Abstract
Background
Itaconic acid (IA) is a versatile platform chemical widely used for the synthesis of various polymers and current methods for IA production based on Aspergillus terreus fermentation are limited in terms of process efficiency and productivity. To construct more efficient IA production strains, A. niger was used as a chassis for engineering IA production by assembling the key components of IA biosynthesis pathways from both A. terreus and Ustilago maydis.
Results
Recombinant A. niger S1596 overexpressing the A. terreus IA biosynthesis genes cadA, mttA, mfsA produced IA of 4.32 g/L, while A. niger S2120 overexpressing the U. maydis IA gene cluster adi1, tad1, mtt1, itp1 achieved IA of 3.02 g/L. Integration of the two IA production pathways led to the construction of A. niger S2083 with IA titers of 5.58 g/L. Increasing cadA copy number in strain S2083 created strain S2209 with titers of 7.99 g/L and deleting ictA to block IA degradation in S2209 created strain S2288 with IA titers of 8.70 g/L. Overexpressing acoA to enhance the supply of IA precursor in strain S2288 generated strain S2444 with IA titers of 9.08 g/L in shake flask.
Conclusion
Recombinant A. niger overexpressing the U. maydis IA biosynthesis pathway was capable of IA accumulation. Combined expression of the two IA biosynthesis pathways from A. terreus and U. maydis in A. niger resulted in much higher IA titers. Furthermore, increasing cadA copy number, deleting ictA to block IA degradation and overexpressing acoA to enhance IA precursor supply all showed beneficial effects on IA accumulation.
Similar content being viewed by others
Background
Itaconic acid (IA), a C5-dicarboxylic acid containing a double bond, is a versatile platform chemical with a wide range of industrial applications such as production of superabsorbents, polyester resins, synthetic latex, surfactants [1] and IA is listed by DOE of U.S. as one of bio-based top 12 building block chemicals [2].
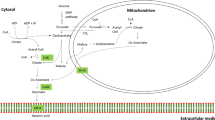
Although IA can be synthesized by conventional chemical means, commercial development is hampered due to the involvement of cumbersome and unsustainable process [3]. Diverse microorganisms are capable of producing IA, including Aspergillus terreus [4], A. flavus [5], Ustilago maydis [6], Candida sp [7] and Pseudozyma antarctica [8] etc. (Table 1). Strains of A. terreus and U. maydis have been extensively investigated for IA production. The metabolic pathways of IA biosynthesis for the two fungal species were distinct (Fig. 1) [3]. A. terreus utilizes cis-aconitate decarboxylase encoded by cadA to convert cis-aconitate to IA, while U. maydis uses two-step reactions involving aconitate-Δ-isomerase encoded by adi1 and trans-aconitate decarboxylase encoded by tad1 to form IA [6, 9]. Mitochondrial carrier proteins MttA and Mtt1 and transporter proteins MfsA and Itp1 are essential components of IA transport and thus the accumulation of IA in fermentation broth. MttA and MfsA genes are clustered in A. terreus, while Mtt1 and Itp1 genes are clustered in U. maydis [3]. MttA and Mtt1 mediate cis-aconitate transport from mitochondria to the cytoplasm, while MfsA and Itp1 are responsible for extracellular secretion of IA [3].
The constructed IA biosynthesis pathway in A. niger. Green represents relevant enzymes from U. maydis. Purple indicates specific enzymes from A. terreus. The “X” indicates gene deletion. For A. niger proteins, AnCexA: citrate exporter, AnOahA: oxaloacetate acetylhydrolase, AnAcoA: aconitase in cytosol; for A.terreus proteins, AtMttA: mitochondrial tricarboxylate transporter, AtCadA: cis-aconitate decarboxylase, AtMfsA: itaconic acid exporter; for U. maydis, UmAdi1: aconitate-Δ-isomerase, UmTad1: trans-aconitate decarboxylase, UmMtt1: mitochondrial tricarboxylate transporter, UmItp1: itaconic acid exporter
Though various microbial strains have been explored for fermentation production of IA [3], A. terreus is still the main source of strains for commercial IA production by fermentation. It was reported that a titer of 80 g/L IA was achieved by a genetically engineered industrial A. terreus with a yield of 0.6 g/g and productivity of 1.1 g/L/h [10]. Glucose is the most commonly used carbon source for IA fermentation by A. terreus, however, the low yield leads to high cost of IA biofabrication [11]. Additionally, much higher titers of IA fermentation process has been documented for engineered U. maydis strains with IA titers of 220 g/L and productivity of 0.73 g/L/h [12], but the low yield of 0.45 g/g prevented the use of U. maydis for IA commercial production [5]. A. niger has numerous desirable traits to be used as a microbial chassis cell for efficient IA production, including a high conversion efficiency from sugar to organic acid, a clear genetic background, low pH tolerance and capable of utilizing cheap and renewable carbon sources [13]. Previously, it was shown that recombinant A. niger overexpressing A. terreus cadA encoding the cis-aconitate decarboxylase was capable of IA production [14]. Moreover, combined overexpression of cadA/ mttA or cadA/ mfsA in A. niger led to enhanced IA production with titers of 1.5 g/L [15]. Overexpressing cadA, mttA and mfsA in an oxaloacetate hydrolase and glucose oxidase deletion mutant A. niger NW186 further boosted IA yields, reaching up to 7.1 g/L [16]. Overexpression of cytosolic citrate synthase (citB) in A. niger carrying the IA-producing gene cluster of A. terreus achieved IA titers of 2 g/L and 26.2 g/L in shake flask and controlled batch cultivation, respectively [17]. Although IA production in A. niger expressing the IA biosynthesis pathway genes from A. terreus were examined by several studies, the effects of introducing genes from U. maydis IA biosynthesis pathway into A. niger on IA production has yet to be reported. It is worth investigating the potential beneficial effects that integrates the positive genes from IA biosynthesis pathways of A. terreus and U. maydis into the genome of A. niger (Fig. 1).
