Abstract
Background
Metabolic syndrome (MS) is associated with increased risks of diabetes and atherosclerotic cardiovascular disease. However, data on the impact of MS and its individual components on subclinical atherosclerosis (SCA) according to diabetes status are scarce.
Methods
Surrogate markers of SCA, brachial–ankle pulse wave velocity (baPWV), and carotid intima–medial thickness (IMT) and plaque were assessed in 2,560 subjects (60 ± 8 years, 33% men) who participated in baseline health examinations for a community-based cohort study.
Results
The participants included 2,149 non-diabetics (84%) and 411 diabetics (16%); 667 non-diabetics (31%) and 285 diabetics (69%) had MS, respectively. Diabetics had significantly higher baPWV and carotid IMT, and more plaques than non-diabetics (p < 0.001, respectively). Individuals with MS had significantly higher baPWV and carotid IMT than those without MS only among non-diabetics (p < 0.001, respectively). Among MS components, increased blood pressure was significantly associated with the exacerbation of all SCA markers in non-diabetics. The number of MS components was significantly correlated with both baPWV and carotid IMT in non-diabetics (baPWV: r = 0.302, p < 0.001; carotid IMT: r = 0.217, p < 0.001). Multiple regression showed both MS and diabetes were significantly associated with baPWV (p < 0.001, respectively), carotid IMT (MS: p < 0.001; diabetes: p = 0.005), and the presence of plaque (MS: p = 0.041; diabetes: p = 0.002).
Conclusions
MS has an incremental impact on SCA in conditions without diabetes. The identification of MS and its individual components is more important for the risk stratification of CVD in non-diabetic individuals.
Similar content being viewed by others
Background
Metabolic syndrome (MS) represents a clustering of several cardiovascular (CV) risk factors including abdominal obesity, impaired glucose intolerance, dyslipidemia, and hypertension, with insulin resistance as a major characteristic [1, 2]. It was recently estimated that MS is common, affecting 24% of adults in the US and 11–19% in Korea [3, 4]. MS is associated with the development of coronary heart disease (CHD) and stroke [5, 6].
MS has been promoted as a means of identifying the risk of diabetes development. A number of different definitions of MS include diabetes as part of the diagnostic criteria of MS. Recently, it has been strongly recommended that conditions with established diabetes or cardiovascular disease (CVD) should be excluded from the definition of MS, because MS is a pre-morbid condition rather than a clinical diagnosis [7]. However, there is a paucity of data supporting this recommendation, especially regarding atherosclerosis. In addition, the effects of MS and its individual components on subclinical atherosclerosis (SCA) according to diabetes status are unknown.
The intima–medial thickness (IMT) and plaque of the carotid artery can be measured noninvasively using high-resolution B-mode ultrasound. Brachial–ankle pulse wave velocity (baPWV) is used as a reproducible index of arterial elasticity and stiffness. Both increased baPWV and thickened IMT of the carotid artery are important surrogate markers of SCA that represent an increased risk of CV events [8, 9]. The present study investigated the effects of MS and its components on SCA according to diabetes status in a sample of 2,560 adults who participated in baseline health examinations for a community-based cohort study.
Methods
Subjects
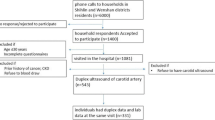
This is a cross-sectional investigation analyzing baseline data collected for a prospective cohort study. We used the data of 2,560 subjects who participated in baseline health examinations for a community-based cohort study in the Seoul area between April 2010 and November 2012. Subjects with a clinical history of CVD, cerebrovascular disease, neurological abnormalities, cerebral hemorrhage, or malignancy were excluded. The study protocol was approved by the local ethics committee of our institution, and informed consent for the procedure was obtained from each individual.
Measurement of biochemical and clinical parameters
All blood samples were obtained after 8 hours of fasting and analyzed for glucose, triglycerides, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol. Height, weight, and waist circumference were measured while subjects wore light clothing and no shoes. Waist circumference was measured at the midpoint between the lower border of the rib cage and iliac crest. Body mass index (BMI) was calculated as weight (kg) ÷ height (m2). MS was defined as when 3 or more of the following were present: (a) abdominal obesity based on waist circumference ≥ 90 cm in males or ≥ 80 cm in females; (b) HDL cholesterol < 40 mg/dL in males or <50 mg/dL in females; (c) fasting triglycerides ≥ 150 mg/dL; (d) blood pressure ≥ 130 mmHg systolic or ≥ 85 mmHg diastolic, or on treatment; and (e) impaired fasting glucose, defined as fasting glucose ≥ 100 mg/dL, based on the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) definition [2]. Diabetes was defined as either fasting glucose ≥ 126 mg/dL, a referral diagnosis of diabetes, or antidiabetic treatment.
