Abstract
Background
To overcome the escalating problems associated with infectious diseases and drug resistance, discovery of new antimicrobials is crucial. The present study aimed to carry out in vitro antimicrobial analysis of 15 medicinal plant species selected according to their traditional medicinal uses in Gurage and Silti Zones, south central Ethiopia.
Methods
Ethanol extracts of various plant parts were investigated for their antimicrobial activity against 20 bacterial and one yeast strains. The minimum inhibitory concentration (MIC) was determined by broth microdilution method.
Results
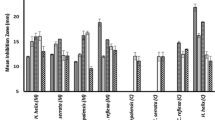
Asparagus africanus, Guizotia schimperi, Lippia adoensis var. adoensis and Premna schimperi were active against Candida albicans, Enterococcus faecalis and Staphylococcus aureus at a concentration of 512 μg/ml or lower. Strong antibacterial activity (MIC ≥ 128 μg/ml) was observed for G. schimperi extract against 17 resistant and sensitive Staphylococcus strains, at a concentration comparable to standard antibiotics. Moreover, this extract showed higher antibacterial activity for the test against S. aureus ATCC 33591, ATCC 33592, SA3 and SA5 strains (128–256 μg/ml) than oxacillin (512 μg/ml).
Conclusions
The study revealed in vitro antibacterial activity of plants used in folk medicine in south central Ethiopia. The usefulness of these plants, in particular of G. schimperi, should be confirmed through further phytochemical and toxicity analyses.
Similar content being viewed by others
Background
Infectious diseases are an important cause of mortality and morbidity, in all regions of the world. The increasing emergence of antimicrobial resistance worsens the impact [1, 2]. It has been shown that risk of negative clinical consequences, mortality, and high treatment costs with drug-resistant bacteria is generally higher compared to patients infected with the same non-resistant bacteria [3]. Increased prevalence of resistant bacteria, together with lack and high cost of new generation drugs has escalated infection-related morbidity and mortality particularly in developing countries like Ethiopia [1, 4].
Numerous biochemical compounds obtained from medicinal plants possess important antimicrobial properties [5]. Application of these compounds is preferred over synthetic drugs as they have long been used in traditional medicine and are considered safe to humans [6]. New and effective antimicrobials identified from plants can consequently be considered in development of new drugs to combat problems associated with drug resistance [7]. Using effective plant extracts to control human diseases has the additional advantage of low production cost, minimal environmental damage and higher accessibility to rural communities [4, 8, 9]. Hence, medicinal plants are expected to be the future alternative source of new antimicrobial products [5, 10].
Treatment of infections with plant-derived compounds is an age-old practice that is globally employed, especially in developing countries [11, 12]. This point applies particularly to Ethiopia, where dramatic geographic, climatic and cultural diversity contribute to a wide range of traditional herbal knowledge and practices by the people [13, 14]. Numerous in vitro studies have been undertaken, and have revealed the antimicrobial potential of herbal medicines traditionally used in various regions of Ethiopia [11, 15–19]. However, many Ethiopian medicinal plants still await scientific validation of their anti-infective properties. The aim of this study was to assess in vitro antimicrobial activity of medicinal plant species selected based on their traditional medicinal uses for infectious diseases treatment in local community of Gurage and Silti Zones, south central Ethiopia. This analysis may also offer a source of information to identify effective medicinal plants against staphylococcal infections and facilitate selection of plants for further phytochemical investigation.
Methods
Selection of plants
Medicinal plants were collected from Gurage and Silti Zones, south central Ethiopia. Specimens were collected, pressed and identified by the first author and Melaku Wondafrash, an expert from the National Herbarium (ETH), through visual comparisons with authenticated plant specimens and using taxonomic keys in the volumes of Flora of Ethiopia and Eritrea [20–25]. Identifications were then authenticated by Prof. Sebsebe Demissew of Addis Ababa University, Ethiopia. Voucher specimens were deposited at the National Herbarium (ETH), Addis Ababa University. Selection of plant species was based on use reports of local informants and traditional herbalists from the study area for treatment of ailments caused by microbial agents. Ethnomedicinal use reports of the 15 medicinal plant species selected, parts used, and route of administration are summarized in Table 1.