In this study, an engineered A. niger strain S1075 deficient in citrate extracellular secretion (cexA deletion) and oxalate production (oahA deletion) was used as starting host strain for assembling the IA biosynthesis pathways. First, cadA, mttA and mfsA from A. terreus were sequentially introduced into S1075 to obtain IA-producing recombinant strain S1596, and adi1, tad1, mtt1 and itp1 from U. maydis were sequentially introduced into A. niger S1075 to obtain IA-producing strain S2120. Second, adi1, tad1 and itp1 were integrated sequentially into strain S1596 to obtain IA-producing strain S2083. Finally, an efficient IA-producing A. niger cell factory S2444 was constructed by increasing cadA gene copy number, overexpressing acoA to enhance supply of IA precursor and deleting ictA to block IA degradation in strain S2083.
Results and discussion
Expression of the A. terreus IA biosynthesis cluster in A. niger S1075
Naturally occurring strains of A. niger are incapable of producing IA due to the absence of cis-aconitate decarboxylase [31]. The fact that engineered A. niger strains expressing a cis-aconitate decarboxylase from A. terreus led to IA accumulation showed the potential of A. niger as a microbial chassis for IA production [14, 29, 31, 32]. In this study, an oxalate-non-producing strain A. niger S422 derived from A. niger ATCC 1015 [33] was used as the parent strain to delete the citrate exporter-coding gene cexA to prevent citrate accumulation during IA fermentation. The resultant citrate-non-producing strain was named S1075. In this study, a cis-aconitate decarboxylase gene cadA from A. terreus was first introduced into A. niger S1075. Thirty-five and ten transformants overexpressing cadA were selected for IA fermentation analysis in the first and second round of screening, respectively. Similar levels of IA accumulation were found in all transformants (Additional file 1: Fig. S2a). The transformant with highest IA titers (1.44 g/L and 2.73 g/L at 4-day and 6-day, respectively) was named as S1361 (Fig. 2a). Our results demonstrated that introduction of cadA to A. niger confers its ability to biosynthesize IA. Similar results were also observed in other engineered A. niger strains [14, 16].
Construction of A. niger cell factory overexpressing specific genes of IA synthesis cluster from A. terreus. a The titer of IA generated by the indicated strains in shake flasks for 4 days and 6 days. b The residual glucose obtained for the indicated strains in shake flasks for 4 days and 6 days. c The determination of expression levels of genes involved in IA production by qRT-PCR in S1596. d Growth of parent strain and mutant strains on PDA plate at 28 °C. Mean and standard deviation values were calculated from 3 independent Erlenmeyer flask samples. The “*” represents statistically significant difference by one-tailed Student’s test. OE represents overexpression
To increase the supply of cis-aconitate for decarboxylation reaction in the cytosol, mttA encoding a mitochondrial tricarboxylate transporter responsible for aconitate transport across mitochondrial membrane was overexpressed in S1361. We next introduced mttA and tested the resulting strain S1486 as described above (Additional file 1: Fig. S3). As shown in Fig. 2a, overexpression of mttA further elevated IA production to 3.01 g/L and 4.08 g/L at 4-day and 6-day, respectively.These results demonstrated that efficient aconitate transport into cytosol benefited IA production, which is consistent with the previous observations that transport of cis-aconitate out of mitochondria is one of the limiting steps in IA production [15].
Furthermore, export of IA out of cytosol is previously shown to be a pivotal step for IA accumulation [15], which is mediated by the major facilitator superfamily protein MfsA as IA exporter in A. terreus. For IA fermentation analysis, transformants overexpressing mfsA were selected as described above, and similar levels of IA accumulation were found in all transformants (Additional file 1: Fig. S4a, b). The transformant with highest IA titer (3.18 g/L and 4.32 g/L at 4-day and 6-day, respectively) was named as S1596 (Fig. 2a). The above results indicated that the gene cluster of IA biosynthesis from A. terreus has a significant positive effect on the accumulation of IA in A. niger [17]. Additionally, as important precursor substances for IA biosynthesis, intracellular cis-aconitate concentration was also determined. As shown in Additional file 1: Table S1, intracellular cis-aconitate slightly increased in S1596 compared with that in A. niger S1075, suggesting that the metabolic flux to IA was enhanced by introducing the gene cluster of IA biosynthesis from A. terreus. Additionally, a small amount of trans-aconitate was formed in A. niger S1596 (Additional file 1: Table S1), which may be due to the lower thermal stability of the cis-aconitate than trans-aconitate, prompting the isomerization of cis-aconitate into trans-aconitate [34]. The results showed that overexpression of cadA, mttA and mfsA leads to the intracellular conversion of cis-aconitate into IA. As the levels of IA accumulation continued to increase, the rates of glucose consumption also increased (Fig. 2b). The results demonstrated that the introduction of cadA, mttA and mfsA genes successfully engineered the metabolic flux to IA, which indicated that the IA biosynthesis pathway derived from A. terreus worked and exhibited great potential for IA production in A. niger chassis. The overexpression of cadA, mttA and mfsA in A. niger S1596 was verified by qRT-PCR (Fig. 2c).
Expression of the U. maydis IA biosynthesis cluster in A. niger S1075
U. maydis is a natural organism to producing dicarboxylic acid using an alternative pathway via trans-aconitate [35]. The aconitate-Δ-isomerase (Adi1) and trans-aconitate decarboxylase (Tad1) from U. maydis are central for IA biosynthesis, and Tad1 is related to 3-carboxy-cis, cis-muconate lactonizing enzyme and Adi1 is a member of the PrpF family [6]. In S. cerevisiae, IA could be synthesized only when the two genes adi1 and tad1 were co-expressed [6]. To evaluate whether introducing the U. maydis gene cluster into A. niger is capable of producing IA, the aconitate-Δ-isomerase gene adi1 and trans-aconitate decarboxylase tad1 were first co-overexpressed in A. niger S1075. According to the method described above, transformants co-overexpressing adi1 and tad1 were selected (Additional file 1: Fig. S5a, b). A. niger S1683 co-overexpressing adi1 and tad1 was shown to be the best IA-producing strain with IA titers of 1.04 g/L and 1.85 g/L IA at 4-day and 6-day, respectively (Fig. 3a), which further illustrated that both adi1 and tad1 played important roles in IA production in A. niger.
