Abstract
Background
This study aimed to assess the long-term effects of tofogliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, on atherosclerosis progression and major clinical parameters in patients with type 2 diabetes lacking an apparent history of cardiovascular disease.
Methods
This was a prospective observational 2-year extension study of the “Using TOfogliflozin for Possible better Intervention against Atherosclerosis for type 2 diabetes patients (UTOPIA)” trial, a 2-year randomized intervention study. The primary endpoints represented changes in the carotid intima-media thickness (IMT). Secondary endpoints included brachial-ankle pulse wave velocity (baPWV) and biomarkers for glucose metabolism, lipid metabolism, renal function, and cardiovascular risks.
Results
The mean IMT of the common carotid artery (IMT-CCA) significantly decreased in both the tofogliflozin (− 0.067 mm, standard error 0.009, p < 0.001) and conventional treatment groups (− 0.080 mm, SE 0.009, p < 0.001) throughout the follow-up period; however, no significant intergroup differences in the changes (0.013 mm, 95% confidence interval (CI) − 0.012 to 0.037, p = 0.32) were observed in a mixed-effects model for repeated measures. baPWV significantly increased in the conventional treatment group (82.7 ± 210.3 cm/s, p = 0.008) but not in the tofogliflozin group (− 17.5 ± 221.3 cm/s, p = 0.54), resulting in a significant intergroup difference in changes (− 100.2 cm/s, 95% CI − 182.8 to − 17.5, p = 0.018). Compared to the conventional treatment group, tofogliflozin significantly improved the hemoglobin A1c and high-density lipoprotein cholesterol levels, body mass index, abdominal circumference, and systolic blood pressure. The frequencies of total and serious adverse events did not vary significantly between the groups.
Conclusions
Tofogliflozin was not associated with improved inhibition of carotid wall thickening but exerted long-term positive effects on various cardiovascular risk factors and baPWV while showing a good safety profile.
Similar content being viewed by others
Background
Sodium-glucose cotransporter 2 (SGLT2) inhibitors lower blood glucose by promoting urinary glucose excretion in an insulin-independent manner. Hence, SGLT2 inhibitors are associated with a lower risk of hypoglycemia than conventional anti-diabetic agents. SGLT2 inhibitors also have pleiotropic effects, such as reducing body weight [1], visceral adipose tissue [2], and blood pressure [3]; improving lipid profile [4]; renal protection [5]; and remarkable cardiovascular protection [6, 7].
Tofogliflozin is an SGLT2 inhibitor with high selectivity for SGLT2. Suzuki et al. measured the sodium-dependent glucose uptake in SGLT2-overexpressing cells and evaluated SGLT2 selectivity against SGLT1, expressed as the ratio of inhibitory concentration 50 (IC50) for each compound. Their study showed that tofogliflozin (2900-fold) was highly selective compared to dapagliflozin (610-fold), canagliflozin (290-fold), ipragliflozin (860-fold), empagliflozin (1100-fold), and luseogliflozin (1600-fold) [8]. Tofogliflozin also has a short elimination half-life (5–6 h) with a high level of urinary excretion [9], which reduces the risk of nocturnal hypoglycemia [10]. In addition to its effect on glycemic control, tofogliflozin improves high-density lipoprotein cholesterol (HDL-C) and triglyceride (TG) levels, decreases body weight and blood pressure, and elevates circulating adiponectin levels [11]. We previously investigated the effect of tofogliflozin on carotid artery intima-media thickness (IMT) [12], which is a marker of atherosclerosis [13], arterial stiffness [14], and patients’ quality of life (QOL) [15], in the “Using TOfogliflozin for Possible better Intervention against Atherosclerosis for type 2 diabetes patients (UTOPIA)” trial. Although tofogliflozin did not delay the progression of IMT thickening, it significantly improved glycemic control, body weight, body mass index (BMI), abdominal circumference, systolic blood pressure, HDL-C, arterial stiffness, and treatment-related QOL [12, 14, 15] during the 2-year intervention period.
In several long-term observational follow-up studies after randomized controlled trials (RCTs) of anti-diabetic agents, it has been shown that emergent risk reductions for diabetic complications were observed during the post-trial follow-up period, highlighting the benefits of early and intensive glycemic control [16]. However, the long-term effects of tofogliflozin on atherosclerosis progression in patients with type 2 diabetes lacking an apparent history of cardiovascular disease have not been investigated. In this prospective observational 2-year extension study of the UTOPIA trial, we aimed to assess the long-term effects of tofogliflozin on the progression of atherosclerosis and major clinical parameters in patients with type 2 diabetes mellitus without an apparent history of cardiovascular disease.
Methods
Study design
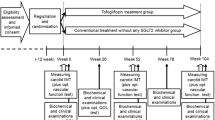
This was a prospective observational 2-year extension study of the UTOPIA trial [17], which was conducted at 22 medical institutions in Japan (Additional file 1). The main UTOPIA study and this extension study were registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR), a non-profit organization in Japan, and met the requirements of the International Committee of Medical Journal Editors (UMIN000017607 and UMIN000032601, respectively). Patient enrollment was conducted between January 2018 and April 2019, and each enrolled patient was observed for 2 years. The study protocol was approved by the Ethical Review Board of Osaka University Hospital and the institutional review board of each participating center according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Ministry of Health, Labour and Welfare in Japan. The study was conducted in accordance with the Declaration of Helsinki, the Ethical Guidelines for Medical and Health Research Involving Human Subjects, and other current legal regulations in Japan. To avoid bias, data management and monitoring were conducted by a third-party entity (Soiken Inc., Toyonaka, Osaka, Japan). All the authors vouch for the completeness and accuracy of the data and the analyses and for the fidelity of the trial to the protocol.