Preparation of plant extracts
Plant materials were air-dried and ground into powder using an electric mill (GM100 Retsch, Germany). Each powdered sample species (15 g) was macerated with 450 ml of 80 % ethanol and placed on a shaker (200 rpm) (GFL3005, Germany) for 24 h. All procedures, stated above, were carried out at room temperature. Extracts were then filtered and concentrated using a rotary vacuum evaporator (R-200 Buchi, Switzerland) at 40 °C. Dried residues were dissolved in 100 % dimethyl sulfoxide (DMSO) to obtain a stock concentration of 51.2 mg/ml, which was kept at −80 °C until use. Dried residue yield figures (%) are shown in Table 1.
Chemicals
Antibiotics ciprofloxacin (purity 99.5 %), oxacillin (purity ≥ 81.5 %), tetracycline (purity ≥ 88 %) and tioconazole (purity 97 %), were purchased from Sigma-Aldrich (Prague, Czech Republic). Potency of the powder was incorporated in the formula for preparation of stock solutions according to EUCAST [26]. DMSO (Penta, Czech Republic), ethanol (Sigma-Aldrich, Czech Republic), and distilled water were used as solvents. Cation-adjusted Mueller-Hinton broth (MHB) (Oxoid, United Kingdom) equilibrated for testing with Tris-buffered saline (Sigma-Aldrich, Czech Republic) was used as a bacterial culture media.
Microorganisms
In this study, 20 bacterial strains and one yeast were tested. The following American Type Culture Collection (ATCC) standard strains were purchased from Oxoid (United Kingdom) for analysis: Candida albicans ATCC 10231, Enterococcus faecalis ATCC 29212, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus (ATCC 25923, ATCC 29213, ATCC 33591, ATCC 33592, ATCC 43300, ATCC BAA 976), and S. epidermidis ATCC 12228. Ten clinical isolates of antibiotic-sensitive as well as antibiotic-resistant S. aureus strains (SA1, SA2, SA3, SA4, SA5, SA6, SA7, SA8, SA9, SA10) were provided by University Hospital in Motol (Prague, Czech Republic). Microorganism cultures were stored in MHB at 4 °C until use. Prior to antimicrobial tests, microorganisms were re-cultured at 37 °C for 24 h (48 h for C. albicans).
Assessment of minimum inhibitory concentrations (MICs)
MICs were determined by the broth microdilution method using 96-well microplates modified according to previous recommendations for effective assessment of the anti-infective potential of natural products [27, 28]. An aliquot of 100 μl of two-fold serial dilutions of each extract was prepared in MBH, equilibrated with Tris-buffered saline, in concentrations ranging 4–512 μg/ml. For inoculum standardization, the turbidity of the bacterial suspension was adjusted to 0.5 McFarland standard (1.5 × 108 CFU/ml) using Densi-La-Meter II (Lachema, Czech Republic) spectrophotometric device. This bacterial suspension was inoculated into each well, and plates were incubated at 37 °C for 24 h (48 h for C. albicans). Microorganism growth was measured as turbidity recorded at 405 nm using the Multiscan Ascent Microplate Reader (Thermo Fisher Scientific, Waltham, MA). The MIC was calculated as the lowest concentration that showed ≥ 80 % reduction of microbial growth compared to extract-free growth control. Antibiotics ciprofloxacin, oxacillin, teteracycline and tioconazole were used as positive controls. Oxacillin and teteracycline were used as markers for methicillin and tetracycline resistance, respectively. Solvents used did not inhibit bacterial growth at concentrations tested. We used S. aureus ATCC 29213 as a quality-control strain for antibiotic susceptibility. Results reported in this study are expressed as the mode of MICs obtained from three independent experiments that were assayed in triplicate.
Results
Extracts from leaves of four species (Asparagus africanus, Guizotia schimperi, Lippia adoensis var. adoensis, Premna schimperi) showed activity against some of the tested microorganisms (Table 2). The extracts were active against C. albicans, E. faecalis and S. aureus at a concentration between 128 and 512 μg/ml. Guizotia schimperi, L. adoensis var. adoensis and P. schimperi showed activity against E. faecalis and S. aureus (MIC range from 128 to 512 μg/ml), whereas A. africanus inhibited growth of E. faecalis (MIC = 512 μg/ml). Candida albicans was susceptible to G. schimperi and L. adoensis var. adoensis at highest concentrations only (MIC = 512 μg/ml). Gram-negative bacteria (E. coli and P. aeruguinosa) were resistant to all ethanol extracts tested in this study.