Measurement of baPWV
All subjects abstained from caffeine-containing food or beverages for at least 45 minutes prior to baPWV measurement. After a subject had been resting in the supine position for at least 5 minutes in a quiet room, blood pressure and baPWV were measured using an automated waveform analyzer (Colin VP-2000, Colin Medical Instruments Corp., Komaki, Japan). Pneumatic cuffs were wrapped around both upper arms and ankles and connected to a plethysmographic sensor to determine the volume pulse waveform; the higher value of blood pressure was used for analysis. The highest value of baPWV measured on either side of each patient was used for analysis.
Measurement of carotid IMT and plaques
Carotid IMT was measured using high-resolution B-mode ultrasonography (Acuson X300, Siemens, USA) with a transducer frequency of 13–15 MHz. Computer-assisted acquisition, processing, B-mode images storage, and calculation of IMT were performed using the Syngo Arterial Health Package (Siemens, USA). Automatic measurements from both common carotid arteries were made at the far wall of the 1-cm segment distal to the carotid bulbs. The mean value of carotid IMT was used for analysis. All carotid IMT measurements were taken at sites free of any discrete plaques. Carotid plaque was defined as the presence of focal wall thickening at least 50% greater than that of the surrounding vessel wall or as a focal region with a carotid IMT greater than 1.5 mm, protruding into the lumen and distinct from the neighboring boundary [10, 11].
Statistical analysis
Clinical and biochemical characteristics are shown according to the presence of diabetes and MS. Values are expressed as mean ± SD. Continuous variables were compared using Student’s t-test, and categorical variables were compared using the χ2 test. Differences in baPWV and carotid IMT with respect to each MS component were tested using ANCOVA separately for individuals with and without diabetes. Correlational analysis between the number of MS components, and baPWV and carotid IMT according to diabetes status was performed using Pearson’s correlation test. Multiple regression analysis was used to evaluate the significance of confounding risk factors for baPWV and carotid IMT. Multiple logistic regression analysis was used to evaluate the significant risk factors for carotid plaques. SPSS version 18 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. All statistical tests were 2-tailed, and p < 0.05 was considered significant.
Results
Clinical characteristics
The clinical characteristics of the 2,560 participants (60 ± 8 years, 33% men) in this study are shown in Table 1. This study included 2,149 non-diabetics (84%) and 411 diabetics (16%). Both non-diabetics and diabetics were classified into 2 subgroups based on the presence of MS. The prevalence of AHA/NHLBI-defined MS in the present study was 40%; 667 non-diabetics (31%) and 285 diabetics (69%) were classified as having MS, respectively.
Differences in SCA parameters according to the presence of MS and diabetes
The mean baPWV, carotid IMT, and prevalence of carotid plaques were significantly higher in diabetics than non-diabetics (baPWV: 1641 ± 284 vs. 1476 ± 250 cm/s, p < 0.001; carotid IMT: 0.84 ± 0.26 vs. 0.76 ± 0.20 mm, p < 0.001; carotid plaques: 36vs.21%, p < 0.001). Subjects with MS had significantly higher baPWV and carotid IMT as well as more plaques than those without MS among only non-diabetics (baPWV: 1562 ± 271 vs. 1438 ± 230 cm/s, p < 0.001; carotid IMT: 0.81 ± 0.22 vs. 0.73 ± 0.19 mm, p < 0.001; carotid plaques: 27 vs. 18%, p < 0.001) (Table 1 and Figure 1). Data related to comparison of SCA parameters between 4 groups are provided in Additional file 1: Table S1.
Impact of individual MS component on SCA parameters according to diabetes status
After adjusting for age, gender, smoking status, LDL, and BMI, MS components including increased blood pressure, triglycerides and fasting glucose as well as decreased HDL were significantly associated with higher baPWV in non-diabetics. MS components including increased waist circumference and blood pressure, and decreased HDL were significantly associated with higher carotid IMT in non-diabetics. Increased blood pressure was significantly associated with a higher risk of carotid plaques and the exacerbation of all parameters of SCA in non-diabetics. However, increased blood pressure was only significantly associated with higher baPWV among all MS components and no components of MS affected other SCA parameters in diabetics (Table 2).
