Abstract
Induced pluripotent stem cells (iPSCs) are undifferentiated stem cells characterized by the ability to differentiate into any cell type in the body. iPSCs are a relatively new and rapidly developing technology in many fields of biology, including developmental anatomy and physiology, pathology, and toxicology. These cells have great potential in research as they are self-renewing and pluripotent with minimal ethical concerns. Protocols for their production have been developed for many domestic animal species, which have since been used to further our knowledge in the progression and treatment of diseases. This research is valuable both for veterinary medicine as well as for the prospect of translation to human medicine. Safety, cost, and feasibility are potential barriers for this technology that must be considered before widespread clinical adoption. This review will analyze the literature pertaining to iPSCs derived from various domestic species with a focus on iPSC production and characterization, applications for tissue and disease research, and applications for disease treatment.
Similar content being viewed by others
Background
Induced pluripotent stem cells (iPSCs) are laboratory-developed pluripotent stem cells generated by the reprogramming of differentiated cells [1]. Takahashi and Yamanaka first discovered somatic cells’ capacity for reprogramming in 2006 after forcing differentiated fibroblast cells to ectopically express four transcription factors associated with pluripotency: Oct4, Sox2, Klf4, and c-Myc, collectively referred to as OSKM [1, 2]. iPSCs have since been of interest to researchers in the fields of toxicology, pathology, virology, developmental anatomy and physiology, amongst others [3,4,5]. iPSCs possess several benefits over other stem cell types such as mesenchymal stromal cells (MSCs) and embryonic stem cells (ESCs). In the context of this review, the term mesenchymal stromal cells has been adopted over mesenchymal stem cells due to the finite self-renewing property of MSCs that does not support the traditionally recognized self-renewing characteristic of stem cells [6]. The versatility of iPSCs may make them preferential over MSCs that are limited in their differentiation potential due to their multipotent nature [7,8,9]. ESCs offer a similar versatility to iPSCs as they are both pluripotent, but not without limitations [8]. ESCs can be obtained from in vivo and in vitro produced embryos at the blastocyst stage [10]. However, technical difficulties have interfered with the isolation and use of ESCs, namely in ungulate species and canines [2, 8, 11, 12]. Oocyte collection for in vitro embryo production is an invasive procedure that has prompted ethical considerations. Disposed reproductive material has been the primary source of oocytes in domestic species obtained from meat processing in livestock or ovariohysterectomies in companion animals [13,14,15]. In vivo protocols may include minimally invasive uterine flushing, often seen in mares [10]. iPSCs provide a more practical alternative to creating ESC-like cells in species where recovery of embryos or in vitro fertilization is difficult or not possible [12]. Unlike ESC lines, autologous iPSC lines can also be produced. This is ideal for transplantation of stem cells and their derivatives as it avoids the immunological complications associated with allogeneic iPSCs. Consequently, iPSCs can be used as an alternative to MSCs and ESCs with the potential for greater research and clinical applicability in domestic species.
While research has focused primarily on human and mice iPSCs, there has been a slow accumulation of iPSC research in domestic animals in the last decade (Fig. 1). iPSC derivation protocols have been developed in species including porcine [16], equine [17], canine [18], bovine [19], galline [20], caprine [21], ovine [22], and feline [23]. Aside from their importance in treating veterinary pathologies, porcine, canine, and equine models have been shown to be valuable for the study and treatment of human disease [24,25,26]. The purpose of this review is to provide an overview of the literature pertaining to current protocols and applications of iPSCs derived from domestic species. This review will address the topics of the development and use of iPSCs for tissue and disease research, their treatment in domestic animals and the barriers to their production and applications.
Cumulative iPSC-Related Publications in Domestic Species, January 2008–March 2020. a Publications regarding induced pluripotent stem cells from January 2008 to March 2020 in domestic animal species including porcine, equine, canine, bovine, galline, caprine, ovine and feline. Increased interest in iPSC research in domestic animals is demonstrated, particularly in the porcine model. b A subset of publications excluding porcine papers to visualize the general positive trend in all other domestic species
iPSC production and characterization
Yamanaka and colleagues’ discovery of iPSCs originated in mice models, followed closely by their derivation from human fibroblasts [1, 27]. Briefly, mice tail fibroblasts or human dermal fibroblasts were cultured then transduced with retroviral vectors containing expression cassettes of the OSKM reprogramming factors, inducing pluripotency in the transduced cells (Fig. 2). Using these protocols as a base, methods have been adapted in order to produce iPSCs in other species.
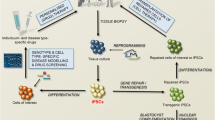
Induced Pluripotent Stem Cell Production and Differentiation. Differentiated cells, e.g. adult fibroblast cells [1], can be reprogrammed via designated reprogramming factors (e.g. Oct4, Sox2, Klf4, and c-Myc), to create iPSCs [2]. Upon exposure to specific differentiation media, iPSCs are capable of differentiating into any cell type of the body, e.g. multipotent neural cells [3]. Under appropriate culture conditions, iPSCs can result in a fully differentiated cell, e.g. a motor neuron [4]. Figure from “Induced pluripotent stem cell model of lysosomal storage disorders,” by Borger DK et al., 2017, Dis Model Mech. 10:691–704, CCBY [28] with minor alterations using Microsoft Word
iPSCs have been developed from porcine [16], equine [17], canine [18], bovine [19], galline [20], caprine [21], ovine [22], and feline [23] tissue. Successful iPSC production from domestic species was first reported in 2009 by Wu and colleagues in porcine, and the field has since expanded to other species (Fig. 1).
iPSCs have been produced from various donor tissue types, transduction systems, and reprogramming factor combinations. In domestic species, iPSCs have been derived from fibroblasts, MSCs and other somatic cell types including epithelial and testicular cells (Table 1). Tissue sources have been obtained from various developmental stages, namely fetal, neonatal, juvenile, and adult. For simplicity, this review has identified any tissue sources obtained from an animal in utero as fetal and those obtained after birth as adult. Deriving iPSCs from adult somatic cells is generally preferable to embryonic derivation due to a higher abundance of cells, easier collection of cells, and the ability to produce autologous iPSC populations for disease treatment. Donor tissue is then cultured and reprogrammed using viral or non-viral vectors containing the designated reprogramming factors. Viral vectors include lentiviruses, oncoviruses, and Sendai viruses, while non-viral vectors include cDNA vectors, minicircles and transposons (Table 1). The selected reprogramming factors typically include OSKM, but other variations have also been explored. Nanog and Lin28 are commonly used in the literature in addition to OSKM, and a small number of papers report the use of other additional transcription factors, such as TERT, and Tet1 (Table 1). More recently, work has been carried out using microRNAs in combination with other factors to achieve pluripotency induction [19, 29, 67]. MicroRNAs alone have only shown partial reprogramming abilities in domestic animals [61].
Following presumed iPSC production, colonies can be analyzed via morphological assessment to select for colonies with the most potential in reprogramming cells to an undifferentiated state. Non-invasive morphological assessment also provides insight into the developmental competence and homogeneity of iPSC colonies. Traditionally, iPSC colonies resemble ESC colonies with well-defined borders and tightly packed cells. More specifically, dome-shaped and flattened colonies are indicators of naïve and primed pluripotency, respectively [111]. Cells in these colonies are expected to have a large nucleus and little cytoplasm [112]. Naïve pluripotency is recognized by characteristic molecular features of the pre-implantation mouse embryonic stem cell, whereas primed pluripotency resembles stem cells of the post-implantation mouse epiblast [113]. Naïve pluripotent stem cells are identified by X chromosome reactivation in females, dependency on leukemia inhibitory factor (LIF) and receptivity to BMP4 to maintain pluripotency, and the transition to a more differentiated state in response to FGF2 and ACTIN/TGFB signalling [58, 114, 115].
Putative iPSCs must then undergo a series of tests to confirm pluripotency (Table 2). In domestic species, pluripotency is often confirmed by the endogenous expression of pluripotency markers, and the formation of in vitro embryoid bodies and in vivo teratomas containing cell types derived from all three germ layers [118]. Chimera formation with germ-line transmission is a less commonly used method in domestic species (as demonstrated in Table 2), but is deemed the gold standard for validating stem cell pluripotency [119].
Although formation of embryoid bodies and teratomas are successful in the majority of papers referenced in this review, many publications lack complete pluripotent characterization of their produced cell lines. Consequently, this review uses the term iPSCs broadly to describe both bona fide iPSCs and iPSC-like cells as some primary research lacks sufficient iPSC characterization to confirm true pluripotency. A common occurrence among studies is the incomplete silencing of exogenous transcription factors. These cell lines may not be truly pluripotent, but are reliant on transgenes to maintain pluripotency. The use of epigenetic modifiers has previously been shown in human and mice cells to increase transgene silencing while maintaining endogenous pluripotency factor expression; results that have now been replicated in the porcine model [65]. This technique could be promising to alleviate these issues, although more work will be required.
As previously mentioned, the gold standard for validating stem cell pluripotency is via chimera formation with germ-line transmission. Chimera formation is defined by the heterogeneous cell population of an early embryo following the injection of iPSCs into the blastocyst. This confirmation method requires iPSCs to integrate into the developing embryo and contribute to all three germ layers and, potentially, germ cells. To confirm germ-line transmission, chimeras are mated with non-chimeras and offspring are assessed for iPSC contribution [116]. Previous research has suggested that the feasibility of chimerism and germ-line transmission is greatly improved with the use of naïve pluripotent stem cells, as opposed to primed pluripotent stem cells [2, 40]. Although porcine naïve iPSC-like cells have been reported, there is little evidence of germline transmission. Analysis of the literature suggested that the generation of chimeras in livestock species is difficult to achieve [20]. Injection of generated iPSCs into an embryo often resulted in limited incorporation [40, 108] and the resulting offspring often were not chimeric [40, 108, 117]. Significant variation in iPSC integration has been shown [19, 112]. To the best of our knowledge, West and colleagues remain the only researchers to successfully realize germline transmission of iPSCs in a domestic species (i.e. porcine) [116]. iPSCs were also tested in cloning transgenic tissue [55, 104] and genomic incorporation of transgenes [60]. It was found that iPSCs could be derived from transgenic organisms, specifically genetically modified pigs designed for xenotransplantation [55]. However, limited developmental potential of embryos past the blastocyst stage was observed [60]. Hence, analysis of germline transmission is currently not feasible in such species. In moving forward with clinical applications of iPSCs in either human or veterinary medicine, being able to truly define cells as iPSCs will be crucial for standardization, quality, and safety assurances.
