Abstract
Microbial cell factories serve as pivotal platforms for the production of high-value natural products, which tend to accumulate on the cell membrane due to their hydrophobic properties. However, the limited space of the cell membrane presents a bottleneck for the accumulation of these products. To enhance the production of intracellular natural products and alleviate the burden on the cell membrane caused by product accumulation, researchers have implemented various membrane engineering strategies. These strategies involve modifying the membrane components and structures of microbial cell factories to achieve efficient accumulation of target products. This review summarizes recent advances in the application of membrane engineering technologies in microbial cell factories, providing case studies involving Escherichia coli and yeast. Through these strategies, researchers have not only improved the tolerance of cells but also optimized intracellular storage space, significantly enhancing the production efficiency of natural products. This article aims to provide scientific evidence and references for further enhancing the efficiency of similar cell factories.
Similar content being viewed by others
Introduction
Microbial cell factories adopt cost-effective and environmentally friendly strategies, utilizing inexpensive renewable resources to produce a wide range of high-value compounds, including fine chemicals, biofuels, natural products, and pharmaceuticals [1,2,3,4]. With the growing demand for enhanced performance of cell factories, the academic community has undertaken extensive and in-depth research efforts. Advanced technologies such as gene editing [5,6,7,8], protein engineering [9,10,11], and dynamic genetic circuits [10,11,12] have been widely applied in the construction and performance optimization of microbial cell factories. Although traditional synthetic biology strategies focus on the construction and optimization of metabolic pathways, such as strengthening central and major metabolic pathways [13,14,15], improving the supply and turnover of cofactors [16, 17], removing competitive metabolic bypaths, and enhancing metabolic flow [18, 19], these methods have shown significant limitations in increasing the yield of many hydrophobic natural products (e.g., steroids, terpenoids, alkaloids, and liposoluble vitamins). In particular, many hydrophobic natural products tend to accumulate in biological membrane structures rather than being secreted into the culture medium, a tendency that, due to the limited space of the cell membrane structure, restricts the effective accumulation of target chemicals. Furthermore, excessive accumulation of these hydrophobic natural products on membrane structures may interfere with the normal physiological functions of the host cells, causing cytotoxicity, thereby worsening the robustness and productive efficiency of the strains. Therefore, engineering the membrane structure of microbial chassis cells to improve cell tolerance and optimize intracellular storage space is crucial for enhancing the capability of microbial cell factories to produce natural products. In recent years, cell membrane engineering, as a strategy for optimizing the performance of cell factories, has gradually attracted widespread attention in the academic community and has led to several successful modification cases. This article reviews the progress of membrane engineering technology research for prokaryotic and eukaryotic microbial cell factories in recent years, summarizes the advantages and limitations of different strategies, and looks forward to new membrane engineering strategies and their potential applications.
Applications of membrane engineering in prokaryotic cell factories: the case of Escherichia coli
Escherichia coli, as a typical representative of prokaryotic cell factories and a member of the Gram-negative bacteria, possesses a cell envelope structure composed of an outer membrane, periplasmic space, and inner membrane. In its natural state, the cell membrane of E. coli is intact, smooth, and evenly distributed. However, according to existing literature, the morphology of the cell membrane can be altered by overexpressing certain endogenous membrane proteins in E. coli. Specifically, the induced expression of ATP synthase (Atp) can induce the formation of membrane cisterns and vesicle structures within the cell [20]; whereas the induction of mannitol permease (MtlA) expression can cause membrane stacking in the vicinity of the inner membrane [21]. Moreover, the induced expression of chemotaxis receptor (Tsr) and sn-glycerol-3-phosphate acyltransferase (PlsB) can promote the formation of a large number of intracellular tubular structure, effectively changing the morphology of the cell membrane [22, 23]. Research by Eriksson et al. further demonstrated that overexpressing the membrane-bound 1,2-diacylglycerol 3-glucosyltransferase from Acholeplasma laidlawii (AlMGS) in E. coli significantly increases the number of intracellular membrane vesicles, an effect far surpassing that of E. coli’s endogenous proteins with similar functions [24, 25]. This finding makes it possible to expand the cell membrane area. Although there is a wide variety of engineering modification strategies for bacterial chassis currently available, the productive performance of chassis strains can still be negatively affected during the fermentation process due to feedback inhibition by products and the accumulation of metabolic by-products [26, 27]. Studies have shown that the toxicity of products is a major factor leading to cell membrane rupture. Therefore, regulating and remodeling the components of the cell membrane to enhance its stability and improve the robustness of the strains has become an important hot topic in current research. The following Table 1 and Fig. 1 summarize main strategies for the engineering modification of E. coli membranes to enhance strain tolerance and improve the performance of cell factories.
Membrane lipid composition engineering
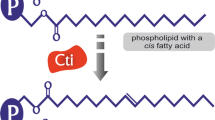
By employing adaptive evolution strategies for the modification of E. coli strains, significant improvements can be made in their growth performance under adverse environmental conditions. Research by Royce et al. revealed that short-term metabolic evolution of E. coli in an octanoic acid environment can increase the ratio of unsaturated to saturated fatty acids (U/S) and the number of acyl chains, thereby reducing the hydrophobicity of the cell surface. In contrast, strains that had not undergone octanoic acid adaptive modification showed increased membrane fluidity and decreased tolerance to the product octanoic acid [28]. Another study has shown that by using evolutionary methods to increase E. coli’s tolerance to exogenous octanoic acid, the evolved strain LAR1, as compared to its parental strain ML115, not only acquired broader tolerance to alcohols and carboxylic acids (including octanoic acid, hexanoic acid, decanoic acid, n-butanol, and isobutanol) but also produced carboxylic acids at a titer 5 times higher than its parent [29]. The acquisition of these evolutionary traits is associated with changes in the membrane phospholipid composition; for example, LAR1 showed a significantly lower saturated:unsaturated (S:U) ratio, decreased C12/C16 and C14/C16 ratios, and an increased average membrane length relative to its parent. Furthermore, Minty et al. [30] reported that an evolved mutant strain, EcNR1 G3.2, which exhibited high fitness under exogenous isobutanol stress, contains mutations in numerous genes and regulators associated with the cell envelope. These include genes for components of the Sec apparatus such as secA and lepB, the membrane proteins regulator hfq, genes related to LPS biosynthesis like fepE and yjgQ, and the regulator of various LPS modification genes phoPQ. Compared to the wild-type, the G3.2 mutant exhibits a significant reduction in cyclopropane fatty acid content, a significant increase in the overall unsaturated/ saturated fatty acid ratio, and an overall increase in cell envelope proteins. Cyclopropane fatty acids (CFAs), as an important component of microbial fatty acids in organisms such as E. coli, play a crucial role in maintaining membrane lipid homeostasis and improving strain acid tolerance [31, 32]. Royce et al. through transcriptome analysis, found that under the stress of octanoic acid, isobutanol, and ethanol, the expression of the cfa gene, which encodes for the CFA synthase, was downregulated. Overexpression of the cfa gene derived from the butanol-tolerant strain Enterococcus faecalis