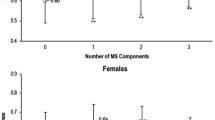
Relationship between the number of MS components and SCA parameters according to diabetes status
The number of MS components was significantly correlated with baPWV and carotid IMT in non-diabetics (baPWV: r = 0.302, p < 0.001; carotid IMT: r = 0.217, p < 0.001). However, it was not significantly correlated with either in diabetics (baPWV: r = 0.022, p = 0.660; carotid IMT: r = −0.003, p = 0.958) (Figure 2).
Impact of MS and diabetes on SCA parameters
Multiple linear regression analysis for baPWV and carotid IMT was performed using age, gender, smoking status, BMI, LDL, MS, and diabetes (Table 3). The results showed that age (β = 14.516, p < 0.001), male sex (β = 52.753, p < 0.001), MS (β = 87.450, p < 0.001), and diabetes (β = 87.408, p < 0.001) were significantly associated with baPWV. Meanwhile, age (β = 0.010, p < 0.001), male sex (β = 0.057, p = 0.001), LDL cholesterol (β = 0.001, p < 0.001), MS (β = 0.042, p < 0.001), and diabetes (β = 0.031, p = 0.005) were significantly associated with carotid IMT. Multiple logistic regression analysis was performed for carotid plaques using the same covariates (Table 3). The results showed that age (odds ratio [OR], 1.08; 95% confidence interval [CI], 1.07–1.10; p < 0.001), male sex (OR, 1.36; 95% CI, 1.00–1.85; p = 0.050), MS (OR, 1.26; 95% CI, 1.01–1.57; p = 0.041), and diabetes (OR, 1.50; 95% CI, 1.17–1.93; p = 0.002) were significantly associated with the prevalence of carotid plaques.
Discussion
To the best of our knowledge, the present study provides the first information on the differential impact of MS on atherosclerotic changes according to diabetes status. The AHA/NHLBI criteria were chosen because they are easy to apply to clinical and epidemiological studies, clearly define each MS component regarding medication status, and follow the current criteria of impaired fasting glucose [12].
Several previous studies assessed the association between MS and atherosclerosis. In the Baltimore Longitudinal Study of Aging (BLSA), Scuteri et al. [13] found that subjects with MS have significantly greater carotid IMT and stiffness than subjects without MS. Nakanish et al. [14] found that clustered features of MS are closely related to the risk of increased aortic PWV in middle-aged Japanese men. However, these studies raised substantial concerns about age-associated increases in vascular stiffness and thickness. Furthermore, they did not consider that MS was not a clinical diagnosis but rather a pre-morbid condition for the development of diabetes, which is closely associated with atherosclerosis. The present study revealed that subjects with MS had greater baPWV, carotid IMT, and plaques than those without MS among non-diabetics. However, this impact of MS on SCA was not observed in subjects with establish diabetes, although both MS and diabetes were independently associated with all vascular parameters after considering risk factors. In addition, the number of MS components was significantly associated with increases in vascular stiffness and thickness in only non-diabetics. Considering the differential impact of MS on SCA according to the presence of diabetes, it might be important to identify the presence of MS in non-diabetic individuals. However, a concurrent diagnosis of MS in individuals with established diabetes might be of little value for the risk stratification of CVD.
The present study identified different impacts of the individual components of MS on SCA, including vascular stiffness and thickness, according to diabetes status. Vascular stiffness reflected by baPWV was influenced by several MS components in non-diabetics, including increased blood pressure, triglyceride, and fasting glucose as well as decreased HDL. However, only increased blood pressure affected vascular stiffness in diabetics. This might be closely associated with the concrete relationship between baPWV and blood pressure irrespective of diabetes status. On the contrary, vascular thickness reflected in carotid IMT and plaque was influenced by MS components, including increased waist circumference and blood pressure, and decreased HDL; however, no MS components significantly affected vascular thickness in diabetics. These results suggest that the progression of atherosclerosis might be directly dependent upon hyperglycemia in patients with established diabetes status [15, 16] but might be influenced by multiple CV risk factors, especially the component of increased blood pressure [17], in patients with a status of MS without diabetes.
