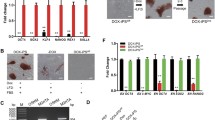
Abstract
It has been demonstrated that naïve and primed pluripotency are determined by different extracellular signals. In this study, we investigated whether intermediate pluripotent states could be available by manipulating the culture condition during the process of generating pig induced pluripotent stem cells (piPSCs). By optimizing the culture condition that efficiently promotes mesenchymal-to-epithelial transition (MET), we found that combination of three growth factors (LIF, FGF2 and BMP4) and two inhibitors (2i: CHIR99021 and SB431542) could generate an intermediate pluripotent state of piPSCs, which were named as LFB2i-piPSCs. The LFB2i-piPSCs are stable and fulfill all the criteria of pluripotency, including expression of pluripotent genes, differentiation into three germ layers via embryoid bodies in vitro and teratoma in vivo. More importantly, the mRNA-sequencing data showed that LFB2i-piPSCs had a mixed transcriptome of naïve and primed pluripotency, which featured by expressing high levels of SOX2, L-MYC and ESRRB and relatively low levels of POU5F1, KLF4 and NANOG. Small RNA sequencing also demonstrated that LFB2i-piPSCs had a mixed microRNA profile of naïve and primed pluripotency, which featured by expressing high levels of miR-302b/367 cluster and miR-106a/363 cluster, and low levels of most let-7 family members and miR-17/92 cluster. Altogether, the LFB2i-piPSCs represent a stable intermediate pluripotent state with unique transcriptome and microRNA signatures. The LFB2i-piPSCs will provide a new tool to explore the mechanisms of pluripotency and reprogramming on pig species.






Similar content being viewed by others
References
Nichols, J., & Smith, A. (2009). Naive and primed pluripotent states. Cell Stem Cell, 4(6), 487–492.
Guo, G., Yang, J., Nichols, J., Hall, J. S., Eyres, I., Mansfield, W., et al. (2009). Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development, 136(7), 1063–1069.
Zhou, H. Y., Li, W. L., Zhu, S. Y., Joo, J. Y., Do, J. T., Xiong, W., et al. (2010). Conversion of mouse epiblast stem cells to an earlier pluripotency state by small molecules. Journal of Biological Chemistry, 285(39), 29676–29680.
Ying, Q. L., Wray, J., Nichols, J., Batlle-Morera, L., Doble, B., Woodgett, J., et al. (2008). The ground state of embryonic stem cell self-renewal. Nature, 453(7194), 519–523.
Chou, Y.F., Chen, H.H., Eijpe, M., Yabuuchi, A., Chenoweth, J.G., Tesar, P., et al. (2008). The Growth Factor Environment Defines Distinct Pluripotent Ground States in Novel Blastocyst-Derived Stem Cells. 135(3), 449–461.
Han, D. W., Greber, B., Wu, G., Tapia, N., Arauzo-Bravo, M. J., Ko, K., et al. (2011). Direct reprogramming of fibroblasts into epiblast stem cells. Nature Cell Biology, 13(1), 66–71.
Roberts, R. M., Telugu, B. P. V. L., & Ezashi, T. (2009). Induced pluripotent stem cells from swine (Sus scrofa) why they may prove to be important. Cell Cycle, 8(19), 3078–3081.
Esteban, M. A., Xu, J., Yang, J., Peng, M., Qin, D., Li, W., et al. (2009). Generation of induced pluripotent stem cell lines from Tibetan miniature pig. Journal of Biological Chemistry, 284(26), 17634–17640.
Wu, Z., Chen, J., Ren, J., Bao, L., Liao, J., Cui, C., et al. (2009). Generation of pig induced pluripotent stem cells with a drug-inducible system. Journal of Molecular Cell Biology, 1(1), 46–54.
Montserrat, N., de Onate, L., Garreta, E., Gonzalez, F., Adamo, A., Eguizabal, C., et al. (2012). Generation of feeder-free pig induced pluripotent stem cells without Pou5f1. Cell Transplantation, 21(5), 815–825.
Cheng, D., Guo, Y. J., Li, Z. Z., Liu, Y. J., Gao, X., Gao, Y., et al. (2012). Porcine induced pluripotent stem cells require LIF and maintain their developmental potential in early stage of embryos. PloS One, 7(12), e51778.
Thomson, A. J., Pierart, H., Meek, S., Bogerman, A., Sutherland, L., Murray, H., et al. (2012). Reprogramming pig fetal fibroblasts reveals a functional LIF signaling pathway. Cellular Reprogramming, 14(2), 112–122.