in E. coli significantly increased the content of CFAs in the membrane, thereby improving the growth status and octanoic acid tolerance of the recombinant strain [33]. In most Gram-negative bacteria, the cytoplasmic cis–trans isomerase can convert unsaturated fatty acids from cis to trans under stress conditions, to maintain cell membrane fluidity. Tan et al. have indicated that overexpressing the cis–trans isomerase Cti from Pseudomonas aeruginosa in E. coli can enhance the rigidity of the cell membrane, reduce its fluidity, and thus increase E. coli’s tolerance and production of octanoic acid [34]. Furthermore, the expression of the cti gene also improves the strain’s tolerance to physical stresses such as low pH, osmotic pressure, high temperature, and to chemical stresses such as short-chain fatty acids, ethanol, organic acids, and aromatic compounds. These results indicate that modifications to the unsaturated fatty acid components in the membrane can affect its fluidity, thereby influencing chassis cells’ tolerance to bulk chemicals. Moreover, the stress tolerance of E. coli can be improved by adjusting the length, number, and conformation of double bonds and rings in the hydrophobic tails of fatty acids within phospholipids [35,36,37]. Phosphatidylserine decarboxylase plays a crucial role in the composition of cell membrane phospholipids. In the research conducted by Tan et al. [38], the overexpression of phosphatidylserine decarboxylase (pssA) resulted in an increased concentration of phosphatidylethanolamine (PE) within the cell membrane phospholipids. Given that the head group of phosphatidylethanolamine has a stronger polarity compared to that of phosphatidylglycerol (PG) and cardiolipin (CL), this led to a decrease in the hydrophobicity of the bacterial surface. Additionally, the composition of the fatty acid tails was also altered. These modifications not only enhanced the production of and tolerance to octanoic acid in E. coli but also increased the strain’s resistance to unfavorable conditions such as acetate, furan, toluene, ethanol, and low pH levels. Research by Wu et al. [39] demonstrated that by combining the knockout of cardiolipin synthesis-related genes clsA, clsB, and clsC with the enhancement of the Regulator of capsule synthesis (RCS) phosphorelay system, the production of colonic acid in E. coli MG1655 was increased by 2.48-fold. These results suggest that altering membrane composition components can effectively confer a strain’s tolerance to chemicals in the environment, providing a theoretical basis for the targeted development of efficient cell factories and the enhancement of production in the future.
Membrane protein engineering
Membrane proteins comprise a range of proteins that perform various critical functions, such as transporters, receptors, enzymes, anchors, and cell adhesion molecules. The homeostasis of these proteins, to some extent, affects the homeostasis of the cell membrane. For example, transporters facilitate the movement of substances across cell membranes. Thus, introducing or enhancing the function of transporters can help reduce the accumulation of toxic products on the cell membrane, increase the robustness of chassis cells, and ultimately improve the productive capacity of cell factories. Korosh et al. [40] incorporated a lysine transport protein (encoded by the ybjE gene) into lysine-producing cells of Synechococcus sp. PCC 7002. This modification resulted in a 1.6-fold increase in the yield per unit biomass compared to the original strain and prevented intracellular lysine accumulation, which is associated with reduced fitness. Wu et al. [41] utilized an autoregulatory system in E. coli chassis to regulate the expression of an active aromatic transporter CouP, enabling it to efficiently transport vanillin across the cell membrane and convert it to catechol. This modification resulted in a 30–40% increase in catechol production compared to the starting strain. Additionally, modulating membrane protein homeostasis through specific reagents to affect membrane permeability is also one of the strategies for increasing production in cell factories. For example, Ca2+ within a physiological concentration range is sufficient to induce clustering of membrane proteins in the plasma membrane through electrostatic interactions [42], and sodium dodecyl sulfate (SDS) acts as a very effective solubilizing agent for a wide range of polypeptides, including membrane proteins. In one case, Chen et al. [43] enhanced cell membrane permeability by adding 0.2 g/dL of CaCl2 and surfactants such as SDS to the culture medium, combined with strategies to boost the supply of key precursors like histidine and methionine, successfully increasing the capacity of E. coli to synthesize unusual sulfur-containing amino acid ergothioneine, reaching a production titer of 243.06 mg/L. Furthermore, regulating the transport of membrane proteins can profoundly affect their homeostasis, which can then be utilized to enhance the production capacity of cell factories. For example, the E. coli soluble protein SecB has been reported involved in the export of periplasmic and outer membrane proteins, and purified SecB has been shown to stimulate protein translocation across E. coli inner membrane vesicles in vitro [44]. Xu et al. [45] utilized protein engineering to create 2800 random mutants of the SecB protein and identified the SecBT10A mutant as having the highest tolerance to n-butanol. This modification led to a 5.3-fold increase in n-butanol tolerance in the E. coli chassis, resulting in n-butanol production reaching 14.58 g/L.
Membrane morphology engineering
Increasing the surface area of the cell membrane is the most direct way to enhance the carrying capacity for hydrophobic compounds. In our prior research, the heterologous expression of AlMGS protein led to an observed increase in cell membrane surface wrinkling [46, 47]. This not only enlarged the surface area of the cell membrane but also provided extra storage space for products such as β-carotene and lycopene, significantly boosting their production levels. Additionally, our study examined the effects of enhancing the cell membrane synthesis pathway on the morphology of carotenoid-producing chassis cells and their capacity for intracellular storage. We found that enhancing the expression of proteins plsB and plsC, which are directly involved in the synthesis of phosphatidylethanolamine, promoted cell membrane synthesis, markedly increasing product yield compared to the initial strain CAR015. By integrating these strategies, a synergistic effect was found in enhancing cell factory performance. Implementing these strategies in the β-carotenoid high-producing strain CAR025 under shake-flask fermentation conditions led to a 39% increase in per unit biomass yield, a strategy applicable to lycopene cell factories as well. Moreover, Yang et al. [48] have reported the implementation of integrated membrane engineering strategies to augment the production of three types of carotenoids and four derivatives of violacein. They modified cell morphology by silencing genes involved in cell division or cell wall metabolism, thereby enlarging the membrane surface area to enhance the accumulation space for hydrophobic products. After fermentation optimization, production of rainbow colorants are significantly enhanced to 322 mg/L of astaxanthin (red), 343 mg/L of β-carotene (orange), 218 mg/L of zeaxanthin (yellow), 1.42 g/L of proviolacein (green), 0.844 g/L of prodeoxyviolacein (blue), 6.19 g/L of violacein (navy), and 11.26 g/L of deoxyviolacein (purple). Additionally, reshaping the morphology of E. coli has also been successfully used to improve the performance of poly-3-hydroxybutyrate (PHB) cell factories. To expand the internal space of cells and mitigate the effects of certain inhibitors on the cell wall, Zhang et al. [49] inhibited enzymes related to cell wall synthesis, such as murD and murE. This flexibility in cell shape increased the possibility for cell size expansion, providing more space for PHB storage, with PHB accumulation reaching 93% of the cell dry weight. Furthermore, to increase cell volume, Elhadi et al. [50] employed CRISPRi technology to suppress the expression of the tubulin protein ftsZ and the cytoskeletal protein mreB, which are associated with cell differentiation and peptidoglycan synthesis, to balance cell growth and morphology regulation. Ultimately, depending on the level of suppression of ftsZ and mreB, an increase in cell volume was observed to various extents. The PHB production by the mutant increased by 71% compared to the control.