Study population
Japanese subjects with type 2 diabetes mellitus who had participated in the main UTOPIA study were asked to participate in this extension study. The sample size of the main UTOPIA study was calculated as follows: the increase in carotid IMT in diabetic patients is estimated to be 0.034 ± 0.054 mm/year (mean ± standard deviation [SD]), and a 1% improvement in hemoglobin A1c (HbA1c) levels improves IMT by 0.02 mm/year [18]. Therefore, to obtain 90% power to detect a difference of 0.04 mm in IMT between the two groups during the 2-year observation period, with an SD of 0.108 mm for the individual difference estimated to be common to both groups and a significance level of 0.05, at least 155 patients in each group must be enrolled. The dropout rate during the 2-year observation period was assumed to be 10%, and the target number of patients to be enrolled was 340 (170 in each group) [17]. As the number of subjects in the Extension study was reduced to 146 in the tofogliflozin group and 145 in the conventional treatment group, the power to detect a difference of 0.04 mm between the groups under the condition of α error = 0.05 was calculated to be 0.88.
The inclusion criterion for this extension study was as follows: subjects who had participated in the main UTOPIA study and provided written informed consent to participate in this extension study after a full explanation of the extension study. The exclusion criterion for this extension study was as follows: subjects deemed unsuitable for participation in this extension study by the investigators. The inclusion criteria for the main study were as follows: (1) Japanese patients with type 2 diabetes mellitus who are not achieving the glycemic control goals described in the “Treatment Guide for Diabetes 2014–2015” edited by the Japan Diabetes Society [19] and have an HbA1c level of < 9% (according to this guide, if it can be achieved with appropriate diet and exercise alone or without side effects such as hypoglycemia while on drug therapy, the target HbA1c level should be < 6%, otherwise < 7% was set to the target value, respectively); (2) no changes (including new prescriptions) in their antidiabetic, antithrombotic, or antihypertensive medications or therapeutic agents for dyslipidemia for at least 12 weeks before signing the consent form; (3) age 30–74 at the time of giving consent; and (4) ability to provide informed consent. The exclusion criteria for the main study were as follows: (1) type 1 or secondary diabetes; (2) in the perioperative period or having a serious infection or injury; (3) a history of myocardial infarction, angina, stroke, or cerebral infarction; (4) severe renal dysfunction or end-stage renal failure (estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2); (5) serious liver function impairment (aspartate aminotransferase ≥ 100 U/L); (6) moderate to severe heart failure (class 3 or worse based on the New York Heart Association Functional Classification); (7) urinary tract or genital infection; (8) pregnancy, possible pregnancy, nursing, or planning to conceive a child; (9) history of hypersensitivity to the study drug; (10) present or past history of a malignant tumor (exceptions: patients not on medication for a malignant tumor with no recurrence of the disease thus far and no recurrence risks during the study were allowed to participate); (11) prohibited from using tofogliflozin; or (12) other ineligibility determined by an investigator.
Randomization and study intervention
As this was a prospective observational extension study of the UTOPIA trial, no randomization was conducted, and no drug or treatment was prohibited or restricted in this study. In the main UTOPIA study, the enrolled subjects were randomly assigned to a tofogliflozin treatment group or a conventional treatment group without SGLT2 inhibitors. In the tofogliflozin group, 20 mg of tofogliflozin once daily was started in addition to the ongoing therapy. However, the addition of an alternative antidiabetic agent (excluding another SGLT2 inhibitor) was permitted 12 weeks after randomization. In the conventional treatment group, either the dosage of the ongoing therapy was increased, or a concomitant oral glucose-lowering drug (excluding any other SGLT2 inhibitor) was added 12 weeks after randomization. Treatment was continued to achieve the target value specified in the Japanese Treatment Guide for Diabetes [19] (generally an HbA1c level < 7.0%) in both groups. In case of hypoglycemia, the dosage of the concomitant oral glucose-lowering drug was titrated.
Observation items and schedule
The enrolled subjects were observed for 104 weeks in the main study followed by additional 104 weeks in this extension study, totaling 208 weeks. In the main study, clinical and biochemical data were collected at 0, 26, 52, 78, and 104 weeks after randomization, and in this extension study, the data were collected at 156 and 208 weeks after the randomization in the main study.
Study outcomes
The primary endpoints in this extension study were the changes in the mean and maximum carotid intima-media thickness of the common carotid artery (IMT-CCA) measured by carotid arterial echography from the baseline (week 0) in the main study to week 208. In particular, the change in the mean of the right and left mean IMT-CCA from baseline to week 208 was considered the most important endpoint in this study. Secondary endpoints included changes in biomarkers of glycemic control (HbA1c and fasting blood glucose), lipids (total cholesterol (TC), HDL-C, TG, and low-density lipoprotein cholesterol (LDL-C)), renal function (serum creatinine, urinary albumin excretion (UAE), and eGFR), and arteriosclerosis and cardiovascular risks (brachial-ankle pulse wave velocity (baPWV) and ankle-brachial index (ABI)), the frequency of cardiovascular events including ischemic heart disease (sudden cardiac death, acute myocardial infarction, unstable angina, and undergoing coronary revascularization), cerebrovascular disorders (cerebral infarction, intracerebral hemorrhage, and subarachnoid hemorrhage), and peripheral arterial disease (arteriosclerosis obliterans and leg amputation), and the frequency of adverse events.
Measurement of carotid IMT
Ultrasonography scans of the carotid artery were performed by expert, specifically-trained sonographers based on the Japan Society of Ultrasonics in Medicine guidelines [20]. To avoid inter-sonographer variability, each participant was examined by the same sonographer with the same equipment (high-resolution B-mode ultrasound scanner equipped with a high-frequency [> 7.5 MHz] linear transducer with a limit of detection of < 0.1 mm) throughout all the visits. Scanning of the extracranial carotid artery was performed bilaterally in different longitudinal and transverse projections, and the site of greatest thickness, including plaque lesions, was sought along the arterial walls. The IMT was measured as the distance between two parallel echogenic lines corresponding to the vascular lumen and the adventitial layer.
