Abstract
Background
An association has been indicated between atopic dermatitis (AD), a prevalent chronic inflammatory skin disease, and diabetes mellitus. However, the exact causal relationship between AD and both type 1 diabetes (T1D) and type 2 diabetes (T2D) remains controversial. This study aimed to explore the causal association between AD and diabetes by Mendelian Randomization (MR) approaches.
Methods
Public genetic summary data for AD was obtained from EAGLE study. Single nucleotide polymorphisms of diabetes were retrieved from four genome-wide association studies that had been performed in European populations. Inverse variance weighted (IVW) in MR analysis was used as the primary means of causality estimation. Several complementary analyses and sensitivity analyses were performed to calculate MR estimates and to enhance the causal inference, respectively. The R package ‘TwoSampleMR’ was used for analysis.
Results
Genetically predicted AD led to a higher risk of T1D (OR, 1.19; 95% CI, 1.05, 1.34; P = 0.006) and T2D (OR, 1.07; 95% CI, 1.02, 1.11; P = 0.003) based on random-effect IVW method. The complementary analyses provided similar positive results. Cochran’s Q test and I2 statistics indicated moderate heterogeneity between AD and both T1D and T2D. No significant horizontal pleiotropy was detected by MR-Egger Intercept p except summary data from FinnGen consortium.
Conclusion
Genetically predicted AD is a risk factor for both T1D and T2D. These findings imply potential shared pathological mechanisms between AD and diabetes, thus suggesting the significance of early clinical diagnosis and prevention of AD in reducing the incidence of diabetes.
Similar content being viewed by others
Introduction
Diabetes is a collection of disorders characterized by impaired glucose metabolism, particularly hyperglycemia, which can cause long-term microvascular complications and non-specific macrovascular complications [1]. The latest International Diabetes Federation report shows that more than 10.5% of adults worldwide are diabetic and expected to account for 12.2% by 2045 [2]. Type 1 diabetes (T1D) is an autoimmune disease characterized by T cell-mediated destruction of pancreatic β cells and absolute deficiency of insulin [3, 4]. The risk of cardiovascular events in patients with T1D is ten times higher than in non-diabetic population [5]. High plasma concentrations of Omega-3 fatty acids in infancy and childhood vitamin D supplementation may reduce the risk of islet autoimmunity [6, 7]. Type 2 diabetes (T2D) is defined as a chronic metabolic disease featured with insulin resistance and deficiency in insulin secretion [8], which is associated with additional metabolic disorders such as dyslipidemia and atherosclerosis [9]. T2D patients carry an essential risk for cardiovascular disease (CVD) [10]. Lifestyle changes can reduce the risk of T2D [11], allowing better diabetes prevention, lower family financial burden and increased life expectancy. Recently, it has been revealed that atopic dermatitis (AD) is related to the risk of T1D and lifetime prediabetes [12, 13].
Previously called atopic eczema, AD is a complex chronic inflammatory skin disease with diverse clinical manifestations and symptoms suffered by approximately 20% of children and 3% of adults worldwide, with the incidence still increasing [14]. AD was proved to be a potential risk factor for several autoimmune diseases (OR = 1.97; 95% CI, 1.93–2.01) including T1D (OR = 1.08; 95% CI, 1.03–1.14) [15]. Wu et al. showed that the prevalence of T1D was significantly higher in patients with AD [13]. In addition, AD directly increased the risk of metabolic diseases especially T2D after adjusting for age, sex, metabolic disorders and other CVD (HR = 2.96; 95% CI, 2.56–3.41, P < 0.001) [16]. In multivariate models controlling for socio-demographic characteristics, smoking history, drinking history and strenuous activity, AD was still associated with a higher risk of diabetes (OR, 1.37; 95% CI, 1.16–1.63) [12]. However, the causal relationship between AD and diabetes remains controversial [17,18,19], which makes it indispensable and significant to verify the relationship between AD and diabetes. In addition, due to residual confounding in observational studies with different ethnicities of the population as well as different sample sizes and data collection methods, there can be bias in the process of deciphering the relationship between AD and diabetes.
As a method in genetic epidemiology, Mendelian randomization (MR) is widely used for its practical and economic advantages. It involves using genetic variants of a disease as instrumental variables (IVs) to explore whether there is a causal relationship between exposure and outcomes [20, 21]. As genetic variants are randomly assigned at meiosis, MR studies are able to reduce the risk of confounding factors and to minimize the susceptibility of reverse causality [20, 21]. In this MR study, we analyzed the summary statistics to explore the causal relationship between AD and diabetes, thus providing new ideas for the management of diabetes. We present a Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) for this MR study (Additional File 1) [22].