Out membrane and associated vesicles engineering
The outer membrane of E. coli consists of various components, including peptidoglycan (PG), lipopolysaccharides (LPS), colanic acid (CA), and outer membrane proteins (Omps), among others. Lipopolysaccharides play a dominant role in the outer layer of the outer membrane in Gram-negative bacteria and are crucial for the formation of other molecules on the outer membrane [51, 52], including flagella, CA, and Omps [53, 54]. It is estimated that each E. coli cell contains approximately 106 LPS molecules and 107 glycerophospholipid molecules [51, 55]. The vast majority of life forms, including eukaryotes, archaea, and bacteria, are capable of producing membrane-bound cellular structures, such as membrane vesicles, microvesicles, exosomes, and virus-like particles [56]. During normal growth, most Gram-negative bacteria naturally secrete Outer Membrane Vesicles (OMVs), which are nanoscale structures ranging from 20 to 250 nm in diameter, formed by the shedding from the cell wall and outward bulging of the outer membrane. Although the exact mechanisms of OMV formation are not fully understood, it is hypothesized that they may arise through three main potential mechanisms: (1) weakened cross-linking interactions between the peptidoglycan layer and the outer membrane; (2) accumulation of peptidoglycan fragments and misfolded proteins in the cytoplasm exerting pressure on the outer membrane, prompting membrane budding and vesicle formation; (3) enrichment of membrane curvature-inducing molecules such as B-band lipopolysaccharide and Pseudomonas quinolone signal (PQS) [57,58,59,60]. Typically, signals inducing OMV formation are closely related to environmental stress encountered by the bacteria, such as temperature stress, amino acid deprivation, phage or antibiotic attack [61, 62]. Additionally, certain culture medium components can also promote OMV production; for instance, hexadecane can induce the production of OMVs in Acinetobacter, while Yersinia spp. can produce outer membrane vesicles under glucose stimulation [63, 64]. OMV formation is also related to the cell’s growth cycle, with organisms like Neisseria meningitidis and Francisella primarily secreting OMVs during the late growth phase, whereas Borrelia burgdorferi and Gallibacterium anatis secrete OMVs during the logarithmic growth phase, and Legionella pneumophila can produce OMVs at various stages of cell growth [65,66,67]. Thus, Gram-negative bacteria can continuously produce OMVs at all stages of growth, and external stimuli can enhance OMV production. Naturally formed OMVs play significant physiological roles, such as reducing the intracellular concentration of organic toxins like toluene, enhancing microbial survival in harsh environments [68]; neutralizing antimicrobial peptides [69]; aiding phage release [70, 71]; removing substances that could burden the cell (such as unfolded cytoplasmic proteins); and facilitating the nucleation process during biofilm formation [72, 73]. Moreover, outer membrane vesicles released from the cell walls of pathogenic bacteria play a key role in the interaction between host and pathogen, including mediating the entry of pathogenic components into host cells and modulating the host’s defense and response capabilities [74,75,76]. As crucial components of the biofilm, OMVs play a vital role in intercellular communication and nutrient acquisition [77, 78].
Endogenous vesicle transport systems play a critical role in improving the transport process of hydrophobic compounds from organelles to the plasma membrane. In recent years, the academic community has delved into strategies for constructing artificial transport systems aimed at facilitating the extracellular transport of intracellular products, thereby indirectly enhancing the continuous production of these products while mitigating adverse effects on the cells. Our previous research focused on increasing the production of carotenoid-producing E. coli by targeting key genes involved in the formation of Outer Membrane Vesicles (OMVs), including those in the Tol-Pal complex (TolR, TolA), affecting the expression and localization of outer membrane proteins (nlpI), and an outer membrane protein (OmpF), to construct an Artificial Membrane Vesicle Transport System (AMVTS) [79]. Our findings indicated that the individual knockout of either tolR or nlpI genes significantly increased β-carotene production. Simultaneous knockout of tolR and nlpI resulted in a 35.6% increase in β-carotene production compared to the starting strain. This may be due to the disruption of these genes affecting the facilitation or integrity of the outer membrane, enhancing the formation of OMVs, and thus enabling the transport of more products through the formed OMVs. Additionally, the work by Yang et al. [48] on enhancing the production of rainbow colorants also employed vesicle engineering strategies. They augmented the generation of inner-membrane vesicles (IMVs) and outer-membrane vesicles (OMVs) within the E. coli chassis cells. The production of these vesicular structures was facilitated by the introduction of the cav1 gene, encoding human caveolin-1 for IMV formation, and the silencing of genes implicated in the formation of OMVs, respectively. Phospholipids, as major components of the outer membrane, including 5% cardiolipin (CL), 70%-80% phosphatidylethanolamine (PE), and 20–25% phosphatidylglycerol (PG), play a crucial role in maintaining the normal function of the outer membrane [80, 81]. In our previous AMVTS research, we also introduced a phospholipid synthesis module to enhance membrane phospholipid synthesis in strains with tolR and nlpI knocked out, thereby exploring its impact on the strain’s efflux capability. The results showed that the carotenoid artificial transport system constructed significantly improved the transport capability of carotenoids in carotenoid-producing chassis, further promoting the intracellular production of carotenoids. This strategy resulted in a 61% increase in yield for CAR025. Similarly, Fordjour et al. [82], by knocking out genes such as TolA-TolQ TolR, successfully constructed a vesicle system in E. coli, thereby improving cell membrane permeability and increasing lycopene production. In application cases aimed at enhancing the PHB production of cell factories, Wang et al. through genomic and transcriptomic analysis, studied flagella assembly in the wild-type E. coli W3110 strain and mutant strains DwaaF, DwaaC, and DwaaG, which only synthesize lipopolysaccharides of varying lengths, concluding that flagella assembly in E. coli depends on the length of lipopolysaccharides; by disrupting all gene clusters related to the polysaccharide part of lipopolysaccharides (LPS), colanic acid (CA), flagella, and/or fimbriae, the outer membrane of E. coli W3110 was successfully modified, creating favorable conditions for PHB production [53, 83]. In addition, Zhang et al. found that disrupting membrane integrity or altering the rigidity of the outer membrane could significantly enhance the PHB production yield in E. coli JM109 [49]. In summary, targeted modifications of the outer membrane of Escherichia coli and the development of artificial vesicle transport systems have proven to be effective strategies for enhancing the production of specific hydrophobic products in recent years, broadening the approach to the development of synthetic biology tools.