Construction of A. niger cell factory expressing relevant genes from U. maydis to produce IA. a The titer of IA generated by the indicated strains in shake flasks for 4 days and 6 days. b The residual glucose obtained for the indicated strains in shake flasks for 4 days and 6 days. c The determination of expression levels of genes involved in IA production by qRT-PCR in S2120. Mean and standard deviation values were calculated from 3 independent Erlenmeyer flask samples. The “*” represents statistically significant difference by one-tailed Student’s test. OE represents overexpression
It is well known that isomerization of cis-aconitate and decarboxylation of trans-aconitate occur in the cytosol, and the putative mitochondrial transporter Mtt1 is the rate-limiting step in the transport of cis-aconitate from mitochondria to cytosol [6]. Thus, we further introduced mtt1 and selected the recombinant strain S1738 with the highest IA titer as described above (Additional file 1: Fig. S6a, b). As shown in Fig. 3a, mtt1 overexpression promotes IA formation in A. niger S1738 with titers of 1.54 g/L and 2.74 g/L at 4-day and 6-day, respectively, indicating an enhanced metabolic flux of cis-aconitate to cytosol and thus an acceleration of bioconversion of cis-aconitate to trans-aconitate.
Considering that a major facilitator superfamily transporter Itp1 was previously shown to be responsible for the IA transport [6], thus we overexpressed itp1 in strain S1738. Transformants overexpressing itp1 were selected as described above, and transformant with highest IA titers named as S2120 (OEadi1, OEtad1, OEmtt1, OEitp1) (Additional file 1: Fig. S7a, b). As shown Fig. 3a, titers of IA in S2120 fermentation broth were 1.82 g/L (0.07 mol/mol) and 3.02 g/L (0.08 mol/mol) at 4-day and 6-day, respectively, which was almost twice that of strain S1683. The results suggested that introduction of itp1 further strengthened the efficiency of IA transport, indicating that IA export was an indispensable factor for the elevation of IA accumulation. Furthermore, compared with A. niger S1075, comparable levels of cis-aconitate and more trans-aconitate were detected in A. niger S2120 (Additional file 1: Table S1), suggesting that the gene cluster of IA biosynthesis from U. maydis successfully enhanced the metabolic flux of cis-aconitate to IA in A. niger through cis/trans-aconitate isomerization. The levels of residual sugar also decreased as IA production increased (Fig. 3b). Overexpression of itp1 may drive the metabolic flux toward IA biosynthesis, making more carbon sources available for IA production. Monitoring of the transcript levels also demonstrated that adi1, tad1, mtt1 and itp1 were overexpressed and have played their expected roles in promoting IA production in strain S2120 (Fig. 3c).
Expression of the U. maydis IA biosynthesis cluster in strain S1596
In order to further improve IA production in A. niger, we investigated the potential beneficial effects for IA synthesis by integrating the two IA biosynthesis routes from A. terreus and U. maydis. Given that IA titers of S1596 (OEcadA, OEmttA, OEmfsA) were significantly higher than that of S2120 (OEadi1, OEtad1, OEmtt1, OEitp1) (Figs. 2a, 3a), adi1 and tad1 were co-introduced into S1596. For IA fermentation analysis, transformants co-overexpressing adi1 and tad1 were selected and tested as described above (Additional file 1: Fig. S8a, b). The transformant with the highest IA titer was selected for further engineering and named as S1779 (OEcadA, OEmttA, OEmfsA, OEadi1, OEtad1) with titers of 3.68 g/L (0.09 mol/mol) and 5.17 g/L (0.11 mol/mol), respectively (Fig. 4a). CAD was a key enzyme for IA production in A. terreus [14], while ADI and TAD played important roles in cis-aconitate isomerization to trans-aconitate as well as IA biosynthesis in U. maydis [6]. The above results showed that co-overexpression of adi1 and tad1 in A. niger cells containing the IA biosynthesis pathway from A. terreus further enhanced the metabolic flux to IA, which exhibited synergistic effects on IA production.
IA production in A. niger S1596 overexpressing genes of the IA sythesis cluster from U. maydis. a The titer of IA produced by the indicated strains in shake flasks for 4 days and 6 days. b The residual glucose obtained for the indicated strains in shake flasks for 4 days and 6 days. Mean and standard deviation values were calculated from 3 independent Erlenmeyer flask samples. The “*” represents statistically significant difference by one-tailed Student’s test. OE represents overexpression
Previous studies demonstrated transporter Itp1 is a rate-limiting step for IA production in U. maydis [6]. Therefore, itp1 was inserted into S1779 in the hope of alleviating this metabolic flux bottleneck. We next introduced itp1 in S1779 and tested the resulting strains S2083 as described above (Additional file 1: Fig. S9a, b). As expected, IA titers in A. niger S2083 increased to 3.97 g/L (0.12 mol/mol) and 5.58 g/L (0.15 mol/mol) at 4-day and 6-day, respectively (Fig. 4a). As shown in Fig. 4b, glucose levels in fermentation broth also decreased with increased IA production, indicating that more carbon sources were directed to the IA biosynthesis pathway in A. niger S2083.