To avoid inter-reader variability, all scans were stored electronically and sent to the IMT evaluation committee. The sent images were inspected in random order by experienced investigators who were not informed of the clinical characteristics of the subjects, using automated digital edge detection software (Intimascope; MediaCross, Tokyo, Japan) [21]. The software system averaged approximately 200 points of the IMT values in the segment 2 cm proximal to the dilation of the carotid sinus (mean-IMT-CCA). In addition, the maximum thicknesses of the intima and media layers, including the plaque lesions, in the common carotid arteries (max-IMT-CCA) were captured separately. The same systematic procedures for analyzing carotid IMT were used in our previous studies [22, 23]. Reproducibility analysis of replicate measurements in the randomly selected 20 subjects yielded absolute mean differences of 0.02 ± 0.01 and 0.01 ± 0.01 for mean-IMT-CCA and max-IMT-CCA, respectively. The intra-observer coefficients of variation for the measurements of mean-IMT-CCA and max-IMT-CCA were 1.1% and 0.7%, respectively.
Statistical analysis
Analyses of the primary and secondary endpoints were performed using data from the full analysis set (FAS) population, including all subjects enrolled in this extension study, and excluding those violating the protocol severely (without written consent, enrollment outside the contract period, etc.). In principle, missing values were not complemented.
Primary analysis was performed using the mixed-effects model for repeated measures (MMRM) with the treatment group, time (week), and interactions between the treatment group and time (week) as fixed effects, and subjects nested by group as random effects. An unstructured covariate was used to model the covariance of within-subject variability. The Kenward-Roger method was used to estimate the degrees of freedom. The sensitivity analysis assessed differences in changes in IMT-CCA from baseline between the two groups using analysis of covariance (ANCOVA) models that included the treatment group, age, sex, baseline IMT-CCA, systolic blood pressure, and administration of statins. For the occurrence of cardiovascular events as a secondary outcome, the time to onset was analyzed using the log-rank test and Cox proportional hazard model.
Baseline and follow-up group comparisons were performed using Student’s t-test or Wilcoxon rank-sum test for continuous variables and Fisher’s exact test or chi-square test for categorical variables. Changes from baseline to treatment visits were assessed using a paired t-test and Wilcoxon signed-rank test within the group. The frequency and proportion of patients reporting AEs were derived from each treatment group and compared using Fisher’s exact test.
All p values were two-sided. Statistical significance was set at p < 0.05. All analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA). All statistical analyses were performed by a third-party entity (Soiken Inc.) under the supervision of an independent biostatistician. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Allocation and patient characteristics
In the main UTOPIA study, 340 participants were randomly allocated to either the tofogliflozin group (n = 169) or the conventional treatment group (n = 171) between January 2016 and November 2016. Of these, 304 completed the follow-up. After the completion of the follow-up in the main UTOPIA study, between June 2018 and April 2019, each participant was asked to participate in this UTOPIA Extension study. Since 13 participants were excluded (6 participants did not give consent to participate in this UTOPIA Extension study, and 7 participants discontinued the hospital visits), 291 participants were enrolled in this UTOPIA Extension study. Of these, 146 and 145 were assigned to the tofogliflozin and conventional treatment groups, respectively, in the UTOPIA Extension study (Fig. 1). The characteristics of patients who participated in the UTOPIA Extension study, as recorded at week 0 (baseline) of the main UTOPIA study, were well balanced between the two groups, except for the use of angiotensin II receptor blockers and eicosapentaenoic acid (Table 1).
Biomarkers for arteriosclerosis
The common mean-IMT-CCA, right max-IMT-CCA, and left max-IMT-CCA at baseline in the tofogliflozin conventional treatment groups are shown in Table 2. Plaques and/or thickened (focal IMT ≥ 1.0 mm) lesions were observed in the right CCA in 208 patients (99 and 109 in the tofogliflozin and conventional treatment groups, respectively), in the left CCA in 218 patients (107 and 111 in the tofogliflozin and conventional treatment groups, respectively), and bilaterally in 147 patients (65 and 82 in the tofogliflozin and conventional treatment groups, respectively).
During the main UTOPIA study (from baseline to week 104), the common mean-IMT-CCA and right and left max-IMT-CCA significantly decreased in both the tofogliflozin and conventional treatment groups, although no significant intergroup differences in the changes were observed in a mixed-effects model for repeated measures. The common mean-IMT-CCA showed a significant reduction throughout the follow-up period (from baseline to the end of the UTOPIA Extension study in both groups (week 208)); however, no significant intergroup differences in the changes were observed (Table 2). In the tofogliflozin group, both the right and left max-IMT-CCA showed a tendency to decrease throughout the follow-up period, but the difference was not statistically significant (p = 0.09 and p = 0.07, respectively). In the conventional treatment group, the right max-IMT-CCA significantly decreased throughout the follow-up period; however, the significant reduction in the left max-IMT-CCA at week 104 disappeared at week 208. No significant intergroup differences in the changes were observed in the right and left max-IMT-CCA throughout the follow-up period. Moreover, ANCOVA models that included treatment group, age, sex, use of insulin, baseline IMT, systolic blood pressure, and administration of statins produced findings that resembled those generated by the mixed-effects models (data not shown).
The mean baPWV significantly increased in the conventional treatment group throughout the follow-up period and was maintained or even decreased in the tofogliflozin group, resulting in a significant intergroup difference in the changes throughout the follow-up period (Table 2).
The ABI did not change significantly throughout the follow-up period in either group. When the ABI was classified into three categories (abnormally low (ABI ≤ 0.9), within the normal range (0.9 < ABI < 1.3), and abnormally high (1.3 ≤ ABI)), the proportion of patients whose ABI was within the normal range was as high as more than 95% at baseline, and no significant intergroup differences were observed throughout the follow-up period (Additional file 2).
Glycemic control
During the main UTOPIA study, HbA1c and fasting blood glucose levels significantly decreased in the tofogliflozin group but not in the conventional treatment group, resulting in a significant intergroup difference in changes. Significant improvements in HbA1c and fasting blood levels were maintained throughout the follow-up period in the tofogliflozin group (Table 3). Significant intergroup differences in the changes in HbA1c levels were also maintained throughout the follow-up period. There was no difference in the proportion of patients treated with other glucose-lowering agents throughout the observation period; however, the tofogliflozin group tended to have fewer patients treated with biguanides and DPP-4 inhibitors than did the conventional treatment group (Additional file 3).