Methods
Study design
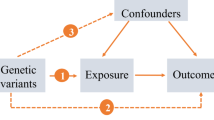
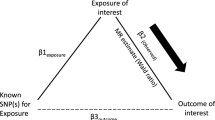
The process of this MR analysis is shown in Fig. 1. Overall, genetic variations were used as IVs to reveal the relationship between AD and both T1D and T2D based on three key hypotheses [23]. Firstly, IVs should directly and significantly affect the risk of AD. Secondly, IVs associated with any potential confounders should be absolutely avoided. Thirdly, IVs should only affect T1D and T2D through AD. Ethical approval and informed consent were obtained in the original studies.
Study design flow diagram of Mendelian randomization (MR). Three key assumptions should be met: Assumption 1: Instrumental variables (IVs) should directly and significantly affect the risk of atopic dermatitis (AD). Assumption 2: IVs associated with any potential confounders should be absolutely avoided. Assumption 3: IVs should only affect type 1 diabetes (T1D) and type 2 diabetes (T2D) through AD. SNPs, single nucleotide polymorphisms
Data sources
Summary statistics and detailed data sources for T1D and T2D in this MR study are provided in Table 1. Summary-level statistics for T1D were derived from two recent large datasets: meta-analysis of genome-wide association study (GWAS) on T1D from Forgetta et al. (9,358 cases and 15,705 controls) [24] and FinnGen consortium (5,928 cases and 183,185 controls). Cases were defined by International Classification of Diseases (ICD)-8 in FinnGen and are available online at https://gwas.mrcieu.ac.uk/datasets/finn-b-E4_DM1/. Summary-level data for T2D were derived from a European-descent meta-analysis (74,124 cases, 824,006 controls) [25] based on 32 studies and another GWAS datasets from Xue et al., including 62,892 cases and 596,424 controls [26].
To investigate whether higher genetical levels of AD increased the odds of T1D and T2D, we selected single nucleotide polymorphisms (SNPs) as IVs for AD identified in a largest GWAS meta-analysis performed by the EArly Genetics & Lifecourse Epidemiology (EAGLE) eczema consortium (21,399 cases, 95,464 controls) [27]. All participants included in this study were of European ancestry. No samples overlap except between EAGLE and Mahajan et al. [25] (Additional File 2: Table S1).
IVs selection
We extracted 21 SNPs with genome-wide significance (P < 5 × 10− 8) in the EAGLE study [27]. An SNP (rs12730935) was removed due to linkage disequilibrium (r2 < 0.01, clump distance < 10,000 kb) [28] based on 1000 genomes European population [29]. SNPs with minor allele frequencies (MAF) < 0.01 also need to be excluded since they usually tend to have low confidence and no SNPs were excluded in this step. To exclude those SNPs with potential confounders, we searched each of these in the PhenoScanner database [30] to satisfy the second fundamental assumption that IVs should avoid being associated with potential confounders. SNP rs4713555 was excluded because it was significantly associated with potential factors for T1D and T2D, including “Medication for cholesterol, blood pressure or diabetes: insulin” (P < 5 × 10− 8). To satisfy the third key assumption that IVs should affect T1D and T2D through AD only, we performed MR-Steiger analysis [31] and removed two SNPs “rs10214237 and rs6827756”, because they were demonstrated to explain more of the outcomes than AD and suggested a reverse causal relationship in Forgetta et al. (Additional File 2: Table S3) [24]. Since two SNPs (rs12188917 and rs6419573) could not be found in Forgetta et al. [24] and Mahajan et al. [25], three SNPs (rs12188917, rs6419573 and rs4809219) could not be found in and Xue et al. [26], we searched proxy-SNPs (r2 > 0.8) from an online website (http://snipa.helmholtz-muenchen.de/snipa3/) as a substitute (rs6596090 for rs12188917, rs1035127 for rs6419573, rs6011018 for rs4809219, respectively). Three SNPs were excluded (rs61813875, rs7127307, rs12951971) since they could neither be found nor replaced in Xue et al. [26].