Tissue and disease research
iPSCs have the potential to be valuable tools for tissue and disease modelling. In vitro differentiation of iPSCs has allowed for study of the developmental processes and pathologies of tissues and may allow for preclinical testing of therapeutic drugs for veterinary and human medicine. With regard to drug screening, there has been success in human and mouse iPSC research in using differentiated iPSC lines to model disease and conduct high-throughput screening of small molecules for their effects on disease progression [120]. This technique allows for testing of potential therapeutics against disease-genotype cells specific to an individual or species without the need for interspecies comparisons or excessive lab animal use. Differentiation into specific cell types has been noted many times in the literature in porcine, equine, canine, galline, and bovine models, which are described below. Although characterization of these differentiation cells is demonstrated by physiological, genetic, or metabolic capacities of cell lines, the degree of differentiation varies from progenitor cells (e.g. neural progenitors [41, 76, 121]), to fully differentiated cell types (e.g. skeletal myocytes [122]). Domestic animal diseases are abundant and have negative health effects for consumers of agricultural animal by-products [123,124,125,126]. Unfortunately, the use of stem cells for research on livestock disease is novel and presently limited in number. The prolonged self-renewing characteristic of iPSCs supports their use in the study of physiology, disease pathology, drug toxicity and vaccine development in domestic species. A summary of veterinary animal iPSC research can be found in Table 3.
Porcine
Porcine iPSCs (piPSCs) have been differentiated into several cell types for research purposes. Currently, they have been used in the production of neural progenitor cells [41, 76, 121], endothelial cells [30], myotubes [134], hepatocytes [50, 78], and vascular smooth muscle cells (VSMCs) [42]. VSMCs in particular have been applied to scaffolds for implantation into immunodeficient mice and successfully formed 3D scaffold-free tissue rings [42].
Equine
Equine iPSCs (eiPSCs) have been differentiated into several cell and tissue types for disease modelling including neurons [86, 127], tendons [128], myotubes [122], and osteoblasts [129]. Functional eiPSC-derived neurons have been produced and were capable of firing action potentials in vitro via functional calcium channels [86]. One paper reported the observation of neurospheres with axonal outgrowths connecting adjacent cells [127]. This paper studied the potential for neurospheres to model West Nile virus (WNV) and Murray Valley encephalitis virus (MVEV), infectious, neurotropic equine diseases [127]. iPSC-derived neurons were successfully infected by WNV and MVEV, which could allow for future research to study mechanisms of these and other infectious diseases and neuropathic conditions.
Musculoskeletal tissue is a major system that would benefit from eiPSC modelling due to the frequency of injuries in competing horses. Artificial tendons derived from iPSCs have been attempted and although two-dimensional assays showed matrix contraction and appropriate gene expression, three-dimensional assays failed to generate functional artificial tendons. ESCs were shown to more efficiently produce functional tendons [128]. Further study is required here as this could be a promising area of regenerative medicine if eiPSC-derived tendons can be improved. Using fibroblast-derived eiPSC lines, researchers induced differentiation into myocytes, the functional unit of muscles. Myotubes demonstrated intracellular calcium release following membrane depolarization [122]. Lastly, functional eiPSC-derived osteoblasts have been reported. These cells expressed genetic markers of osteoblasts and were shown to produce hydroxyapatite and calcium matrices, highly specific characteristics of bone tissue [129]. Artificial production of bone may allow for study of bone physiology and diseases but may also benefit veterinary treatment of fractures and other pathologies.
Wound management is a common problem in equine medicine, and skin grafting, the ideal treatment, is often not possible due to a low supply of donor tissues [135]. One paper described a protocol where eiPSCs were differentiated into keratinocytes (eiPSC-KCs) to produce skin grafts. The eiPSC-KCs were likened to both progenitor and primary keratinocyte-like cells, potentially indicative of epidermal basal stem cell identity, ideal for in vivo wound management [84].
Canine
MSCs derived from canine iPSCs (ciPSCs) have been proposed as an intermediate stage to developing canine models of musculoskeletal tissues through chondrogenic and osteogenic pathways [130]. ciPSCs were differentiated into MSCs and subsequently differentiated into chondrocytes and osteoblasts in three-dimensional hydrogel culture conditions. Researchers proposed these three-dimensional cultures as effective models for studying canine osteoarthritis in order to develop MSC-based therapies and further model human degenerative joint disease [130].
A novel protocol has been published to generate functional canine platelets to treat thrombocytopenia, a canine and human clotting disorder. ciPSCs were differentiated into mature megakaryocytes which could be induced to release functional platelets [131]. This could serve as an alternative treatment to blood transfusion, the only effective therapy currently available.
Galline
Galline iPSCs (giPSCs) have been used in studying viral infection and replication [100, 132, 133]. Newcastle disease (NDV) is a common avian viral disease often found in domestic poultry [132]. Studies have demonstrated that giPSCs are capable of NDV infection [132, 133], and that viable cells displayed increased tolerability but not immunity to the virus [133]. giPSCs could also be used to produce replication-incompetent viruses, such as the highly pathogenic H5 avian influenza viruses [136]. Replication-incompetent viruses were produced with the goose influenza H5 gene and were incorporated into giPSCs. Using these cells, the virus was further transduced into a bladder cancer-derived cell line and could be inactivated by formaldehyde [100]. The use of giPSCs for vaccine production may be beneficial over chick embryos or eggs due to a decreased risk of contamination [100]. These results suggest that giPSCs have the potential to produce inactive viruses for vaccine production.
Bovine
Limited disease modelling has been observed with bovine iPSCs (biPSCs); however, current efforts have demonstrated their potential application in toxicological studies to elucidate the effects of toxic environmental compounds. Cattle can be used to investigate the negative effects of environmental endocrine disrupting compounds (EDCs) on humans and livestock species as the potential for harmful chemicals leaching into waterways and soils has become a prominent concern [137]. Despite a lack of clinical evidence, it has been proposed that EDCs can affect the reproductive functioning of cattle, greatly impacting agricultural production [138]. Bovine iPSCs have been applied to research the EDCs phthalate esters [94]. It was found that phthalate esters significantly downregulated androgen receptors of iPSCs, which supported apoptosis [94]. Such studies introduced biPSCs as a feasible tool in studying the effects of endocrine disruptors and other chemicals on cell populations. biPSCs have also been differentiated into epithelial-like cells that phenotypically resembled mammary cells [97]. These cells could further be investigated for their application in tissue regeneration for oncology patients who have undergone a mastectomy.
Disease treatment
Although research of specific pathologies is generally limited to single publications, the use of iPSCs to treat diseases and injuries in animals is growing and will likely be integrated into veterinary practice in the future. The field of domestic animal regenerative medicine may also provide models for human pathologies. Stem cell research that was once conducted on rodents is now growing in dogs and pigs [139, 140], species shown to be better models for human disease [25, 139, 140]. Table 4 summarizes the current research for iPSC-based treatments in domestic animals, which for the purpose of this review, includes all in vivo applications of iPSCs and their derivatives.
Porcine
Pigs are the most frequently used model of disease in domestic species. Porcine iPSCs have been employed in the study of tissue regeneration in bone [24, 141], muscle [51, 142], and nervous tissue [54, 143]. The findings in a majority of the articles published confirms that piPSCs are capable of integrating into tissue at the site of implantation [142, 143] and are capable of cueing endogenous pathways to upregulate [51], thus improving conditions at the site of tissue damage or death.
In a study of bone regeneration, piPSC-derived osteoblast-like cells were able to improve the trabecular and cortical bone structures of fractured tibias [141]. In a similar study, partial tibial cartilage regeneration at the transplantation site was observed with the regenerated cartilage originating from iPSCs [24].
Other studies examined the beneficial treatment effect of iPSCs on chronic myocardial ischemia [51] and infarction [142]. Regenerative therapy of cardiac tissue in porcine models involved direct injection of undifferentiated piPSCs into myocardium [51, 142]. The treatment was found to significantly decrease the infarction area, decrease regional perfusion, and increase angiogenesis with local incorporation of piPSCs into myocardium and blood vessel without tumor formation [142]. A similar study found small tumor formations that eventually arrested in growth [51]. While the research suggested variations in the grafting capabilities of these piPSCs into host tissues, there was an identified increase in smooth muscle actin, indicating piPSCs interact in some form with host tissue. In brief, piPSCs have been shown to contribute to myocardium regeneration.
piPSCs were also applied to regenerate nerve tissue. Researchers differentiated porcine iPSCs into neural progenitor cells (NPCs) and rod photoreceptors in vitro, then successfully implanted them into the site of cell damage. piPSCs not only incorporated into the host tissue at the site of implantation, but further extended beyond the grafted region long-term [54, 143]. The results suggest that piPSCs are capable of effectively integrating into host tissue, making them a candidate for clinical application.
Equine
Two papers have been published describing an in vivo application of eiPSCs for the treatment of musculoskeletal injuries in equines [87, 144]. In the first paper, published in 2016, muscle injuries were induced in a GFP mouse model by injecting notexin, a myotoxic venom, along with an injection of eiPSCs. It was reported that these muscles saw an increase in myofiber production, and since eiPSCs were non-GFP reporting, it was shown that muscle fibers originating from the eiPSCs were produced. Undifferentiated cells remained in the muscle, indicating the dangerous potential for cancer formation [87].
To safeguard against potential cancer formation, another paper differentiated eiPSCs into MSCs prior to injection, reducing the risk of undesired proliferation. The eiPSC-MSCs were then injected into horses with various musculoskeletal disorders including fractures, tendonitis, osteochondrosis, and osteoarthritis. Improvements were observed including reduced lameness fever and fracture lines, although some horses also experienced hot flush and edema [144]. Although successful, this paper indicates a need for further development of less immune-reactive therapies.