Renal function and lipid metabolism
Serum creatinine levels significantly increased and the eGFR significantly decreased in both groups throughout the follow-up period, but no significant intergroup differences in the changes were observed (Table 3).
Total cholesterol and LDL-C levels significantly decreased in the conventional treatment group but not in the tofogliflozin group throughout the follow-up period, although no significant intergroup differences in the changes were observed (Table 3). HDL-C levels significantly increased in both groups throughout the main UTOPIA study and the follow-up period, and a significant intergroup difference in changes was observed at week 208; the increase was larger in the tofogliflozin group than in the conventional treatment group. Triglyceride levels did not change significantly in either group throughout the follow-up period.
BMI and blood pressure
The BMI was significantly decreased in the tofogliflozin group throughout the follow-up period but did not change significantly during the main UTOPIA study. However, in the conventional treatment group, the BMI decreased significantly from baseline to the end of the UTOPIA Extension study, resulting in a significant intergroup difference in changes throughout the follow-up period (Table 3). Waist circumference was significantly decreased in the tofogliflozin group throughout the follow-up period but was unchanged in the conventional treatment group. There was a significant intergroup difference in changes throughout the follow-up period.
Systolic and diastolic blood pressure were significantly decreased throughout the follow-up period in the tofogliflozin group, but no significant intragroup change in blood pressure was observed throughout the follow-up period in the conventional treatment group. Although a significant intergroup difference in the changes in systolic blood pressure was observed throughout the follow-up period, no significant intergroup difference in the changes in diastolic blood pressure was observed throughout the follow-up period.
Safety
The frequency of cardiovascular events was compared between the groups. The frequency of the predefined composite endpoint comprising cardiovascular mortality, acute myocardial infarction, unstable angina, and heart failure was significantly lower in the tofogliflozin group than in the conventional treatment group in the log-rank test (Additional file 4. The frequency of study agent-related events (hypoglycemia, urinary tract and genital infection, skin manifestations, and body fluid reduction) (Additional file 5) and renal events (Additional file 6) did not significantly differ between the groups.
The adverse events (AEs) that occurred during the study are listed in Additional file 7. During the follow-up period, 88 participants (60.3%) in the tofogliflozin group and 96 participants (66.2%) in the conventional treatment group developed AEs. Furthermore, 45 participants (30.8%) in the tofogliflozin group and 47 participants (32.4%) in the conventional treatment group developed serious AEs. The overall incidence of AEs and serious AEs as well as the incidence of each AE did not vary significantly between the groups.
Discussion
This UTOPIA Extension study showed that tofogliflozin was not associated with improved inhibition of carotid wall thickening but induced long-term attenuation of baPWV progression and improvement of HbA1c and HDL-C levels, BMI, waist circumference, and systolic blood pressure while maintaining a good safety profile. This study is the first to demonstrate that the beneficial effect of SGLT2 inhibitors on arterial stiffness lasts for a long time (more than 4 years).
The main UTOPIA study was the first RCT to assess the effect of SGLT2 inhibitors on carotid atherosclerosis, which demonstrated a significant decrease in IMT with tofogliflozin treatment. Similarly, in this UTOPIA Extension study, a significant reduction in the mean IMT-CCA and a tendency towards decreased right and left max-IMT-CCA were maintained throughout the follow-up period in the tofogliflozin treatment group (Table 2). However, as a significant IMT reduction was also observed in the control group, no significant intergroup difference was detected in both the main and extended UTOPIA studies. Thus, the series of UTOPIA studies have not demonstrated a beneficial effect of tofogliflozin on carotid atherosclerosis.
One possible explanation for these findings is that the effect of tofogliflozin on the atherosclerotic changes that occur in the large arteries, including the carotid arteries, is limited. Indeed, a sub-analysis of the main UTOPIA trial has recently shown that the tissue characteristics of the carotid arterial wall did not change in either the tofogliflozin or conventional treatment groups during a 104-week treatment period [24]. In contrast, Irace et al. reported a significant decrease in carotid IMT after only 3 months of treatment with empagliflozin, another SGLT2 inhibitor [25]. They suggested that this early reduction in IMT may be related to hemodynamic changes that occur during empagliflozin treatment. These findings may be consistent with the fact that although RCTs of SGLT2 inhibitors reported decreased cardiovascular events [6, 7], these treatments did not reduce atherothrombotic events such as myocardial infarction and stroke [6, 7, 26].
It is also possible that the beneficial effects of tofogliflozin on the carotid wall, if any, were masked by the administration of additional antidiabetic, antihypertensive, and antilipidemic agents. Biguanides [27, 28], dipeptidyl peptidase-4 inhibitors [22, 23], angiotensin II receptor blockers [29, 30], and antilipidemic agents [31, 32] were reported to have inhibitory effects on the progression of IMT thickening, and more than half the participants in the UTOPIA Extension study used these drugs. Moreover, there was a difference between the two groups in the rates of biguanide and dipeptidyl peptidase-4 inhibitor use during the observation period, although the difference was not statistically significant. Similarly, there were imbalances in the administration of angiotensin II receptor blockers (37.7% and 50.3% in the tofogliflozin and conventional treatment groups at baseline (p = 0.034), respectively) and lipid-lowering agents (49.3% and 60.0% in the tofogliflozin and conventional treatment groups at baseline (p = 0.08), respectively), and these imbalances were observed throughout the study period. Further trials are required to evaluate the effect of SGLT2 inhibitors on IMT progression in first-line therapy with SGLT2 inhibitors.
In contrast, the increase in baPWV, an indicator of arterial stiffness, was significantly attenuated with tofogliflozin treatment but not with conventional treatment in both the UTOPIA main and extension studies (Table 2). baPWV is associated with various cardiovascular risk factors [33, 34] and could be a predictor of future cardiovascular events. Evidence on the effect of SGLT2 inhibitors on arterial stiffness remains controversial. Some studies reported beneficial effects of other SGLT2 inhibitors similar to those observed in the UTOPIA main and extension studies using other indices of arterial stiffness [35,36,37], whereas others reported no benefit of SGLT2 inhibitors on arterial stiffness [38]. However, the observation periods in these previous studies were relatively short.