We calculated the R2 and F statistic to assess the presence of weak IVs. It is generally accepted that the F statistics higher than the threshold of 10 indicates a low risk of weak IV bias. F = R2(N − 2)/(1 - R2) [32], where R2 indicates the degree of explanation of AD by IVs [33], N indicates the sample size. These 19 SNPs collectively explained 6.76% of the genetic variance of AD. All F statistics are higher than 10, indicating the absence of any weak IVs (Additional File 2: Table S2). Ultimately, we obtained 19 SNPs as IVs for this MR analysis, while only 17 SNPs for Forgetta et al. [24], 16 SNPs for Xue et al. [26]. (Additional File 2: Table S2 and 3).
Statistical analysis
The associations of SNP-AD and SNP-diabetes were combined into one ratio to estimate causal effects. Inverse variance weighting (IVW) of different models was the predominant approach for this MR analysis [34], which provides the highest statistical power when the three key MR assumptions mentioned earlier are met and is more reliable for estimation when there is heterogeneity among SNPs. In addition, to make the results more reliable and robust, we performed a set of complementary analyses. Even if up to 50% of the information in the analysis comes from invalid IVs, the weighted median method still allows the results to be an unbiased estimate of causality [35]. Simple median method with equal weights was also used to estimate causality [35]. Due to its robustness in identifying pleiotropy, the MR-robust adjusted profile score (MR-Raps) was well received [36]. MR-PRESSO outlier test can detect outliers thus providing a more precisely MR estimation after removing them [37]. Scatter plots were provided to describe the causal relationship between genetically predicted AD and both T1D and T2D. Two-sided P < 0.05 was considered statistically significant. For multiple comparisons, Bonferroni correction was performed (P < 0.05/2).
Based on a type I error rate threshold of 0.05, power calculations were performed by calculating each study’s sample size, the proportion of cases and the explanation of variance by mRND [38].
Sensitivity analyses
Heterogeneity among IVs was assessed by the calculation of Cochran’s Q and I2 statistics [39]. A Cochran’s Q P value of < 0.025 (0.05/2) or I2 statistic > 25% implies a heterogeneity that cannot be ignored [39], then IVW random-effects was considered to estimate the MR results with; otherwise, the IVW fixed-effects model was used [40]. MR-Egger regression was used to calculate the horizontal pleiotropy by estimating the intercept based on weighted linear regression of SNP-diabetes genetic susceptibility on SNP-AD associations [41]. A P value < 0.025 (0.05/2) of MR-Egger regression implies a potential bias in the IVW estimates. Meta-analysis combining the results of two T1D datasets and two T2D datasets respectively was performed by random-effects model without any significant heterogeneity (I2 = 0%, P = 0.50 for T1D; I2 = 0%, P = 0.82 for T2D) for the sake of estimating a more robust result. Leave-one-out method possesses powerful features to detect the bias of any single SNP on MR results [42]. SNPs strongly and independently influenced causality by leave-one-out method were retained.
R packages ‘TwoSampleMR’ [43], ‘MR-PRESSO’ [37] and ‘mr.raps’ [36] were used for this MR analysis. All statistical analyses for this study were performed in R software (version 4.1.3).
Results
Causal estimates between AD and the risk of diabetes
Main findings are presented in Fig. 2. The random-effects IVW analysis indicated that genetically predicted AD was positively associated with increased risk of T1D (OR, 1.24; 95% confidence interval (CI), 1.04, 1.49; P = 0.018) in Forgetta et al. Despite the null causal relationship found between AD and T1D in FinnGen consortium, meta-analysis by combining Forgetta et al. and FinnGen consortium reinforced the positive causal relationship (OR, 1.19; 95% CI, 1.05, 1.34; P = 0.006) (Fig. 2). We used the same approach to analyze the causal relationship between AD and T2D. Genetically predicted AD led to a higher risk of T2D in Xue et al. by random-effects IVW (OR, 1.07; 95% CI, 1.02, 1.14; P = 0.013). AD presented a suggestive significance for the risk of developing T2D in Mahajan et al. (OR, 1.06; 95% CI, 1.00, 1.13; P = 0.036). Meta-analysis by combining Xue et al. and Mahajan et al. consolidated the result (OR, 1.07; 95% CI, 1.02, 1.11; P = 0.003) (Fig. 2). Complementary analyses showed a consistent causal direction with the random-effects IVW analysis (Table 2; Fig. 3).