MS has recently been promoted as a means of identifying the risk of diabetes development. Gupta et al. [18] found that both impaired fasting glucose and MS can predict the risk of new-onset diabetes and that MS is a better predictor of the risk of new-onset diabetes in hypertensive patients. In contrast, Stern et al. [19] reported that MS is inferior to the Framingham Risk Score, an established predictive model for either type 2 diabetes or CVD. In the present study, although we did not analyze the significance of MS as a predictor of type 2 diabetes development, diabetics had a significantly greater risk of SCA than non-diabetics, independent of MS status. These results suggest that diabetes strongly influences atherosclerosis independent of MS and highlight importance of identifying the new development of diabetes in non-diabetics with MS.
MS and diabetes share many common characteristics; 65–85% of diabetic individuals have MS [20–22]. However, only a few studies have examined the effect of the combination of MS and diabetes on the risk of CVD, and their results are inconsistent. Malik et al. [23] showed that individuals with MS but not diabetes have increased risks of CHD and CVD, and that diabetes predicts CHD, CVD, and overall mortality. Alexander et al. [21] reported that the prevalence of CHD is substantially higher in subjects with both diabetes and MS than in those with only diabetes. Tong et al. [22] showed that the presence of MS is associated with an increased risk of CHD in Chinese individuals with diabetes. On the contrary, Church et al. [24] reported that the presence of diabetes is associated with a 3-fold greater CVD mortality risk and that MS status does not affect this risk in men from the Aerobics Center Longitudinal Study (ACLS). In addition, while MS and diabetes confer an increased risk of CVD, recent evidence suggests that subjects with these conditions have a wide range of increased risks [25–27]. Malik et al. [26] reported that subjects with MS or diabetes have low risks of CHD when carotid IMT or coronary artery calcium (CAC) is not elevated. Furthermore, they reported that CAC predicts CVD and CHD events better than carotid IMT. Wong et al. [27] reported that subjects with MS and diabetes have a greater incidence and progression of CAC than those without these conditions; moreover, progression also predicts CHD events in those with MS and diabetes. The evaluation of baPWV and carotid IMT in the present study might be insufficient to stratify the CV risk in diabetic individuals because these SCA markers were not significantly different between diabetics with and without MS. Therefore, further investigations might be required for complete CV risk stratification and should include the assessment of morphological and functional vascular damage as well as serological markers in patients with MS and diabetes.
MS is a pre-morbid condition rather than a clinical diagnosis and has been advocated as a useful clinical tool for predicting diabetes and CVD. Although a number of different definitions of MS include diabetes as a diagnostic criterion of MS, the World Health Organization (WHO) strongly recommended that the conditions of established diabetes or CVD should be excluded in the definition of MS and proposed research that justifies the inclusion of type 2 diabetes in the definition [7]. Given the current controversy over the definition of the MS, the present result that diabetes strongly influences SCA irrespective of the presence of MS is good evidence arguing against the inclusion of patients with established type 2 diabetes in the domain of MS.
This study has some limitations. First, the criteria of MS might be dependent on race and ethnicity [28]. However, the present study included only a Korean population. Second, the impact of MS on the progression of atherosclerosis might somewhat differ according to age group [29]. However, no sub-analysis of SCA according to age group was not performed because the participants of this study were relatively older. Third, a previous study reported that dynamic endurance training favorably affects most of the CV risk factors related to MS [30]. However, we did not evaluate the physical activity of participants. Fourth, there were relatively few subjects with diabetes compared to those without diabetes because our study was a community-based cohort study. Fifth, we could not eliminate the possible effects of underlying disease and medication for hypertension, dyslipidemia, and diabetes on atherosclerosis because of the observational design of this study. Finally, we did not evaluate the degree of hyperglycemic control using HbA1c in diabetic patients. Further prospective studies with larger sample sizes are required to address these issues.
Conclusions
In conclusion, MS has an incremental impact on SCA in conditions without diabetes. The identification of MS and its individual components is more important for the risk stratification of CVD in non-diabetic individuals.