Applications of membrane engineering in eukaryotic cell factories: the case of yeast hosts
Eukaryotic cells show significant distinctions from prokaryotic cells in membrane phospholipid composition. The complexity of eukaryotic cell membranes is higher, featuring the evolution of steroid molecules, viewed as an adaptation to rising levels of oxygen in Earth’s atmosphere [84]. Yeast cells, for example, manage their membrane fluidity and stability by producing ergosterol, indicating fungi’s evolutionary adjustment to environmental shifts [85]. Eukaryotic cell membranes consist of a broad array of glycerophospholipids, including phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, phosphatidylinositol, and phosphatidic acid. Phosphatidylcholine, making up more than 50% of the phospholipids in most eukaryotic cell membranes, underscores its crucial role in membrane structure and functionality [86]. Moreover, eukaryotes have a sophisticated internal membrane system, encompassing organelles with internal membranes such as mitochondria, endoplasmic reticulum, and Golgi apparatus, with the endoplasmic reticulum serving as the main site for lipid synthesis [87]. The mammalian Golgi apparatus not only synthesizes sphingolipids but also contributes to the production of complex lipids like sphingomyelin (SM), glucosylceramide (GlcCer), lactosylceramide (LacCer), and more structured glycosphingolipids [88]. The asymmetric distribution and transfer of lipids, causing lipid imbalance, are vital for membrane curvature induction and vesicle budding, crucial for packaging and transporting cellular materials [67, 89]. Diverse membrane systems in eukaryotes showcase variation in composition and transport mechanisms. Even within the same type of organelle, compositional variances can be observed across different eukaryotic levels, for instance, in cardiolipin content between mammalian and yeast mitochondria. Such differences in organelle membrane architecture significantly impact the synthesis and storage efficiency of heterologous natural products in microbial systems, mirroring eukaryotic cells’ evolutionary responses and adaptations to environmental challenges. Table 2 and Fig. 2 outlines strategies for enhancing cell factory performance via yeast membrane engineering alterations.
Cell membrane engineering
Rodríguez et al. [90] expressed two sunflower (Helianthus annuus) oleic acid delta-12 desaturases, encoded by FAD2-1 and FAD2-3, in the wild-type yeast strain W303-1A (trp1) and analyzed their effects on growth and stress tolerance. The result showed that by adjusting the concentration of monounsaturated fatty acids in Saccharomyces cerevisiae and converting them to dienoic acids, it is possible to synergistically enhance the fluidity of the cell membrane, thereby improving the yeast strain’s tolerance to freezing stress and NaCl stress. The engineered strain overexpressing FAD2 showed a survival rate of 90% after 35 days at 20 °C, compared to only 50% for the wild-type strain. Zhu et al. [91] revealed that sphingolipid acyl chain elongase (ELO2) is related to osmotic tolerance. Overexpression of ELO2 in Saccharomyces cerevisiae increases the content of long-chain fatty acids in sphingolipid, thereby enhancing the integrity of cell membrane and the cell’s tolerance to osmotic pressure. Addressing the problem of decreased membrane fluidity and insufficient secretion efficiency caused by β-carotene, Bu and others intervened by overexpressing the stearoyl-CoA desaturase (OLE1) [92]. Under these conditions, cell membrane fluidity was enhanced, and the secretion capacity for β-carotene was improved. Compared to the starting strain YBX-01, the extracellular secretion and intracellular production of β-carotene by strain YBX-20 increased by 5.80 and 1.71 times, respectively. Similarly, to alleviate the cytotoxicity of lycopene, Hong et al. [93] increased the content of unsaturated fatty acids in the cell membrane and enhanced cell protection and product yield by overexpressing the stearoyl-CoA desaturase encoded by OLE1 and the transcription factor encoded by STB5 involved in NADPH generation. Compared to the starting strain, the production of lycopene was successfully increased by 74.6 times. Chen et al. [27] by modifying the membrane composition of S. cerevisiae, including regulating stress response factors and synthesizing sterols and phospholipids-related factors (such as INO2) to enhance lycopene production, increased the lycopene yield of the engineered strain by 22 times compared to the starting strain.
Lipid droplet engineering
Lipid droplets (LDs) are spherical organelles surrounded by a monolayer of phospholipids, with over 200 different structural and functional proteins localized on their surface, such as acyl-CoA diacylglycerol acyltransferase (DGAT) and Perilipin, and a core of neutral lipids like triacylglycerols (TAGs), which are stored in lipid droplets mainly in the form of lipid esters. LDs serve as storage sites for lipophilic compounds. Matthäus et al. [94] found that by enhancing the formation of LDs in Y. lipolytica and overexpressing the rate-limiting genes for isoprenoid biosynthesis, GGS1 and HMG1, the cell’s capacity to store lycopene was improved, subsequently increasing lycopene synthesis with a yield reaching 16 mg/g of dry cell weight (DCW). Similarly, by overexpressing diacylglycerol acyltransferase (DGA1) in S. cerevisiae to increase its intracellular storage capacity and enhancing the expression of the key mevalonate (MVA) pathway gene acetyl-CoA carboxylase (ACC1), the engineered strain’s fermentation yield of α-amyrin was increased by 106 times compared to the wild-type strain [95]. Additionally, by overexpressing the fatty acid desaturase (OLE1) in S. cerevisiae and knocking out the seipin gene (FLD1), which regulates LDs size, lycopene production reached 73.3 mg/g DCW [96]. Xia et al. [97], by regulating the expression of triglycerides in LDs and the ERG9 protein, achieved yeast cell β-carotene production of 11.4 mg/g DCW and 142 mg/L, which was 107.3% and 49.5% higher than the original strain, respectively, thereby alleviating the stress on the cell membrane caused by β-carotene accumulation. Lin et al. [98] developed a protein scaffold system to organize and bring together multiple enzymes involved in yeast ester biosynthesis on the surface of intracellular LDs. This setup mimics the cell’s natural organization, aiming to improve metabolic pathways that can become inefficient when such organization is lost. By using the plant protein oleosin and cohesin-dockerin interaction pairs, they managed to gather the enzymes near the location of the key enzyme, alcohol-o-acetyltransferase (Atf1), which is crucial for the process. This close arrangement of enzymes boosted the efficiency of the ester biosynthesis pathway. By testing different scaffold designs and levels of pathway expression, they found a setup that doubled the production rate of ethyl acetate compared to the unorganized pathway. This approach shows promise for optimizing other metabolic pathways, especially those involving reactions on lipid droplets or membranes. Shi et al. [99] co-localized protopanaxadiol synthase (PPDS), involved in ginsenoside biosynthesis, with its substrate dammarenediol II in LDs, enhancing the conversion rate of dammarenediol II to protopanaxadiol in yeast by 3.9 times to 86%.