Increasing the copy number of cadA in strain S2083
Increased copy number of cadA was shown to be beneficial for IA accumulation [36]. The titers of IA in all transformants with introduction of multiple copies of cadA exhibited higher titers of IA than A. niger S2083 (Additional file 1: Fig. S10a). After a second round of screening, the transformant with highest IA titer was selected and named as S2209 [OEcadA (multiple copies), OEmttA, OEmfsA, OEadi1, OEtad1, OEitp1] (Additional file 1: Fig. S10b). As shown Fig. 5a, titers of IA in A.niger S2209 fermentation broth were 7.04 g/L and 7.99 g/L at 4-day and 6-day, respectively. It has been reported that the expression level of CAD was proportional to the IA production [16, 23]. Indeed, a similar phenomenon was observed in this study, suggesting that the conversion cis-aconitate to IA was a rate-limiting step for IA biosynthesis in the heterogenously hybrid cell factory. Additionally, the concentration of glucose also decreased with increased IA production (Fig. 5b). In conclusion, the incorporation of multiple copies of cadA into A. niger promoted the decarboxylation reaction of aconitic acid, thus enhancing its biosynthesis of IA.
IA production in strain S2083 with increased cadA gene copy number. a The titer of IA generated by the indicated strains in shake flasks for 4 days and 6 days. b The residual glucose obtained for the indicated strains in shake flasks for 4 days and 6 days. Mean and standard deviation values were calculated from 3 independent Erlenmeyer flask samples. The “*” represents statistically significant difference by one-tailed Student’s test. OE represents overexpression
Reduced byproduct itaconyl-CoA accumulation in strain S2209
Itaconyl-CoA transferase (IctA) was shown to convert IA to itaconyl-CoA in A. niger, the step of IA degradation pathway [37]. To prevent the IA degradation, ictA encoding itaconyl-CoA transferase was deleted in S2209 to obtain recombinant strain S2288 [OEcadA (multiple copies), OEmttA, OEmfsA, OEadi1, OEtad1, OEitp1, ΔictA]. As shown in Fig. 6a, deletion of ictA resulted in IA accumulation with titers of 7.68 g/L and 8.70 g/L at 4-day and 6-day, respectively, indicating that ictA deletion is beneficial for IA accumulation. Additionally, Fig. 6b showed that the levels of residual sugar for strain S2288 was lower than that of S2209 in shake flask fermentation (Fig. 6b). These results indicate that blocking itaconyl-CoA formation likely directed more carbon for IA biosynthesis.
IA production in strain S2209 with ictA deletion. a The titer of IA generated by the indicated strains in shake flasks for 4 days and 6 days. b The residual glucose obtained for the indicated strains in shake flasks for 4 days and 6 days. Mean and standard deviation values were calculated from 3 independent Erlenmeyer flask samples. The “*” represents statistically significant difference by one-tailed Student’s test. OE represents overexpression. Δ represents deletion
Construction of an efficient IA-producing recombinant strain S2444
Cis-aconitic acid as a precursor of IA is formed by the dehydration of citric acid under the action of aconitase encoded by acoA [2]. Aconitase is mainly found in mitochondria, while cis-aconitic acid decarboxylase, aconitate-Δ-isomerase and trans-aconitate decarboxylase are located in the cytosol [35]. The positive effect of overexpression of the aconitase was also shown in A. niger [29] and E. coli [38]. Thus, acoA was overexpressed in the cytosol of S2288 to obtain more efficient IA-producing strain. As mentioned above, we next introduced the gene acoA into S2288 to create recombinant strain S2444 (Additional file 1: Fig. S11a, b). The overexpression of acoA enabled further accumulation of IA in A. niger S2444 with titers of 8.53 g/L and 9.08 g/L, respectively (Fig. 7a). A. terreus is a natural producer of IA. However, the relative high price of IA produced by A. terreus was partly due to its high purity requirements of industrial cultivation medium and sophisticated control of fermentation processes [39]. Recently, U. maydis was reported to be capable of IA accumulation with long fermentation period and low productivity [12]. By contrast, A. niger is an outstanding workhorse of organic acid production with low requirements for fermentation nutrition and excellent acid resistance. Additionally, A. niger is a robust cell factory for the accumulation of citric acid, an important precursor of IA biosynthesis, with high productivity and low cost.
IA production in strain S2288 overexpressing acoA in cytosol. a The titer of IA generated by the indicated strains in shake flasks for 4 days and 6 days. b The residual glucose obtained for the indicated strains in shake flasks for 4 days and 6 days. c The biomass measured by collecting mycelium of 6-days cultured in IA fermentation medium. d The determination of expression levels of genes involved in IA production by qRT-PCR in the control strain S2083 and strain S2444. Mean and standard deviation values were calculated from 3 independent Erlenmeyer flask samples. The “*” represents statistically significant difference by one-tailed Student’s test. OE represents overexpression. Δ represents deletion
acoA overexpression strain S2444 exhibited similar growth to S1075, S1596 and S2120 on PDA medium (Fig. 2d). Moreover, the glucose in S2444 fermentation broth was almost depleted (Fig. 7b), indicating that more carbon was converted to IA. These results were similar to previous studies, in which malic acid production was improved by reinforcing glycolytic pathway and intensifying glucose transport [40]. Compared with the starting strain S2288, the biomass of strain S2444 increased by 28.20% in shake flask fermentation (Fig. 7c). Additionally, we analyzed the transcript levels for 3 genes involved in IA production. Compared with strain S2083 overexpressing cadA and acoA, the expression levels of these two genes were increased by 2.23 and 1.55 folds in S2444, respectively, and transcripts for ictA were not detected (Fig. 7d).