Host immune responses are a major concern for clinical use of iPSCs, especially in species like horses where allogeneic cell use would be ideal. Further to the example above, another paper tested the immune potential of in vivo transplantation of allogeneic eiPSCs. Injected cells induced a minor, focal inflammatory response, but cellular signs of chronic inflammation persisted until the end of the study period 30 days after grafting. Undifferentiated cells have reduced expression of MHC surface proteins, but upon differentiation in vivo, these proteins increase, stimulating an increased immune reaction [145]. Although these cells were undifferentiated, the risk of immune response is significant and must still be addressed in differentiated eiPSCs, especially if this response increases with differentiation prior to implantation.
Canine
Fewer developments have been made in ciPSC research, but a 2011 paper showed the potential for ciPSCs to be used for ischemic tissue damage treatment, both in hind limb ischemia and cardiac infarction mouse models [92]. ciPSCs were differentiated into endothelial cells (ciPSC-ECs), then injected into mice models. In hindlimb ischemia mice, ciPSC-ECs were shown to significantly improve revascularization in the compromised tissue. In cardiac infarction models, ciPSC-ECs were shown to engraft onto the heart muscle itself and improve cardiac contractility. In both models it was demonstrated that donor cells were lost over time, indicating a possible need for repeated treatments. Nevertheless, the lack of recurring original symptoms of ischemia suggests the capability of these cells to induce long-lasting, persistent effects in tissues following their disappearance [92].
Barriers
Safety
There are several concerns to be resolved before in vivo use of iPSCs can be justified. Immune reactivity is one concern in the use of allogenic cells that has been discussed earlier in this view. Aside from the formation of undesired cell types, the most significant risk is in vivo tumorigenesis due to the proliferative potential of iPSCs. Current research has demonstrated that differentiation of iPSCs and purification of differentiated cellular products prior to implantation can reduce tumour formation [146]. Alternatively, tumour formation has been addressed in mice models with the application of “suicide genes”. Using a drug-inducible suicide system, apoptosis of iPSC-derived cells can be initiated with exposure to a particular drug [147]. This system would allow for the complete inactivation of iPSC derivatives in the event of aberrant growth or modification.
Immune reaction to transplanted iPSCs is another safety concern for clinical application. The use of autologous transplants would mitigate these effects, although is not realistic for commercialization of treatments due to prohibitively high costs. Research is being conducted into a cellular “cloaking” system that would allow cells to go undetected by the immune system of the host. Modification of allogeneic cells by altering MHC and HLA antigens has also shown potential to eliminate immune reaction in human iPSCs xenotransplantation studies [148]. However, this system is not without limitations; it is dangerous to create cell populations that cannot be controlled by the host immune system. Introducing a system that combines the drug-inducible suicide system and the cloaking system could potentially resolve this issue.
Many transduction systems used for iPSC production have inherent safety concerns due to their random integration into the host genome. Random integration can lead to disruptions in host genes and an increased risk of oncogenicity. A non-genome integrating Sendai virus system [149, 150] allows for the production of transgene-free iPSCs while maintaining a high reprogramming efficiency [149]. Similarly, a non-viral system that operates on the use of piggyBac transposons can create transgene-free iPSCs via excision from the genome following iPSC generation [151]. Originally described in human models, these systems have since been applied to domestic species, including dogs [88, 89], chickens [20], and cattle [152]. The wide range of reprogramming system options is beyond the scope of this article but have been reviewed elsewhere [153,154,155].
Technical barriers
Researchers have relied on precedent methods of human and mouse models to generate iPSCs in domestic animals [49, 114, 156]. An issue seen in many domestic models is the retention of pluripotent transgene expression; a situation that allows for the maintenance of pluripotency, or in many cases a pseudo-pluripotent state, that can interfere with differentiation. Most iPSCs derived from domestic species have been generated by viral integration of human or murine reprogramming transgenes that remain expressed [82, 83, 85, 86, 91]. The continuous expression of these transgenes suggests an incomplete epigenetic remodeling with OKSM factors alone and a greater need for understanding and optimizing the pluripotency induction process in domestic species. The use of non-viral vectors may prove effective in iPSC production, while overcoming the issue of transgene expression. Unfortunately, there is limited research on the application of iPSCs for disease research with the use of non-viral vectors. Yu and colleagues remain the only research group to successfully generate iPSCs using non-viral minicircles capable of generating chimeric chicks [20]. As few researchers have confirmed pluripotency by means of chimerism, confirmation of bona fide iPSCs has been limited. Often, cells believed to be iPSCs are iPSC-like cells as there are technical difficulties in yielding bona fide iPSCs that can maintain pluripotency independent of doxycycline [80]. Bona fide iPSCs remain difficult to obtain and further investigation into true iPSC production is required.
Despite significant species conservation of pluripotency genes, some divergence of the core pluripotency genes have been identified between mammals [157]. For example, the use of OSKM, Lin-28 and Nanog has been well established in porcine models, while other species are still under investigation, e.g. felids where OSKM plus NANOG may be required [23, 158]. It may be necessary to modify existing methods of achieving pluripotency, such as including additional reprogramming factors or developing different culture conditions to overcome species-specific reprogramming barriers.
Cost
Cost is another barrier to the application of iPSCs in domestic species due to laborious production. As previously mentioned, iPSCs from domestic animals have technical barriers limiting yield. As a result, more reagents, tissue, time, and labour are required for sufficient production [159]. Further costs have been associated with autologous iPSC treatment due to the production and maintenance of many cell lines and associated labour costs.
Future directions
Organoids, three-dimensional cell cultures that demonstrate characteristic development, anatomy, and physiology of a tissue, are currently an undeveloped tool in iPSCs derived from domestic species. Organoids have recently been developed from human iPSCs, which suggests the possibility of producing any organ of the body under the appropriate conditions. The use of iPSCs derived from domestic animals for organoid production could also be applied to veterinary medicine. Similar to human iPSCs, two-dimensional models have limitations in drug screen and assessing disease progression as they do not resemble in vivo conditions like organoids. Hence, virologists and drug developers can use them to better understand the mechanisms of disease or drug actions [160]. Several researchers have already exemplified the use of human embryonic stem cell-derived organoids in detecting harmful effects of toxins on the functionality and morphology of the organoids [161, 162], and the ability of organoids to be derived from tumorigenic tissue for drug testing [163]. Nevertheless, these studies have not yet been investigated in iPSC-derived organoids.
CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats-associated protein 9) mediated gene editing has been applied to iPSC research to correct or induce genetic mutations in iPSC lines, primarily in the study of monogenic diseases. Figure 3 demonstrates the potential research and clinical applications of CRISPR/Cas9-edited iPSCs in domestic species. Extensive research has been done in human models and has been reviewed previously [164, 165]. Although the use of genome editing technologies has been limited in domestic species, a single report of successful CRISPR editing of bovine iPSCs has been published [166]. Genome editing of iPSCs in combination with chimera generation provide the potential for transgenic animal development. In agricultural animals, the artificial introduction of valuable traits e.g. therapeutic proteins in milk, decreased waste product, and disease resistance, could be invaluable to the farming industry [20, 167]. Economically, this would require germline transmission, which has seen little success in domestic species as compared to rodents [20, 116, 168]. Further research is required to fully understand the feasibility, safety and ethical implications of germline transmission of genetically modified iPSCs from domestic species.
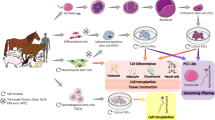
Potential Use of Domestic Animal iPSCs for Drug Discovery, Disease Modelling and Cell Replacement Therapy. Induced pluripotent stem cell (iPSCs) can be generated from healthy animals (e.g. dogs) for allogeneic cell transplantation of therapeutic cell types/tissue indicative of the disease. Alternatively, iPSCs can be generated from animals harbouring a genetic disorder and through CRISPR/Cas9-mediated genome editing technologies these genetic mutations can be corrected so that differentiated cell products from these iPSCs can be utilized in autologous cell replacement therapies. In addition, both the genetically mutated iPSCs and their genome-corrected iPSCs can be compared and contrasted for disease modelling purposes. This disease-in-a-dish could be potentially use as a high throughput screening system to discover novel drug candidates. Figure by Dean H. Betts (Adobe Photoshop)
Proteomic, metabolic, and methylomic analysis of iPSCs have limited acknowledgement and investigation in research. However, there have been recent efforts to investigate the proteins and sites of methylation of human iPSCs and their derivatives. The investigation of -omics in iPSCs can assist in confirming how completely iPSCs have been reprogrammed to an undifferentiated stem cell state and resemble ESCs. Studying proteins and sites of methylation have clinical applications in autologous cell replacement therapy [169]. Aside from one study investigating the effects of epigenetic modifiers on silencing on exogenous transcription in piPSCs [65], epigenetics is an unexplored area of iPSC research in domestic species. In humans, research has shown isogenic iPSC populations and similar epigenetic markers of hiPSCs and hESCs [170]. Such results further emphasize the potential applicability of iPSCs in disease research and as a substitute for ESCs.
Although there is a steadily growing number of publications pertaining to porcine, equine, and canine models, the numbers are much fewer for cattle, goats, chickens, and cats. Hence, new research initiatives should further investigate these species for their potential application in the fields of disease modelling, treatment, and enhancement of production animals.
Conclusion
Induced pluripotent stem cells are an innovative tool that hold great potential in contributing to veterinary medicine. Protocols for the production of iPSCs in some domestic species have been well-defined and have prompted research into their many potential applications. iPSC cultures have allowed for the production of tissues that can be studied for their physiological use and disease pathologies. Further, iPSCs themselves may be used in the future for the treatment of various diseases seen by veterinary practitioners. Although achievements have been made, a great deal of work is still required before these techniques can be clinically applied.
Availability of data and materials
All data analyzed during this study is included in this published article.
Abbreviations
- biPSCs:
-
Bovine induced pluripotent stem cells
- ciPSCs:
-
Canine induced pluripotent stem cells
- ciPSCs-ECs:
-
Canine induced pluripotent stem cells differentiated into endothelial cells
- CRISPR/Cas9:
-
Clustered regularly interspaced short palindromic repeats-associated protein 9
- EDCs:
-
Endocrine disrupting compounds
- eiPSCs:
-
Equine induced pluripotent stem cells
- eiPSC-KCs:
-
Equine induced pluripotent stem cells differentiated into keratinocytes
- ESCs:
-
Embryonic stem cells
- giPSCs:
-
Galline induced pluripotent stem cells
- iPSCs:
-
Induced pluripotent stem cells
- MSCs:
-
Mesenchymal stromal cells
- MVEV:
-
Murray Valley encephalitis virus
- NDV:
-
Newcastle disease virus
- NPCs:
-
Neural progenitor cells
- OSKM:
-
Oct4, Sox2, Klf4, and c-Myc
- piPSCs:
-
Porcine induced pluripotent stem cells
- VSMCs:
-
Vascular smooth muscle cells
- WNV:
-
West Nile virus
References
Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126(4):663–76 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0092867406009767. Cited 2020 May 14.