Scatter plot of the MR estimates for the association of AD with the risk of T1D and T2D based on Forgetta et al. (A), FinnGen (B), Mahajan et al. (C) and Xue et al. (D). AD, atopic dermatitis; T1D, type 1 diabetes; T2D, type 2 diabetes; MR-Raps, MR Robust adjusted profile score; MR-PERSSO, MR Pleiotropy Residual Sum and Outlier
Three SNPs with significant pleiotropy were identified by MR-PRESSO outliers test (rs2212434, rs2041733, rs4809219) in Mahajan et al. (Additional File 2: Table S4). After removing these outliers, we performed a replicate analysis (OR, 1.08; 95% CI, 1.03, 1.13; P = 0.002), and found that the results maintained the same direction without any horizontal pleiotropy by MR-Egger intercept (Additional File 2: Table S5).
Sensitivity analyses of MR
Cochran’s Q and I2 statistics indicated moderate heterogeneity between AD and both T1D and T2D (PCochran’s Q < 0.025 or I2 > 25%) (Table 3). However, we did not detect horizontal pleiotropy by MR-Egger Intercept p (threshold set at P < 0.025) except for FinnGen (Table 3, Additional File: Table S4). In leave-one-out analysis, several SNPs crossed the zero line after being removed (in Mahajan et al. and Xue et al.), while removing rs6419573 did not cause such a change (in FinnGen), indicating an individual SNP-driven causal estimation of genetically predicted AD on the risk of T1D and T2D. Attention needs to be directed to the robustness of causal relationships and result interpretations with caution (Fig. 4).
Leave-one-out plots for the MR analyses of AD on both T1D and T2D based on (A) Forgetta et al., (B) FinnGen, (C) Mahajan et al. and (D) Xue et al. Leave-one-out sensitivity analysis possesses powerful features to detect the bias of any single SNP on MR results. AD, atopic dermatitis; T1D, type 1 diabetes; T2D, type 2 diabetes; SNPs, single nucleotide polymorphisms
Power calculations of MR
This study had a sufficient power (> 80%) to detect OR of 1.24 for T1D based on Forgetta et al. (Power = 99%), OR of 1.06 for T2D based on Mahajan et al. (Power = 98%), and OR of 1.07 for T2D based on Xue et al. (Power = 99%). However, it did not provide enough confidence (Power = 78%) to calculate the OR of 1.14 for T1D based on the FinnGen consortium.
Discussion
To our knowledge, it is the first study to systematically explore the causal relationship between AD and diabetic risk by the approach of MR. We found that AD could increase the risk of both T1D and T2D in the European population.
AD is a chronic inflammatory skin disease primarily driven by T helper (Th) 2 and characterized by frequent episodes of persistent pruritus. Its growing prevalence causes a huge skin health burden and family financial burden both in the pediatric and adult populations [44]. The pathogenesis of AD is complex and usually involves the interaction between genetic susceptibility, skin barrier abnormalities, immune dysfunction and environmental factors [45]. Reduced expression of the protein filaggrin induced by mutations in FLG gene is found in 50% of AD patients [46, 47], which increases the risk of early-onset AD and is recognized as a major genetic predisposing factor for AD [48]. Lower levels of total ceramides accelerate water loss from the stratum corneum of skin among AD patients [49]. In the acute phase, AD is characterized by Th2 polarization [50, 51]. Pro-inflammatory cytokines induced by epidermal barrier damage activate innate immune components, leading to massive production of Th2 cytokines such as IL-4, IL-5 and IL-13 [52, 53].
As an autoimmune disease mediated primarily by Th1, T1D also displays association with AD by accumulating evidences. A large case-control study from Sweden including 104,832 AD cases and 1,022,435 controls showed that AD was significantly associated with multiple autoimmune disorders including T1D [15]. Wu et al. showed a higher prevalence of T1D in AD patients by analyzing 41,950 cases and 167,800 controls from the National Health Insurance Research Database (NHIRD) of Taiwan [13]. Several potential mechanisms have been proposed to explain these findings. As a Th2 cytokine, IL-4 contributes to autoimmune diabetes through increased expression of self-antigens in pancreatic islets [54]. Anderson et al. considered that β-cell destruction in T1D is a Th2-, not a Th1-mediated event [55]. Recent studies suggest that IL-4, IL-17 and IL-33 are simultaneously involved in the pathogenesis of AD and T1D by regulating autoimmune responses, implying the possibility of shared pathological process between AD and T1D [56]. As it is difficult to explain the association between T1D and AD by the traditional Th1/Th2 paradigm, an upgraded model with sophisticated T cell functional compartmentation may help to illustrate the underlying mechanism [57, 58].