Abbreviations
- ACLS:
-
Aerobics Center Longitudinal Study
- AHA/NHLBI:
-
American Heart Association/National Heart, Lung, and Blood Institute
- baPWV:
-
Brachial–ankle Pulse Wave Velocity
- BLSA:
-
Baltimore Longitudinal Study of Aging
- BMI:
-
Body Mass Index
- CAC:
-
Coronary Artery Calcium
- CHD:
-
Coronary Heart Disease
- CI:
-
Confidence Interval
- CV:
-
Cardiovascular
- CVD:
-
Cardiovascular Disease
- DBP:
-
Diastolic Blood Pressure
- FBS:
-
Fasting Blood Sugar
- HDL:
-
High-Density Lipoprotein
- IMT:
-
Intima–Medial Thickness
- LDL:
-
Low-Density Lipoprotein
- MS:
-
Metabolic Syndrome
- OR:
-
Odd Ratio
- SBP:
-
Systolic Blood Pressure
- SCA:
-
Subclinical Atherosclerosis
- SD:
-
Standard Deviation
- WHO:
-
World Health Organization.
References
NCEP: Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). J Am Med Assoc. 2001, 285: 2486-2497. 10.1001/jama.285.19.2486.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F: Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005, 112: 2735-2752. 10.1161/CIRCULATIONAHA.105.169404.
Ford ES, Giles WH, Dietz WH: Prevalence of the metabolic syndrome among US adults. J Am Med Assoc. 2002, 287: 356-359. 10.1001/jama.287.3.356.
Park JS, Park HD, Yun JW, Jung CH, Lee WY, Kim SW: Prevalence of the metabolic syndrome as defined by NCEP-ATPIII among the urban Korean population. Korean J Med. 2002, 63: 290-298.
Klein BE, Klein R, Lee KE: Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in beaver Dam. Diabetes Care. 2002, 25: 1790-1794. 10.2337/diacare.25.10.1790.
Nakanishi N, Takatorige T, Fukuda H, Shirai K, Li W, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K: Components of the metabolic syndrome as predictors of cardiovascular disease and type 2 diabetes in middle-aged Japanese men. Diabetes Res Clin Pract. 2004, 64: 59-70. 10.1016/j.diabres.2003.10.001.
Simmons RK, Alberti KG, Gale EA, Colagiuri S, Tuomilehto J, Qiao Q, Ramachandran A, Tajima N, Brajkovich Mirchov I, Ben-Nakhi A, Reaven G, Hama Sambo B, Mendis S, Roglic G: The metabolic syndrome: useful concept or clinical tool? report of a WHO expert consultation. Diabetologia. 2010, 53: 600-605. 10.1007/s00125-009-1620-4.
Yamashina A, Tomiyama H, Arai T, Hirose K, Koji Y, Hirayama Y, Yamamoto Y, Hori S: Brachial-ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res. 2003, 26: 615-622. 10.1291/hypres.26.615.
Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, Azen SP: The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998, 128: 262-269.
Roman MJ, Naqvi TZ, Gardin JM, Gerhard-Herman M, Jaff M, Mohler E: Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: a report from the American society of echocardiography and the society of vascular medicine and biology. J Am Soc Echocardiogr. 2006, 19: 943-954. 10.1016/j.echo.2006.04.020.
Hunt KJ, Sharrett AR, Chambless LE, Folsom AR, Evans GW, Heiss G: Acoustic shadowing on B-mode ultrasound of the carotid artery predicts CHD. Ultrasound Med Biol. 2001, 27: 357-365. 10.1016/S0301-5629(00)00353-7.
The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003, 260 (Suppl 1): S5-S20.
Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, Lakatta EG: Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol. 2004, 43: 1388-1395. 10.1016/j.jacc.2003.10.061.
Nakanishi N, Suzuki K, Tatara K: Clustered features of the metabolic syndrome and the risk for increased aortic pulse wave velocity in middle-aged Japanese men. Angiology. 2003, 54: 551-559. 10.1177/000331970305400504.
Larsen JR, Brekke M, Bergengen L, Sandvik L, Arnesen H, Hanssen KF, Dahl-Jorgensen K: Mean HbA1c over 18 years predicts carotid intima media thickness in women with type 1 diabetes. Diabetologia. 2005, 48: 776-779. 10.1007/s00125-005-1700-z.
Sander D, Schulze-Horn C, Bickel H, Gnahn H, Bartels E, Conrad B: Combined effects of hemoglobin A1c and C-reactive protein on the progression of subclinical carotid atherosclerosis: the INVADE study. Stroke. 2006, 37: 351-357. 10.1161/01.STR.0000199034.26345.bc.
Czernichow S, Bertrais S, Blacher J, Oppert JM, Galan P, Ducimetière P, Hercberg S, Safar M, Zureik M: Metabolic syndrome in relation to structure and function of large arteries: a predominant effect of blood pressure. A report from the SU.VI.MAX. Vascular study. Am J Hypertens. 2005, 18: 1154-1160. 10.1016/j.amjhyper.2005.04.006.