Ezashi, T., Telugu, B. P., Alexenko, A. P., Sachdev, S., Sinha, S., & Roberts, R. M. (2009). Derivation of induced pluripotent stem cells from pig somatic cells. Proceedings of the National Academy of Sciences of the United States of America, 106(27), 10993–10998.
West, F. D., Terlouw, S. L., Kwon, D. J., Mumaw, J. L., Dhara, S. K., Hasneen, K., et al. (2010). Porcine induced pluripotent stem cells produce chimeric offspring. Stem Cells and Development, 19(8), 1211–1220.
West, F. D., Uhl, E. W., Liu, Y., Stowe, H., Lu, Y., Yu, P., et al. (2011). Brief report: chimeric pigs produced from induced pluripotent stem cells demonstrate germline transmission and no evidence of tumor formation in young pigs. Stem Cells, 29(10), 1640–1643.
Plath, K., & Lowry, W. E. (2011). Progress in understanding reprogramming to the induced pluripotent state. Nature Reviews. Genetics, 12(4), 253–265.
Li, R. H., Liang, J. L., Ni, S., Zhou, T., Qing, X. B., Li, H. P., et al. (2010). A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell, 7(1), 51–63.
Samavarchi-Tehrani, P., Golipour, A., David, L., Sung, H. K., Beyer, T. A., Datti, A., et al. (2010). Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell, 7(1), 64–77.
Chen, J., Liu, J., Yang, J., Chen, Y., Ni, S., Song, H., et al. (2011). BMPs functionally replace Klf4 and support efficient reprogramming of mouse fibroblasts by Oct4 alone. Cell Research, 21(1), 205–212.
Gao, Y., Guo, Y. J., Duan, A. Q., Cheng, D., Zhang, S. Q., & Wang, H. Y. (2014). Optimization of culture conditions for maintaining porcine induced pluripotent stem cells. DNA and Cell Biology, 33(1), 1–11.
Zhang, S., Chen, S., Li, W., Guo, X., Zhao, P., Xu, J., et al. (2011). Rescue of ATP7B function in hepatocyte-like cells from Wilson’s disease induced pluripotent stem cells using gene therapy or the chaperone drug curcumin. Human Molecular Genetics, 20(16), 3176–3187.
Rodriguez, A., Allegrucci, C., & Alberio, R. (2012). Modulation of pluripotency in the porcine embryo and iPS cells. PloS One, 7(11), e49079.
Fujishiro, S., Nakano, K., Mizukami, Y., Azami, T., Arai, Y., Matsunari, H., et al. (2013). Generation of naive-like porcine-induced pluripotent stem cells capable of contributing to embryonic and fetal development. Stem Cells and Development, 22(3), 473–482.
Maherali, N., & Hochedlinger, K. (2009). Tgfbeta signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Current Biology, 19(20), 1718–1723.
Xiao, S., Xie, D., Cao, X. Y., Yu, P. F., Xing, X. Y., Chen, C. C., et al. (2012). Comparative epigenomic annotation of regulatory DNA. Cell, 149(6), 1381–1392.
Cao, S., Han, J., Wu, J., Li, Q., Liu, S., Zhang, W., et al. (2014). Specific gene-regulation networks during the pre-implantation development of the pig embryo as revealed by deep sequencing. BMC Genomics, 15, 4.
Telugu, B. P., Ezashi, T., Sinha, S., Alexenko, A. P., Spate, L., Prather, R. S., et al. (2011). Leukemia inhibitory factor (LIF)-dependent, pluripotent stem cells established from inner cell mass of porcine embryos. Journal of Biological Chemistry, 286(33), 28948–28953.
Buganim, Y., Faddah, D. A., Cheng, A. W., Itskovich, E., Markoulaki, S., Ganz, K., et al. (2012). Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell, 150(6), 1209–22.
Hassani, S. N., Totonchi, M., Gourabi, H., Scholer, H. R., & Baharvand, H. (2014). Signaling roadmap modulating naive and primed pluripotency. Stem Cells and Development, 23(3), 193–208.
Heinrich, E. M., & Dimmeler, S. (2012). MicroRNAs and stem cells control of pluripotency, reprogramming, and lineage commitment. Circulation Research, 110(7), 1014–1022.
Worringer, K. A., Rand, T. A., Hayashi, Y., Sami, S., Takahashi, K., Tanabe, K., et al. (2014). The let-7/LIN-41 pathway regulates reprogramming to human induced pluripotent stem cells by controlling expression of prodifferentiation genes. Cell Stem Cell, 14(1), 40–52.
Tsukiyama, T., & Ohinata, Y. (2014). A modified EpiSC culture condition containing a GSK3 inhibitor can support germline-competent pluripotency in mice. PloS One, 9(4), e95329.
