentiation: Novel effects on signaling molecules for a putative Cdk2 inhibitor. Cell Signal. 2020;71: 109555.
Abdalla AN, Abdallah ME, Aslam A, Bader A, Vassallo A, Tommasi ND, et al. Synergistic anti leukemia effect of a novel Hsp90 and a Pan cyclin dependent kinase inhibitors. Molecules. 2020;25(9):2220.
Zhang Q, Miao S, Han X, Li C, Zhang M, Cui K, et al. MicroRNA-3619-5p suppresses bladder carcinoma progression by directly targeting β-catenin and CDK2 and activating p21. Cell Death Dis. 2018;9(10):1–13.
Jung JH, You S, Oh JW, Yoon J, Yeon A, Shahid M, et al. Integrated proteomic and phosphoproteomic analyses of cisplatin-sensitive and resistant bladder cancer cells reveal CDK2 network as a key therapeutic target. Cancer Lett. 2018;437:1–12.
Liu X, Liu S, Piao C, Zhang Z, Zhang X, Jiang Y, et al. Non-metabolic function of MTHFD2 activates CDK2 in bladder cancer. Cancer Sci. 2021;112(12):4909.
Jin X, Ge L-P, Li D-Q, Shao Z-M, Di G-H, Xu X-E, et al. LncRNA TROJAN promotes proliferation and resistance to CDK4/6 inhibitor via CDK2 transcriptional activation in ER+ breast cancer. Mol Cancer. 2020;19(1):1–18.
Aziz D, Portman N, Fernandez KJ, Lee C, Alexandrou S, Llop-Guevara A, et al. Synergistic targeting of BRCA1 mutated breast cancers with PARP and CDK2 inhibition. NPJ Breast Cancer. 2021;7(1):1–14.
Jian Y, Huang X, Fang L, Wang M, Liu Q, Xu H, et al. Actin-like protein 6A/MYC/CDK2 axis confers high proliferative activity in triple-negative breast cancer. J Exp Clin Cancer Res. 2021;40(1):1–18.
Satriyo PB, Su CM, Ong JR, Huang W-C, Fong I-H, Lin C-C, et al. 4-Acetylantroquinonol B induced DNA damage response signaling and apoptosis via suppressing CDK2/CDK4 expression in triple negative breast cancer cells. Toxicol Appl Pharmacol. 2021;422: 115493.
Al-Sanea MM, Obaidullah AJ, Shaker ME, Chilingaryan G, Alanazi MM, Alsaif NA, et al. A new CDK2 inhibitor with 3-hydrazonoindolin-2-one scaffold endowed with anti-breast cancer activity: design, synthesis, biological evaluation, and in silico insights. Molecules. 2021;26(2):412.
Feng J, Wen T, Li Z, Feng L, Zhou L, Yang Z, et al. Cross-talk between the ER pathway and the lncRNA MAFG-AS1/miR-339-5p/CDK2 axis promotes progression of ER+ breast cancer and confers tamoxifen resistance. Aging (Albany NY). 2020;12(20):20658.
Zhang X, Zhao Y, Wang C, Ju H, Liu W, Zhang X, et al. Rhomboid domain-containing protein 1 promotes breast cancer progression by regulating the p-Akt and CDK2 levels. Cell Commun Signal. 2018;16(1):1–15.
Blain SW. Targeting p27 tyrosine phosphorylation as a modality to inhibit CDK4 and CDK2 and cause cell cycle arrest in breast cancer cells. Oncoscience. 2018;5(5–6):144.
Bi Y, Guo S, Xu X, Kong P, Cui H, Yan T, et al. Decreased ZNF750 promotes angiogenesis in a paracrine manner via activating DANCR/miR-4707-3p/FOXC2 axis in esophageal squamous cell carcinoma. Cell Death Dis. 2020;11(4):1–17.
Patel P, Tsiperson V, Gottesman SR, Somma J, Blain SW. Dual inhibition of CDK4 and CDK2 via targeting p27 tyrosine phosphorylation induces a potent and durable response in breast cancer cells. Mol Cancer Res. 2018;16(3):361–77.
Wang Y, Chen Y, Cheng X, Zhang K, Wang H, Liu B, et al. Design, synthesis and biological evaluation of pyrimidine derivatives as novel CDK2 inhibitors that induce apoptosis and cell cycle arrest in breast cancer cells. Bioorg Med Chem. 2018;26(12):3491–501.
Abd El-Sattar NE, Badawy EH, AbdEl-Hady WH, Abo-Alkasem MI, Mandour AA, Ismail NS. Design and synthesis of new CDK2 inhibitors containing thiazolone and thiazolthione scafold with apoptotic activity. Chem Pharm Bull. 2021;69(1):106–17.
He Z, Zhang R, Jiang F, Zhang H, Zhao A, Xu B, et al. FADS1-FADS2 genetic polymorphisms are associated with fatty acid metabolism through changes in DNA methylation and gene expression. Clin Epigenetics. 2018;10(1):113.
Sang X, Belmessabih N, Wang R, Stephen P, Lin S-X. CRIF1-CDK2 interface inhibitors enhance taxol inhibition of the lethal triple-negative breast cancer. Cancers. 2022;14(4):989.
Scott GK, Chu D, Kaur R, Malato J, Rothschild DE, Frazier K, et al. ERpS294 is a biomarker of ligand or mutational ERα activation and a breast cancer target for CDK2 inhibition. Oncotarget. 2017;8(48):83432.
Hur S, Kim JH, Yun J, Ju YW, Han JM, Heo W, et al. Protein Phosphatase 1H, cyclin-dependent kinase inhibitor p27, and cyclin-dependent kinase 2 in paclitaxel resistance for triple negative breast cancers. J Breast Cancer. 2020;23(2):162.
Rao SS, Stoehr J, Dokic D, Wan L, Decker JT, Konopka K, et al. Synergistic effect of eribulin and CDK inhibition for the treatment of triple negative breast cancer. Oncotarget. 2017;8(48):83925.
Qu C, Zhu W, Dong K, Pan Z, Chen Y, Chen X, et al. Inhibitory effect of hydroxysafflor yellow B on the proliferation of human breast cancer MCF-7 cells. Recent Pat Anti-Cancer Drug Discov. 2019;14(2):187–97.
Ismail MM, Soliman DH, Sabour R, Farrag AM. Synthesis of new arylazopyrazoles as apoptosis inducers: candidates to inhibit proliferation of MCF-7 cells. Arch Pharm. 2021;354(1):2000214.
Huang S-W, Sun M-T, Lee W-S, Su Y-S, Lee Y-T, Chiang M-H, et al. Cancer as an infectious disease: a different treatment alternative using a combination of tigecycline and pyrvinium pamoate–an example of breast cancer. J Microbiol Immunol Infect. 2022;55(1):51–9.
Zheng Q, Zhang J, Zhang T, Liu Y, Du X, Dai X, et al. Hsa_circ_0000520 overexpression increases CDK2 expression via miR-1296 to facilitate cervical cancer cell proliferation. J Transl Med. 2021;19(1):1–16.
Qu X, Zhu L, Song L, Liu S. circ_0084927 promotes cervical carcinogenesis by sponging miR-1179 that suppresses CDK2, a cell cycle-related gene. Cancer Cell Int. 2020;20(1):1–17.
Zhou M, Yang Z, Wang D, Chen P, Zhang Y. The circular RNA circZFR phosphorylates Rb promoting cervical cancer progression by regulating the SSBP1/CDK2/cyclin E1 complex. J Exp Clin Cancer Res. 2021;40(1):1–18.
Abd El-Karim SS, Syam YM, El Kerdawy AM, Abdelghany TM. New thiazol-hydrazono-coumarin hybrids targeting human cervical cancer cells: Synthesis, CDK2 inhibition, QSAR and molecular docking studies. Bioorg Chem. 2019;86:80–96.