Gupta AK, Prieto-Merino D, Dahlöf B, Sever PS, Poulter NR: Metabolic syndrome, impaired fasting glucose and obesity, as predictors of incident diabetes in 14120 hypertensive patients of ASCOT-BPLA: comparison of their relative predictability using a novel approach. Diabet Med. 2011, 28: 941-947. 10.1111/j.1464-5491.2011.03330.x.
Stern MP, Williams K, González-Villalpando C, Hunt KJ, Haffner SM: Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease?. Diabetes Care. 2004, 27: 2676-2681. 10.2337/diacare.27.11.2676.
Koehler C, Ott P, Benke I, Hanefeld M: Comparison of the prevalence of the metabolic syndrome by WHO, AHA/NHLBI, and IDF definitions in a German population with type 2 diabetes: the diabetes in Germany (DIG) study. Horm Metab Res. 2007, 39: 632-635. 10.1055/s-2007-985816.
Alexander CM, Landsman PB, Teutsch SM, Haffner SM: NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003, 52: 1210-1214. 10.2337/diabetes.52.5.1210.
Tong PC, Kong AP, So WY, Yang X, Ho CS, Ma RC, Ozaki R, Chow CC, Lam CW, Chan JC, Cockram CS: The usefulness of the international diabetes federation and the national cholesterol education Program’s adult treatment panel III definitions of the metabolic syndrome in predicting coronary heart disease in subjects with type 2 diabetes. Diabetes Care. 2007, 30: 1206-1211. 10.2337/dc06-1484.
Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR: Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in united states adults. Circulation. 2004, 110: 1245-1250. 10.1161/01.CIR.0000140677.20606.0E.
Church TS, Thompson AM, Katzmarzyk PT, Sui X, Johannsen N, Earnest CP, Blair SN: Metabolic syndrome and diabetes, alone and in combination, as predictors of cardiovascular disease mortality among men. Diabetes Care. 2009, 32: 1289-1294. 10.2337/dc08-1871.
Bulugahapitiya U, Siyambalapitiya S, Sithole J, Idris I: Is diabetes a coronary risk equivalent? systematic review and metaanalysis. Diabet Med. 2009, 26: 142-148. 10.1111/j.1464-5491.2008.02640.x.
Malik S, Budoff MJ, Katz R, Blumenthal RS, Bertoni AG, Nasir K, Szklo M, Barr RG, Wong ND: Impact of subclinical atherosclerosis on cardiovascular disease events in individuals with metabolic syndrome and diabetes: the multi-ethnic study of atherosclerosis. Diabetes Care. 2011, 34: 2285-2290. 10.2337/dc11-0816.
Wong ND, Nelson JC, Granston T, Bertoni AG, Blumenthal RS, Carr JJ, Guerci A, Jacobs DR, Kronmal R, Liu K, Saad M, Selvin E, Tracy R, Detrano R: Metabolic syndrome, diabetes, and incidence and progression of coronary calcium: the multiethnic study of atherosclerosis study. JACC Cardiovasc Imaging. 2012, 5: 358-366. 10.1016/j.jcmg.2011.12.015.
Gurka MJ, Ice CL, Sun SS, Deboer MD: A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovasc Diabetol. 2012, 11: 128-10.1186/1475-2840-11-128.
Herder M, Arntzen KA, Johnsen SH, Mathiesen EB: The metabolic syndrome and progression of carotid atherosclerosis over 13 years. The tromsø study. Cardiovasc Diabetol. 2012, 11: 77-10.1186/1475-2840-11-77.
Pattyn N, Cornelissen VA, Eshghi SR, Vanhees L: The effect of exercise on the cardiovascular risk factors constituting the metabolic syndrome: a meta-analysis of controlled trials. Sports Med. 2013, 43: 121-133. 10.1007/s40279-012-0003-z.
Acknowledgements
This research was supported by the Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (2012027176).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interest.
Authors’ contributions
All authors listed in the manuscript participated in the design of the study and in writing the manuscript. KW and SS performed the statistical analysis. All authors read and approved the final manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Won, KB., Chang, HJ., Kim, HC. et al. Differential impact of metabolic syndrome on subclinical atherosclerosis according to the presence of diabetes. Cardiovasc Diabetol 12, 41 (2013). https://doi.org/10.1186/1475-2840-12-41
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2840-12-41