The identification and functional analysis of key genes for IA biosynthesis and the metabolic engineering in the hosts, including A. terreus, U. maydis etc., provided valuable insights for guiding the construction of an A. niger cell factory for efficient IA production. Theoretically, the extrapolations are often tentative, organism specific and purely qualitative. Therefore, quantitative metabolic flux predictions based on the genetic modifications of the key components of IA biosynthesis and secretion pathways would further guide the rational design of designer strains of A. niger for more efficient IA production. Although directing more metabolic flux to citric acid and/or aconitic acid has been shown to enhance IA accumulation in recombinant A. niger, a quantitative model relating metabolic flux to IA titers is still lacking. Construction of a quantitative metabolic flux prediction model for IA titers will entail the measurement of the concentrations of key metabolites including citrate, cis-aconitate in both cytoplasm and mitochondria, as well as quantitative measurements of the expression levels of key relevant genes in the IA biosynthesis and secretion pathways of in engineered A. niger strains, thus accelerating the design and engineering of more efficient IA production strains using the A. niger chassis. Additionally, MES was used to maintain the pH in IA production in flask cultures in this study. Considering the cost of using high concentrations of MES, recently we further evaluated the potential of the recombinant strains to synthesize IA in fermentation tanks in which the pH was adjusted in real time by feeding NaOH. Our primary data indicated that the recombinant strains, such as S2444, produced titers of IA using NaOH as pH neutralizer comparable to that of using MES (data not reported). Thus, NaOH would provide a practical means to maintain a desirable pH for IA fermentation with our engineered strains. In fact, NaOH is widely used as a pH neutralizer in industry due to its efficiency and low cost.
Conclusion
A. niger is a powerful and versatile cell factory for industrial production of organic acids such as citric acid and malic acid. Engineering efficient A. niger cell factories for development of fermentation for commercial IA production represents an important research direction. Given that natural A. niger strains do not produce IA, engineering A. niger for efficient IA production depends on the construction of artificial IA biosynthesis route by recruiting and tuning of key components of IA synthetic pathways from other microorganisms. In this study, genes from IA biosynthesis clusters of A. terreus and U. maydis were overexpressed individually or in various combinations in A. niger. Our results showed that overexpressing adi1, tad1, mtt1 and itp1 from the U. maydis IA biosynthesis gene cluster enabled the production in A. niger, and combined expression of the two distinct IA biosynthesis gene clusters in strain S2083 (OEcadA, OEmttA, OEmfsA, OEadi1, OEtad1, OEitp1) demonstrated higher IA production than expression of either of the two biosynthesis routes. Furthermore, increasing the cadA copy number, blocking the IA degradation by ictA deletion and overexpressing acoA in A. niger all contributed to enhanced IA production in an additive manner, as shown in the recombinant strain S2444 [OEcadA (multiple copies), OEmttA, OEmfsA, OEadi1, OEtad1, OEitp1, ΔictA, OEacoA] achieving IA titers of 9.08 g/L.
Materials and methods
Strains and culture conditions
A. niger S422 derived from A. niger ATCC 1015 [33] was used as the parent strain to delete the citrate exporter-coding gene cexA to prevent citrate accumulation during IA fermentation. The resultant citrate-non-producing strain was named S1075. A. niger strains were grown at 28 °C on potato dextrose agar (PDA) supplemented with or without 250 μg/mL hygromycin B as previously described [40]. Selection for A. niger transformants was performed in the medium A composed of 30 g/L malt extract, 5 g/L tryptone, 218 g/L sorbitol, 20 g/L agar [41]. The recombination of loxP-hph-loxP loci was achieved in the PDA plates supplemented with 10 μg/mL doxycycline (DOX) as previously reported with slight modifications. Escherichia coli JM109 used for gene cloning was cultured at 37 °C in Luria Bertani (LB) medium [42]. A. niger mutants obtained in this study are listed in Table 2.
Plasmid construction
To express cadA derived from A. terreus in A. niger, cadA was amplified by PCR with primers 3501/3502 using pLH257 as a template, which contains the cadA gene synthesized by GENEWIZ. The Kpn I-digested PCR product was purified and ligated to the downstream of glyceraldehyde-3-phosphate dehydrogenase promoter (PgpdA) of pLH454. The resultant recombinant plasmid was named pLH786. The same strategy was applied to construction of recombinant plasmids for expression of mttA, mfsA, mtt1, itp1 and co-expression of adi1 and tad1, respectively. The resultant recombinant plasmids were named pLH850, pLH862, pLHl023, pLH991 and pLH941, respectively. The expression of mttA, mfsA, mtt1 and adi1 was controlled by PgpdA, while the expression of tad1 and itp1 were under the control of promoter PpkiA, a pyruvate kinase promoter of A. niger [40]. The cytoplasmic expression of AcoA was constructed by removing its putative mitochondrial localization signal with a 24 amino acid N-terminal, and the resultant recombinant plasmid carrying the acoA expression cassette was named pLH780. The ictA gene deletion plasmid was constructed by the same method as previously described [40]. Briefly, the 5’ and 3’ flanking sequences of ictA were respectively amplified with primers 3522/3523 and primers 3524/3525 using A. niger genomic DNA as a temple. The relevant sequences were digested and integrated into the flanks of loxP-hph-loxP to obtain plasmid pLH1008. The same strategy was applied to the construction of recombinant plasmids for deletion of cexA and oahA, respectively. All primers used in this study are listed in Additional file 1: Table S2.
Transformation of A. niger
Protoplast preparation was performed as previously described [43]. A. niger spores (1 × 108) were inoculated into 250 mL of liquid fermentation medium (20 g/L glucose, 10 g/L yeast extract, 1 g/L MgSO4, 1 g/L KH2PO4) and cultivated for 16 ~ 18 h at 28 °C. The mycelia were harvested by centrifugation at 12,000 rpm for 15 min and washed with sterile water. Protoplast formation was achieved in 10 mL of MC buffer (7.35 g/L CaCl2, 3.90 g/L Mes, 52.19 g/L KCl, pH5.8) containing cell wall digesting enzymes including lysing enzymes from Trichodema harzianum (Sigma®, L1412), yatalase (Takara) and ß-glucuronidase from Helix pomatia (Sigma G7017). Incubation was performed at 37 °C with 120 rpm for 4 h. To the resulting protoplasts, 10 mL cold STC buffer (218.6 g/L sorbitol, 7.35 g/L CaCl2, 1.21 g/L Tris, pH7.5) was added and centrifuged at 2,300 rpm at 4 °C for 10 min, followed by resuspending twice with STC buffer and directly used for transformation of A. niger. Plasmid DNA (50 μg) was mixed with 200 μL of protoplast suspension and 660 μL PEG solution (600 g/L PEG6000, 147.02 g/L CaCl2), and incubated on ice for 20 min, followed by addition of 2 mL PEG solution and incubation for 10 min at room temperature. The protoplast mixture was spread on the medium A with 4 mL of STC buffer and 4 mL of the medium B composed of 30 g/L malt extract, 5 g/L tryptone, 218 g/L sorbitol, 10 g/L agar, and cultivated for 4 ~ 6 days at 28 °C. Fungal colonies appearing on PDA supplemented with 250 μg/mL hygromycin B were selected and further verified by PCR analysis using specific primers [40].