Saqub H, Proetsch-Gugerbauer H, Bezrookove V, Nosrati M, Vaquero EM, de Semir D, et al. Dinaciclib, a cyclin-dependent kinase inhibitor, suppresses cholangiocarcinoma growth by targeting CDK2/5/9. Sci Rep. 2020;10(1):1–13.
Peng X, Pan K, Zhao W, Zhang J, Yuan S, Wen X, et al. NPTX1 inhibits colon cancer cell proliferation through down-regulating cyclin A2 and CDK2 expression. Cell Biol Int. 2018;42(5):589–97.
Samir N, George RF, Elrazaz EZ, Ayoub IM, Shalaby EM, Plaisier JR, et al. Synthesis of some tropane-based compounds targeting colon cancer. Future Med Chem. 2020;12(23):2123–40.
Zhou X, Li S, Ma T, Zeng J, Li H, Liu X, et al. MEX3A knockdown inhibits the tumorigenesis of colorectal cancer via modulating CDK2 expression. Exp Ther Med. 2021;22(5):1–8.
Somarelli JA, Roghani RS, Moghaddam AS, Thomas BC, Rupprecht G, Ware KE, et al. A precision medicine drug discovery pipeline identifies combined CDK2 and 9 inhibition as a novel therapeutic strategy in colorectal cancer. Mol Cancer Ther. 2020;19(12):2516–27.
Zhang J, Cui K, Huang L, Yang F, Sun S, Bian Z, et al. SLCO4A1-AS1 promotes colorectal tumourigenesis by regulating Cdk2/c-Myc signalling. J Biomed Sci. 2022;29(1):1–17.
Tang Z, Li L, Tang Y, Xie D, Wu K, Wei W, et al. CDK 2 positively regulates aerobic glycolysis by suppressing SIRT 5 in gastric cancer. Cancer Sci. 2018;109(8):2590–8.
Wang Y, Jiang R, Wang Q, Li Y, Sun Z, Zhao H. Silencing LINC01021 inhibits gastric cancer through upregulation of KISS1 expression by blocking CDK2-dependent phosphorylation of CDX2. Mol Ther Nucleic Acids. 2021;24:832–44.
Chen C, Lei J, Zheng Q, Tan S, Ding K, Yu C. Poly (rC) binding protein 2 (PCBP 2) promotes the viability of human gastric cancer cells by regulating CDK2. FEBS Open Bio. 2018;8(5):764–73.
Cheng A-Y, Chien Y-C, Lee H-C, Hsieh Y-H, Yu Y-L. Water-extracted Ganoderma lucidum induces apoptosis and S-phase arrest via cyclin-CDK2 pathway in glioblastoma cells. Molecules. 2020;25(16):3585.
Guo E, Liang C, He X, Song G, Liu H, Lv Z, et al. Long noncoding RNA LINC00958 accelerates gliomagenesis through regulating miR-203/CDK2. DNA Cell Biol. 2018;37(5):465–72.
Gao T, Gu G, Tian J, Zhang R, Zheng X, Wang Y, et al. LncRNA HSP90AA1-IT1 promotes gliomas by targeting miR-885-5p-CDK2 pathway. Oncotarget. 2017;8(43):75284.
Zhu Y, Ke K-B, Xia Z-K, Li H-J, Su R, Dong C, et al. Discovery of vanoxerine dihydrochloride as a CDK2/4/6 triple-inhibitor for the treatment of human hepatocellular carcinoma. Mol Med. 2021;27(1):1–14.
Liang Y, Fan Y, Liu Y, Fan H. HNRNPU promotes the progression of hepatocellular carcinoma by enhancing CDK2 transcription. Exp Cell Res. 2021;409(1): 112898.
Yang A-L, Wu Q, Hu Z-D, Wang S-P, Tao Y-F, Wang A-M, et al. A network pharmacology approach to investigate the anticancer mechanism of cinobufagin against hepatocellular carcinoma via downregulation of EGFR-CDK2 signaling. Toxicol Appl Pharmacol. 2021;431:115739.
Liang XH, Feng ZP, Liu FQ, Yan R, Yin LY, Shen H, et al. MAPRE1 promotes cell cycle progression of hepatocellular carcinoma cells by interacting with CDK2. Cell Biol Int. 2020;44(11):2326–33.
Huang S, Zhang C, Sun C, Hou Y, Zhang Y, Tam NL, et al. Obg-like ATPase 1 (OLA1) overexpression predicts poor prognosis and promotes tumor progression by regulating P21/CDK2 in hepatocellular carcinoma. Aging (Albany NY). 2020;12(3):3025.
Wei W, Huang X, Shen X, Lian J, Chen Y, Wang W, et al. Overexpression of IncRNA TPT1-AS1 suppresses hepatocellular carcinoma cell proliferation by downregulating CDK2. Crit Rev™ Eukaryot Gene Expr. 2022;32.
Kang J, Huang X, Dong W, Zhu X, Li M, Cui N. Long non-coding RNA LINC00630 facilitates hepatocellular carcinoma progression through recruiting transcription factor E2F1 to up-regulate cyclin-dependent kinase 2 expression. Hum Exp Toxicol. 2021;40(12_suppl):S257–68.
Ghasemi H, Jamshidi A, Ghatee MA, Mazhab-Jafari K, Khorasani M, Rahmati M, et al. PPARγ activation by pioglitazone enhances the anti-proliferative effects of doxorubicin on pro-monocytic THP-1 leukemia cells via inducing apoptosis and G2/M cell cycle arrest. J Receptors Signal Transduct. 2021. https://doi.org/10.1080/10799893.2021.1988972.
Almehmadi SJ, Alsaedi AM, Harras MF, Farghaly TA. Synthesis of a new series of pyrazolo [1, 5-a] pyrimidines as CDK2 inhibitors and anti-leukemia. Bioorg Chem. 2021;117: 105431.
Xin X, Lu Y, Xie S, Chen Y, Jiang X, Song S, et al. miR-155 accelerates the growth of human liver cancer cells by activating CDK2 via targeting H3F3A. Mol Ther Oncolytics. 2020;17:471–83.
Yu D, Li Y, Zhong M. MicroRNA-597 inhibits NSCLC progression through negatively regulating CDK2 expression. Eur Rev Med Pharmacol Sci. 2020;24(8):4288–97.
Shen Z, Wang J, Ke K, Chen R, Zuo A, Zhang R, et al. Polyphyllin I, a lethal partner of Palbociclib, suppresses non-small cell lung cancer through activation of p21/CDK2/Rb pathway in vitro and in vivo. Cell Cycle. 2021;20(23):2494–506.
Li Z, Zhang Y, Zhou Y, Wang F, Yin C, Ding L, et al. Tanshinone IIA suppresses the progression of lung adenocarcinoma through regulating CCNA2-CDK2 complex and AURKA/PLK1 pathway. Sci Rep. 2021;11(1):1–12.
Kawakami M, Mustachio LM, Rodriguez-Canales J, Mino B, Roszik J, Tong P, et al. Next-generation CDK2/9 inhibitors and anaphase catastrophe in lung cancer. JNCI J Natl Cancer Inst. 2017;109(6).
Lee H, Lee H-J, Bae IJ, Kim JJ, Kim S-H. Inhibition of STAT3/VEGF/CDK2 axis signaling is critically involved in the antiangiogenic and apoptotic effects of arsenic herbal mixture PROS in non-small lung cancer cells. Oncotarget. 2017;8(60): 101771.
Chorner PM, Moorehead RA. A-674563, a putative AKT1 inhibitor that also suppresses CDK2 activity, inhibits human NSCLC cell growth more effectively than the pan-AKT inhibitor, MK-2206. PLoS ONE. 2018;13(2): e0193344.
Bolin S, Borgenvik A, Persson CU, Sundström A, Qi J, Bradner JE, et al. Combined BET bromodomain and CDK2 inhibition in MYC-driven medulloblastoma. Oncogene. 2018;37(21):2850–62.
Mohammed ER, Elmasry GF. Development of newly synthesised quinazolinone-based CDK2 inhibitors with potent efficacy against melanoma. J Enzyme Inhib Med Chem. 2022;37(1):686–700.