Ezashi T, Telugu B, Roberts RM. Induced Pluripotent Stem Cells from Pigs and Other Ungulate Species: An Alternative to Embryonic Stem Cells? Reprod Domest Anim. 2012;47(s4):92–7. https://doi.org/10.1111/j.1439-0531.2012.02061.x Cited 2020 May 14.
Scott CW, Peters MF, Dragan YP. Human induced pluripotent stem cells and their use in drug discovery for toxicity testing. Toxicol Lett. 2013;219(1):49–58. https://doi.org/10.1016/j.toxlet.2013.02.020.
Kuadkitkan A, Wikan N, Smith DR. Induced pluripotent stem cells: A new addition to the virologists armamentarium. J Virol Methods. 2016;235:191–5. https://doi.org/10.1016/j.jviromet.2016.03.009.
Nelson T, Martinez-Fernandez A, Terzic A. Induced pluripotent stem cells: developmental biology to regenerative medicine. Nat Rev Cardiol. 2010;7(12):700–10.
Lindner U, Kramer J, Rohwedel J, Schlenke P. Mesenchymal stem or stromal cells: Toward a better understanding of their biology? Transfus Med Hemother. 2010;37(2):75–83.
Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12(2):126–31 Available from: http://www.nature.com/articles/nrm3049. Cited 2020 May 14.
Pieri NCG, de Souza AF, Botigelli RC, Machado LS, Ambrosio CE, dos Santos Martins D, et al. Stem cells on regenerative and reproductive science in domestic animals. Vet Res Commun. 2019;43(1):7–16. https://doi.org/10.1007/s11259-019-9744-6 Cited 2020 May 14.
Zomer HD, Vidane AS, Gonçalves NN, Ambrósio CE. Mesenchymal and induced pluripotent stem cells: general insights and clinical perspectives. Stem Cells Cloning. 2015;8:125–34 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4592031/. Cited 2020 May 25.
Tecirlioglu RT, Trounson AO. Embryonic stem cells in companion animals (horses, dogs and cats): Present status and future prospects. Reprod Fertil Dev. 2007;19(6):740–7.
Sandmaier SES, Nandal A, Powell A, Garrett W, Blomberg L, Donovan DM, et al. Generation of induced pluripotent stem cells from domestic goats. Mol Reprod Dev. 2015;82(9):709–21. https://doi.org/10.1002/mrd.22512 Cited 2020 May 14.
Chow L, Johnson V, Regan D, Wheat W, Webb S, Koch P, et al. Safety and immune regulatory properties of canine induced pluripotent stem cell-derived mesenchymal stem cells. Stem Cell Res. 2017;25:221–32 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1873506117302404. Cited 2020 May 18.
Goszczynski DE, Cheng H, Demyda-Peyrás S, Medrano JF, Wu J, Ross PJ. In vitro breeding: Application of embryonic stem cells to animal production. Biol Reprod. 2019;100(4):885–95.
Wu X, Song M, Yang X, Liu X, Liu K, Jiao C, et al. Establishment of bovine embryonic stem cells after knockdown of CDX2. Sci Rep. 2016;6:1–12. https://doi.org/10.1038/srep28343.
Nagashima JB, Sylvester SR, Nelson JL, Cheong SH, Mukai C, Lambo C, et al. Live Births from Domestic Dog (Canis familiaris) Embryos Produced by In Vitro Fertilization. PLoS One. 2015;10(12):e0143930. https://doi.org/10.1371/journal.pone.0143930 Cited 2020 May 18.
Ezashi T, Telugu BPVL, Alexenko AP, Sachdev S, Sinha S, Roberts RM. Derivation of induced pluripotent stem cells from pig somatic cells. Proc Natl Acad Sci. 2009;106(27):10993–8. https://doi.org/10.1073/pnas.0905284106 Cited 2020 May 14.
Nagy K, Sung H-K, Zhang P, Laflamme S, Vincent P, Agha-Mohammadi S, et al. Induced Pluripotent Stem Cell Lines Derived from Equine Fibroblasts. Stem Cell Rev Rep. 2011;7(3):693–702. https://doi.org/10.1007/s12015-011-9239-5 Cited 2020 May 14.
Shimada H, Nakada A, Hashimoto Y, Shigeno K, Shionoya Y, Nakamura T. Generation of canine induced pluripotent stem cells by retroviral transduction and chemical inhibitors. Mol Reprod Dev. 2010;77(1):2–2. https://doi.org/10.1002/mrd.21117 Cited 2020 May 14.
Bai C, Li X, Gao Y, Yuan Z, Hu P, Wang H, et al. Melatonin improves reprogramming efficiency and proliferation of bovine-induced pluripotent stem cells. J Pineal Res. 2016;61(2):154–67. https://doi.org/10.1111/jpi.12334 Cited 2020 May 17.
Yu P, Lu Y, Jordan BJ, Liu Y, Yang J, Hutcheson JM, et al. Nonviral Minicircle Generation of Induced Pluripotent Stem Cells Compatible with Production of Chimeric Chickens. Cell Reprogram. 2014;16(5):366–78. https://doi.org/10.1089/cell.2014.0028 Cited 2020 May 17.
Song H, Li H, Huang M, Xu D, Gu C, Wang Z, et al. Induced pluripotent stem cells from goat fibroblasts. Mol Reprod Dev. 2013;80(12):1009–17. https://doi.org/10.1002/mrd.22266 Cited 2020 May 14.
Bao L, He L, Chen J, Wu Z, Liao J, Rao L, et al. Reprogramming of ovine adult fibroblasts to pluripotency via drug-inducible expression of defined factors. Cell Res. 2011;21(4):600–8 Available from: http://www.nature.com/articles/cr20116. Cited 2020 May 14.
Zhou R, Comizzoli P, Keefer CL. Endogenous pluripotent factor expression after reprogramming cat fetal fibroblasts using inducible transcription factors. Mol Reprod Dev. 2019;86(11):1671–81. https://doi.org/10.1002/mrd.23257 Cited 2020 May 14.
Uto S, Nishizawa S, Hikita A, Takato T, Hoshi K. Application of induced pluripotent stem cells for cartilage regeneration in CLAWN miniature pig osteochondral replacement model. Regen Ther. 2018;9:58–70 Available from: https://linkinghub.elsevier.com/retrieve/pii/S2352320418300105. Cited 2020 May 17.
Van Steenbeek FG, Hytönen MK, Leegwater PAJ, Lohi H. The canine era: the rise of a biomedical model. Anim Genet. 2016;47(5):519–27. https://doi.org/10.1111/age.12460 Cited 2020 May 14.
Smith RK, Garvican ER, Fortier LA. The current “state of play” of regenerative medicine in horses: what the horse can tell the human. Regen Med. 2014;9(5):673–85.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131(5):861–72 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0092867407014717.Cited 2020 May 18.
Borger DK, McMahon B, Lal TR, Serra-Vinardell J, Aflaki E, Sidransky E. Induced pluripotent stem cell models of lysosomal storage disorders. DMM Dis Model Mech. 2017;10(6):691–704.
Ma K, Song G, An X, Fan A, Tan W, Tang B, et al. miRNAs promote generation of porcine-induced pluripotent stem cells. Mol Cell Biochem. 2014;389(1–2):209–18. https://doi.org/10.1007/s11010-013-1942-x Cited 2020 May 22.
Wei R, Lv J, Li X, Li Y, Xu Q, Jin J, et al. Derivation of endothelial cells from porcine induced pluripotent stem cells by optimized single layer culture system. J Vet Sci. 2020;21(1):e9. https://doi.org/10.4142/jvs.2020.21.e9 Cited 2020 May 17.
Ma Y, Yu T, Cai Y, Wang H. Preserving self-renewal of porcine pluripotent stem cells in serum-free 3i culture condition and independent of LIF and b-FGF cytokines. Cell Death Dis. 2018;4(1):21 Available from: http://www.nature.com/articles/s41420-017-0015-4. Cited 2020 May 22.
Zhong L, Mu H, Wen B, Zhang W, Wei Q, Gao G, et al. Long non-coding RNAs involved in the regulatory network during porcine pre-implantation embryonic development and iPSC induction. Sci Rep. 2018;8(1):6649. https://doi.org/10.1038/s41598-018-24863-5 Cited 2020 May 17.
Zhang W, Wang H, Zhang S, Zhong L, Wang Y, Pei Y, et al. Lipid Supplement in the Cultural Condition Facilitates the Porcine iPSC Derivation through cAMP/PKA/CREB Signal Pathway. Int J Mol Sci. 2018;19(2):509 Available from: http://www.mdpi.com/1422-0067/19/2/509. Cited 2020 May 22.
Zhang S, Guo Y, Cui Y, Liu Y, Yu T, Wang H. Generation of Intermediate Porcine iPS Cells Under Culture Condition Favorable for Mesenchymal-to-Epithelial Transition. Stem Cell Rev Rep. 2015;11(1):24–38. https://doi.org/10.1007/s12015-014-9552-x Cited 2020 May 22.
Wang J, Gu Q, Hao J, Jia Y, Xue B, Jin H, et al. Tbx3 and Nr5α2 Play Important Roles in Pig Pluripotent Stem Cells. Stem Cell Rev Rep. 2013;9(5):700–8. https://doi.org/10.1007/s12015-013-9439-2 Cited 2020 May 22.
Cheng D, Guo Y, Li Z, Liu Y, Gao X, Gao Y, et al. Porcine Induced Pluripotent Stem Cells Require LIF and Maintain Their Developmental Potential in Early Stage of Embryos. PLoS One. 2012;7(12):e51778. https://doi.org/10.1371/journal.pone.0051778 Cited 2020 May 22.