Construction of target gene overexpression and deletion strains
Gene deletion and overexpression strains of A. niger were obtained by the polyethylene glycol (PEG)-mediated protoplast transformation system as described previously [43]. Briefly, plasmid DNA harboring cadA was integrated into A. niger S1075 genome through PEG-protoplast transformation. Transformants were selected on medium A supplemented with 250 μg/mL hygromycin B at 28 °C for 3 ~ 5 days and cadA integration event was determined by PCR analysis using primers 3501/641, and the generated OEcadA with loxP-hph-loxP cassette was named S1361. qRT-PCR was used to further determine gene overexpression or knockout in the mutants. As previously described, the hygromycin B sensitive strains were selected on PDA plates supplemented with 10 μg/mL DOX and verified via PCR analysis. The same strategy was used to construct mttA, mfsA, adi1, tad1, mtt1, itp1 and acoA overexpression strains and ictA deletion strains. All strains in this study are listed in Table 2.
Shake flask cultivation
For A. niger cultivation, 1 × 108 spores were added to 50 mL IA fermentation medium in 250 mL baffled Erlenmeyer flask and cultivated at 35 °C on rotary shaker of 250 rpm for 6 days. The medium used IA fermentation was previously described Vogel’s medium with slight modification [32]. IA fermentation medium was composed of 100 g/L glucose, 2.5 g/L Na3-citrate × 2H2O, 5 g/L KH2PO4, 2 g/L NH4NO3, 0.2 g/L MgSO4 × 7H2O, 0.1 g/L CaCl2 × H2O, 0.5 mg/L citric acid × H2O, 0.5 mg/L ZnSO4 × 7H2O, 0.1 mg/L Fe(NH4)2(SO4)2 × 7H2O, 0.025 mg/L CuSO4 × 5H2O, 0.005 mg/L MnSO4 × H2O, 0.005 mg/L H3BO3, 0.005 g/L Na2MoO4 × 2H2O, and 0.005 mg/L biotin without MnSO4. Furthermore, IA fermentation medium contains 1 M morpholinoethanesulfonic acid (MES), and the starting pH of the medium was 6.5.
Measurement of organic acid and glucose concentrations
The IA was detected using high-performance liquid chromatography (HPLC). The samples were centrifuged at 12,000 rpm. The supernatant was then diluted to 1:10 with ultrapure water and filtered through a 0.22 μm filter. The processed solution was measured by HPLC (Agilent 1260 VWD UV detector) applying Aminex HPX-87H column (Bio-Rad) thermostated at 65 °C [40]. This solution was monitored at wavelength 210 nm using a UV detector, with 5 mM H2SO4 as the mobile phase running at 0.6 mL/min based on the methods described previously [40]. The concentrations of each glucose were determined using SBA-40E biosensor analyzer (Biology Institute of Shandong Academy of Sciences, Jinan, China) [40].
Biomass measurement
Biomass analysis was determined according to the literature with slight modification [44]. Briefly, 10 mL of cultures in shake flasks was harvested at the end of fermentation. Then, collected samples were washed with distilled water and filtered, and dried at 85 °C to a constant weight to obtain cell dry weight.
RNA purification and transcription analysis
Spores (1 × 108) of A. niger strains were added to 50 mL IA fermentation medium in 250 mL Erlenmeyer flasks at 35 °C, 250 rpm. Mycelia were collected after 6 days of cultivation and ground with liquid nitrogen. Extractions of total RNA were performed using E.A.N.A.™ Fungal RNA Kit (Omega Bio-tek, Inc.) and treated with OBI DNase I (Omega Bio-tek, Inc.) according to the manufacturer’s instructions. The cDNA was obtained by PrimeScriptTMRT reagent Kit with gDNA Eraser Perfect Real Time (TaKaRa Biomedical Technology Co., Ltd). Quantitative real-time PCR (qRT-PCR) was performed on the Applied Biosystems StepOne TM Real-Time PCR System Thermal Cycling Block according to the manufacturer’s protocol. The calculated threshold cycle (Ct) values of the beta-actin gene was used as the corresponding endogenous reference for normalization of the results. The relative gene expression levels between mutant strain and the parent strain were analyzed using the qualified Ct (2−ΔΔCt) [40]. Primers used in this experiment are listed Additional file 1: Table S2.
Statistical analysis
All experiments were conducted in triplicate and data were expressed in mean values and standard deviations. Results were analyzed via a software of GraphPad Prism 8, and p < 0.05 was regarded as statistically significant.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- cadA :
-
Cis-Aconitate decarboxylase gene
- mttA :
-
Mitochondrial transporter gene
- mfsA :
-
A putative major facilitator superfamily transporter gene
- adi1 :
-
Aconitate-Δ-isomerase gene
- tad1 :
-
Trans-Aconitate decarboxylase gene
- mtt1 :
-
Mitochondrial transporter gene
- itp1 :
-
Itaconate transporter protein gene
- cexA :
-
Citrate exporter gene
- oahA :
-
Oxaloacetate acetylhydrolase gene
- acoA :
-
Aconitase gene
- ictA :
-
Itaconyl-CoA transferase gene
- PgpdA:
-
Glyceraldehyde-3-phosphate dehydrogenase promoter
- OE:
-
Overexpression
- IA:
-
Itaconic acid
- Ct:
-
The calculated threshold cycle
- PDA:
-
Potato dextrose agar
- LB:
-
Luria Bertani
- PEG:
-
The polyethylene glycol
- MES:
-
Morpholinoethanesulfonic acid
- DOX:
-
Doxycycline
- HPLC:
-
High-performance liquid chromatography
References
Mitsuyasu O, Dwiarti L, Shin K, Park EY. Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl Microbiol Biotechnol. 2009;84:597–606.