Chang, K. H., & Li, M. (2013). Clonal isolation of an intermediate pluripotent stem cell state. Stem Cells, 31(5), 918–927.
Gafni, O., Weinberger, L., Mansour, A. A., Manor, Y. S., Chomsky, E., Ben-Yosef, D., et al. (2013). Derivation of novel human ground state naive pluripotent stem cells. Nature, 504(7479), 282–6.
Ware, C. B., Nelson, A. M., Mecham, B., Hesson, J., Zhou, W. Y., Jonlin, E. C., et al. (2014). Derivation of naive human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 111(12), 4484–4489.
Xu, J., Lamouille, S., & Derynck, R. (2009). TGF-beta-induced epithelial to mesenchymal transition. Cell Research, 19(2), 156–172.
Ichida, J. K., Blanchard, J., Lam, K., Son, E. Y., Chung, J. E., Egli, D., et al. (2009). A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell, 5(5), 491–503.
Nakagawa, M., Koyanagi, M., Tanabe, K., Takahashi, K., Ichisaka, T., Aoi, T., et al. (2008). Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nature Biotechnology, 26(1), 101–106.
Brons, I. G., Smithers, L. E., Trotter, M. W., Rugg-Gunn, P., Sun, B., de Sousa, C., Lopes, S. M., et al. (2007). Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature, 448(7150), 191–5.
Yang, J. Y., Mumaw, J. L., Liu, Y. B., Stice, S. L., & West, F. D. (2013). SSEA4-positive pig induced pluripotent stem cells are primed for differentiation into neural cells. Cell Transplantation, 22(6), 945–959.
Festuccia, N., Osorno, R., Halbritter, F., Karwacki-Neisius, V., Navarro, P., Colby, D., et al. (2012). Esrrb is a direct nanog target gene that can substitute for nanog function in pluripotent cells. Cell Stem Cell, 11(4), 477–490.
Feng, B., Jiang, J., Kraus, P., Ng, J. H., Heng, J. C., Chan, Y. S., et al. (2009). Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nature Cell Biology, 11(2), 197–203.
Martello, G., Sugimoto, T., Diamanti, E., Joshi, A., Hannah, R., Ohtsuka, S., et al. (2012). Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell, 11(4), 491–504.
Greve, T. S., Judson, R. L., & Blelloch, R. (2013). microRNA control of mouse and human pluripotent stem cell behavior. Annual Review of Cell and Developmental Biology, 29, 213–39.
Acknowledgments
We thank Dr. West for providing the RNA lysate of FGF2-piPSCs. This work was supported by the National Natural Science Foundation of China (No. 31371505, 31301218); the National Basic Research Program of China (2011CBA01002) and the Northwest A&F University research start-up grant (No. 201104050355).
Author Contribution
S.Z., Y.G.: conception and design, collection and/or assembly of data, data analysis and interpretation, and manuscript writing; S.Z. and Y.G. contributed equally to this article. Y.L.: analysis of RNA-seq data; Y.C.: analysis of small RNA-seq data; T.Y.: collection and/or assembly of data; H.W.: conception and design, financial support, administrative support, data analysis and interpretation, manuscript writing, and final approval of manuscript.
Conflict of Interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
(A) Genomic DNA PCR analysis of transgene integration in two piPSC lines LFB2i-piPSC-1 and LFB2i-piPSC-2. (B) Quantitative RT-PCR analysis of endogenous expression of POU5F1, SOX2, NANOG and ESRRB in LFB2i-piPSC-1 and LFB2i-piPSC-2. The y-axis represents the fold change vs. PEFs. (C) Karyotype analysis of LFB2i-piPSC-2. (D) Growth curve of LFB2i-piPSC-1 and PEFs. (GIF 74 kb)
Figure S2
RT-PCR analysis of genes expression in embryoid bodies (EBs) derived from LFB2i-piPSCs. The undifferentiated cells were used as negative control. NESTIN is for ectoderm marker; AFP for endoderm marker; DESMIN for mesoderm marker. GAPDH is used as internal control. (GIF 20 kb)
Table S1
(DOC 36 kb)
Table S2
(DOC 32 kb)
Table S3
(XLS 1326 kb)
Table S4
(XLS 449 kb)
Table S5
(XLS 400 kb)
Table S6
(XLS 21 kb)
Table S7
(XLS 35 kb)
Table S8
(XLS 18 kb)
Rights and permissions
About this article
Cite this article
Zhang, S., Guo, Y., Cui, Y. et al. Generation of Intermediate Porcine iPS Cells Under Culture Condition Favorable for Mesenchymal-to-Epithelial Transition. Stem Cell Rev and Rep 11, 24–38 (2015). https://doi.org/10.1007/s12015-014-9552-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-014-9552-x