The results of the UTOPIA main and extension studies indicate that tofogliflozin could have different effects on IMT and baPWV. IMT and baPWV are both indicators of atherosclerosis; however, they are believed to reflect different aspects of cardiovascular risk. Carotid IMT primarily reflects the degree of atherosclerosis, particularly structural changes that develop in large arteries [39]. In contrast, baPWV reflects deterioration in the elasticity of arterial walls due to functional and structural arteriosclerotic changes that occur in the area extending from the aorta to the arteries of the extremities [40, 41]. Loss of arterial wall elasticity increases vascular wall stress, resulting in atherosclerosis, elevated left ventricular afterload, left ventricular diastolic dysfunction, and coronary perfusion disorder. Therefore, baPWV may reflect not only atherosclerotic changes but also the risk for various cardiovascular disorders, including cardiac dysfunction.
Recently, there has been a growing number of research reports suggesting a cardiovascular protective effect of tofogliflozin. Kishima et al. conducted a prospective RCT to compare the suppressive effects of tofogliflozin and anagliptin, a DPP-4 inhibitor, on atrial fibrillation recurrence after catheter ablation in patients with type 2 diabetes mellitus. In their study, greater suppression of atrial fibrillation recurrence was achieved with tofogliflozin than with anagliptin [42]. In addition, Joki et al. reported that tofogliflozin treatment ameliorated right heart overload and pulmonary vascular remodeling in an experimental rodent model [43]. To compare subsequent cardiovascular risks, including heart failure, myocardial infarction, angina pectoris, stroke, and atrial fibrillation, following the use of different SGLT2 inhibitors, Suzuki et al. analyzed a large-scale real-world dataset comprising approximately 25,000 patients with diabetes mellitus who were newly prescribed SGLT2 inhibitors (empagliflozin, dapagliflozin, canagliflozin, ipragliflozin, tofogliflozin, and luseogliflozin) in Japan. The risk for subsequent development of these outcomes was comparable between the individual SGLT2 inhibitors [44]. Despite the lack of RCTs on tofogliflozin with atherosclerotic cardiovascular disease or heart failure as primary outcomes and, thus, the lack of sufficient evidence, it is likely that tofogliflozin, like other SGLT2 inhibitors, has a beneficial effect on cardiovascular disease, particularly on cardiac function and heart failure.
In this study, the tofogliflozin group showed significantly lower HbA1c levels at week 208 compared with baseline, and this reduction in HbA1c levels was significantly greater than that in the conventional treatment group. Throughout the observation period, there was no difference between the treatment groups in the proportion of patients concomitantly receiving other diabetes medications. This suggests that the effect of tofogliflozin on glycemic control lasts at least 4 years. Although tofogliflozin did not affect serum TC, LDL-C, and TG levels, it elevated serum HDL-C levels compared to conventional treatment, even during the UTOPIA Extension study (Table 3). As low HDL-C levels are considered a cardiovascular risk factor, the long-lasting effect of tofogliflozin on serum HDL-C levels, which persisted for years, may lead to cardiovascular protection. Tofogliflozin treatment was also associated with a significant reduction in body weight, waist circumference, and systolic and diastolic blood pressure, consistent with previous reports indicating the favorable hemodynamic effects of SGLT2 inhibitors.
Tofogliflozin did not affect renal function biomarkers (serum creatinine, UAE, and eGFR) in this study. This was not consistent with previous reports demonstrating the renal benefits of SGLT2 inhibitors [5, 7, 45]. In a previous study, the renal benefits of SGLT2 inhibitors were more prominent in patients with a lower eGFR at baseline than in others [46]; hence, one reason for this inconsistency might be the relatively normal to mildly-impaired renal function of patients in this study: the baseline eGFR was 80.6 ± 20.7 mL/min/1.73 m2 and 81.6 ± 24.6 mL/min/1.73 m2 in the tofogliflozin and conventional treatment groups, respectively.
Regarding safety, during the 2-year intervention with tofogliflozin and 2-year extensional observation, the frequency of all adverse events did not vary between the groups. However, the frequency of the composite cardiovascular endpoint comprising cardiovascular mortality, acute myocardial infarction, unstable angina, and heart failure was significantly lower in the tofogliflozin group than in the conventional treatment group (Additional file 4), suggesting a long-term effect of tofogliflozin on the suppression of cardiovascular events.
Collectively, these results indicate that tofogliflozin treatment improves various cardiovascular risk factors and that such beneficial effects persist for at least 4 years, suggesting that tofogliflozin should be used early in diabetes management.
This study had several limitations. First, the main UTOPIA study was not a double-blind, placebo-controlled trial but rather a prospective, open-label, randomized trial with a blinded endpoint. In addition, the UTOPIA Extension study is a prospective observational study without any restriction in medication in both groups. Although the assessment of endpoints was blinded and conducted by expert committees, the study design may have caused unexpected bias. Second, all participants in this study were Japanese. Therefore, generalizing the results of this study to other racial or ethnic groups should be approached with caution. Finally, we did not correct for multiplicity in this study. This may lead to false-positive results.
Conclusions
This is the first study to demonstrate that tofogliflozin is not associated with improved inhibition of carotid wall thickening but exerts long-term positive effects on various cardiovascular risk factors including HbA1c and HDL-C levels, BMI, waist circumference, systolic blood pressure, and baPWV while showing a good safety profile.
Availability of data and materials
The datasets generated and/or analyzed during our study are available from the corresponding author upon reasonable request.