Zhao M, Lu X, Zong H, Li J, Zhuge B. Itaconic acid production in microorganisms. Biotechnol Lett. 2018;40:455–64.
Deeksha G, Vinod K, Khare SK. Recent advances in itaconic acid production from microbial cell factories. Biocatal Agric Biotechnol. 2021;36: 102130.
Krull S, Hevekerl A, Kuenz A, Prüße U. Process development of itaconic acid production by a natural wild type strain of Aspergillus terreus to reach industrially relevant final titers. Appl Microbiol Biotechnol. 2017;101:4063–72.
Wierckx N, Agrimi G, Lübeck PS, Steiger MG, Mira NP, Punt PJ. Metabolic specialization in itaconic acid production: a tale of two fungi. Curr Opin Biotechnol. 2020;62:153–9.
Geiser E, Przybilla SK, Friedrich A, Buckel W, Wierckx N, Blank LM, et al. Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate. Microb Biotechnol. 2015;9:116–26.
Takeshi T, Sugisawa T, Ishidori T, Nakahara T, Sugiyama J. Itaconic acid fermentation by a yeast belonging to the genus Candida. Agri Biol Chem. 1981;45:475–9.
William EL, Kurtzman CP, Kuo TM. Production of itaconic acid by Pseudozyma antarctica NRRL Y-7808 under nitrogen-limited growth conditions. Enzyme Microb Technol. 2006;39:824–7.
Kanamasa S, Dwiarti L, Okabe M, Park EY. Cloning and functional characterization of the cis-aconitic acid decarboxylase (CAD) gene from Aspergillus terreus. Appl Microbiol Biotechnol. 2008;80:223–9.
Huang X, Lu X, Li Y, Li X, Li JJ. Improving itaconic acid production through genetic engineering of an industrial Aspergillus terreus strain. Microb Cell Fact. 2014;13:1.
Klement T, Büchs J. Itaconic acid-a biotechnological process in change. Bioresource Technol. 2013;135:422–31.
Tehrani HH, Becker J, Bator I, Saur K, Meyer S, Lóia ACR, et al. Integrated strain- and process design enable production of 220 g L−1 itaconic acid with Ustilago maydis. Biotechnol Biofuels. 2019. https://doi.org/10.1186/s13068-019-1605-6.
Steiger MG, Blumhoff ML, Mattanovich D, Sauer M. Biochemistry of microbial itaconic acid production. Front Microbiol. 2013. https://doi.org/10.3389/fmicb.2013.00023.
Li A, Luijk NV, Beek MT, Caspers M, Punt P, Werf MVD. A clone-based transcriptomics approach for the identification of genes relevant for itaconic acid production in Aspergillus. Fungal Genet Biol. 2011;48:602–11.
Li A, Pfelzer N, Zuijderwijk R, Brickwedde A, Zeijl CV, Punt P. Reduced by-product formation and modified oxygen availability improve itaconic acid production in Aspergillus niger. Appl Microbiol Biotechnol. 2013;97:3901–11.
Straat LVD, Vernooij M, Lammers M, Berg WVD, Schonewille T, Cordewener J, et al. Expression of the Aspergillus terreus itaconic acid biosynthesis cluster in Aspergillus niger. Microb Cell Fact. 2014;13(1):11.
Hossain AH, Li A, Brickwedde A, Wilms L, Caspers M, Overkamp K, et al. Rewiring a secondary metabolite pathway towards itaconic acid production in Aspergillus niger. Microb Cell Fact. 2016. https://doi.org/10.1186/s12934-016-0527-2.
Saha BC, Kennedy GJ, Qureshi N, Bowman MJ. Production of itaconic acid from pentose sugars by Aspergillus terreus. Biotechnol Prog. 2017;33:1059–67.
Kuenz A, Gallenmüller Y, Willke T, Vorlop KD. Microbial production of itaconic acid: developing a stable platform for high product concentrations. Appl Microbiol Biotechnol. 2012;96:1209–16.
Hevekerl A, Kuenz A, Vorlop KD. Filamentous fungi in microtiter plates—an easy way to optimize itaconic acid production with Aspergillus terreus. Appl Microbiol Biotechnol. 2014;98:6983–9.
Hevekerl A, Kuenz A, Vorlop KD. Influence of the pH on the itaconic acid production with Aspergillus terreus. Appl Microbiol Biotechnol. 2014;98:10005–12.
Zambanini T, Tehrani HH, Geiser E, Merker D, Schleese S, Krabbe J, et al. Efficient itaconic acid production from glycerol with Ustilago vetiveriae TZ1. Biotechnol Biofuels. 2017. https://doi.org/10.1186/s13068-017-0809-x.
Okamoto S, Chin T, Hiratsuka K, Aso Y, Tanaka Y, Takahashi T, et al. Production of itaconic acid using metabolically engineered Escherichia coli. J Gen Appl Microbiol. 2014;60:191–7.
Harder BJ, Bettenbrock K, Klamt S. Model-based metabolic engineering enables high yield itaconic acid production by Escherichia coli. Metab Eng. 2016;38:29–37.
Blazeck J, Miller J, Pan A, Gengler J, Holden C, Jamoussi M, et al. Metabolic engineering of Saccharomyces cerevisiae for itaconic acid production. Appl Microbiol Biotechnol. 2014;98:8155–64.
Young EM, Zhao Z, Gielesen BEM, Wu L, Gordon DB, Roubos JA, et al. Iterative algorithm-guided design of massive strain libraries, applied to itaconic acid production in yeast. Metab Eng. 2018;48:33–43.