Fan A, Ma K, An X, Ding Y, An P, Song G, et al. Effects of TET1 knockdown on gene expression and DNA methylation in porcine induced pluripotent stem cells. Reproduction. 2013;146(6):569–79 Available from: https://rep.bioscientifica.com/view/journals/rep/146/6/569.xml. Cited 2020 May 22.
Esteban MA, Xu J, Yang J, Peng M, Qin D, Li W, et al. Generation of Induced Pluripotent Stem Cell Lines from Tibetan Miniature Pig. J Biol Chem. 2009;284(26):17634–40 Available from: http://www.jbc.org/lookup/doi/10.1074/jbc.M109.008938. Cited 2020 May 22.
Ruan WM, Han JY, Li P, Cao SY, An Y, Lim B, et al. A novel strategy to derive iPS cells from porcine fibroblasts. Sci China Life Sci. 2011;54(6):553–9.
Fujishiro SH, Nakano K, Mizukami Y, Azami T, Arai Y, Matsunari H, et al. Generation of naive-like porcine-induced pluripotent stem cells capable of contributing to embryonic and fetal development. Stem Cells Dev. 2013;22(3):473–82. https://doi.org/10.1089/scd.2012.0173 Cited 2020 May 17.
Kim E, Kim M, Hwang S, Kim J, Lee G, Park YS, et al. Neural induction of porcine-induced pluripotent stem cells and further differentiation using glioblastoma-cultured medium. J Cell Mol Med. 2019;23(3):2052–63. https://doi.org/10.1111/jcmm.14111 Cited 2020 May 22.
Luo J, Qin L, Kural MH, Schwan J, Li X, Bartulos O, et al. Vascular smooth muscle cells derived from inbred swine induced pluripotent stem cells for vascular tissue engineering. Biomaterials. 2017;147:116–32 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0142961217306026. Cited 2020 May 17.
Canizo JR, Vazquez Echegaray C, Klisch D, Aller JF, Paz DA, Alberio RHR, et al. Exogenous human OKSM factors maintain pluripotency gene expression of bovine and porcine iPS-like cells obtained with STEMCCA delivery system. BMC Res Notes. 2018;11(1):509. https://doi.org/10.1186/s13104-018-3627-8 Cited 2020 May 17.
Kim E, Zheng Z, Jeon Y, Jin YX, Hwang SU, Cai L, et al. An Improved System for Generation of Diploid Cloned Porcine Embryos Using Induced Pluripotent Stem Cells Synchronized to Metaphase. PLoS One. 2016;11(7):e0160289. https://doi.org/10.1371/journal.pone.0160289 Cited 2020 May 17.
Choi K-H, Park J-K, Son D, Hwang JY, Lee D-K, Ka H, et al. Reactivation of Endogenous Genes and Epigenetic Remodeling Are Barriers for Generating Transgene-Free Induced Pluripotent Stem Cells in Pig. PLoS One. 2016;11(6):e0158046. https://doi.org/10.1371/journal.pone.0158046 Cited 2020 May 22.
Rodríguez A, Allegrucci C, Alberio R. Modulation of Pluripotency in the Porcine Embryo and iPS Cells. PLoS One. 2012;7(11):e49079. https://doi.org/10.1371/journal.pone.0049079 Cited 2020 May 22.
Setthawong PP, Tharasanit T, Techakumphu M. Effects of activin A on the pluripotency of induced pluripotent stem cells derived from porcine Sertoli cells. Thai J Vet Med. 2019;49(2):183–91.
Setthawong P, Phakdeedindan P, Tiptanavattana N, Rungarunlert S, Techakumphu M, Tharasanit T. Generation of porcine induced-pluripotent stem cells from Sertoli cells. Theriogenology. 2019;127:32–40 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0093691X1831094X. Cited 2020 May 14.
Montserrat N, Bahima EG, Batlle L, Häfner S, Rodrigues AMC, González F, et al. Generation of Pig iPS Cells: A Model for Cell Therapy. J Cardiovasc Transl Res. 2011;4(2):121–30. https://doi.org/10.1007/s12265-010-9233-3 Cited 2020 May 17.
Ao Y, Mich-Basso JD, Lin B, Yang L. High efficient differentiation of functional hepatocytes from porcine induced pluripotent stem cells. PLoS One. 2014;9(6):1–11. https://doi.org/10.1371/journal.pone.0100417 Cited 2020 May 17.
Zhou Y, Wang S, Yu Z, Hoyt, Robert FJ, Hunt T, Kindzelski B, et al. Induced Pluripotent Stem Cell Transplantation in the Treatment of Porcine Chronic Myocardial Ischemia. Ann Thorac Surg. 2014;98(6):2130–7 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0003497514014374. Cited 2020 May 17.
Yang J-R, Shiue Y-L, Liao C-H, Lin S-Z, Chen L-R. Establishment and Characterization of Novel Porcine Induced Pluripotent Stem Cells Expressing hrGFP. Cloning Stem Cells. 2009;11(2):235–44.
Secher JO, Ceylan A, Mazzoni G, Mashayekhi K, Li T, Muenthaisong S, et al. Systematic in vitro and in vivo characterization of Leukemia-inhibiting factor- and Fibroblast growth factor-derived porcine induced pluripotent stem cells. Mol Reprod Dev. 2017;84(3):229–45. https://doi.org/10.1002/mrd.22771 Cited 2020 May 22.
Strnadel J, Carromeu C, Bardy C, Navarro M, Platoshyn O, Glud AN, et al. Survival of syngeneic and allogeneic iPSC–derived neural precursors after spinal grafting in minipigs. Sci Transl Med. 2018;10(440):eaam6651. https://doi.org/10.1126/scitranslmed.aam6651 Cited 2020 May 17.
Kwon DJ, Hwang I-S, Kim H-R, Kim Y-R, Oh KB, Ock S-A, et al. Aberrant methylation of Meg3 in alpha1,3-galactosyltransferase knockout pig induced pluripotent stem cells. Animal Cells Syst (Seoul). 2016;20(3):130–9. https://doi.org/10.1080/19768354.2016.1191543 Cited 2020 May 17.
Kwon D-J, Jeon H, Oh KB, Ock S-A, Im G-S, Lee S-S, et al. Generation of Leukemia Inhibitory Factor-Dependent Induced Pluripotent Stem Cells from the Massachusetts General Hospital Miniature Pig. Biomed Res Int. 2013;2013:1–11 Available from: http://www.hindawi.com/journals/bmri/2013/140639/. Cited 2020 May 22.
West FD, Terlouw SL, Kwon DJ, Mumaw JL, Dhara SK, Hasneen K, et al. Porcine induced pluripotent stem cells produce chimeric offspring. Stem Cells Dev. 2010;19(8):1211–20 https://go-gale-com.subzero.lib.uoguelph.
Zhang Y, Wei C, Zhang P, Li X, Liu T, Pu Y, et al. Efficient Reprogramming of Naïve-Like Induced Pluripotent Stem Cells from Porcine Adipose-Derived Stem Cells with a Feeder-Independent and Serum-Free System. PLoS One. 2014;9(1):e85089. https://doi.org/10.1371/journal.pone.0085089 Cited 2020 May 17.
Wu Z, Chen J, Ren J, Bao L, Liao J, Cui C, et al. Generation of Pig Induced Pluripotent Stem Cells with a Drug-Inducible System. J Mol Cell Biol. 2009;1(1):46–54. https://doi.org/10.1093/jmcb/mjp003 Cited 2020 May 17.
Kim SJ, Kwon HS, Kwon DK, Koo OJ, Moon JH, Park EJ, et al. Production of Transgenic Porcine Embryos Reconstructed with Induced Pluripotent Stem-Like Cells Derived from Porcine Endogenous Factors Using piggyBac System. Cell Reprogram. 2019;21(1):26–36. https://doi.org/10.1089/cell.2018.0036 Cited 2020 May 17.
Qiao S, Deng Y, Li S, Yang X, Shi D, Li X. Partially Reprogrammed Induced Pluripotent Stem Cells Using MicroRNA Cluster miR-302s in Guangxi Bama Minipig Fibroblasts. Cell Reprogram. 2019;21(5):229–37. https://doi.org/10.1089/cell.2019.0035 Cited 2020 May 22.
Xu J, Yu L, Guo J, Xiang J, Zheng Z, Gao D, et al. Generation of pig induced pluripotent stem cells using an extended pluripotent stem cell culture system. Stem Cell Res Ther. 2019;10(1):193. https://doi.org/10.1186/s13287-019-1303-0 Cited 2020 May 22.
Li D, Secher J, Hyttel P, Ivask M, Kolko M, Hall VJ, et al. Generation of transgene-free porcine intermediate type induced pluripotent stem cells. Cell Cycle. 2018;17(23):2547–63. https://doi.org/10.1080/15384101.2018.1548790 Cited 2020 May 17.
Kwon D-J, Hwang I-S, Kwak T-U, Yang H, Park M-R, Ock S-A, et al. Effects of Cell Cycle Regulators on the Cell Cycle Synchronization of Porcine induced Pluripotent Stem Cells. Dev Reprod. 2017;21(1):47–54 Available from: http://www.ksdb.org/archive/view_article?pid=dr-21-1-47. Cited 2020 May 22.
Mao J, Zhang Q, Deng W, Wang H, Liu K, Fu H, et al. Epigenetic Modifiers Facilitate Induction and Pluripotency of Porcine iPSCs. Stem Cell Rep. 2017;8(1):11–20 Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213671116302752. Cited 2020 May 22.
Fukuda T, Tani T, Haraguchi S, Donai K, Nakajima N, Uenishi H, et al. Expression of Six Proteins Causes Reprogramming of Porcine Fibroblasts Into Induced Pluripotent Stem Cells With Both Active X Chromosomes. J Cell Biochem. 2017;118(3):537–53. https://doi.org/10.1002/jcb.25727 Cited 2020 May 17.
Zhang W, Zhong L, Wang J, Han J. Distinct MicroRNA Expression Signatures of Porcine Induced Pluripotent Stem Cells under Mouse and Human ESC Culture Conditions. PLoS One. 2016;11(7):e0158655. https://doi.org/10.1371/journal.pone.0158655 Cited 2020 May 22.