Roy T, Boateng ST, Banang-Mbeumi S, Singh PK, Basnet P, Chamcheu R-CN, et al. Synthesis, inverse docking-assisted identification and in vitro biological characterization of Flavonol-based analogs of fisetin as c-Kit, CDK2 and mTOR inhibitors against melanoma and non-melanoma skin cancers. Bioorg Chem 2021;107:104595.
Bo L, Wei B, Wang Z, Kong D, Gao Z, Miao Z. Bioinformatics analysis of the CDK2 functions in neuroblastoma. Mol Med Rep. 2018;17(3):3951–9.
Han Y, Wei Y, Yao J, Chu Y-Y, Li C-W, Hsu JL, et al. Inhibition of CDK2 reduces EZH2 phosphorylation and reactivates ERα expression in high-grade serous ovarian carcinoma. Am J Cancer Res. 2020;10(4):1194.
He Y, Wei L, Zhang S, Liu H, Fang F, Li Y. LncRNA PLAC2 positively regulates CDK2 to promote ovarian carcinoma cell proliferation. Cancer Manage Res. 2020;12:5713.
Duan P-j, Zhao J-h, Xie L-l. Cul4B promotes the progression of ovarian cancer by upregulating the expression of CDK2 and CyclinD1. J Ovarian Res. 2020;13(1):1–10.
Ding C-H, Yin C, Chen S-J, Wen L-Z, Ding K, Lei S-J, et al. The HNF1α-regulated lncRNA HNF1A-AS1 reverses the malignancy of hepatocellular carcinoma by enhancing the phosphatase activity of SHP-1. Mol Cancer. 2018;17(1):1–14.
Chen T, Liu L, Zou Y, Hu X, Zhang W, Zhou T, et al. Nobiletin downregulates the SKP2-p21/p27-CDK2 axis to inhibit tumor progression and shows synergistic effects with palbociclib on renal cell carcinoma. Cancer Biol Med. 2021;18(1):227.
Xu C, Zheng J. siRNA against TSG101 reduces proliferation and induces G0/G1 arrest in renal cell carcinoma–involvement of c-myc, cyclin E1, and CDK2. Cell Mol Biol Lett. 2019;24(1):1–9.
Tan G, Zhang G-Y, Xu J, Kang C-W, Yan Z-K, Lei M, et al. PLA2G10 facilitates the cell-cycle progression of soft tissue leiomyosarcoma cells at least by elevating cyclin E1/CDK2 expression. Biochem Biophys Res Commun. 2020;527(2):525–31.
Wang F, Li Z, Zhou J, Wang G, Zhang W, Xu J, et al. SIRT1 regulates the phosphorylation and degradation of P27 by deacetylating CDK2 to promote T-cell acute lymphoblastic leukemia progression. J Exp Clin Cancer Res. 2021;40(1):1–16.
Costa C, Wang Y, Ly A, Hosono Y, Murchie E, Walmsley CS, et al. PTEN loss mediates clinical cross-resistance to CDK4/6 and PI3Kα inhibitors in breast cancer. Cancer Discov. 2020;10(1):72–85.
Guenther LM, Dharia NV, Ross L, Conway A, Robichaud AL, Catlett JL, et al. A combination CDK4/6 and IGF1R inhibitor strategy for Ewing sarcoma. Clin Cancer Res. 2019;25(4):1343–57.
Xia Z-K, Wang W, Qiu J-G, Shi X-N, Li H-J, Chen R, et al. Discovery of a new CDK4/6 and PI3K/AKT multiple kinase inhibitor aminoquinol for the treatment of hepatocellular carcinoma. Front Pharmacol. 2021;12.
Bazzar W, Bocci M, Hejll E, Högqvist Tabor V, Hydbring P, Grandien A, et al. Pharmacological inactivation of CDK2 inhibits MYC/BCL-XL-driven leukemia in vivo through induction of cellular senescence. Cell Cycle. 2021;20(1):23–38.
Gao Y, Wang H, Zhong A, Yu T. Expression and prognosis of CyclinA and CDK2 in patients with advanced cervical cancer after chemotherapy. Cell Mol Biol (Noisy-le-grand). 2020;66(3):85–91.
Liu H, Weng J. A comprehensive bioinformatic analysis of cyclin-dependent kinase 2 (CDK2) in glioma. Gene. 2022;822: 146325.
Dong W, Zhu H, Gao H, Shi W, Zhang Y, Wang H, et al. Expression of cyclin E/Cdk2/p27Kip1 in growth hormone adenomas. World Neurosurg. 2019;121:e45–53.
Lian J, Zhang X, Lu Y, Hao S, Zhang Z, Yang Y. Expression and significance of LncRNA-MINCR and CDK2 mRNA in primary hepatocellular carcinoma. Comb Chem High Throughput Screening. 2019;22(3):201–6.
Liu T-T, Li R, Huo C, Li J-P, Yao J, Ji X-L, et al. Identification of CDK2-related immune forecast model and ceRNA in lung adenocarcinoma, a pan-cancer analysis. Front Cell Dev Biol. 2021. https://doi.org/10.3389/fcell.2021.682002.
Cao T, Xiao T, Huang G, Xu Y, Zhu JJ, Wang K, et al. CDK3, target of miR-4469, suppresses breast cancer metastasis via inhibiting Wnt/β-catenin pathway. Oncotarget. 2017;8(49):84917.
Zhang X, Zhang B, Zhang P, Lian L, Li L, Qiu Z, et al. Norcantharidin regulates ERα signaling and tamoxifen resistance via targeting miR-873/CDK3 in breast cancer cells. PLoS ONE. 2019;14(5): e0217181.
Li W, Zheng Z, Chen H, Cai Y, Xie W. Knockdown of long non-coding RNA PVT1 induces apoptosis and cell cycle arrest in clear cell renal cell carcinoma through the epidermal growth factor receptor pathway. Oncol Lett. 2018;15(5):7855–63.
Cui J, Yang Y, Li H, Leng Y, Qian K, Huang Q, et al. MiR-873 regulates ERα transcriptional activity and tamoxifen resistance via targeting CDK3 in breast cancer cells. Oncogene. 2015;34(30):3895–907.
Lu J, Zhang ZL, Huang D, Tang N, Li Y, Peng Z, et al. Cdk3-promoted epithelial-mesenchymal transition through activating AP-1 is involved in colorectal cancer metastasis. Oncotarget. 2016;7(6):7012.
Liu Z-H, Yang S-Z, Li W-Y, Dong S-Y, Zhou S-Y, Xu S. CircRNA_141539 can serve as an oncogenic factor in esophageal squamous cell carcinoma by sponging miR-4469 and activating CDK3 gene. Aging (Albany NY). 2021;13(8):12179.
Zhang Z, Huang A, Zhang A, Zhou C. HuR promotes breast cancer cell proliferation and survival via binding to CDK3 mRNA. Biomed Pharmacother. 2017;91:788–95.
Zheng L, Li X, Meng X, Chou J, Hu J, Zhang F, et al. Competing endogenous RNA networks of CYP4Z1 and pseudogene CYP4Z2P confer tamoxifen resistance in breast cancer. Mol Cell Endocrinol. 2016;427:133–42.
Wang P, Chen S, Fang H, Wu X, Chen D, Peng L, et al. miR-214/199a/199a* cluster levels predict poor survival in hepatocellular carcinoma through interference with cell-cycle regulators. Oncotarget. 2016;7(1):929.
Sugimori N, Espinoza JL, Trung LQ, Takami A, Kondo Y, An DT, et al. Paraptosis cell death induction by the thiamine analog benfotiamine in leukemia cells. PLoS ONE. 2015;10(4): e0120709.
Zhang Y, Yang L, Ling C, Heng W. HuR facilitates cancer stemness of lung cancer cells via regulating miR-873/CDK3 and miR-125a-3p/CDK3 axis. Biotech Lett. 2018;40(4):623–31.