Intracellular membrane engineering
Due to the imbalance between the protein synthesis load on the endoplasmic reticulum (ER) and its folding capacity triggering the unfolded protein response, normalization of ER function necessitates size adjustments. In the research conducted by Kim et al. [100], a significant increase in squalene production was achieved, reaching 634 mg/L, which was a 71-fold increase compared to the control group, by combining ER expansion with other metabolic engineering strategies. Liu et al. [101] expanded the ER in S. cerevisiae by knocking out the phosphatidic acid phosphatase PAH1 gene and overexpressing the transcription factor INO2, thereby increasing the production of soyasaponin by 20% compared to the control strain. Similarly, Arendt et al. [102] knocked out PAH1 gene in S. cerevisiae using CRISPR-Cas9, the ER membrane was expanded and the production of β-amyrin, medicagenic acid, and medicagenic-28-o-glucoside was increased 8 times, 6 times and 16 times compared with the original strain, respectively. In the work of Zhang et al. [103], the DHCR7 from Danio rerio and POX2 were overexpressed in Y. lipolytica for building planticized membrane, resulting the production of campesterol to 942 mg/L in 5L bioreactor. Similarly, Qian et al.[104] deleted ERG5 and overexpressed of two copies of dhcr7 in Y. lipolytica, the campesterol content reached 837 mg/L, which was 3.7-fold compared with the initial strain. Furthermore, Guerfal et al. [105] increased the production of intracellular membranes and the expression of membrane proteins in yeast by interfering with key enzymatic steps in lipid synthesis; by modulating lipid synthesis and cell morphology, the production of the triterpenoid compound lupeol in Y. lipolytica was optimized, significantly enhancing yield through this strategy.
Conclusion and future perspectives
Against the backdrop of rapid developments in synthetic biology and systems biology, microbial cell factories have shown immense application potential in producing high-value hydrophobic compounds. Membrane engineering, as a key technology, aims to enhance the production capacity and tolerance of cells towards these compounds by finely regulating microbial membrane structure and function, and has achieved significant results in optimizing the performance of microbial cell factories. Various engineering tools, mainly including genome editing (CRISPR-Cas9, CRISPRi) [49, 50, 101], adaptive laboratory evolution (ALE) [29, 33, 90, 91], pathway engineering (gene fine-tuning, multiple genomic integrations) [27, 96, 103] have been employed to achieve the goals of membrane engineering. The application of transcending membrane barriers is manifested not only in enhancing strain tolerance and robustness to improve the production efficiency of cell factories but also involves optimizing the accumulation and storage of target products on cell membranes to alleviate the cellular burden and stress caused by excessive accumulation.
However, although current research has demonstrated that strategies such as modifying cell membrane protein expression, adjusting lipid composition, and reshaping membrane structure can significantly enhance the efficiency of product production, these modification strategies still face numerous challenges in practical applications. Firstly, the biology of cell membranes is extremely complex, and minor changes in membrane composition can have profound effects on the cell’s overall function and stability. Thus, how to precisely regulate the modification of cell membranes to achieve a balance between increasing the production of hydrophobic compounds without disturbing the cell’s normal physiological functions represents a significant challenge in current research. Additionally, different hydrophobic compounds have varying effects on cell membranes, requiring personalized membrane engineering designs and optimizations for each target product, increasing the complexity and difficulty of research. Secondly, although membrane engineering modifications have improved microbial cell tolerance to hydrophobic compounds, efficient secretion of the products remains a challenge. For many hydrophobic compounds, their production efficiency is limited due to the toxicity caused by accumulation within the cell and the inefficiency of traditional secretion pathways. Therefore, designing new efficient secretion systems to facilitate the transport of hydrophobic compounds from the cell to the external environment is a key direction for the future development of membrane engineering. Moreover, scaling up from laboratory to industrial-scale production poses another significant challenge. Membrane engineering strategies validated under laboratory conditions are often difficult to apply directly to large-scale production processes. This is not only because the physical and chemical conditions during scaling up may affect the production performance of the modified cells but also because factors such as cost-effectiveness, production stability, and environmental sustainability need to be considered in large-scale production processes. Therefore, successfully translating membrane engineering techniques from the laboratory to industrial applications requires further research and innovation.
Facing these challenges, future research needs to delve into multiple areas (Fig. 3). On one hand, building on previous studies on regulating cell membrane synthesis pathways for the accumulation and storage of hydrophobic compounds like carotenoids, it is deemed necessary to conduct more comprehensive and in-depth studies on cell membrane components, especially the synthesis pathways of central phospholipids and phosphatidylglycerol. By more detailed single-gene or multi-gene regulation of different cell membrane component synthesis pathways and establishing the connection between key genes regulating membrane component synthesis pathways and the production of hydrophobic compounds, it might reveal mechanisms of hydrophobic compound accumulation on cell membranes and identify potentially relevant structures associated with cellular synthesis pathways capable of storing hydrophobic compounds, thereby finding more effective methods to improve the intracellular production, accumulation, and storage of hydrophobic compounds. On the other hand, developing new efficient secretion systems to facilitate the effective secretion of hydrophobic compounds will be key to improving production efficiency. Additionally, given the differences in cell membrane components and their proportions between prokaryotic and eukaryotic cells, which may affect the efficacy of the same compound in two types of cell factories differently, it is particularly important to deeply study the cell membrane synthesis pathways of prokaryotic and eukaryotic chassis cells, combined with a detailed analysis and discussion on the structural characteristics and synthetic properties of target compounds.
In summary, although the application of membrane engineering in microbial cell factories faces challenges, with the continuous advancement of science and technology and the deepening of interdisciplinary collaboration, its prospects in the field of biomanufacturing remain very broad. Through in-depth research and technological innovation, the future will enable more efficient, stable, and environmentally friendly biological production of hydrophobic natural products, making a greater contribution to the sustainable development of human society.
Data availability
No datasets were generated or analysed during the current study.
References
Choi KR, Jang WD, Yang D, Cho JS, Park D, Lee SY. Systems metabolic engineering strategies: integrating systems and synthetic biology with metabolic engineering. Trends Biotechnol. 2019;37(8):817–37.
Lee SY, Kim HU, Chae TU, Cho JS, Kim JW, Shin JH, Kim DI, Ko Y-S, Jang WD, Jang Y-S. A comprehensive metabolic map for production of bio-based chemicals. Nat Catal. 2019;2:18–33.
Luo ZW, Ahn JH, Chae TU, Choi SY, Park SY, Choi Y, Kim J, Prabowo CPS, Lee JA, Yang D, et al. Metabolic engineering of Escherichia coli. Metab Eng. 2021;13:339–402.
Yang D, Prabowo CPS, Eun H, Park SY, Cho I, Jiao S, Lee SY. Escherichia coli as a platform microbial host for systems metabolic engineering. Essays Biochem. 2021;65(2):225–46.
Cho JS, Choi KR, Prabowo CPS, Shin JH, Yang D, Jang J, Lee SY. CRISPR/Cas9-coupled recombineering for metabolic engineering of Corynebacterium glutamicum. Metab Eng. 2017;42:157–67.
Yan M-Y, Yan H, Ren G-X, Zhao J, Guo X-P, Sun Y-C. CRISPR-Cas12a-assisted recombineering in bacteria. Appl Environ Microb. 2017;83(17):e00947-e1017.
Banno S, Nishida K, Arazoe T, Mitsunobu H, Kondo A. Deaminase-mediated multiplex genome editing in Escherichia coli. Nat Microbiol. 2018;3(4):423–9.
Liu Z, Dong H, Cui Y, Cong L, Zhang D. Application of different types of CRISPR/Cas-based systems in bacteria. Microb Cell Fact. 2020;19(1):172.