Wang J, Wei R, Bou G, Liu Z. Tbx3 and Nr5α2 improve the viability of porcine induced pluripotent stem cells after dissociation into single cells by inhibiting RHO-ROCK-MLC signaling. Biochem Biophys Res Commun. 2015;456(3):743–9. https://doi.org/10.1016/j.bbrc.2014.12.041 Cited 2020 May 22.
Petkov S, Hyttel P, Niemann H. The Small Molecule Inhibitors PD0325091 and CHIR99021 Reduce Expression of Pluripotency-Related Genes in Putative Porcine Induced Pluripotent Stem Cells. Cell Reprogram. 2014;16(4):235–40. https://doi.org/10.1089/cell.2014.0010 Cited 2020 May 22.
Gao Y, Guo Y, Duan A, Cheng D, Zhang S, Wang H. Optimization of Culture Conditions for Maintaining Porcine Induced Pluripotent Stem Cells. DNA Cell Biol. 2014;33(1):1–11.
Ji G, Ruan W, Liu K, Wang F, Sakellariou D, Chen J, et al. Telomere Reprogramming and Maintenance in Porcine iPS Cells. PLoS One. 2013;8(9):e74202. https://doi.org/10.1371/journal.pone.0074202 Cited 2020 May 22.
Park K-M, Cha S-H, Ahn C, Woo H-M. Generation of porcine induced pluripotent stem cells and evaluation of their major histocompatibility complex protein expression in vitro. Vet Res Commun. 2013;37(4):293–301. https://doi.org/10.1007/s11259-013-9574-x Cited 2020 May 22.
Petkov S, Hyttel P, Niemann H. The Choice of Expression Vector Promoter Is an Important Factor in the Reprogramming of Porcine Fibroblasts into Induced Pluripotent Cells. Cell Reprogram. 2013;15(1):1–8. https://doi.org/10.1089/cell.2012.0053 Cited 2020 May 22.
Kues WA, Herrmann D, Barg-Kues B, Haridoss S, Nowak-Imialek M, Buchholz T, et al. Derivation and Characterization of Sleeping Beauty Transposon-Mediated Porcine Induced Pluripotent Stem Cells. Stem Cells Dev. 2013;22(1):124–35.
Liu Y, Yang JY, Lu Y, Yu P, Dove CR, Hutcheson JM, et al. α -1,3-Galactosyltransferase Knockout Pig Induced Pluripotent Stem Cells: A Cell Source for the Production of Xenotransplant Pigs. Cell Reprogram. 2013;15(2):107–16. https://doi.org/10.1089/cell.2012.0062 Cited 2020 May 22.
Yang J-Y, Mumaw JL, Liu Y, Stice SL, West FD. SSEA4-Positive Pig Induced Pluripotent Stem Cells are Primed for Differentiation into Neural Cells. Cell Transplant. 2013;22(6):945–59. https://doi.org/10.3727/096368912X657279 Cited 2020 May 22.
Gu M, Nguyen PK, Lee AS, Xu D, Hu S, Plews JR, et al. Microfluidic Single-Cell Analysis Shows That Porcine Induced Pluripotent Stem Cell–Derived Endothelial Cells Improve Myocardial Function by Paracrine Activation. Circ Res. 2012;111(7):882–93. https://doi.org/10.1161/CIRCRESAHA.112.269001 Cited 2020 May 17.
Aravalli RN, Cressman ENK, Steer CJ. Hepatic differentiation of porcine induced pluripotent stem cells in vitro. Vet J. 2012;194(3):369–74 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1090023312002158. Cited 2020 May 17.
Liu K, Ji G, Mao J, Liu M, Wang L, Chen C, et al. Generation of Porcine-Induced Pluripotent Stem Cells by Using OCT4 and KLF4 Porcine Factors. Cell Reprogram. 2012;14(6):505–13. https://doi.org/10.1089/cell.2012.0047 Cited 2020 May 25.
Hall VJ, Kristensen M, Rasmussen MA, Ujhelly O, Dinnyés A, Hyttel P. Temporal Repression of Endogenous Pluripotency Genes during Reprogramming of Porcine Induced Pluripotent Stem Cells. Cell Reprogram. 2012;14(3):204–16. https://doi.org/10.1089/cell.2011.0089 Cited 2020 May 22.
Montserrat N, de Oñate L, Garreta E, Gonzãlez F, Adamo A, Eguizãbal C, et al. Generation of Feeder-Free Pig Induced Pluripotent Stem Cells without Pou5f1. Cell Transplant. 2012;21(5):815–25. https://doi.org/10.3727/096368911X601019 Cited 2020 May 22.
Khodadadi K, Sumer H, Pashaiasl M, Lim S, Williamson M, Verma PJ. Induction of Pluripotency in Adult Equine Fibroblasts without c-MYC. Stem Cells Int. 2012;2012:1–9 Available from: http://www.hindawi.com/journals/sci/2012/429160/. Cited 2020 May 18.
Breton A, Sharma R, Diaz AC, Parham AG, Graham A, Neil C, et al. Derivation and characterization of induced pluripotent stem cells from equine fibroblasts. Stem Cells Dev. 2012;22(4):611–21.
Aguiar C, Therrien J, Lemire P, Segura M, Smith LC, Theoret CL. Differentiation of equine induced pluripotent stem cells into a keratinocyte lineage. Equine Vet J. 2016;48(3):338–45. https://doi.org/10.1111/evj.12438 Cited 2020 May 17.
Whitworth DJ, Ovchinnikov DA, Sun J, Fortuna PRJ, Wolvetang EJ. Generation and characterization of leukemia inhibitory factor-dependent equine induced pluripotent stem cells from adult dermal fibroblasts. Stem Cells Dev. 2014;23(13):1515–23.
Sharma R, Livesey MR, Wyllie DJA, Proudfoot C, Whitelaw CBA, Hay DC, et al. Generation of functional neurons from feeder-free, keratinocyte-derived equine induced pluripotent stem cells. Stem Cells Dev. 2014;23(13):1523–34.
Lee E-M, Kim A-Y, Lee E-J, Park J-K, Park S-I, Cho S-G, et al. Generation of Equine-Induced Pluripotent Stem Cells and Analysis of Their Therapeutic Potential for Muscle Injuries. Cell Transplant. 2016;25(11):2003–16. https://doi.org/10.3727/096368916X691691 Cited 2020 May 17.
Tsukamoto M, Nishimura T, Yodoe K, Kanegi R, Tsujimoto Y, Alam ME, et al. Generation of footprint-free canine induced pluripotent stem cells using auto-erasable sendai virus vector. Stem Cells Dev. 2018;27(22):1577–86.
Gonçalves NJN, Bressan FF, Roballo KCS, Meirelles FV, Xavier PLP, Fukumasu H, et al. Generation of LIF-independent induced pluripotent stem cells from canine fetal fibroblasts. Theriogenology. 2017;92:75–82 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0093691X17300249. Cited 2020 May 18.
Koh S, Thomas R, Tsai S, Bischoff S, Lim J-H, Breen M, et al. Growth requirements and chromosomal instability of induced pluripotent stem cells generated from adult canine fibroblasts. Stem Cells Dev. 2013;22(6):951–63.
Luo J, Suhr ST, Chang EA, Wang K, Ross PJ, Nelson LL, et al. Generation of leukemia inhibitory factor and basic fibroblast growth factor-dependent induced pluripotent stem cells from canine adult somatic cells. Stem Cells Dev. 2011;20(10):1669–78.
Lee AS, Xu D, Plews JR, Nguyen PK, Nag D, Lyons JK, et al. Preclinical Derivation and Imaging of Autologously Transplanted Canine Induced Pluripotent Stem Cells. J Biol Chem. 2011;286(37):32697–704. https://doi.org/10.1074/jbc.M111.235739 Cited 2020 May 17.
Baird A, Barsby T, Guest D. Derivation of canine induced pluripotent stem cells. Reprod Domest Anim. 2015;50(4):669–76.
Wang SSWW, Wang SSWW, Wu DC, Lin YC, Ku CC, Wu CC, et al. Androgen receptor-mediated apoptosis in bovine testicular induced pluripotent stem cells in response to phthalate esters. Cell Death Dis. 2013;4(11):1–11 Available from: http://www.nature.com/articles/cddis2013420. Cited 2020 May 17.
Pillai VV, Kei TG, Reddy SE, Das M, Abratte C, Cheong SH, et al. Induced pluripotent stem cell generation from bovine somatic cells indicates unmet needs for pluripotency sustenance. Anim Sci J. 2019;90(9):1149–60. https://doi.org/10.1111/asj.13272 Cited 2020 May 17.
Talbot NC, Sparks WO, Phillips CE, Ealy AD, Powell AM, Caperna TJ, et al. Bovine trophectoderm cells induced from bovine fibroblasts with induced pluripotent stem cell reprogramming factors: Induced bovine trophectoderm cells. Mol Reprod Dev. 2017;84(6):468–85. https://doi.org/10.1002/mrd.22797 Cited 2020 May 17.
Cravero D, Martignani E, Miretti S, Accornero P, Pauciullo A, Sharma R, et al. Generation of Induced Pluripotent Stem Cells from Bovine Epithelial Cells and Partial Redirection Toward a Mammary Phenotype In Vitro. Cell Reprogram. 2015;17(3):211–20. https://doi.org/10.1089/cell.2014.0087 Cited 2020 May 17.
Lei L, Li L, Du F, Chen CH, Wang H, Keefer CL. Monitoring bovine fetal fibroblast reprogramming utilizing a bovine NANOG promoter-driven EGFP reporter system. Mol Reprod Dev. 2013;80(3):193–203. https://doi.org/10.1002/mrd.22147 Cited 2020 May 17.
Cao H, Yang P, Pu Y, Sun X, Yin H, Zhang Y, et al. Characterization of bovine induced pluripotent stem cells by lentiviral transduction of reprogramming factor fusion proteins. Int J Biol Sci. 2012;8(4):498–511 Available from: http://www.ijbs.com/v08p0498.htm. Cited 2020 May 17.
Liou JF, Wu WR, Chen LR, Shiue YL. Establishment of an induced pluripotent cell line from Taiwan black silkie chick embryonic fibroblasts for replication-incompetent virus production. Sci Rep. 2019;9(1):15745 Available from: http://www.nature.com/articles/s41598-019-52282-7. Cited 2020 May 17.