Zhao Y, Guo Q, Chen J, Hu J, Wang S, Sun Y. Role of long non-coding RNA HULC in cell proliferation, apoptosis and tumor metastasis of gastric cancer: a clinical and in vitro investigation. Oncol Rep. 2014;31(1):358–64.
Xiao T, Zhu J, Huang S, Peng C, He S, Du J, et al. Phosphorylation of NFAT3 by CDK3 induces cell transformation and promotes tumor growth in skin cancer. Oncogene. 2017;36(20):2835–45.
Nakatani K, Matsuo H, Harata Y, Higashitani M, Koyama A, Noura M, et al. Inhibition of CDK4/6 and autophagy synergistically induces apoptosis in t (8; 21) acute myeloid leukemia cells. Int J Hematol. 2021;113(2):243–53.
Rubio C, Martínez-Fernández M, Segovia C, Lodewijk I, Suarez-Cabrera C, Segrelles C, et al. CDK4/6 inhibitor as a novel therapeutic approach for advanced bladder cancer independently of RB1 status. Clin Cancer Res. 2019;25(1):390–402.
Li Z, Razavi P, Li Q, Toy W, Liu B, Ping C, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the hippo pathway. Cancer Cell. 2018;34(6):893-905.e8.
Asghar US, Barr AR, Cutts R, Beaney M, Babina I, Sampath D, et al. Single-cell dynamics determines response to CDK4/6 inhibition in triple-negative breast cancer. Clin Cancer Res. 2017;23(18):5561–72.
Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548(7668):471–5.
Oh SJ, Cho H, Kim S, Noh KH, Song K-H, Lee H-J, et al. Targeting cyclin D-CDK4/6 sensitizes immune-refractory cancer by blocking the SCP3–NANOG axis. Can Res. 2018;78(10):2638–53.
Shen W-C, Shi Y-W. Effect of MiR-142-3p targeting HOXA5 on proliferation, cycle arrest and apoptosis of acute B lymphocytic leukemia cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29(4):1085–92.
Zhang L, Wang X, Wu J, Xiao R, Liu J. MiR-335-3p inhibits cell proliferation and induces cell cycle arrest and apoptosis in acute myeloid leukemia by targeting EIF3E. Biosci Biotechnol Biochem. 2021;85(9):1953–61.
Wu F, Yin C, Qi J, Duan D, Jiang X, Yu J, et al. miR-362-5p promotes cell proliferation and cell cycle progression by targeting GAS7 in acute myeloid leukemia. Hum Cell. 2020;33(2):405–15.
Zhang T, Wang J, Zhai X, Li H, Li C, Chang J. MiR-124 retards bladder cancer growth by directly targeting CDK4. Acta Biochim Biophys Sin. 2014;46(12):1072–9.
Chen S, Wang W, Lin G, Zhong S. MicroRNA-195 inhibits epithelial-mesenchymal transition via downregulating CDK4 in bladder cancer. Int J Clin Exp Pathol. 2018;11(8):3891.
Cao Z, Xu L, Zhao S, Zhu X. The functions of microRNA-124 on bladder cancer. Onco Targets Ther. 2019;12:3429.
Ge Q, Wang C, Chen Z, Li F, Hu J, Ye Z. The suppressive effects of miR-1180–5p on the proliferation and tumorigenicity of bladder cancer cells. 2017.
Alves CL, Ehmsen S, Terp MG, Portman N, Tuttolomondo M, Gammelgaard OL, et al. Co-targeting CDK4/6 and AKT with endocrine therapy prevents progression in CDK4/6 inhibitor and endocrine therapy-resistant breast cancer. Nat Commun. 2021;12(1):1–15.
Teo ZL, Versaci S, Dushyanthen S, Caramia F, Savas P, Mintoff CP, et al. Combined CDK4/6 and PI3Kα inhibition is synergistic and immunogenic in triple-negative breast cancer. Can Res. 2017;77(22):6340–52.
Zhu X, Chen L, Huang B, Li X, Yang L, Hu X, et al. Efficacy and mechanism of the combination of PARP and CDK4/6 inhibitors in the treatment of triple-negative breast cancer. J Exp Clin Cancer Res. 2021;40(1):1–18.
Zhang Z, Li J, Ou Y, Yang G, Deng K, Wang Q, et al. CDK4/6 inhibition blocks cancer metastasis through a USP51-ZEB1-dependent deubiquitination mechanism. Signal Transduct Target Ther. 2020;5(1):1–13.
Kharenko OA, Patel RG, Calosing C, van der Horst EH. Combination of ZEN-3694 with CDK4/6 inhibitors reverses acquired resistance to CDK4/6 inhibitors in ER-positive breast cancer. Cancer Gene Ther. 2021:1–11.
Dang F, Nie L, Zhou J, Shimizu K, Chu C, Wu Z, et al. Inhibition of CK1ε potentiates the therapeutic efficacy of CDK4/6 inhibitor in breast cancer. Nat Commun. 2021;12(1):1–15.
Charles A, Bourne CM, Korontsvit T, Aretz ZE, Mun SS, Dao T, et al. Low-dose CDK4/6 inhibitors induce presentation of pathway specific MHC ligands as potential targets for cancer immunotherapy. Oncoimmunology. 2021;10(1):1916243.
O’Brien NA, McDermott MS, Conklin D, Luo T, Ayala R, Salgar S, et al. Targeting activated PI3K/mTOR signaling overcomes acquired resistance to CDK4/6-based therapies in preclinical models of hormone receptor-positive breast cancer. Breast Cancer Res. 2020;22(1):1–17.
Zhang H, Wang J, Li J, Zhou X, Yin L, Wang Y, et al. HMGB1 is a key factor for tamoxifen resistance and has the potential to predict the efficacy of CDK4/6 inhibitors in breast cancer. Cancer Sci. 2021;112(4):1603–13.
Romero-Pozuelo J, Figlia G, Kaya O, Martin-Villalba A, Teleman AA. Cdk4 and Cdk6 couple the cell-cycle machinery to cell growth via mTORC1. Cell Rep. 2020;31(2): 107504.
Tian C, Wei Y, Li J, Huang Z, Wang Q, Lin Y, et al. A Novel CDK4/6 and PARP dual inhibitor ZC-22 effectively suppresses tumor growth and improves the response to cisplatin treatment in breast and ovarian cancer. Int J Mol Sci. 2022;23(5):2892.
Kartika ID, Kotani H, Iida Y, Koyanagi A, Tanino R, Harada M. Protective role of cytoplasmic p21Cip1/Waf1 in apoptosis of CDK4/6 inhibitor-induced senescence in breast cancer cells. Cancer Med. 2021;10(24):8988–99.
Feng T, Xu D, Tu C, Li W, Ning Y, Ding J, et al. MiR-124 inhibits cell proliferation in breast cancer through downregulation of CDK4. Tumor Biol. 2015;36(8):5987–97.
Li Q, Liu J, Jia Y, Li T, Zhang M. miR-623 suppresses cell proliferation, migration and invasion through direct inhibition of XRCC5 in breast cancer. Aging (Albany NY). 2020;12(11):10246.
Wu J, Xu W, Ma L, Sheng J, Ye M, Chen H, et al. Formononetin relieves the facilitating effect of lncRNA AFAP1-AS1-miR-195/miR-545 axis on progression and chemo-resistance of triple-negative breast cancer. Aging (Albany NY). 2021;13(14):18191.
Feng T, Shao F, Wu Q, Zhang X, Xu D, Qian K, et al. miR-124 downregulation leads to breast cancer progression via LncRNA-MALAT1 regulation and CDK4/E2F1 signal activation. Oncotarget. 2016;7(13):16205.
Li D, Song H, Wu T, Xie D, Hu J, Zhao J, et al. MiR-519d-3p suppresses breast cancer cell growth and motility via targeting LIM domain kinase 1. Mol Cell Biochem. 2018;444(1):169–78.