Zhu Z, Zhou YJ, Krivoruchko A, Grininger M, Zhao ZK, Nielsen J. Expanding the product portfolio of fungal type I fatty acid synthases. Nat Chem Biol. 2017;13(4):360–2.
Rossini E, Gajewski J, Klaus M, Hummer G, Grininger M. Analysis and engineering of substrate shuttling by the acyl carrier protein (ACP) in fatty acid synthases (FASs). bioRxiv. 2018;54(82):11606–9.
Dinh CV, Prather KLJ. Development of an autonomous and bifunctional quorum-sensing circuit for metabolic flux control in engineered Escherichia coli. Proc Natl Acad Sci USA. 2019;116(51):25562–8.
Doong SJ, Gupta A, Prather KLJ. Layered dynamic regulation for improving metabolic pathway productivity in Escherichia coli. Proc Natl Acad Sci USA. 2018;115(12):2964–9.
Yuan LZ, Rouvière PE, Larossa RA, Suh W. Chromosomal promoter replacement of the isoprenoid pathway for enhancing carotenoid production in E. coli. Metab Eng. 2006;8(1):79–90.
Yoon SH, Park HM, Kim JE, Lee SH, Choi MS, Kim JY, Oh DK, Keasling JD, Kim SW. Increased beta-carotene production in recombinant Escherichia coli harboring an engineered isoprenoid precursor pathway with mevalonate addition. Biotechnol Prog. 2007;23(3):599–605.
Zhao J, Li Q, Sun T, Zhu X, Xu H, Tang J, Zhang X, Ma Y. Engineering central metabolic modules of Escherichia coli for improving β-carotene production. Metab Eng. 2013;17:42–50.
Jung J, Lim JH, Kim SY, Im D-K, Seok JY, Lee S-JV, Oh MK, Jung GY. Precise precursor rebalancing for isoprenoids production by fine control of gapA expression in Escherichia coli. Metab Eng. 2016;38:401–8.
Wu Y, Yan P, Li Y, Liu X, Wang Z, Chen T, Zhao X. Enhancing β-carotene production in Escherichia coli by perturbing central carbon metabolism and improving the NADPH supply. Front Bioeng Biotechnol. 2020;9(8):585.
Guo JY, Hu KL, Bi CH, Li QY, Zhang XL. Construction of an alternative glycerol-utilization pathway for improved β-carotene production in Escherichia coli. J Ind Microbiol Biotechnol. 2018;45(8):697–705.
Shi S, Chang Y, Yu J, Chen H, Wang Q, Bi Y. Identification and functional analysis of two novel genes-geranylgeranyl pyrophosphate synthase gene (AlGGPPS) and isopentenyl pyrophosphate isomerase gene (AlIDI)-from aurantiochytrium limacinum significantly enhance de novo β-carotene biosynthesis in Escherichia coli. Mar Drugs. 2023;21(4):249.
von Meyenburg K, Jørgensen BB, van Deurs B. Physiological and morphological effects of overproduction of membrane-bound ATP synthase in Escherichia coli K-12. EMBO J. 1984;3(8):1791–7.
van Weeghel RP, Keck W, Robillard GT. Regulated high-level expression of the mannitol permease of the phosphoenolpyruvate-dependent sugar phosphotransferase system in Escherichia coli. Proc Natl Acad Sci USA. 1990;87(7):2613–7.
Herskovits AA, Shimoni E, Minsky A, Bibi E. Accumulation of endoplasmic membranes and novel membrane-bound ribosome-signal recognition particle receptor complexes in Escherichia coli. J Cell Biol. 2002;159(3):403–10.
Wilkison WO, Walsh JP, Corless JM, Bell RM. Crystalline arrays of the Escherichia coli sn-glycerol-3-phosphate acyltransferase, an integral membrane protein. J Biol Chem. 1986;261(21):9951–8.
Eriksson HM, Wessman P, Ge C, Edwards K, Wieslander A. Massive formation of intracellular membrane vesicles in Escherichia coli by a monotopic membrane-bound lipid glycosyltransferase. J Biol Chem. 2009;284(49):33904–14.
Ge C, Gómez-Llobregat J, Skwark MJ, Ruysschaert JM, Wieslander AK, Lindén M. Membrane remodeling capacity of a vesicle-inducing glycosyltransferase. FEBS J. 2014;281(16):3667–84.
Jarboe LR, Liu P-H, Royce LA. Engineering inhibitor tolerance for the production of biorenewable fuels and chemicals. Curr Opin Chem Eng. 2011;1:38–42.
Chen Y, Xiao W, Wang Y, Liu H, Li X, Yuan Y. Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering. Microb Cell Fact. 2016;15(1):113.
Royce LA, Liu P-H, Stebbins MJ, Hanson BC, Jarboe LR. The damaging effects of short chain fatty acids on Escherichia coli membranes. Appl Microbiol Biotechnol. 2013;97(18):8317–27.
Royce LA, Yoon JM, Chen Y, Rickenbach E, Shanks JV, Jarboe LR. Evolution for exogenous octanoic acid tolerance improves carboxylic acid production and membrane integrity. Metab Eng. 2015;29:180–8.
Minty JJ, Lesnefsky AA, Lin F, Chen YC, Zaroff TA, Veloso A, Xie B, McConnell CA, Ward RJ, Schwartz DR, et al. Evolution combined with genomic study elucidates genetic bases of isobutanol tolerance in Escherichia coli. Microb Cell Fact. 2011;10:18.
Shabala L, Ross T. Cyclopropane fatty acids improve Escherichia coli survival in acidified minimal media by reducing membrane permeability to H+ and enhanced ability to extrude H+. Res Microbiol. 2008;159(6):458–61.
Zhang Y-M, Rock CO. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol. 2008;6(3):222–33.
Royce LA, Boggess E, Fu Y, Liu P-H, Shanks JV, Dickerson JA, Jarboe LR. Transcriptomic analysis of carboxylic acid challenge in Escherichia coli: beyond membrane damage. PLoS ONE. 2014;9(2): e89580.
Tan Z, Yoon JM, Nielsen DR, Shanks JV, Jarboe LR. Membrane engineering via trans unsaturated fatty acids production improves Escherichia coli robustness and production of biorenewables. Metab Eng. 2016;35:105–13.
Luo LH, Seo PS, Seo JW, Heo SY, Kim DH, Kim CH. Improved ethanol tolerance in Escherichia coli by changing the cellular fatty acids composition through genetic manipulation. Biotechnol Lett. 2009;31:1867–71.
Lennen RM, Pfleger BF. Modulating membrane composition alters free fatty acid tolerance in Escherichia coli. PLoS ONE. 2013;8(1): e54031.
Sherkhanov S, Korman TP, Bowie JU. Improving the tolerance of Escherichia coli to medium-chain fatty acid production. Metab Eng. 2014;25:1–7.
Tan Z, Khakbaz P, Chen Y, Lombardo J, Yoon JM, Shanks JV, Klauda JB, Jarboe LR. Engineering Escherichia coli membrane phospholipid head distribution improves tolerance and production of biorenewables. Metab Eng. 2017;44:1–12.