Lu Y, West FD, Jordan BJ, Jordan ET, West RC, Yu P, et al. Induced Pluripotency in Chicken Embryonic Fibroblast Results in a Germ Cell Fate. Stem Cells Dev. 2014;23(15):1755–64.
Katayama M, Hirayama T, Tani T, Nishimori K, Onuma M, Fukuda T. Chick derived induced pluripotent stem cells by the poly-cistronic transposon with enhanced transcriptional activity. J Cell Physiol. 2018;233(2):990–1004. https://doi.org/10.1002/jcp.25947 Cited 2020 May 17.
Fuet A, Montillet G, Jean C, Aubel P, Kress C, Rival-Gervier S, et al. NANOG Is Required for the Long-Term Establishment of Avian Somatic Reprogrammed Cells. Stem Cell Rep. 2018;11(5):1272–86 Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213671118303904.
Song H, Li H, Huang M, Xu D, Wang Z, Wang F. Big animal cloning using transgenic induced pluripotent stem cells: A case study of goat transgenic induced pluripotent stem cells. Cell Reprogram. 2016;18(1):37–47. https://doi.org/10.1089/cell.2015.0035 Cited 2020 May 17.
Chen H, Zuo Q, Wang Y, Song J, Yang H, Zhang Y, et al. Inducing goat pluripotent stem cells with four transcription factor mRNAs that activate endogenous promoters. BMC Biotechnol. 2017;17(1):11. https://doi.org/10.1186/s12896-017-0336-7 Cited 2020 May 17.
Chu Z, Niu B, Zhu H, He X, Bai C, Li G, et al. PRMT5 enhances generation of induced pluripotent stem cells from dairy goat embryonic fibroblasts via down-regulation of p53. Cell Prolif. 2015;48(1):29–38. https://doi.org/10.1111/cpr.12150 Cited 2020 May 17.
Tai D, Liu P, Gao J, Jin M, Xu T, Zuo Y, et al. Generation of Arbas Cashmere Goat Induced Pluripotent Stem Cells Through Fibroblast Reprogramming. Cell Reprogram. 2015;17(4):297–305. https://doi.org/10.1089/cell.2014.0107 Cited 2020 May 22.
Sartori C, DiDomenico AI, Thomson AJ, Milne E, Lillico SG, Burdon TG, et al. Ovine-induced pluripotent stem cells can contribute to chimeric lambs. Cell Reprogram. 2012;14(1):8–19. https://doi.org/10.1089/cell.2011.0050 Cited 2020 May 17.
German SD, Campbell KHS, Thornton E, McLachlan G, Sweetman D, Alberio R. Ovine Induced Pluripotent Stem Cells Are Resistant to Reprogramming after Nuclear Transfer. Cell Reprogram. 2015;17(1):19–27. https://doi.org/10.1089/cell.2014.0071 Cited 2020 May 22.
Liu J, Balehosur D, Murray B, Kelly JM, Sumer H, Verma PJ. Generation and characterization of reprogrammed sheep induced pluripotent stem cells. Theriogenology. 2012;77(2):338–346.e1 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0093691X11004092. Cited 2020 May 17.
Ghimire S, Van Der Jeught M, Neupane J, Roost MS, Anckaert J, Popovic M, et al. Comparative analysis of naive, primed and ground state pluripotency in mouse embryonic stem cells originating from the same genetic background. Sci Rep. 2018;8(1):1–11.
Nagasaka R, Matsumoto M, Okada M, Sasaki H, Kanie K, Kii H, et al. Visualization of morphological categories of colonies for monitoring of effect on induced pluripotent stem cell culture status. Regen Ther. 2017;6:41–51. https://doi.org/10.1016/j.reth.2016.12.003.
Nichols J, Smith A. Naive and Primed Pluripotent States. Cell Stem Cell. 2009;4(6):487–92. https://doi.org/10.1016/j.stem.2009.05.015.
Soto DA, Ross PJ. Pluripotent stem cells and livestock genetic engineering. Transgenic Res. 2016;25(3):289–306. https://doi.org/10.1007/s11248-016-9929-5 Cited 2020 May 17.
Telugu BPVL, Ezashi T, Roberts RM. Porcine induced pluripotent stem cells analogous to nave and primed embryonic stem cells of the mouse. Int J Dev Biol. 2010;54(11–12):1703–11 Available from: http://www.intjdevbiol.com/paper.php?doi=103200bt. Cited 2020 May 17.
West FD, Uhl EW, Liu Y, Stowe H, Lu Y, Yu P, et al. Brief report: Chimeric pigs produced from induced pluripotent stem cells demonstrate germline transmission and no evidence of tumor formation in young pigs. Stem Cells. 2011;29(10):1640–3. https://doi.org/10.1002/stem.713 Cited 2020 May 17.
Zhang W, Pei Y, Zhong L, Wen B, Cao S, Han J. Pluripotent and metabolic features of two types of porcine iPSCs derived from defined mouse and human ES cell culture conditions. PLoS One. 2015;10(4):e0124562. https://doi.org/10.1371/journal.pone.0124562 Cited 2020 May 17.
Martí M, Mulero L, Pardo C, Morera C, Carrió M, Laricchia-Robbio L, et al. Characterization of pluripotent stem cells. Nat Protoc. 2013;8(2):223–53 Available from: http://www.nature.com/articles/nprot.2012.154. Cited 2020 May 17.
Mascetti VL, Pedersen RA. Contributions of Mammalian Chimeras to Pluripotent Stem Cell Research. Cell Stem Cell. 2016;19(2):163–75. https://doi.org/10.1016/j.stem.2016.07.018.
Kim J, Lana B, Torelli S, Ryan D, Catapano F, Ala P, et al. A new patient‐derived iPSC model for dystroglycanopathies validates a compound that increases glycosylation of α‐dystroglycan. EMBO Rep. 2019;20(11):1–15.
Webb RL, Gallegos-Cárdenas A, Miller CN, Solomotis NJ, Liu HX, West FD, et al. Pig Induced Pluripotent Stem Cell-Derived Neural Rosettes Parallel Human Differentiation into Sensory Neural Subtypes. Cell Reprogram. 2017;19(2):88–94. https://doi.org/10.1089/cell.2016.0057 Cited 2020 May 17.
Amilon KR, Cortes-Araya Y, Moore B, Lee S, Lillico S, Breton A, et al. Generation of Functional Myocytes from Equine Induced Pluripotent Stem Cells. Cell Reprogram. 2018;20(5):275–81. https://doi.org/10.1089/cell.2018.0023 Cited 2020 May 17.
Arzt J, Belsham GJ, Lohse L, Bøtner A, Stenfeldt C. Transmission of Foot-and-Mouth Disease from Persistently Infected Carrier Cattle to Naive Cattle via Transfer of Oropharyngeal Fluid. mSphere. 2018;3(5):e00365-18 Available from: https://msphere.asm.org/content/3/5/e00365-18. Cited 2020 May 17.
Rahimi P, Sohrabi A, Ashrafihelan J, Edalat R, Alamdari M, Masoudi M, et al. Emergence of African Swine Fever Virus, Northwestern Iran. Emerg Infect Dis. 2010;16(12):1946–8 Available from: http://wwwnc.cdc.gov/eid/article/16/12/10-0378_article.htm. Cited 2020 May 17.
Taylor RA, Condoleo R, Simons RRL, Gale P, Kelly LA, Snary EL. The Risk of Infection by African Swine Fever Virus in European Swine Through Boar Movement and Legal Trade of Pigs and Pig Meat. Front Vet Sci. 2020;6(January):486. https://doi.org/10.3389/fvets.2019.00486/full Cited 2020 May 17.
Zhao K, He W, Xie S, Song D, Lu H, Pan W, et al. Highly Pathogenic Fowlpox Virus in Cutaneously Infected Chickens, China. Emerg Infect Dis. 2014;20(7):1208–10 Available from: http://wwwnc.cdc.gov/eid/article/20/7/13-1118_article.htm. Cited 2020 May 17.
Fortuna PRJ, Bielefeldt-Ohmann H, Ovchinnikov DA, Wolvetang EJ, Whitworth DJ. Cortical neurons derived from equine induced pluripotent stem cells are susceptible to neurotropic flavivirus infection and replication: an in vitro model for equine neuropathic diseases. Stem Cells Dev. 2018;27(10):704–15.
Bavin EP, Smith O, Baird AEG, Smith LC, Guest DJ. Equine induced pluripotent stem cells have a reduced tendon differentiation capacity compared to embryonic stem cells. Front Vet Sci. 2015;2:55.
Baird A, Lindsay T, Everett A, Iyemere V, Paterson YZ, McClellan A, et al. Osteoblast differentiation of equine induced pluripotent stem cells. Biol Open. 2018;7(5):bio033514. https://doi.org/10.1242/bio.033514 Cited 2020 May 17.
Whitworth DJ, Frith JE, Frith TJR, Ovchinnikov DA, Cooper-White JJ, Wolvetang EJ. Derivation of mesenchymal stromal cells from canine induced pluripotent stem cells by inhibition of the TGFβ/Activin Signaling Pathway. Stem Cells Dev. 2014;23(24):3021–33.
Nishimura T, Hatoya S, Kanegi R, Sugiura K, Wijewardana V, Kuwamura M, et al. Generation of functional platelets from canine induced pluripotent stem cells. Stem Cells Dev. 2013;22(14):2026–35.
Shittu I, Zhu Z, Lu Y, Hutcheson JM, Stice SL, West FD, et al. Development, characterization and optimization of a new suspension chicken-induced pluripotent cell line for the production of Newcastle disease vaccine. Biologicals. 2016;44(1):24–32 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1045105615001074. Cited 2020 May 17.
Susta L, He Y, Hutcheson JM, Lu Y, West FD, Stice SL, et al. Derivation of chicken induced pluripotent stem cells tolerant to Newcastle disease virus-induced lysis through multiple rounds of infection. Virol J. 2016;13(1):1–15. https://doi.org/10.1186/s12985-016-0659-3 Cited 2020 May 17.
Genovese NJ, Domeier TL, Telugu BPVL, Roberts RM. Enhanced Development of Skeletal Myotubes from Porcine Induced Pluripotent Stem Cells. Sci Rep. 2017;7(1):41833 Available from: http://www.nature.com/articles/srep41833. Cited 2020 May 22.