Our findings in exploring the causal relationship between AD and T2D are consistent with several previous studies. Results from a National Health Interview Survey (NHIS) showed that AD increases the risk of lifetime prodromal diabetes [12]. Compared to controls, AD patients had a significantly higher risk of metabolic disorders such as hyperlipidemia and T2D [16]. Kok et al. found a significant association between moderate to severe AD and metabolic complications such as hypertension, hyperlipidemia, and T2D [59]. The exact mechanism how AD increases the risk of T2D is not clear. Chronic low-grade inflammation and immune system activation may function in the pathogenesis of obesity-related metabolic disorders [60,61,62,63]. T2D patients carry an elevated incidence of filaggrin null mutations, which is highly consistent with what happens in AD [64]. T2D patients expressed higher levels of IL-4 and IL-5 in their serum, suggesting the role of Th2-mediated inflammatory responses [65]. In addition, IL-17 exacerbated the inflammatory state in T2D, and IL-13 was significantly elevated in the serum of insulin-resistant patients [66, 67]. Based on above evidences, both AD and T2D have similar over-production of cytokines and inflammatory mediators, which may account for the increased risk of T2D in AD patients.
However, controversies still exist concerning the association between AD and diabetes. A population-based cohort study showed that AD did not increase the risk of T1D [68]. Schmitt et al. concluded that AD was associated with a reduced risk of T1D [18]. Andersen et al. showed adult AD patients either treated as inpatients or outpatients are unrelated to risk of new-onset T2D [69]. A cross-sectional study from Canada even showed that AD is a protective factor for T2D [70]. These inconsistent results could be attributed to several aspects of reasons. First, observational studies have their intrinsic limitations of selection and information biases that may lead to inaccurate results. Second, the conventional Th1/Th2 model may be insufficient to describe the immune dysregulation of T1D and AD. Third, environmental factors are involved in the occurrence of T2D, while MR study is based on the genetic level. Finally, MR study explains the lifetime effect of AD on diabetes, whereas observational studies are usually based on a limited period. Though we used the MR-Steiger method to exclude potential reverse causal confounding, it only supported a uni-directional causality and failed to explore the risk of AD in T1D patients. Therefore, a series of subsequent studies are still needed to investigate the relationship between AD and diabetes.
Generally, the IVW method provides the highest statistical power than other MR approaches in cases where the three key assumptions of MR are met without any significant pleiotropy among SNPs [34]. Most MR studies consider IVW as a primary analysis method [71], which becomes more persuasive after meta-analysis [72]. Several complementary analyses including weighted median, simple median and MR-raps between AD and T1D indicated a null causal association, which required us to pay more attention to the robustness of the results. However, all of complementary analyses provided consistent beta direction, which was strictly required by researchers in most MR studies [73, 74].
MR study is an emerging method utilizing genetic variations to explore the causal relationship between exposure and outcomes. The present study was designed upon the MR framework and showed distinct advantages. Environmental factors including diet, air pollutant and microbiota are involved in the onset of AD, which are inevitable confounders in observational studies and may contribute to the controversial causal relationship between AD and diabetes [75]. However, the instrumental variables consisting of SNPs overcame the causality effect limited to a span of time in observational studies, excluded the potential interference of residual confounders and were not affected by reverse causality with MR-Steiger analysis in current MR study, leading to more credible results and more convincing clinical guidance. All studies used for the analysis were based on European ancestry, thus avoiding causal bias due to ethnic differences.
The present study should be viewed in the light of its limitations. A nonlinear association between AD severity and diabetes could be neglected since our study was based on summary data. Whereas sensitivity analyses were performed, we need to be aware of the heterogeneity among SNPs. Horizontal pleiotropies were detected and the statistical power threshold of 80% of the association between AD and T1D from FinnGen was failed to reach. Several SNPs could drive the results separately and sample overlap existed between EAGLE and Mahajan et al. These factors may lead to bias in estimating causality and need our attention. MR-Egger was abandoned for use to estimate causality since the algorithm would lead to overly wide CIs, potentially leading to incorrect conclusions.
Conclusion
As the first Mendelian randomization study to explore the causal association between AD and diabetes, the present study advocated that AD contributed to the occurrence of both T1D and T2D. These findings imply potential shared pathological processes underlying AD and diabetes and suggest that early prevention and diagnosis of AD may reduce the risk of developing T1D and T2D.