Abbreviations
- ABI:
-
Ankle-brachial index
- AE:
-
Adverse events
- ANCOVA:
-
Analysis of covariance
- baPWV:
-
Brachial-ankle pulse wave velocity
- BMI:
-
Body mass index
- eGFR:
-
Estimated glomerular filtration rate
- HbA1c:
-
Hemoglobin A1c
- HDL-C:
-
High-density lipoprotein cholesterol
- IMT:
-
Intima-media thickness
- LDL-C:
-
Low-density lipoprotein cholesterol
- SD:
-
Standard deviation
- SGLT2:
-
Sodium-glucose cotransporter 2
- TG:
-
Triglyceride
- UAE:
-
Urinary albumin excretion
References
Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, phase 3 study. Curr Med Res Opin. 2014;30:1245–55.
Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–31.
Seino Y, Kaku K, Inagaki N, Haneda M, Sasaki T, Fukatsu A, et al. Fifty-two-week long-term clinical study of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Endocr J. 2015;62:593–603.
Sakai S, Kaku K, Seino Y, Inagaki N, Haneda M, Sasaki T, et al. Efficacy and safety of the SGLT2 inhibitor luseogliflozin in Japanese patients with type 2 diabetes mellitus stratified according to baseline body mass index: pooled analysis of data from 52-week phase III trials. Clin Ther. 2016;38:843-62.e9.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Suzuki M, Honda K, Fukazawa M, Ozawa K, Hagita H, Kawai T, et al. Tofogliflozin, a potent and highly specific sodium/glucose cotransporter 2 inhibitor, improves glycemic control in diabetic rats and mice. J Pharmacol Exp Ther. 2012;341:692–701.
Kasahara-Ito N, Fukase H, Ogama Y, Saito T, Ohba Y, Shimada S, et al. Pharmacokinetics and pharmacodynamics of tofogliflozin (a selective SGLT2 inhibitor) in healthy male subjects. Drug Res. 2017;67:349–57.
Takeishi S, Tsuboi H, Takekoshi S. Comparison of tofogliflozin 20 mg and ipragliflozin 50 mg used together with insulin glargine 300 U/mL using continuous glucose monitoring (CGM): a randomized crossover study. Endocr J. 2017;64:995–1005.
Kaku K, Watada H, Iwamoto Y, Utsunomiya K, Terauchi Y, Tobe K, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol. 2014;13:65.
Katakami N, Mita T, Yoshii H, Shiraiwa T, Yasuda T, Okada Y, et al. Tofogliflozin does not delay progression of carotid atherosclerosis in patients with type 2 diabetes: a prospective, randomized, open-label, parallel-group comparative study. Cardiovasc Diabetol. 2020;19:110.
Katakami N, Mita T, Gosho M, Takahara M, Irie Y, Yasuda T, et al. Clinical utility of carotid ultrasonography in the prediction of cardiovascular events in patients with diabetes: a combined analysis of data obtained in five longitudinal studies. J Atheroscler Thromb. 2018;25:1053–66.
Katakami N, Mita T, Yoshii H, Shiraiwa T, Yasuda T, Okada Y, et al. Effect of tofogliflozin on arterial stiffness in patients with type 2 diabetes: prespecified sub-analysis of the prospective, randomized, open-label, parallel-group comparative UTOPIA trial. Cardiovasc Diabetol. 2021;20:4.
Katakami N, Mita T, Yoshii H, Shiraiwa T, Yasuda T, Okada Y, et al. The influence of tofogliflozin on treatment-related quality of life in patients with type 2 diabetes mellitus. Diabetes Ther. 2021;12:2499–515.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
Katakami N, Mita T, Yoshii H, Shiraiwa T, Yasuda T, Okada Y, et al. Rationale, design, and baseline characteristics of the UTOPIA trial for preventing diabetic atherosclerosis using an SGLT2 inhibitor: a prospective, randomized, open-label, parallel-group comparative study. Diabetes Ther. 2017;8:999–1013.
Yokoyama H, Katakami N, Yamasaki Y. Recent advances of intervention to inhibit progression of carotid intima-media thickness in patients with type 2 diabetes mellitus. Stroke. 2006;37:2420–7.
Araki E, Inagaki N, Inoguchi T, Utsunomiya K, Tanizawa Y, Nakamura J, et al. The Japan Diabetes Society: treatment guide for diabetes 2014–2015. Tokyo: Bunkodo Co., Ltd.; 2014.
Terminology and Diagnostic Criteria Committee, Japan Society of Ultrasonics in Medicine. Subcommittee for preparing guidelines for ultrasound diagnosis of carotid artery: standard method for ultrasound evaluation of carotid artery lesions. Jpn J Med Ultrason. 2009;36:501–18.
Yanase T, Nasu S, Mukuta Y, Shimizu Y, Nishihara T, Okabe T, et al. Evaluation of a new carotid intima-media thickness measurement by B-mode ultrasonography using an innovative measurement software. Intimascope Am J Hypertens. 2006;19:1206–12.
Mita T, Katakami N, Shiraiwa T, Yoshii H, Onuma T, Kuribayashi N, et al. Sitagliptin attenuates the progression of carotid intima-media thickening in insulin-treated patients with type 2 diabetes: the Sitagliptin Preventive Study of Intima-Media Thickness Evaluation (SPIKE): a randomized controlled trial. Diabetes Care. 2016;39:455–64.
Mita T, Katakami N, Yoshii H, Onuma T, Kaneto H, Osonoi T, et al. Alogliptin, a dipeptidyl peptidase 4 inhibitor, prevents the progression of carotid atherosclerosis in patients with type 2 diabetes: the Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A). Diabetes Care. 2016;39:139–48.
Katakami N, Mita T, Maeda N, Sato Y, Watada H, Shimomura I, et al. Evaluation of the effect of tofogliflozin on the tissue characteristics of the carotid wall-a sub-analysis of the UTOPIA trial. Cardiovasc Diabetol. 2022;21:19.
Irace C, Casciaro F, Scavelli FB, Oliverio R, Cutruzzolà A, Cortese C, et al. Empagliflozin influences blood viscosity and wall shear stress in subjects with type 2 diabetes mellitus compared with incretin-based therapy. Cardiovasc Diabetol. 2018;17:52.
Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME study. Diabetes Care. 2016;39:717–25.