Stashak TS, Theoret CL. Equine Wound Management. Ames: Wiley-Blackwell; 2008.
Sutton TC. The Pandemic Threat of Emerging H5 and H7 Avian Influenza Viruses. Viruses. 2018;10(9) Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6164301/. Cited 2020 May 23.
Magnusson U, Persson S. Endocrine Disruptors in Domestic Animal Reproduction: A Clinical Issue? Reprod Domest Anim. 2015;50:15–9.
Wang X, Shang L, Wang J, Wu N, Wang S. Effect of phthalate esters on the secretion of prostaglandins (F2α and E2) and oxytocin in cultured bovine ovarian and endometrial cells. Domest Anim Endocrinol. 2010;39(2):131–6. https://doi.org/10.1016/j.domaniend.2010.03.002.
Lian Q, Zhang YY, Zhang J, Zhang HK, Wu X, Zhang YY, et al. Functional Mesenchymal Stem Cells Derived From Human Induced Pluripotent Stem Cells Attenuate Limb Ischemia in Mice. Circulation. 2010;121(9):1113–23. https://doi.org/10.1161/CIRCULATIONAHA.109.898312 Cited 2020 May 17.
Zhu Y, Wu X, Liang Y, Gu H, Song K, Zou X, et al. Repair of cartilage defects in osteoarthritis rats with induced pluripotent stem cell derived chondrocytes. BMC Biotechnol. 2016;16(1):78. https://doi.org/10.1186/s12896-016-0306-5 Cited 2020 May 17.
Liao YJ, Tang PC, Chen YH, Chu FH, Kang TC, Chen LR, et al. Porcine induced pluripotent stem cell-derived osteoblast-like cells prevent glucocorticoid-induced bone loss in Lanyu pigs. PLoS One. 2018;13(8):1–21. https://doi.org/10.1371/journal.pone.0202155 Cited 2020 May 17.
Li X, Zhang F, Song G, Gu W, Chen M, Yang B, et al. Intramyocardial Injection of Pig Pluripotent Stem Cells Improves Left Ventricular Function and Perfusion: A Study in a Porcine Model of Acute Myocardial Infarction. PLoS One. 2013;8(6):e66688. https://doi.org/10.1371/journal.pone.0066688 Cited 2020 May 17.
Zhou L, Wang W, Liu Y, De Castro JF, Ezashi T, Telugu BPVL, et al. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem Cells. 2011;29(6):972–80. https://doi.org/10.1002/stem.637 Cited 2020 May 17.
Chung M-J, Park S, Son J-Y, Lee J-Y, Yun HH, Lee E-J, et al. Differentiation of equine induced pluripotent stem cells into mesenchymal lineage for therapeutic use. Cell Cycle. 2019;18(21):2954–71. https://doi.org/10.1080/15384101.2019.1664224 Cited 2020 May 17.
Aguiar C, Theoret C, Smith O, Segura M, Lemire P, Smith LC. Immune potential of allogeneic equine induced pluripotent stem cells. Equine Vet J. 2015;47(6):708–14.
Bavin EP, Atkinson F, Barsby T, Guest DJ. Scleraxis is essential for tendon differentiation by equine embryonic stem cells and in equine fetal tenocytes. Stem Cells Dev. 2017;26(6):441–50.
Liang Q, Monetti C, Shutova MV, Neely EJ, Hacibekiroglu S, Yang H, et al. Linking a cell-division gene and a suicide gene to define and improve cell therapy safety. Nature. 2018;563(7733):701–4 Available from: http://www.nature.com/articles/s41586-018-0733-7. Cited 2020 May 17.
Xu H, Wang B, Ono M, Kagita A, Fujii K, Sasakawa N, et al. Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility. Cell Stem Cell. 2019;24(4):566–578.e7 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1934590919300475. Cited 2020 May 17.
Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on sendai virus, an RNA virus that does not integrate into the host genome. Proc Japan Acad Ser B, Phys Biol Sci. 2009;85(8):348–62.
Lieu PT, Fontes A, Vemuri MC, Macarthur CC. Generation of induced pluripotent stem cells with CytoTune, a non-integrating sendai virus. Methods Mol Biol. 2013;997:45–56.
Woodard LE, Wilson MH. piggyBac-ing models and new therapeutic strategies. Trends Biotechnol. 2015;33(9):525–33 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4663986/. Cited 2020 May 18.
Kawaguchi T, Cho D, Hayashi M, Tsukiyama T, Kimura K, Matsuyama S, et al. Derivation of Induced Trophoblast Cell Lines in Cattle by Doxycycline-Inducible piggyBac Vectors. PLoS One. 2016;11(12):e0167550. https://doi.org/10.1371/journal.pone.0167550 Cited 2020 May 17.
Malik N, Rao MS. A Review of the Methods for Human iPSC Derivation. Totowa: Humana Press; 2013. p. 23–33. https://doi.org/10.1007/978-1-62703-348-0_3. Cited 2020 May 17.
Shao L, Wu W-S. Gene-delivery systems for iPSC cell generation. Expert Opin Biol Ther. 2010;10(2):231–42.
Zhang M, Niibe K, Kondo T, Kamano Y, Saeki M, Egusa H. Gene Delivery and Expression Systems in Induced Pluripotent Stem Cells. In: Interface Oral Health Science 2016. Singapore: Springer Singapore; 2017. p. 121–33. https://doi.org/10.1007/978-981-10-1560-1_11. Cited 2020 May 24.
Wei C, Li X, Zhang P, Zhang Y, Liu T, Jiang S, et al. Characterization of porcine partially reprogrammed iPSCs from adipose-derived stem cells. Reproduction. 2015;149(5):485–96 Available from: https://rep.bioscientifica.com/view/journals/rep/149/5/485.xml. Cited 2020 May 17.
Bernardo AS, Jouneau A, Marks H, Kensche P, Kobolak J, Freude K, et al. Mammalian embryo comparison identifies novel pluripotency genes associated with the naïve or primed state. Biol Open. 2018;7(8):bio.033282.
Verma R, Liu J, Holland MK, Temple-Smith P, Williamson M, Verma PJ. Nanog Is an Essential Factor for Induction of Pluripotency in Somatic Cells from Endangered Felids. Biores Open Access. 2013;2(1):72–6 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3569963/. Cited 2020 May 18.
Beers J, Linask KL, Chen JA, Siniscalchi LI, Lin Y, Zheng W, et al. A cost-effective and efficient reprogramming platform for large-scale production of integration-free human induced pluripotent stem cells in chemically defined culture. Sci Rep. 2015;5 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4464084/. Cited 2020 May 18.
Cota-Coronado A, Durnall JC, Díaz NF, Thompson LH, Díaz-Martínez NE. Unprecedented Potential for Neural Drug Discovery Based on Self-Organizing hiPSC Platforms. Molecules. 2020;25(5):1150 Available from: https://www.mdpi.com/1420-3049/25/5/1150. Cited 2020 May 24.
Calderon-Gierszal EL, Prins GS. Directed differentiation of human embryonic stem cells into prostate organoids in vitro and its perturbation by low-dose bisphenol a exposure. PLoS One. 2015;10(7):1–20.
Sandström J, Eggermann E, Charvet I, Roux A, Toni N, Greggio C, et al. Development and characterization of a human embryonic stem cell-derived 3D neural tissue model for neurotoxicity testing. Toxicol in Vitro. 2017;38:124–35. https://doi.org/10.1016/j.tiv.2016.10.001.
Broutier L, Mastrogiovanni G, Verstegen MMA, Francies HE, Gavarró LM, Bradshaw CR, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23(12):1424–35.
Jehuda R Ben, Shemer Y, Binah O. Genome Editing in Induced Pluripotent Stem Cells using CRISPR/Cas9 Modeling Human Disease in a Dish Using Induced Pluripotent Stem Cells (iPSCs). Stem Cell Reviews and Reports. 2015;14:323–36.
Argani H. Genome engineering for stem cell transplantation. Exp Clin Transplant. 2019;17:31–7.
Heo YT, Quan X, Xu YN, Baek S, Choi H, Kim N-H, et al. CRISPR/Cas9 Nuclease-Mediated Gene Knock-In in Bovine-Induced Pluripotent Cells. Stem Cells Dev. 2015;24(3):393–402.
de Pessôa LV F, Bressan FF, Freude KK. Induced pluripotent stem cells throughout the animal kingdom: Availability and applications. World J Stem Cells. 2019;11(8):491–505 Available from: https://www.wjgnet.com/1948-0210/full/v11/i8/491.htm. Cited 2020 May 17.
Chen C-H, Su Y-H, Lee K-H, Chuang C. Germline Competent Pluripotent Mouse Stem Cells Generated by Plasmid Vectors. Anim Biotechnol. 2016;27(3):157–65. https://doi.org/10.1080/10495398.2016.1140056 Cited 2020 May 24.
Requena J, Alvarez-Palomo AB, Codina-Pascual M, Delgado-Morales R, Moran S, Esteller M, et al. Global Proteomic and Methylome Analysis in Human Induced Pluripotent Stem Cells Reveals Overexpression of a Human TLR3 Affecting Proper Innate Immune Response Signaling. Stem Cells. 2019;37(4):476–88.
de Boni L, Gasparoni G, Haubenreich C, Tierling S, Schmitt I, Peitz M, et al. DNA methylation alterations in iPSC- and hESC-derived neurons: Potential implications for neurological disease modeling. Clin Epigenetics. 2018;10(1):1–13.
Acknowledgements
Not applicable.
Funding
Dr. Koch’s work is supported by numerous granting agencies including Equine Guelph, Pet-Trust, Natural Sciences and Engineering Research Council of Canada, Morris Animal Foundation, Canadian Foundation of Innovation, and the Ontario Ministry of Innovation.
Author information
Authors and Affiliations
Contributions
RS and SP contributed to the conception of the review, data collection and analysis, and drafting of the manuscript. TK and KR contributed to the conception of the review. RS, SP, TK, KR, and DBs contributed to the manuscript revision. RS, SP, KR, TK, and DB read and approved the final manuscript.
Author’s information
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Scarfone, R.A., Pena, S.M., Russell, K.A. et al. The use of induced pluripotent stem cells in domestic animals: a narrative review. BMC Vet Res 16, 477 (2020). https://doi.org/10.1186/s12917-020-02696-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-020-02696-7