Data Availability
The summary statistics of GWAS for atopic dermatitis are derived from a GWAS meta-analysis conducted by EAGLE consortium (https://www.eagle-consortium.org/); Full GWAS summary statistics for T1D are publicly available through FinnGen consortium (https://www.finngen.fi/en) and Forgetta et al. (Pubmed ID:32,005,708);
Summary level data for T2D can be accessed from Mahajan et al. (Pubmed ID:30,297,969) and Xue et al. (Pubmed ID:30,054,458)
Abbreviations
- AD:
-
Atopic dermatitis
- T1D:
-
Type 1 diabetes
- T2D:
-
Type 2 diabetes
- MR:
-
Mendelian randomization
- OR:
-
Odds ratio
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- GWAS:
-
Genome-wide association study
- SNPs:
-
Single nucleotide polymorphisms
- IVs:
-
Instrumental variables
- IVW:
-
Inverse variance weighted
- CVD:
-
Cardiovascular disease
- MAF:
-
Minor allele frequencies
- MR-PRESSO:
-
MR-pleiotropy residual sum and outlier
- MR-Raps:
-
MR-robust adjusted profile score
- Th:
-
T helper cell
References
Nathan DM. Diagnosing diabetes mellitus - best practices still unclear. Nat Rev Endocrinol. 2018;14(10):572–3.
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32(4):457–67.
Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–300.
Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care. 2006;29(11):2528–38.
Norris JM, Lee H-S, Frederiksen B, Erlund I, Uusitalo U, Yang J, et al. Plasma 25-Hydroxyvitamin D concentration and risk of Islet Autoimmunity. Diabetes. 2018;67(1):146–54.
Niinistö S, Takkinen H-M, Erlund I, Ahonen S, Toppari J, Ilonen J, et al. Fatty acid status in infancy is associated with the risk of type 1 diabetes-associated autoimmunity. Diabetologia. 2017;60(7):1223–33.
Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes Mellitus. Int J Mol Sci. 2020;21(17):6275.
Battisti WP, Palmisano J, Keane WE. Dyslipidemia in patients with type 2 diabetes. Relationships between lipids, kidney disease and cardiovascular disease. Clin Chem Lab Med. 2003;41(9):1174–81.
Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421.
Bellou V, Belbasis L, Tzoulaki I, Evangelou E. Risk factors for type 2 diabetes mellitus: an exposure-wide umbrella review of meta-analyses. PLoS ONE. 2018;13(3):e0194127.
Silverberg JI, Greenland P. Eczema and cardiovascular risk factors in 2 US adult population studies. J Allergy Clin Immunol. 2015;135(3):721–8e6.
Wu L-C, Hwang C-Y, Chung P-I, Hua T-C, Chen Y-D, Chu S-Y, et al. Autoimmune disease comorbidities in patients with atopic dermatitis: a nationwide case-control study in Taiwan. Pediatr Allergy Immunol. 2014;25(6):586–92.
Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(Suppl 1):8–16.
Ivert LU, Wahlgren CF, Lindelöf B, Dal H, Bradley M, Johansson EK. Association between atopic dermatitis and autoimmune diseases: a population-based case-control study. Br J Dermatol. 2021;185(2):335–42.
Jung HJ, Lee DH, Park MY, Ahn J. Cardiovascular comorbidities of atopic dermatitis: using National Health Insurance data in Korea. Allergy Asthma Clin Immunol. 2021;17(1):94.
Andersen YMF, Egeberg A, Gislason GH, Skov L, Thyssen JP. Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol. 2017;76(2):274–80e1.
Schmitt J, Schwarz K, Baurecht H, Hotze M, Fölster-Holst R, Rodríguez E, et al. Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. J Allergy Clin Immunol. 2016;137(1):130–6.
Rosenbauer J, Herzig P, Giani G. Atopic eczema in early childhood could be protective against type 1 diabetes. Diabetologia. 2003;46(6):784–8.
Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–63.
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–R98.
Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of Observational Studies in Epidemiology using mendelian randomization: the STROBE-MR Statement. JAMA. 2021;326(16):1614–21.
Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG. Using published data in mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30(7):543–52.
Forgetta V, Manousaki D, Istomine R, Ross S, Tessier M-C, Marchand L, et al. Rare genetic variants of large effect influence risk of type 1 diabetes. Diabetes. 2020;69(4):784–95.
Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50(11):1505–13.
Xue A, Wu Y, Zhu Z, Zhang F, Kemper KE, Zheng Z, et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun. 2018;9(1):2941.
Paternoster L, Standl M, Waage J, Baurecht H, Hotze M, Strachan DP, et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat Genet. 2015;47(12):1449–56.