Peng X, Yan B, Shen Y. MiR-1301-3p inhibits human breast cancer cell proliferation by regulating cell cycle progression and apoptosis through directly targeting ICT1. Breast Cancer. 2018;25(6):742–52.
Flaxman SR, Bourne RR, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–34.
Achari C, Winslow S, Ceder Y, Larsson C. Expression of miR-34c induces G2/M cell cycle arrest in breast cancer cells. BMC Cancer. 2014;14(1):1–9.
Wang Z, Ren C, Yang L, Zhang X, Liu J, Zhu Y, et al. Silencing of circular RNA_0000326 inhibits cervical cancer cell proliferation, migration and invasion by boosting microRNA-338-3p-dependent down-regulation of CDK4. Aging (Albany NY). 2021;13(6):9119.
Xiao H, Zeng J, Li H, Chen K, Yu G, Hu J, et al. MiR-1 downregulation correlates with poor survival in clear cell renal cell carcinoma where it interferes with cell cycle regulation and metastasis. Oncotarget. 2015;6(15):13201.
Gu Y, Niu S, Wang Y, Duan L, Pan Y, Tong Z, et al. DMDRMR-mediated regulation of m6A-modified CDK4 by m6A reader IGF2BP3 drives ccRCC progression. Can Res. 2021;81(4):923–34.
Xiao H, Xiao W, Cao J, Li H, Guan W, Guo X, et al. miR-206 functions as a novel cell cycle regulator and tumor suppressor in clear-cell renal cell carcinoma. Cancer Lett. 2016;374(1):107–16.
Sun W, Nie W, Wang Z, Zhang H, Li Y, Fang X. Lnc HAGLR promotes colon cancer progression through sponging miR-185-5p and activating CDK4 and CDK6 in vitro and in vivo. Onco Targets Ther. 2020;13:5913.
Yang H, Lin J, Jiang J, Ji J, Wang C, Zhang J. miR-20b-5p functions as tumor suppressor microRNA by targeting cyclinD1 in colon cancer. Cell Cycle. 2020;19(21):2939–54.
Ma X, Luo J, Zhang Y, Sun D, Lin Y. LncRNA MCM3AP-AS1 upregulates CDK4 by sponging miR-545 to suppress G1 arrest in colorectal cancer. Cancer Manage Res. 2020;12:8117.
Zhu X, Ma S-P, Yang D, Liu Y, Wang Y-P, Lin T, et al. miR-142–3p suppresses cell growth by targeting CDK4 in colorectal cancer. Cell Physiol Biochem. 2018;51(4):1969–81.
Lulla AR, Slifker MJ, Zhou Y, Lev A, Einarson MB, Dicker DT, et al. miR-6883 family miRNAs target CDK4/6 to induce G1 phase cell-cycle arrest in colon cancer cells. Can Res. 2017;77(24):6902–13.
Zhang T, Cai X, Li Q, Xue P, Chen Z, Dong X, et al. Hsa-miR-875-5p exerts tumor suppressor function through down-regulation of EGFR in colorectal carcinoma (CRC). Oncotarget. 2016;7(27):42225.
Zheng Z, Hong D, Zhang X, Chang Y, Sun N, Lin Z, et al. uc. 77-downregulation promotes colorectal cancer cell proliferation by inhibiting FBXW8-mediated CDK4 protein degradation. Front Oncol. 2021;11:1419.
Wu C-L, Shan T-D, Han Y, Kong Y, Li Y-B, Peng X-G, et al. Long intergenic noncoding RNA 00665 promotes proliferation and inhibits apoptosis in colorectal cancer by regulating miR-126-5p. Aging (Albany NY). 2021;13(10):13571.
Zheng Z, Cui H, Wang Y, Yao W. Downregulation of RPS15A by miR-29a-3p attenuates cell proliferation in colorectal carcinoma. Biosci Biotechnol Biochem. 2019;83(11):2057–64.
Ding C, Wei R, Rodríguez RA, Mullor MDMR. LncRNA PCAT-1 plays an oncogenic role in epithelial ovarian cancer by modulating cyclinD1/CDK4 expression. Int J Clin Exp Pathol. 2019;12(6):2148.
Lang B, Zhao S. miR-486 functions as a tumor suppressor in esophageal cancer by targeting CDK4/BCAS2. Oncol Rep. 2018;39(1):71–80.
Zhang Y, Wang Q, Li H, Ye T, Gao F, Liu Y. miR-124 radiosensitizes human esophageal cancer cell TE-1 by targeting CDK4. Genet Mol Res. 2016;15(2):1–10.
Jiang S, Zhao C, Yang X, Li X, Pan Q, Huang H, et al. miR-1 suppresses the growth of esophageal squamous cell carcinoma in vivo and in vitro through the downregulation of MET, cyclin D1 and CDK4 expression. Int J Mol Med. 2016;38(1):113–22.
Lei X, Yang S, Yang Y, Zhang J, Wang Y, Cao M. Long noncoding RNA DLX6-AS1 targets miR-124-3p/CDK4 to accelerate Ewing’s sarcoma. Am J Transl Res. 2019;11(10):6569.
Qian Y, Wu X, Wang H, Hou G, Han X, Song W. PAK1 silencing is synthetic lethal with CDK4/6 inhibition in gastric cancer cells via regulating PDK1 expression. Hum Cell. 2020;33(2):377–85.
Lin A, Bu W, Wang P, Gao J, Yang J, Ding F, et al. miR-449a/b negatively regulates E2F1 to suppress proliferation of gastric cancer cells. Nan Fang yi ke da xue xue bao J South Med Univ. 2020;40(1):13–9.
Luo D, Fan H, Ma X, Yang C, He Y, Ge Y, et al. miR-1301-3p promotes cell proliferation and facilitates cell cycle progression via targeting SIRT1 in gastric cancer. Front Oncol. 2021;11.
Lin X-M, Wang Z-J, Lin Y-X, Chen H. Decreased exosome-delivered miR-486-5p is responsible for the peritoneal metastasis of gastric cancer cells by promoting EMT progress. World J Surg Oncol. 2021;19(1):1–10.
Sun C, Zhang S, Liu C, Liu X. Curcumin promoted miR-34a expression and suppressed proliferation of gastric cancer cells. Cancer Biother Radiopharm. 2019;34(10):634–41.
Zhang Q, Feng Y, Liu P, Yang J. MiR-143 inhibits cell proliferation and invasion by targeting DNMT3A in gastric cancer. Tumor Biol. 2017;39(7):1010428317711312.
Liao A, Tan G, Chen L, Zhou W, Hu H. RASSF1A inhibits gastric cancer cell proliferation by miR-711-mediated downregulation of CDK4 expression. Oncotarget. 2016;7(5):5842.
Mi Y, Li Y, He Z, Chen D, Hong Q, You J. Upregulation of linc-ROR promotes the proliferation, migration, and invasion of gastric cancer cells through miR-212-3p/FGF7 axis. Cancer Manage Res. 2021;13:899.
Zhao Z, Wang L, Song W, Cui H, Chen G, Qiao F, et al. Reduced miR-29a-3p expression is linked to the cell proliferation and cell migration in gastric cancer. World J Surg Oncol. 2015;13(1):1–7.
Lin Z, Zhou Z, Guo H, He Y, Pang X, Zhang X, et al. Long noncoding RNA gastric cancer-related lncRNA1 mediates gastric malignancy through miRNA-885-3p and cyclin-dependent kinase 4. Cell Death Dis. 2018;9(6):1–16.
Li M, Xiao A, Floyd D, Olmez I, Lee J, Godlewski J, et al. CDK4/6 inhibition is more active against the glioblastoma proneural subtype. Oncotarget. 2017;8(33):55319.
Olmez I, Zhang Y, Manigat L, Benamar M, Brenneman B, Nakano I, et al. Combined c-Met/Trk inhibition overcomes resistance to CDK4/6 inhibitors in glioblastoma. Can Res. 2018;78(15):4360–9.