Blazeck J, Hill A, Jamoussi M, Pan A, Miller J, Alper HS. Metabolic engineering of Yarrowia lipolytica for itaconic acid production. Metab Eng. 2015;32:66–73.
Otten A, Brocker M, Bott M. Metabolic engineering of Corynebacterium glutamicum for the production of itaconate. Metab Eng. 2015;30:156–65.
Blumhoff ML, Steiger MG, Mattanovich D, Sauer M. Targeting enzymes to the right compartment: metabolic engineering for itaconic acid production by Aspergillus niger. Metab Eng. 2013;19:26–32.
Yin X, Shin HD, Li J, Du G, Liu L, Chen J, Kivisaar M. Pgas, a low-pH-induced promoter, as a tool for dynamic control of gene expression for metabolic engineering of Aspergillus niger. Appl Environ Microbiol. 2017. https://doi.org/10.1128/AEM.03222-16.
Timothy C, Cairns LB, Meyer V. Something old, something new: challenges and developments in Aspergillus niger biotechnology. Essays Biochem. 2021;65:213–24.
Blumhoff M, Steiger MG, Marx H, Mattanovich D, Sauer M. Six novel constitutive promoters for metabolic engineering of Aspergillus niger. Appl Microbiol Biotechnol. 2012;97:259–67.
Xu Y, Shan L, Zhou Y, Xie Z, Ball AS, Cao W, et al. Development of a Cre-loxP-based genetic system in Aspergillus niger ATCC1015 and its application to construction of efficient organic acid-producing cell factories. Appl Microbiol Biotechnol. 2019;103:8105–14.
Wyrzykowski D, Hebanowska E, Wiczk GN, Makowski M, Chmurzyński L. Thermal behaviour of citric acid and isomeric aconitic acids. J Therm Anal Calorim. 2011;104(2):731–5.
Bafana R, Pandey RA. New approaches for itaconic acid production: bottlenecks and possible remedies. Crit Rev Biotechnol. 2017;38:68–82.
Kim J, Seo HM, Bhatia SK, Song HS, Kim JH, Jeon JM, et al. Production of itaconate by wholecell bioconversion of citrate mediated by expression of multiple cis-aconitate decarboxylase (cadA) genes in Escherichia coli. Sci Rep. 2016;7:39768.
Hossain AH, Beek AT, Punt PJ. Itaconic acid degradation in Aspergillus niger: the role of unexpected bioconversion pathways. Fungal Biol Biotechnol. 2019. https://doi.org/10.1186/s40694-018-0062-5.
Vuoristo KS, Mars AE, Sangra JV, Springer J, Eggink G, Sanders JPM, et al. Metabolic engineering of itaconate production in Escherichia coli. Appl Microbiol Biotechnol. 2014;99:221–8.
Kuenz A, Krull S. Biotechnological production of itaconic acid—things you have to know. Appl Microbiol Biotechnol. 2018;102:3901–14.
Xu YX, Zhou YT, Cao W, Liu H. Improved production of malic acid in Aspergillus niger by abolishing citric acid accumulation and enhancing glycolytic flux. ACS Synth Biol. 2020;9:1418–25.
Zhang J, Liu C, Xie Y, Li N, Ning Z, Du N, et al. Enhancing fructooligosaccharides production by genetic improvement of the industrial fungus Aspergillus niger ATCC 20611. J Biotechnol. 2017;249:25–33.
Cao W, Yan L, Li M, Liu X, Xu Y, Xie Z, et al. Identification and engineering a C4-dicarboxylate transporter for improvement of malic acid production in Aspergillus niger. Appl Microbiol Biotechnol. 2020;104:9773–83.
Liu Z, Friesen TL. Polyethylene glycol (PEG)-mediated transformation in filamentous fungal pathogens. Methods Mol Biol. 2012;835:365.
Brown SH, Bashkirova L, Berka R, Chandler T, Doty T, McCall K, et al. Metabolic engineering of Aspergillus oryzae NRRL 3488. Appl Microbiol Biotechnol. 2013;97:89.
Acknowledgements
The authors would like to thank Dr. Jiao Liu, Weixia Gao and Zhoujie Xie for their guidance on experiment. Thanks to Prof. Xiaoguang Liu for his valuable comments.
Funding
This study was supported by the National Key Research and Development Program of China (2021YFC2100700) and the Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-KJGG-006).
Author information
Authors and Affiliations
Contributions
HL and WC designed the experiments. YW and WC performed most of the experiments. YW, YG, WC and HL analyzed the results. YW, WC and HL wrote the manuscript with the contribution from all other authors. All work was under the supervision of HL. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Additional file figures. Figure S1. HPLC analysis of extracellular oxalic acid and citric acid in A. niger S1075; Figure S2. Screening of transformants of A. niger S1075 with cadA overexpression; Figure S3. Screening of transformants of A. niger S1361 with mttA overexpression; Figure S4. Screening of transformants of A. niger S1486 with mfsA overexpression; Figure S5. Screening of transformants of A. niger S1075 with adi1 and tad1 co-overexpression; Figure S6. Screening of transformants of A. niger S1683 with mtt1 overexpression; Figure S7. Screening of transformants of A. niger S1738 with itp1 overexpression; Figure S8. Screening of transformants of A. niger S1596 with adi1 and tad1 co-overexpression; Figure S9. Screening of transformants of A. niger S1779 with itp1 overexpression; Figure S10. Screening of transformants of A. niger S2083 with increased cadA gene copy number; Figure S11. Screening of transformants of A. niger S2288 with acoA overexpression. Additional file tables. Table S1. Extracellular organic acid (mM) in different strains at 4-day and 6-day fermentation; Table S2. Primers used in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Y., Guo, Y., Cao, W. et al. Synergistic effects on itaconic acid production in engineered Aspergillus niger expressing the two distinct biosynthesis clusters from Aspergillus terreus and Ustilago maydis. Microb Cell Fact 21, 158 (2022). https://doi.org/10.1186/s12934-022-01881-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-022-01881-7