Katakami N, Yamasaki Y, Hayaishi-Okano R, Ohtoshi K, Kaneto H, Matsuhisa M, et al. Metformin or gliclazide, rather than glibenclamide, attenuate progression of carotid intima-media thickness in subjects with type 2 diabetes. Diabetologia. 2004;47:1906–13.
Matsumoto K, Sera Y, Abe Y, Tominaga T, Yeki Y, Miyake S. Metformin attenuates progression of carotid arterial wall thickness in patients with type 2 diabetes. Diabet Res Clin Pract. 2004;64:225–8.
Mortsell D, Malmqvist K, Held C, Kahan T. Irbesartan reduces common carotid artery intima-media thickness in hypertensive patients when compared with atenolol: the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) study. J Intern Med. 2007;261:472–9.
Ono H, Minatoguchi S, Watanabe K, Yamada Y, Mizukusa T, Kawasaki H, et al. Candesartan decreases carotid intima-media thickness by enhancing nitric oxide and decreasing oxidative stress in patients with hypertension. Hypertens Res. 2008;31:271–9.
Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation. 2002;106:2055–60.
MacMahon S, Sharpe N, Gamble G, Hart H, Scott J, Simes J, et al. Effects of lowering average or below-average cholesterol levels on the progression of carotid atherosclerosis: results of the LIPID Atherosclerosis substudy. Circulation. 1998;97:1784–90.
Won KB, Chang HJ, Kim HC, Jeon K, Lee H, Shin S, et al. Differential impact of metabolic syndrome on subclinical atherosclerosis according to the presence of diabetes. Cardiovasc Diabetol. 2013;12:41.
Ohnishi H, Saitoh S, Takagi S, Ohata J, Isobe T, Kikuchi Y, et al. Pulse wave velocity as an indicator of atherosclerosis in impaired fasting glucose: the Tanno and Sobetsu study. Diabetes Care. 2003;26:437–40.
Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:1180–93.
Striepe K, Jumar A, Ott C, Karg MV, Schneider MP, Kannenkeril D, et al. Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation. 2017;136:1167–9.
Pfeifer M, Townsend RR, Davies MJ, Vijapurkar U, Ren J. Effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on blood pressure and markers of arterial stiffness in patients with type 2 diabetes mellitus: a post hoc analysis. Cardiovasc Diabetol. 2017;16:29.
Kario K, Okada K, Murata M, Suzuki D, Yamagiwa K, Abe Y, et al. Effects of luseogliflozin on arterial properties in patients with type 2 diabetes mellitus: the multicenter, exploratory LUSCAR study. J Clin Hypertens. 2020;22:1585–93.
Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation. 2011;124:489–532.
Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18:3S-10S.
Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605.
Kishima H, Mine T, Fukuhara E, Kitagaki R, Asakura M, Ishihara M. Efficacy of sodium-glucose cotransporter 2 inhibitors on outcomes after catheter ablation for atrial fibrillation. JACC Clin Electrophysiol. 2022;8:1393–404.
Joki Y, Konishi H, Takasu K, Minamino T. Tofogliflozin, a sodium-glucose cotransporter 2 inhibitor, improves pulmonary vascular remodeling due to left heart disease in mice. J Cardiol. 2023;81:347–55.
Suzuki Y, Kaneko H, Okada A, Itoh H, Matsuoka S, Fujiu K, et al. Comparison of cardiovascular outcomes between SGLT2 inhibitors in diabetes mellitus. Cardiovasc Diabetol. 2022;21:67.
Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704.
Jardine MJ, Zhou Z, Mahaffey KW, Oshima M, Agarwal R, Bakris G, et al. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: a secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol. 2020;31:1128–39.
Acknowledgements
The authors thank all the staff and patients who participated in this study. The authors gratefully acknowledge the assistance of D. Takayama, H. Yamada, and A. Yoneda (Soiken Inc., Tokyo, Japan) and thank Editage (www.editage.com) for English language editing.
Funding
Financial support for this study, including fees for the assistance and processing of the article by the journal, was provided by Kowa Co., Ltd., Tokyo, Japan. The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript. Naoto Katakami and Tomoya Mita contributed to the conception and design of the study, development and amendment of the study protocol, subject enrollment and study implementation, data collection, discussion, writing, reviewing, and editing of this manuscript. Hirotaka Watada and Iichiro Shimomura supervised the conception, design, discussion, and reviewing of this manuscript. Other authors contributed to subject enrollment and study implementation, data collection, discussion, and review of this manuscript. All authors approved the final version of the manuscript. Naoto Katakami is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethical Review Board of Osaka University Hospital (approval number: 17367, date of approval: 2018/3/29) and the institutional review board of each participating center according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Ministry of Health, Labour and Welfare in Japan. The study was conducted in accordance with the Declaration of Helsinki of 1964 (revised in 2013), the Ethical Guidelines for Medical and Health Research Involving Human Subjects, and other current legal regulations in Japan. Informed consent was obtained from all patients for inclusion in the study.
Consent for publication
Not applicable.