Moradimotlagh A, Arefian E, Valojerdi RR, Ghaemi S, Adegani FJ, Soleimani M. MicroRNA-129 inhibits glioma cell growth by targeting CDK4, CDK6, and MDM2. Mol Ther Nucleic Acids. 2020;19:759–64.
Deng X, Ma L, Wu M, Zhang G, Jin C, Guo Y, et al. miR-124 radiosensitizes human glioma cells by targeting CDK4. J Neurooncol. 2013;114(3):263–74.
Qiu S, Huang D, Yin D, Li F, Li X, Kung H-F, et al. Suppression of tumorigenicity by microRNA-138 through inhibition of EZH2-CDK4/6-pRb-E2F1 signal loop in glioblastoma multiforme. Biochimica et Biophysica Acta (BBA)-Mol Basis Dis. 2013;1832(10):1697–707.
Wang R, Zhang S, Chen X, Li N, Li J, Jia R, et al. EIF4A3-induced circular RNA MMP9 (circMMP9) acts as a sponge of miR-124 and promotes glioblastoma multiforme cell tumorigenesis. Mol Cancer. 2018;17(1):1–12.
Cao Y, Li X, Kong S, Shang S, Qi Y. CDK4/6 inhibition suppresses tumour growth and enhances the effect of temozolomide in glioma cells. J Cell Mol Med. 2020;24(9):5135–45.
Bao XA, Peng Y, Shen J, Yang L. Sevoflurane inhibits progression of glioma via regulating the HMMR antisense RNA 1/microRNA-7/cyclin dependent kinase 4 axis. Bioengineered. 2021;12(1):7893–906.
Zhou X, Xia Y, Li L, Zhang G. MiR-101 inhibits cell growth and tumorigenesis of Helicobacter pylori related gastric cancer by repression of SOCS2. Cancer Biol Ther. 2015;16(1):160–9.
Lyu J, Miao Y, Yu F, Chang C, Guo W, Zhu H. CDK4 and TERT amplification in head and neck mucosal melanoma. J Oral Pathol Med. 2021;50(10):971–8.
Hu Q, Peng J, Jiang L, Li W, Su Q, Zhang J, et al. Metformin as a senostatic drug enhances the anticancer efficacy of CDK4/6 inhibitor in head and neck squamous cell carcinoma. Cell Death Dis. 2020;11(10):1–16.
Digiacomo G, Fumarola C, La Monica S, Bonelli MA, Cretella D, Alfieri R, et al. Simultaneous combination of the CDK4/6 inhibitor palbociclib with regorafenib induces enhanced anti-tumor effects in hepatocarcinoma cell lines. Front Oncol. 2020:1880.
Bin X, Chen Y, Ma J, Tang R, Zhao Z, Wang K, et al. circ_0001588 induces the malignant progression of hepatocellular carcinoma by modulating miR-874/CDK4 signaling. J Immunol Res. 2021;2021.
Guan Z, Tan J, Gao W, Li X, Yang Y, Li X, et al. Circular RNA hsa_circ_0016788 regulates hepatocellular carcinoma tumorigenesis through miR-486/CDK4 pathway. J Cell Physiol. 2019;234(1):500–8.
Li W, Jiang H. Up-regulation of miR-498 inhibits cell proliferation, invasion and migration of hepatocellular carcinoma by targeting FOXO3. Clin Res Hepatol Gastroenterol. 2020;44(1):29–37.
Zhang Y, Zhang H, Wu S. LncRNA-CCDC144NL-AS1 promotes the development of hepatocellular carcinoma by inducing WDR5 expression via sponging miR-940. J Hepatocell Carcinoma. 2021;8:333.
Zheng S-Z, Sun P, Wang J-P, Liu Y, Gong W, Liu J. MiR-34a overexpression enhances the inhibitory effect of doxorubicin on HepG2 cells. World J Gastroenterol. 2019;25(22):2752.
Furuta M, Kozaki K-I, Tanimoto K, Tanaka S, Arii S, Shimamura T, et al. The tumor-suppressive miR-497-195 cluster targets multiple cell-cycle regulators in hepatocellular carcinoma. PLoS ONE. 2013;8(3):e60155.
Li M, Chen H, Xia L, Huang P. Circular RNA circSP3 promotes hepatocellular carcinoma growth by sponging microRNA-198 and upregulating cyclin-dependent kinase 4. Aging (Albany NY). 2021;13(14):18586.
Wu S, Wu Z, Xu H, Zhang J, Gu W, Tan X, et al. miR-34a-5p inhibits the malignant progression of KSHV-infected SH-SY5Y cells by targeting c-fos. PeerJ. 2022;10: e13233.
Böhm MJ, Marienfeld R, Jäger D, Mellert K, von Witzleben A, Brüderlein S, et al. Analysis of the CDK4/6 cell cycle pathway in leiomyosarcomas as a potential target for inhibition by palbociclib. Sarcoma. 2019;2019.
Wright GM, Gimbrone NT, Sarcar B, Percy TR, Gordián ER, Kinose F, et al. CDK4/6 inhibition synergizes with inhibition of P21-Activated Kinases (PAKs) in lung cancer cell lines. PLoS ONE. 2021;16(6): e0252927.
Qin Q, Li X, Liang X, Zeng L, Wang J, Sun L, et al. CDK4/6 inhibitor palbociclib overcomes acquired resistance to third-generation EGFR inhibitor osimertinib in non-small cell lung cancer (NSCLC). Thorac Cancer. 2020;11(9):2389–97.
Xing Z, Zhang Z, Gao Y, Zhang X, Kong X, Zhang J, et al. The lncRNA LINC01194/miR-486-5p axis facilitates malignancy in non-small cell lung cancer via regulating CDK4. Onco Targets Ther. 2020;13:3151.
Xu X, Tao R, Sun L, Ji X. Exosome-transferred hsa_circ_0014235 promotes DDP chemoresistance and deteriorates the development of non-small cell lung cancer by mediating the miR-520a-5p/CDK4 pathway. Cancer Cell Int. 2020;20(1):1–15.
Li D, Li D-Q, Liu D, Tang X-J. MiR-613 induces cell cycle arrest by targeting CDK4 in non-small cell lung cancer. Cell Oncol. 2016;39(2):139–47.
Feng H, Ge F, Du L, Zhang Z, Liu D. MiR-34b-3p represses cell proliferation, cell cycle progression and cell apoptosis in non-small-cell lung cancer (NSCLC) by targeting CDK4. J Cell Mol Med. 2019;23(8):5282–91.
Wang Q, PM K. CircRNA_001010 adsorbs miR-5112 in a sponge form to promote proliferation and metastasis of non-small cell lung cancer (NSCLC). Eur Rev Med Pharmacol Sci. 2020;24(8):4271–80.
Qin Y, Zhou X, Huang C, Li L, Liu H, Liang N, et al. Lower miR-340 expression predicts poor prognosis of non-small cell lung cancer and promotes cell proliferation by targeting CDK4. Gene. 2018;675:278–84.
Shao Y, Shen Y-Q, Li Y-L, Liang C, Zhang B-J, Lu S-D, et al. Direct repression of the oncogene CDK4 by the tumor suppressor miR-486-5p in non-small cell lung cancer. Oncotarget. 2016;7(23):34011.
Sun C-C, Li S-J, Li D-J. Hsa-miR-134 suppresses non-small cell lung cancer (NSCLC) development through down-regulation of CCND1. Oncotarget. 2016;7(24):35960.
Zhou H, Huang Z, Chen X, Chen S. miR-98 inhibits expression of TWIST to prevent progression of non-small cell lung cancers. Biomed Pharmacother. 2017;89:1453–61.
Jin J-J, Liu Y-H, Si J-M, Ni R, Wang J. Overexpression of miR-1290 contributes to cell proliferation and invasion of non small cell lung cancer by targeting interferon regulatory factor 2. Int J Biochem Cell Biol. 2018;95:113–20.
Yu H, Chen Y, Jiang P. Circular RNA HIPK3 exerts oncogenic properties through suppression of miR-124 in lung cancer. Biochem Biophys Res Commun. 2018;506(3):455–62.