Wu J, Huang M, Zhan Y, Liu M, Hu X, Wu Y, Qiao J, Wang Z, Li H, Wang J, Wang X. Regulating cardiolipin biosynthesis for efficient production of colanic acid in Escherichia coli. J Agric Food Chem. 2023;71(22):8516–26.
Korosh TC, Markley AL, Clark RL, McGinley LL, McMahon KD, Pfleger BF. Engineering photosynthetic production of l-lysine. Metab Eng. 2017;44:273–83.
Wu W, Liu F, Singh S. Toward engineering E. coli with an autoregulatory system for lignin valorization. Proc Natl Acad Sci USA. 2018;115(12):2970–5.
Zilly FE, Halemani ND, Walrafen D, Spitta L, Schreiber A, Jahn R, Lang T. Ca2+ induces clustering of membrane proteins in the plasma membrane via electrostatic interactions. EMBO J. 2011;30(7):1209–20.
Chen J, Wang Y, Du G, Kang Z. Optimizing precursor supply and cell membrane permeability to enhance ergothionein synthesis in Escherichia coli. J Food Biotechnol. 2022;41(8):43–52.
Kumamoto CA. SecB protein: a cytosolic export factor that associates with nascent exported proteins. J Bioenerg Biomembr. 1990;22:337–51.
Xu G, Wu A, Xiao L, Han R, Ni Y. Enhancing butanol tolerance of Escherichia coli reveals hydrophobic interaction of multi-tasking chaperone SecB. Biotechnol Biofuels. 2019;28(12):164.
Wu T, Ye L, Zhao D, Li S, Li Q, Zhang B, Bi C, Zhang X. Membrane engineering—a novel strategy to enhance the production and accumulation of β-carotene in Escherichia coli. Metab Eng. 2017;43(Pt A):85–91.
Wu T, Ye L, Zhao D, Li S, Li Q, Zhang B, Bi C. Engineering membrane morphology and manipulating synthesis for increased lycopene accumulation in Escherichia coli cell factories. 3 Biotech. 2018;8(6):269.
Yang D, Park SY, Lee SY. Production of rainbow colorants by metabolically engineered Escherichia coli. Adv Sci. 2021;8(13):2100743.
Zhang XC, Guo Y, Liu X, Chen XG, Wu Q, Chen GQ. Engineering cell wall synthesis mechanism for enhanced PHB accumulation in E. coli. Metab Eng. 2018;45:32–42.
Elhadi D, Lv L, Jiang XR, Wu H, Chen GQ. CRISPRi engineering E. coli for morphology diversification. Metab Eng. 2016;38:358–69.
Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700.
Wang X, Quinn PJ. Lipopolysaccharide: biosynthetic pathway and structure modification. Prog Lipid Res. 2010;49(2):97–107.
Wang J, Ma W, Fang Y, Zhang H, Liang H, Li Y, Wang X. Truncating the structure of lipopolysaccharide in Escherichia coli can effectively improve poly-3-hydroxybutyrate production. ACS Synth Biol. 2020;9(5):1201–15.
Wang Z, Wang J, Ren G, Li Y, Wang X. Deletion of the genes waaC, waaF, or waaG in Escherichia coli W3110 disables the flagella biosynthesis. J Basic Microb. 2016;56(9):1021–35.
Emiola A, Andrews SS, Heller C, George J. Crosstalk between the lipopolysaccharide and phospholipid pathways during outer membrane biogenesis in Escherichia coli. P Natl Acad Sci USA. 2016;113(11):3108–13.
Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13(10):605–19.
Wensink J, Witholt B. Outer-membrane vesicles released by normally growing Escherichia coli contain very little lipoprotein. Eur J Biochem. 1981;116(2):331–5.
Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–84.
Kulkarni HM, Jagannadham MV. Biogenesis and multifaceted roles of outer membrane vesicles from gram-negative bacteria. Microbiology. 2014;160(Pt 10):2109–21.
Haurat MF, Elhenawy W, Feldman MF. Prokaryotic membrane vesicles: new insights on biogenesis and biological roles. Biol Chem. 2015;396(2):95–109.
Kadurugamuwa JL, Beveridge TJ. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177(14):3998–4008.
van de Waterbeemd B, Zomer G, van den Ijssel J, van Keulen L, Eppink MH, van der Ley P, van der Pol LA. Cysteine depletion causes oxidative stress and triggers outer membrane vesicle release by Neisseria meningitidis; implications for vaccine development. PLoS ONE. 2013;8(1): e54314.
Kolodziejek AM, Caplan AB, Bohach GA, Paszczynski AJ, Minnich SA, Hovde CJ. Physiological levels of glucose induce membrane vesicle secretion and affect the lipid and protein composition of Yersinia pestis cell surfaces. Appl Environ Microbiol. 2013;79(14):4509–14.
Eddy JL, Gielda LM, Caulfield AJ, Rangel SM, Lathem WW. Production of outer membrane vesicles by the plague pathogen Yersinia pestis. PLoS ONE. 2014;9: e107002.
Galka F, Wai SN, Kusch H, Engelmann S, Hecker M, Schmeck B, Hippenstiel S, Uhlin BE, Steinert M. Proteomic characterization of the whole secretome of Legionella pneumophila and functional analysis of outer membrane vesicles. Infect Immun. 2008;76(5):1825–36.
Charon NW, Goldstein SF, Marko M, Hsieh C, Gebhardt LL, Motaleb MA, Wolgemuth CW, Limberger RJ, Rowe N. The flat-ribbon configuration of the periplasmic flagella of Borrelia burgdorferi and its relationship to motility and morphology. J Bacteriol. 2009;191(2):600–7.
Bager RJ, Persson G, Nesta B, Soriani M, Serino L, Jeppsson M, Nielsen TK, Bojesen AM. Outer membrane vesicles reflect environmental cues in Gallibacterium anatis. Vet Microbiol. 2013;167(3–4):565–72.
Kobayashi H, Uematsu K, Hirayama H, Horikoshi K. Novel toluene elimination system in a toluene-tolerant microorganism. J Bacteriol. 2000;182(22):6451–5.
Manning AJ, Kuehn MJ. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011;11:258.
Loeb MR. Bacteriophage T4-mediated release of envelope components from Escherichia coli. J Virol. 1974;13(3):631–41.
Loeb MR, Kilner J. Release of a special fraction of the outer membrane from both growing and phage T4-infected Escherichia coli B. Biochim Biophys Acta. 1978;514(1):117–27.
Schooling SR, Beveridge TJ. Membrane vesicles: an overlooked component of the matrices of biofilms. J Bacteriol. 2006;188(16):5945–57.
McBroom AJ, Kuehn MJ. Release of outer membrane vesicles by gram-negative bacteria is a novel envelope stress response. Mol Microbiol. 2007;63(2):545–58.
Kato S, Kowashi Y, Demuth DR. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb Pathog. 2002;32(1):1–13.
Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. Embo J. 2004;23(23):4538–49.
Choi SJ, Kim MH, Jeon J, Kim OY, Choi Y, Seo J, Hong SW, Lee WH, Jeon SG, Gho YS, et al. Active immunization with extracellular vesicles derived from staphylococcus aureus effectively protects against staphylococcal lung infections, mainly via Th1 cell-mediated immunity. PLoS ONE. 2015;10(9): e0136021.
Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol. 2015;13(10):620–30.
Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 2018;17(1):13–24.
Wu T, Li S, Ye L, Zhao D, Fan F, Li Q, Zhang B, Bi C, Zhang X. Engineering an artificial membrane vesicle trafficking system (AMVTS) for the excretion of β-carotene in Escherichia coli. ACS Synth Biol. 2019;8(5):1037–46.
Renner LD, Weibel DB. Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes. Proc Natl Acad Sci USA. 2011;108(15):6264–9.
Tan BK, Bogdanov M, Zhao J, Dowhan W, Raetz CRH, Guan Z. Discovery of a cardiolipin synthase utilizing phosphatidylethanolamine and phosphatidylglycerol as substrates. Proc Natl Acad Sci USA. 2012;109(41):16504–9.
Fordjour E, Bai Z, Li S, Li S, Sackey I, Yang Y, Liu CL. Improved membrane permeability via hypervesiculation for in situ recovery of lycopene in Escherichia coli. ACS Synth Biol. 2023;12(9):2725–39.
Wang J, Ma W, Fang Y, Zhang H, Liang H, Liu H, Wang T, Chen S-W, Ji J, Wang X. Engineering the outer membrane could facilitate better bacterial performance and effectively enhance poly-3-hydroxybutyrate accumulation. Appl Environ Microb. 2021;87(23): e0138921.
Gold DA, Caron AM, Fournier GP, Summons RE. Paleoproterozoic sterol biosynthesis and the rise of oxygen. Nature. 2017;543(7645):420–3.
Klose C, Surma MA, Gerl MJ, Meyenhofer F, Shevchenko A, Simons K. Flexibility of a eukaryotic lipidome— insights from yeast lipidomics. PLoS ONE. 2012;7(4): e35063.
van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9(2):112–24.
Bell RM, Ballas LM, Coleman RA. Lipid topogenesis. J Lipid Res. 1981;22(3):391–403.
Henneberry AL, Wright MM, McMaster CR. The major sites of cellular phospholipid synthesis and molecular determinants of fatty acid and lipid head group specificity. Mol Biol Cell. 2002;13(9):3148–61.
Pomorski T, Menon AK. Lipid flippases and their biological functions. Cell Mol Life Sci. 2006;63(24):2908–21.
Rodríguez-Vargas S, Sánchez-García A, Martínez-Rivas JM, Prieto JA, Randez-Gil F. Fluidization of membrane lipids enhances the tolerance of Saccharomyces cerevisiae to freezing and salt stress. Appl Environ Microbiol. 2007;73(1):110–6.
Zhu G, Yin N, Luo Q, Liu J, Chen X, Liu L, Wu J. Enhancement of sphingolipid synthesis improves osmotic tolerance of Saccharomyces cerevisiae. Appl Environ Microbiol. 2020;86(8):e02911-e2919.
Bu X, Lin JY, Cheng J, Yang D, Duan CQ, Koffas M, Yan GL. Engineering endogenous ABC transporter with improving ATP supply and membrane flexibility enhances the secretion of β-carotene in Saccharomyces cerevisiae. Biotechnol Biofuels. 2020;13:168.
Hong JB, Park S-H, Kim S, Kim S-W, Hahn J-S. Efficient production of lycopene in Saccharomyces cerevisiae by enzyme engineering and increasing membrane flexibility and NAPDH production. Appl Micro Biot. 2019;103(1):211–23.
Matthäus F, Ketelhot M, Gatter M, Barth G. Production of lycopene in the non-carotenoid-producing yeast Yarrowia lipolytica. Appl Environ Microbiol. 2014;80(5):1660–9.
Yu Y, Rasool A, Liu H, Lv B, Chang P, Song H, Wang Y, Li C. Engineering Saccharomyces cerevisiae for high yield production of α-amyrin via synergistic remodeling of α-amyrin synthase and expanding the storage pool. Metab Eng. 2020;62:72–83.
Ma T, Shi B, Ye Z, Li X, Liu M, Chen Y, Xia J, Nielsen J, Deng Z, Liu T. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metab Eng. 2019;52:134–42.
Bu X, Lin JY, Duan CQ, Koffas MAG, Yan GL. Dual regulation of lipid droplet-triacylglycerol metabolism and ERG9 expression for improved β-carotene production in Saccharomyces cerevisiae. Microb Cell Fact. 2022;21(1):3.
Lin JL, Zhu J, Wheeldon I. Synthetic protein scaffolds for biosynthetic pathway colocalization on lipid droplet membranes. ACS Synth Biol. 2017;6(8):1534–44.
Shi Y, Wang D, Li R, Huang L, Dai Z, Zhang X. Engineering yeast subcellular compartments for increased production of the lipophilic natural products ginsenosides. Metab Eng. 2021;67:104–11.
Kim JE, Jang IS, Son SH, Ko YJ, Cho BK, Kim SC, Lee JY. Tailoring the Saccharomyces cerevisiae endoplasmic reticulum for functional assembly of terpene synthesis pathway. Metab Eng. 2019;56:50–9.
Liu Q, Liu Y, Li G, Savolainen O, Chen Y, Nielsen J. De novo biosynthesis of bioactive isoflavonoids by engineered yeast cell factories. Nat Commun. 2021;12(1):6085.
Arendt P, Miettinen K, Pollier J, De Rycke R, Callewaert N, Goossens A. An endoplasmic reticulum-engineered yeast platform for overproduction of triterpenoids. Metab Eng. 2017;40:165–75.
Zhang Y, Wang Y, Yao M, Liu H, Zhou X, Xiao W, Yuan Y. Improved campesterol production in engineered Yarrowia lipolytica strains. Biotechnol Lett. 2017;39(7):1033–9.
Qian YD, Tan SY, Dong GR, Niu YJ, Hu CY, Meng YH. Increased campesterol synthesis by improving lipid content in engineered Yarrowia lipolytica. Appl Microbiol Biotechnol. 2020;104(16):7165–75.
Guerfal M, Claes K, Knittelfelder O, De Rycke R, Kohlwein SD, Callewaert N. Enhanced membrane protein expression by engineering increased intracellular membrane production. Microb Cell Fact. 2013;12:122.
Acknowledgements
No applicable.
Funding
This work was supported by the Youth Program of National Natural Science Foundation of China (Grant Numbers: 21908168), the General Program of National Natural Science Foundation of China (Grant Numbers: 31870122), the Education Commission Scientific Research Project of Tianjin (Grant Number: 2022KJ004) and the National College Student Innovation Project of China (Grant Numbers: 202310069006).
Author information
Authors and Affiliations
Contributions
TW, JJJ and JZL prepared a draft of the manuscript. TW and HYZ finalized the manuscript. Haihua Ruan supervised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, T., Jiang, J., Zhang, H. et al. Transcending membrane barriers: advances in membrane engineering to enhance the production capacity of microbial cell factories. Microb Cell Fact 23, 154 (2024). https://doi.org/10.1186/s12934-024-02436-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-024-02436-8