Competing interests
Naoto Katakami was a staff member of the endowed chair established by funds from Kowa Co. Ltd. and has received lecture fees from Astellas Pharma Inc., AstraZeneca K.K., Daiichi Sankyo Inc., Eli Lilly Japan K. K, Kowa Company Ltd., Kyowa Hakko Kirin Co. Ltd., Mitsubishi Tanabe Pharma Co., Nippon Boehringer Ingelheim Co., Novartis Pharmaceuticals, Novo Nordisk Pharma, Ono Pharmaceutical Co., Taisho Toyama Pharmaceutical Co., Takeda Pharmaceutical Co., Teijin Pharma, Sanofi-Aventis, and Sumitomo Pharma Co. Tomoya Mita has received lecture fees from Kissei Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ono Pharmaceutical Co., Ltd., and Teijin Pharma Company, scholarship donations from MSD K.K., Astellas Pharma Inc., AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., Sanofi-Aventis K.K., Daiichi Sankyo Company, Limited, Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Terumo Corporation, Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Pfizer Japan Inc., Benefit One Health Care Inc., Mochida Pharmaceutical Co., Ltd., and Nitto Boseki Co., Ltd. as well as endowed chair funding from MSD K.K. and Takeda Pharmaceutical Company Limited. Tetsuyuki Yasuda has received lecture fees from Eli Lilly Japan K.K., Sumitomo Pharma, Novo Nordisk Pharma Ltd., and Teijin Pharma. Yosuke Okada has received lecture fees from MSD K.K., Mitsubishi Tanabe Pharma Corporation, Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., Novartis Pharmaceuticals Corp., Bayer Holding Ltd., Kowa Pharmaceutical Co. Ltd., Sanofi-Aventis K.K., and Sumitomo Pharma. Hideaki Kaneto has received lecture fees from Nippon Boehringer Ingelheim Co., Ltd., Sanofi-Aventis K.K., Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited, Daiichi Sankyo Company, Limited, Mitsubishi Tanabe Pharma Corporation, AstraZeneca K.K., Astellas Pharma Inc., Novartis Pharmaceuticals Co., and Sumitomo Pharma Co.; scholarship donations from Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co., Ltd., Sumitomo Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., Sanofi-Aventis K.K., Eli Lilly Japan K.K., Astellas Pharma Inc., Daiichi Sankyo Company, Limited, Mitsubishi Tanabe Pharma Corporation, MSD K.K., Takeda Pharmaceutical Company Limited, AstraZeneca K.K., Mochida Pharmaceutical Co., Ltd., Taisho Pharmaceutical Co., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., and Abbott Co. Ltd; and research funding from Taisho Pharmaceutical Co., Sumitomo Pharma Co., and Nippon Boehringer Ingelheim Co., Ltd. Nobuichi Kuribayashi has received lecture fees from Novo Nordisk Pharma Ltd. Satoshi Kawashima has received lecture fees from Sanofi-Aventis K.K., Sumitomo Pharma Co., Kowa Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo Co., and Terumo Corporation. Yasunori Sato has received lecture fees from Mochida Pharmaceutical Co., Ltd, Eisai Co., Ltd., and Eli Lilly and Company. Hirotaka Watada has received honoraria for lectures for Bayer Pharma Japan, Teijin Pharma Ltd., MSD, Sanofi-Aventis K.K., Novo Nordisk, Nippon Boehringer Ingelheim, Eli Lilly, Sumitomo Pharma, Mitsubishi Tanabe Pharma, Daiichi Sankyo Company, Ltd, Abbott, Kowa Co., Ltd., Taisho Pharmaceutical, Astellas Pharma, Kissei Pharmaceutical Co., Ltd., AstraZeneca K.K., Ono Pharmaceutical Co. Ltd. Sanwa Kagaku, Takeda Pharmaceuticals, and research activities for Takeda Pharmaceuticals, Nippon Boehringer Ingelheim, Kissei Pharmaceutical, Novo Nordisk, Mitsubishi Tanabe Pharma, Lifescan Japan, Kyowa Kirin, Sumitomo Pharma, Eli Lilly, Teijin Pharma, Taisho Pharmaceutical, Abbott, Ono Pharmaceutical Co. Ltd., Soiken Inc., Sanwa Kagaku, and Kowa. Iichiro Shimomura has received lecture fees from Amgen Astellas Biopharma K.K., Astellas Pharma Inc., AstraZeneca K.K., Covidien Japan Inc., Daiichi Sankyo Co., Eli Lilly Japan K.K, MSD K.K., KOBAYASHI Pharmaceutical Co., Ltd., Kowa Company, Ltd., Kyowa Kirin Co., Ltd., Takeda Pharma K.K., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Nippon Boehringer Ingelheim Co., Nippon Chemiphar Co., Ltd., Novartis Pharma K.K., Novo Nordisk Pharma, Ono Pharmaceutical Co., Rohto Pharmaceutical Co., Ltd., Sanofi K.K., Sanwa Kagaku Kenkyusho Co., Sumitomo Pharma Co., Taisho Pharmaceutical Co. Ltd., Teijin Pharma,; and research funds from Astellas Pharma Inc., Cancerscan Inc., Daiichi Sankyo Co., Dainippon Sumitomo Pharma Co., Eli Lilly Japan K.K, Japan Agency for Medical Research and Development (AMED), Kaken Pharmaceutical Co., KOBAYASHI Pharmaceutical Co., Ltd., Kowa Company, Ltd., Kubarahonke Co., Ltd., Kyowa Kirin Co., Ltd., Mitsubishi Tanabe Pharma Co., MSD K.K, Novartis Pharma K.K., Novo Nordisk Pharma, Ono Pharmaceutical Co., Rohto Pharmaceutical Co., Ltd. Sanofi K.K., Shionogi & Co., Takeda Pharma K.K., Teijin Pharma; and scholarship donations from Daiichi Sankyo Co., Kowa Company, Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Novo Nordisk Pharma, Sumitomo Pharma Co., Takeda Pharma K.K., and Teijin Pharma. Hidenori Yoshii, Toshihiko Shiraiwa, Akira Kurozumi, Masahiro Hatazaki, Takeshi Osonoi, Tsunehiko Yamamoto, Kazuhisa Maeda, Hiroki Yokoyama, Keisuke Kosugi, Ketaro Ohtoshi, Isao Hayashi, Satoru Sumitani, Mamiko Tsugawa, Kayoko Ryomoto, Ken Kato, and Tadashi Nakamura declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
List of study investigators.
Additional file 2.
Effects of tofogliflozin on ABI.
Additional file 3.
Changes in concomitantly used glucose-lowering agent.
Additional file 4.
Frequency of cardiovascular events.
Additional file 5.
Frequency of study agent-related events.
Additional file 6.
Frequency of renal events.
Additional file 7.
Adverse events.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Katakami, N., Mita, T., Yoshii, H. et al. Tofogliflozin long-term effects on atherosclerosis progression and major clinical parameters in patients with type 2 diabetes mellitus lacking a history of cardiovascular disease: a 2-year extension study of the UTOPIA trial. Cardiovasc Diabetol 22, 143 (2023). https://doi.org/10.1186/s12933-023-01879-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-01879-4