Wei F, Wang M, Li Z, Wang Y, Zhou Y. miR‑593 inhibits proliferation and invasion and promotes apoptosis in non‑small cell lung cancer cells by targeting SLUG‑associated signaling pathways Corrigendum in/https://doi.org/10.3892/mmr. 2021.12555. Mol Med Rep. 2019;20(6):5172–82.
Sherman EJ, Mitchell DC, Garner AL. The RNA-binding protein SART3 promotes miR-34a biogenesis and G1 cell cycle arrest in lung cancer cells. J Biol Chem. 2019;294(46):17188–96.
Cheng R, Zhang G, Bai Y, Zhang F, Zhang G. LncRNA SENCR promotes cell proliferation and progression in non-small-cell lung cancer cells via sponging miR-1-3p. Cell Cycle. 2021;20(14):1402–14.
Du B, Wang Z, Zhang X, Feng S, Wang G, He J, et al. MicroRNA-545 suppresses cell proliferation by targeting cyclin D1 and CDK4 in lung cancer cells. PLoS ONE. 2014;9(2): e88022.
Sun H, Han X, Zhong M, Yu D. Linc00703 suppresses non-small cell lung cancer progression by modulating cyclinD1/CDK4 expression. Eur Rev Med Pharmacol Sci. 2020;24(11):6131–8.
Zhang S, Wenjia X, Gaochao D, Weizhang X, Ming L, Lin X. Cyclic RNA molecule circ_0007766 promotes the proliferation of lung adenocarcinoma cells by up-regulating the expression of Cyclin D1/CyclinE1/CDK4. Zhongguo Fei Ai Za Zhi. 2019;22(5).
Lukoseviciute M, Maier H, Poulou-Sidiropoulou E, Rosendahl E, Holzhauser S, Dalianis T, et al. Targeting PI3K, FGFR, CDK4/6 signaling pathways together with cytostatics and radiotherapy in two medulloblastoma Cell lines. Front Oncol. 2021;11.
Zhao L, Chen T, Tang X, Li S, Liang R, Wang Y. Medulloblastoma malignant biological behaviors are associated with HOTAIR/miR-483–3p/CDK4 axis. Ann Transl Med. 2020;8(14):886.
Yang Y, Cui H, Wang X. Downregulation of EIF5A2 by miR-221-3p inhibits cell proliferation, promotes cell cycle arrest and apoptosis in medulloblastoma cells. Biosci Biotechnol Biochem. 2019;83(3):400–8.
AbuHammad S, Cullinane C, Martin C, Bacolas Z, Ward T, Chen H, et al. Regulation of PRMT5–MDM4 axis is critical in the response to CDK4/6 inhibitors in melanoma. Proc Natl Acad Sci. 2019;116(36):17990–8000.
Santiappillai NT, Abuhammad S, Slater A, Kirby L, McArthur GA, Sheppard KE, et al. CDK4/6 inhibition reprograms mitochondrial metabolism in BRAFV600 melanoma via a p53 dependent pathway. Cancers. 2021;13(3):524.
Posch C, Sanlorenzo M, Ma J, Kim ST, Zekhtser M, Ortiz-Urda S. MEK/CDK4, 6 co-targeting is effective in a subset of NRAS, BRAF and ‘wild type’melanomas. Oncotarget. 2018;9(79):34990.
Kollmann K, Briand C, Bellutti F, Schicher N, Blunder S, Zojer M, et al. The interplay of CDK4 and CDK6 in melanoma. Oncotarget. 2019;10(14):1346.
Teh JL, Erkes DA, Cheng PF, Tiago M, Wilski NA, Field CO, et al. Activation of CD8+ T cells contributes to anti-tumor effects of CDK4/6 inhibitors plus MEK inhibitors. Cancer Immunol Res. 2020;8(9):1114–21.
Hayes TK, Luo F, Cohen O, Goodale AB, Lee Y, Pantel S, et al. A functional landscape of resistance to MEK1/2 and CDK4/6 inhibition in NRAS-mutant melanoma. Can Res. 2019;79(9):2352–66.
Bian D, Wu Y, Song G. Novel circular RNA, hsa_circ_0025039 promotes cell growth, invasion and glucose metabolism in malignant melanoma via the miR-198/CDK4 axis. Biomed Pharmacother. 2018;108:165–76.
Georgantas RW III, Streicher K, Luo X, Greenlees L, Zhu W, Liu Z, et al. Micro RNA-206 induces G 1 arrest in melanoma by inhibition of CDK 4 and C yclin D. Pigment Cell Melanoma Res. 2014;27(2):275–86.
Zhang M, Zhao X, Cai X, Wang P, Yu M, Wei Z. Knockdown of long non-coding RNA plasmacytoma variant translocation 1 inhibits cell proliferation while promotes cell apoptosis via regulating miR-486-mediated CDK4 and BCAS2 in multiple myeloma. Ir J Med Sci (1971). 2020;189(3):825–34.
Cao Y, Shi X, Liu Y, Xu R, Ai Q. MicroRNA-338-3p inhibits proliferation and promotes apoptosis of multiple myeloma cells through targeting Cyclin-dependent kinase 4. Oncol Res. 2018;27(1):117.
Wu H, Xia L, Xu H. Role of FUS-CHOP in myxoid liposarcoma via miR-486/CDK4 axis. Biochem Genet. 2021:1–12.
Jiang Q, Zhang Y, Zhao M, Li Q, Chen R, Long X, et al. miR-16 induction after CDK4 knockdown is mediated by c-Myc suppression and inhibits cell growth as well as sensitizes nasopharyngeal carcinoma cells to chemotherapy. Tumor Biol. 2016;37(2):2425–33.
Lv LY, Wang YZ, Zhang Q, Zang HR, Wang XJ. miR-539 induces cell cycle arrest in nasopharyngeal carcinoma by targeting cyclin-dependent kinase 4. Cell Biochem Funct. 2015;33(8):534–40.
Wang C, Mao C, Lai Y, Cai Z, Chen W. MMP1 3′ UTR facilitates the proliferation and migration of human oral squamous cell carcinoma by sponging miR-188-5p to up-regulate SOX4 and CDK4. Mol Cell Biochem. 2021;476(2):785–96.
Kang Y, Zhang Y, Sun Y. MicroRNA-198 suppresses tumour growth and metastasis in oral squamous cell carcinoma by targeting CDK4. Int J Oncol. 2021;59(1):1–13.
Zhang W, Hong W. Upregulation of miR-519d-3p inhibits viability, proliferation, and G1/S cell cycle transition of oral squamous cell carcinoma cells through targeting CCND1. Cancer Biother Radiopharmac. 2020.
Shang A, Lu W-Y, Yang M, Zhou C, Zhang H, Cai Z-X, et al. miR-9 induces cell arrest and apoptosis of oral squamous cell carcinoma via CDK 4/6 pathway. Artif Cells Nanomed Biotechnol. 2018;46(8):1754–62.
Wang W-T, Qi Q, Zhao P, Li C-Y, Yin X-Y, Yan R-B. miR-590-3p is a novel microRNA which suppresses osteosarcoma progression by targeting SOX9. Biomed Pharmacother. 2018;107:1763–9.
Jia F, Zhang Z, Zhang X. MicroRNA-338-3p inhibits tumor growth and metastasis in osteosarcoma cells by targeting RUNX2/CDK4 and inhibition of MAPK pathway. J Cell Biochem. 2019;120(4):6420–30.
Cheng S, Zheng J, Liu X, Shi J, Gong F, Zhang X, et al. Knockdown of 91 H suppresses the tumorigenesis of osteosarcoma via inducing methylation of CDK4 promoter. Technol Cancer Res Treat. 2021;20:1533033821990006.
Zhang Q-F, Li J, Jiang K, Wang R, Ge J-L, Yang H, et al. CDK4/6 inhibition promotes immune infiltration in ovarian cancer and synergizes with PD-1 blockade in a B cell-dependent manner. Theranostics. 2020;10(23):10619.