Zha L-F, Dong J-T, Wang J-L, Chen Q-W, Wu J-F, Zhou Y-C, et al. Effects of Insomnia on Peptic Ulcer Disease using mendelian randomization. Oxid Med Cell Longev. 2021;2021:2216314.
Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–73.
Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35(22):4851–3.
Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13(11):e1007081.
Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for mendelian randomization. Stat Methods Med Res. 2017;26(5):2333–55.
Shim H, Chasman DI, Smith JD, Mora S, Ridker PM, Nickerson DA, et al. A multivariate genome-wide association analysis of 10 LDL subfractions, and their response to statin treatment, in 1868 Caucasians. PLoS ONE. 2015;10(4):e0120758.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–65.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some Invalid Instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14.
Zhao Q, Wang J, Hemani G, Bowden J, Small DS. Statistical inference in two-sample summary-data mendelian randomization using robust adjusted profile score. The Annals of Statistics. 2020;48(3):1742–69.
Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Brion M-JA, Shakhbazov K, Visscher PM. Calculating statistical power in mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497–501.
Greco MFD, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926–40.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data mendelian randomization. Stat Med. 2017;36(11):1783–802.
Burgess S, Thompson SG. Interpreting findings from mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89.
Wang Y, Guo P, Liu L, Zhang Y, Zeng P, Yuan Z. Mendelian randomization highlights the causal role of normal thyroid function on blood lipid profiles. Endocrinology. 2021;162(5):bqab037.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408.
Drucker AM, Wang AR, Li W-Q, Sevetson E, Block JK, Qureshi AA. The Burden of atopic dermatitis: Summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26–30.
Bieber T. Atopic dermatitis. N Engl J Med. 2008;358(14):1483–94.
Baurecht H, Irvine AD, Novak N, Illig T, Bühler B, Ring J, et al. Toward a major risk factor for atopic eczema: meta-analysis of filaggrin polymorphism data. J Allergy Clin Immunol. 2007;120(6):1406–12.
Palmer CNA, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441–6.
Luukkonen TM, Kiiski V, Ahola M, Mandelin J, Virtanen H, Pöyhönen M, et al. The value of FLG null mutations in Predicting Treatment response in atopic dermatitis: an observational study in finnish patients. Acta Derm Venereol. 2017;97(4):456–63.
Danso M, Boiten W, van Drongelen V, Gmelig Meijling K, Gooris G, El Ghalbzouri A, et al. Altered expression of epidermal lipid bio-synthesis enzymes in atopic dermatitis skin is accompanied by changes in stratum corneum lipid composition. J Dermatol Sci. 2017;88(1):57–66.
Grewe M, Walther S, Gyufko K, Czech W, Schöpf E, Krutmann J. Analysis of the cytokine pattern expressed in situ in inhalant allergen patch test reactions of atopic dermatitis patients. J Invest Dermatol. 1995;105(3):407–10.
Taha RA, Leung DY, Ghaffar O, Boguniewicz M, Hamid Q. In vivo expression of cytokine receptor mRNA in atopic dermatitis. J Allergy Clin Immunol. 1998;102(2):245–50.
Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96(1):2–7.
Tsakok T, Woolf R, Smith CH, Weidinger S, Flohr C. Atopic dermatitis: the skin barrier and beyond. Br J Dermatol. 2019;180(3):464–74.
Falcone M, Yeung B, Tucker L, Rodriguez E, Krahl T, Sarvetnick N. IL-4 triggers autoimmune diabetes by increasing self-antigen presentation within the pancreatic islets. Clin Immunol. 2001;98(2):190–9.
Anderson JT, Cornelius JG, Jarpe AJ, Winter WE, Peck AB. Insulin-dependent diabetes in the NOD mouse model. II. Beta cell destruction in autoimmune diabetes is a TH2 and not a TH1 mediated event. Autoimmunity. 1993;15(2):113–22.
Shruthi S, Mohan V, Amutha A, Aravindhan V. Increased serum levels of novel T cell cytokines IL-33, IL-9 and IL-17 in subjects with type-1 diabetes. Cytokine. 2016;86:6–9.
Tzeng ST, Hsu SG, Fu LS, Chi CS. Prevalence of atopy in children with type 1 diabetes mellitus in central Taiwan. J Microbiol Immunol Infect. 2007;40(1):74–8.
Kero J, Gissler M, Hemminki E, Isolauri E. Could TH1 and TH2 diseases coexist? Evaluation of asthma incidence in children with coeliac disease, type 1 diabetes, or rheumatoid arthritis: a register study. J Allergy Clin Immunol. 2001;108(5):781–3.