The ethanol extract of G. schimperi, which showed strong activity against E. faecalis and S. aureusas as compared with other plant extracts, was subjected to further antibacterial analysis against 16 standard and clinical isolates of staphylococcal strains. The clinical isolates were resistant to either oxacillin (MIC ≥ 4 μg/ml) or tetracycline (MIC ≥ 16 μg/ml). Three isolates (SA2, SA3 and SA9) were resistant to both antibiotics, and can be considered as multidrug-resistant strains. Strong antibacterial activity was observed for G. schimperi extract against all strains tested at concentrations of 128–256 μg/ml (Table 3). Moreover, this extract showed higher antibacterial activity for tests against S. aureus ATCC 33591, ATCC 33592, SA3 and SA5 strains (128–256 μg/ml) than oxacillin (512 μg/ml). The same MIC values (128 μg/ml) were obtained for G. schimperi extract as for tetracycline in the test against ATCC 33592.
Discussion
The extracts tested in the present study revealed the potential of traditional medicinal plants in searching for novel pharmaceuticals. We explored 15 plants used in the Gurage and Silti Zones of Ethiopia. Gram-positive bacteria were more sensitive to the medicinal plant extracts tested than Gram-negative bacteria, consistent to previous findings [29, 30]. The G. schimperi extract inhibited all standard and clinical isolates of S. aureus tested. The latter bacterium has been stated as one of the leading causes of human infections, causing significant nosocomial illness, generally via hospital-acquired infections [31]. It occurs commonly in Ethiopia, and shows high levels of resistance to commonly-used antibiotics [2]. In this study, antibacterial activity was most pronounced against ATCC 33591, ATCC 33592, SA3 and SA5, with G. schimperi exhibiting higher activity (MIC ≤ 256 μg/ml) than oxacillin (MIC = 512 μg/ml). Togan et al. [32] described possible differences in susceptibility patterns between standard and clinical strains, in which clinical strains may represent current isolates responsible for clinical disease and spread of resistance.
To the best of our knowledge, no studies related to antimicrobial activity of G. schimperi (synonym of G. scabra subsp. schimperi) have been published previously. This annual weed named “Mocho” by the local people is very close taxonomically to G. abyssinica and G. scabra [24]. It is most likely the wild progenitor of G. abyssinica, cultivated for its edible seeds and known for its medicinal uses [33]. Chemical analysis of essential oils from G. scabra leaves collected from Rwanda has characterized germacrene-D, limonene and diterpenes as the principal constituents. These components have been shown to exhibit several medicinal properties [34, 35]. From a chemotaxonomic point of view, different plant species in a genus often share similar chemical components [36]. In view of these facts, the inhibition exhibited by G. schimperi against standard and clinical isolates in particularly at comparable concentration to standard antibiotics is very promising for phytomedicine development, so phytochemical investigation of G. schimperi leaves is needed to identify their antimicrobial active constituents.
Antimicrobial analysis of L. adoensis var. adoensis extract showed activity against C. albicans, E. faecalis and S. aureus. However, no activity against E. coli or P. aeruginosa. In other studies, petroleum ether, chloroform, acetone and methanol extracts of L. adoensis var. adoensis showed significant activity against E. coli and P. aeruginosa [17] but were inactive against C. albicans [16]. Wasihun et al. [17] reported presence of secondary metabolites of L. adoensis responsible for its antimicrobial activity. The latter authors further showed that, non-polar fractions have relatively better antimicrobial activity compared to polar fractions. Motamedi et al. [37] report that solubility of active principles in plant materials varies according to extraction solvent used, which may relate to differences in antimicrobial effect of plant extracts [38]. Hence, the extraction solvents used in this study could have caused variation in the antimicrobial activity results.
Asparagus africanus extracts showed activity against E. faecalis. Asparagus spp. contain steroidal saponins as major bioactive constituents besides others including, such as flavonoids, resins and tannins [39]. Our results could reflect the bioactive constituents mentioned above. Madikizela et al. [40] applied the broth microdilution method and reported A. africanus ethanol extract as inactive against S. aureus, which complements our results. We found that P. schimperi inhibited growth of E. faecalis and S. aureus. Habtemariam et al. [19]reported a novel diterpene in leaves as active against S. aureus, which might explain the antibacterial activity of P. schimperi in our study.