Liu C, Huang Y, Cui Y, Zhou J, Qin X, Zhang L, et al. The immunological role of CDK4/6 and potential mechanism exploration in ovarian cancer. Front Immunol. 2021;12.
Ho C-M, Chang T-H, Yen T-L, Hong K-J, Huang S-H. Collagen type VI regulates the CDK4/6-p-Rb signaling pathway and promotes ovarian cancer invasiveness, stemness, and metastasis. Am J Cancer Res. 2021;11(3):668.
Liu G, Sun Y, Ji P, Li X, Cogdell D, Yang D, et al. MiR-506 suppresses proliferation and induces senescence by directly targeting the CDK4/6–FOXM1 axis in ovarian cancer. J Pathol. 2014;233(3):308–18.
Salvador-Barbero B, Álvarez-Fernández M, Zapatero-Solana E, El Bakkali A, del Camino MM, López-Casas PP, et al. CDK4/6 inhibitors impair recovery from cytotoxic chemotherapy in pancreatic adenocarcinoma. Cancer Cell. 2020;37(3):340-53. E6.
Zhang B, Li D, Jin X, Zhang K. The CDK4/6 inhibitor PD0332991 stabilizes FBP1 by repressing MAGED1 expression in pancreatic ductal adenocarcinoma. Int J Biochem Cell Biol. 2020;128: 105859.
Willobee BA, Gaidarski AA, Dosch AR, Castellanos JA, Dai X, Mehra S, et al. Combined blockade of MEK and CDK4/6 pathways induces senescence to improve survival in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2021;20(7):1246–56.
Huang F, Tang J, Zhuang X, Zhuang Y, Cheng W, Chen W, et al. MiR-196a promotes pancreatic cancer progression by targeting nuclear factor kappa-B-inhibitor alpha. PLoS ONE. 2014;9(2): e87897.
Wu C, Ma L, Wei H, Nie F, Ning J, Jiang T. MiR-1256 inhibits cell proliferation and cell cycle progression in papillary thyroid cancer by targeting 5-hydroxy tryptamine receptor 3A. Hum Cell. 2020;33(3):630–40.
Li S, Wang C, Yu X, Wu H, Hu J, Wang S, et al. miR-3619-5p inhibits prostate cancer cell growth by activating CDKN1A expression. Oncol Rep. 2017;37(1):241–8.
Wei W-R, Zeng G-J, Liu C, Zou B-W, Li L. Overexpression of miR-96 promotes cell proliferation by targeting FOXF2 in prostate cancer. Int J Clin Exp Pathol. 2017;10(7):7596.
Fu X, Wang D, Shu T, Cui D, Fu Q. LncRNA NR2F2-AS1 positively regulates CDK4 to promote cancer cell proliferation in prostate carcinoma. Aging Male. 2020;23(5):1073–9.
Shi X, Li H, Shi A, Yao H, Ke K, Dong C, et al. Discovery of rafoxanide as a dual CDK4/6 inhibitor for the treatment of skin cancer. Oncol Rep. 2018;40(3):1592–600.
Ohara M, Saito K, Kageyama K, Terai M, Cheng H, Aplin AE, et al. Dual targeting of CDK4/6 and cMET in metastatic uveal melanoma. Cancers. 2021;13(5):1104.
Teh JL, Purwin TJ, Han A, Chua V, Patel P, Baqai U, et al. Metabolic adaptations to MEK and CDK4/6 cotargeting in uveal melanoma. Mol Cancer Ther. 2020;19(8):1719–26.
Matsuo H, Nakatani K, Harata Y, Higashitani M, Ito Y, Inagami A, et al. Efficacy of a combination therapy targeting CDK4/6 and autophagy in a mouse xenograft model of t (8; 21) acute myeloid leukemia. Biochem Biophys Rep. 2021;27: 101099.
Sinclair WD, Cui X. The effects of HER2 on CDK4/6 activity in breast cancer. Clin Breast Cancer. 2022;22(3):e278–85.
Chen J, Wu W, He X, Jia L, Yang J, Si X, et al. Exosomal miR-122-5p is related to the degree of myelosuppression caused by chemotherapy in patients with colorectal cancer. Cancer Manage Res. 2021;13:8329.
Decker T, Seifert R, Bichler M, Birtel A, Fischer G, Nonnenbroich C, et al. Elective discontinuation of CDK4/6 inhibitors in patients with metastatic hormone receptor-positive, her-2-negative breast cancer: a retrospective single-center experience. Oncol Res Treat. 2021;44(9):443–9.
Han Y, Wang J, Wang Z, Xu B. Comparative efficacy and safety of CDK4/6 and PI3K/AKT/mTOR inhibitors in women with hormone receptor-positive, HER2-negative metastatic breast cancer: a systematic review and network meta-analysis. Curr Probl Cancer. 2020;44(6): 100606.
Lin M, Chen Y, Jin Y, Hu X, Zhang J. Comparative overall survival of CDK4/6 inhibitors plus endocrine therapy vs. endocrine therapy alone for hormone receptor-positive, HER2-negative metastatic breast cancer. J Cancer. 2020;11(24):7127.
Collins JM, Nordstrom BL, McLaurin KK, Dalvi TB, McCutcheon SC, Bennett JC, et al. A real-world evidence study of CDK4/6 inhibitor treatment patterns and outcomes in metastatic breast cancer by germline BRCA mutation status. Oncol Ther. 2021;9(2):575–89.
Palleschi M, Maltoni R, Ravaioli S, Vagheggini A, Mannozzi F, Fanini F, et al. Ki67 and PR in patients treated with CDK4/6 inhibitors: a real-world experience. Diagnostics. 2020;10(8):573.
Cook MM, Al Rabadi L, Kaempf AJ, Saraceni MM, Savin MA, Mitri ZI. Everolimus plus exemestane treatment in patients with metastatic hormone receptor-positive breast cancer previously treated with CDK4/6 inhibitor therapy. Oncologist. 2021;26(2):101–6.
Ding W, Li Z, Wang C, Ruan G, Chen L, Tu C. The CDK4/6 inhibitor in HR-positive advanced breast cancer: a systematic review and meta-analysis. Medicine. 2018;97(20):e10746.
Nguyen LV, Searle K, Jerzak KJ. Central nervous system-specific efficacy of CDK4/6 inhibitors in randomized controlled trials for metastatic breast cancer. Oncotarget. 2019;10(59):6317.
Del Re M, Crucitta S, Lorenzini G, De Angelis C, Diodati L, Cavallero D, et al. PI3K mutations detected in liquid biopsy are associated to reduced sensitivity to CDK4/6 inhibitors in metastatic breast cancer patients. Pharmacol Res. 2021;163: 105241.
Desnoyers A, Nadler MB, Kumar V, Saleh R, Amir E. Comparison of treatment-related adverse events of different Cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer: a network meta-analysis. Cancer Treat Rev. 2020;90: 102086.
Daniell KM, Bardia A, Sun F, Roberts SA, Brunelle CL, Gillespie TC, et al. Incidence of peripheral edema in patients receiving PI3K/mTOR/CDK4/6 inhibitors for metastatic breast cancer. Breast Cancer Res Treat. 2019;175(3):649–58.
Acknowledgements
The authors would like to thank the clinical Research Development Unit (CRDU) of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran for their support, cooperation and assistance throughout the period of study.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MT and AB designed and supervised the study. SGF and NG wrote the draft and revised it. TK, NAD, BMH and PD collected the data and designed the figures and tables. All the authors read the submitted version and approved it.
Corresponding authors
Ethics declarations
Ethics approval and consent to Participant
Not applicable.
Consent of publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ghafouri-Fard, S., Khoshbakht, T., Hussen, B.M. et al. A review on the role of cyclin dependent kinases in cancers. Cancer Cell Int 22, 325 (2022). https://doi.org/10.1186/s12935-022-02747-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02747-z