Kok WL, Yew YW, Thng TG. Comorbidities Associated with Severity of atopic dermatitis in Young Adult Males: a National Cohort Study. Acta Derm Venereol. 2019;99(7):652–6.
Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–801.
Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107.
Chawla A, Nguyen KD, Goh YPS. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11(11):738–49.
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97.
Thyssen JP, Linneberg A, Carlsen BC, Johansen JD, Engkilde K, Hansen T, et al. A possible association between a dysfunctional skin barrier (filaggrin null-mutation status) and diabetes: a cross-sectional study. BMJ Open. 2011;1(1):e000062.
Sokolova RN, Yankova RK, Abadjieva TI, Popova TA, Ivanovska MV, Murdjeva MA, et al. Association between type 2 diabetes, obesity and key Immunological Components of IgE-mediated inflammation. Folia Med (Plovdiv). 2017;59(2):159–64.
Abdel-Moneim A, Bakery HH, Allam G. The potential pathogenic role of IL-17/Th17 cells in both type 1 and type 2 diabetes mellitus. Biomed Pharmacother. 2018;101:287–92.
Martínez-Reyes CP, Gómez-Arauz AY, Torres-Castro I, Manjarrez-Reyna AN, Palomera LF, Olivos-García A, et al. Serum levels of Interleukin-13 increase in subjects with insulin resistance but do not correlate with markers of Low-Grade systemic inflammation. J Diabetes Res. 2018;2018:7209872.
de Lusignan S, Alexander H, Broderick C, Dennis J, McGovern A, Feeney C, et al. Atopic dermatitis and risk of autoimmune conditions: Population-based cohort study. J Allergy Clin Immunol. 2022;150(3):709–13.
Andersen YMF, Egeberg A, Gislason GH, Skov L, Knop FK, Thyssen JP. Adult atopic dermatitis and the risk of type 2 diabetes. J Allergy Clin Immunol. 2017;139(3):1057–9.
Drucker AM, Qureshi AA, Dummer TJB, Parker L, Li WQ. Atopic dermatitis and risk of hypertension, type 2 diabetes, myocardial infarction and stroke in a cross-sectional analysis from the Canadian Partnership for Tomorrow Project. Br J Dermatol. 2017;177(4):1043–51.
Lin Z, Deng Y, Pan W. Combining the strengths of inverse-variance weighting and Egger regression in mendelian randomization using a mixture of regressions model. PLoS Genet. 2021;17(11):e1009922.
Chen H, Chen S, Ye H, Guo X. Protective Effects of circulating TIMP3 on coronary artery disease and myocardial infarction: a mendelian randomization study. J Cardiovasc Dev Dis. 2022;9(8):277.
Venkatesh SS, Ferreira T, Benonisdottir S, Rahmioglu N, Becker CM, Granne I, et al. Obesity and risk of female reproductive conditions: a mendelian randomisation study. PLoS Med. 2022;19(2):e1003679.
Cheng F, Luk AO, Shi M, Huang C, Jiang G, Yang A, et al. Shortened leukocyte telomere length is Associated with Glycemic Progression in Type 2 diabetes: a prospective and mendelian randomization analysis. Diabetes Care. 2022;45(3):701–9.
Schuler CF 4th, Billi AC, Maverakis E, Tsoi LC, Gudjonsson JE. Novel insights into atopic dermatitis. J Allergy Clin Immunol. 2022. https://doi.org/10.1016/j.jaci.2022.10.023. Online ahead of print.
Acknowledgements
The authors sincerely thank the EAGLE consortium, HERMES consortium, FinnGen consortium, Forgetta et al., Mahajan et al., and Xue et al. for providing summary statistics. We would like to express our gratitude to Dr. Heng Chen from Zhejiang University for his MR guidance.
Funding
This work was supported by National Natural Science Foundation of China (No. 82071991).
Author information
Authors and Affiliations
Contributions
Study conception and design: Feiwei Lu; data analyses: Feiwei Lu; draft preparation: Feiwei Lu, Boting Wu; supervision of the study: Yongshi Wang; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable. Ethical approval and informed consent for studies included in the analyses were provided in the original publications.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, F., Wu, B. & Wang, Y. Mendelian randomization indicates that atopic dermatitis contributes to the occurrence of diabetes. BMC Med Genomics 16, 132 (2023). https://doi.org/10.1186/s12920-023-01575-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-023-01575-y