Extracts of Solanum incanum fruit (methanol, hexane and chloroform) tested by disc diffusion and broth dilution techniques showed no activity against E. coli, S. aureus and P. aeruginosa [41], matching our findings. Alamri and Moustafa [42] applied agar well diffusion to test ethanol extracts of S. incanum fruit, and found it very active against S. aureus, with less activity against P. aeruginosa and E. coli. In a similar study, phenolic compounds were isolated from S. incanum fruits, which could be responsible for inhibition of S. aureus [42]. Concentration of active principles in plants may vary with climate and across geography [15]. Moreover, different methodologies may contribute to differences in antibacterial activity, particularly in the case of our S. incanum fruit extracts.
In the present study, some of the plant species tested on antimicrobial activity showed no inhibition within the applied concentration ranges. Known medicinal plants, such as Apodytes dimidiata (bark), Olinia rochetiana (bark) and Polygala sadebeckiana (root), have been claimed to be medicinally useful by local communities of the study area and in previous scientific studies [29, 30, 43]. The methanol extracts of O. rochetiana bark exhibited antiviral activity against measles virus [43], whereas the anticancer agent, camptothecine, was isolated from the bark of A. dimidiata [44]. For P. sadebeckiana, apart from the ethnomedicinal uses reported by Hailemariam et al. [45] in Ethiopia (the root being used to cure liver disease, abdominal distention and snake bite), no information was found on its medicinal use and antimicrobial effects. It is further also possible that ethanol extract, plants that showed no inhibition, is only active at higher concentrations than the starting concentration (512 μg/ml) used in our study. In general, the disparities between our findings and others may result from differences in chemical composition of extracts, effects of secondary metabolites including antiviral properties [46], geographic variation in antimicrobial properties, or methodological considerations. Scientific testing of medicinal properties thus need to consider these diverse factors, such that application of different testing methods and extraction solvents is important. Regarding species that resulted inactive in this study, despite strong claims of medicinal value, further analyses are needed before more conclusions can be drawn.
Conclusions
The present study revealed the potential of some traditional medicinal plants to be used as sources of antimicrobials. The usefulness of these plants, in particular of G. schimperi, should be confirmed through further phytochemical and toxicity analyses.
References
Mulu A, Moges F, Tessema B, Kassu A. Pattern and multiple drug resistance of bacterial pathogens isolated from wound infection at University of Gondar Teaching Hospital, Northwest Ethiopia. Ethiop Med J. 2006;44(2):125–31.
Olivier C, Williams-Jones B, Doize B, Ozdemir V. Containing global antibiotic resistance: ethical drug promotion in the developing world. In: Sosa A et al., editors. Antibiotic resistance in developing countries. New York: Springer; 2010. p. 505–24.
World Health Organization (WHO). Antimicrobial Resistance. In: Fact SheetNo 194. 2014. http://www.who.int/mediacentre/factsheets/fs194/en/. Accessed 07 June 2015.WHO.
Borkotoky R, Kalita MP, Barooah M, Bora SS, Goswami C. Evaluation and screening of antimicrobial activity of some important medicinal plants of Assam. IJOART. 2013;2(4):132–9.
Abdallah EM. Plants: an alternative source for antimicrobials. J Appl Pharmaceut Sci. 2011;1(6):16–20.
Rakholiya K, Kaneria M, Desai D, Chanda S. Antimicrobial activity of decoction extracts of residual parts (seed and peels) of Mangifera indica L. var. Kesar against pathogenic and food spoilage microorganism. In: Mendez-Vilas A, editor. Microbial pathogens and strategies for combating them: Science, Technology and Education. FORMATEX. 2013. p. 850–6.
Bonjar GHS. Screening for antibacterial properties of some Iranian plants against two strains of Escherichia coli. Asian J Plant Sci. 2004;3:310–4.
Ray AB, Sarma BK, Singh UP. Medicinal properties of plants: antifungal, antibacterial and antiviral activities. Lucknow: International Book Distributing Co; 2004.
Sharma BC. In-vitro antibacterial activity of certain folk medicinal plants from Darjeeling Himalayas used to treat microbial infections. J Pharmacogn Phytochem. 2013;2(4):1–4.
Savoia D. Plant-derived antimicrobial compounds: alternatives to antibiotics. Future Microbiol. 2012;7(8):979–90.
Geyid A, Abebe D, Debella A, Makonnen Z, Aberra F, Teka F, et al. Screening of some medicinal plants of Ethiopia for their anti-microbial properties and chemical profiles. J Ethnopharmacol. 2005;97:421–7.
De Albuquerque UP. Re-examining hypotheses concerning the use and knowledge of medicinal plants: a study in the Caatinga vegetation of NE Brazil. J Ethnobiol Ethnomed. 2006;2:30.
Demissew S, Dagne E. Basic and applied research in medicinal plants. In: Zewdu M, Demissie A, editors. Conservation and sustainable use of medicinal plants in Ethiopia proceeding of the national workshop on biodiversity conservation and sustainable use of medicinal plants in Ethiopia. Addis Ababa: Institute of Biodiversity Conservation and Research; 2001.
Giday M. An ethnobotanical study of medicinal plants used by the Zay people in Ethiopia. Skriftserie. 2001;3:81–99.
Taye B, Giday M, Animut A, Seid J. Antibacterial activities of selected medicinal plants in traditional treatment of human wounds in Ethiopia. Asian Pac J Trop Biomed. 2011;1(5):370–5.
Tadeg H, Mohammed E, Asres K, Gebre-Mariam T. Antimicrobial activities of some selected traditional Ethiopian medicinal plants used in the treatment of skin disorders. J Ethnopharmacol. 2005;100:168–75.
Wasihun Y, Adraro T, Ali S. Evaluation of antibacterial activity and phytochemical constituents of leaf extract of Lippia adoensis. APJEE. 2014;1(1):45–53.
Tafesse G, Mekonnen Y, Makonnen E. Antifertility effect of aqueous and ethanol extracts of the leaves and roots of Asparagus africanus in rats. Afr Health Sci. 2006;6(2):81–5.
Habtemariam S, Gray AI, Halbert GW, Waterman PG. A novel antibacterial diterpene from Premna schimperi. Planta Med. 1990;56:187–9.
Hedberg I, Edwards S, eds. Flora of Ethiopia and Eritrea. Pittosporaceae to Araliaceae. Volume 3. The National Herbarium, Addis Ababa, Ethiopia, and Department of Systematic Botany, Uppsala, Sweden; 1989.
Edwards S, Tadesse M, Hedberg I, eds. Flora of Ethiopia and Eritrea. Canellaceae to Euphorbiaceae. Volume 2. Issue part 2. The National Herbarium, Addis Ababa, Ethiopia, and Department of Systematic Botany, Uppsala, Sweden; 1995.
Edwards S, Demissew S, Hedberg I, eds. Flora of Ethiopia and Eritrea. Hydrocharitaceae to Arecaceae. Volume 6. The National Herbarium, Addis Ababa, Ethiopia, and Department of Systematic Botany, Uppsala, Sweden; 1997.
Edwards S, Tadesse M, Demissew S, Hedberg I, eds. Flora of Ethiopia and Eritrea. Magnoliaceae to Flacourtiaceae. Volume 2. Issue part 1. The National Herbarium, Addis Ababa, Ethiopia, and Department of Systematic Botany, Uppsala, Sweden; 2000.
Hedberg I, Friis I, Edwards S, eds. Flora of Ethiopia and Eritrea. Asteraceae (Compositae). Volume 4. Issue part 2. The National Herbarium, Addis Ababa, Ethiopia, and Department of Systematic Botany, Uppsala, Sweden; 2004.
Hedberg I, Kelbessa Ensermu, Edwards S, Demissew Sebsebe, Persson E, eds. Flora of Ethiopia and Eritrea. Gentianaceae to Cyclocheilaceae. Volume 5. The National Herbarium, Addis Ababa, Ethiopia, and Department of Systematic Botany, Uppsala, Sweden; 2006.
European Committee for Antimicrobial Susceptibility Testing (EUCAST). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin Micro Inf. 2003;9(8):1–7.
CLSI (Clinical and Laboratory Standards Institute). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. 8th ed. Wayne: Document M7-A8; 2009.
Cos P, Vlietinck AJ, Vanden Berghe D, Maes L. Anti-infective potential of natural products: how to develop a stronger in-vitro ‘proof-of-concept’. J Ethnopharmacol. 2006;106(3):290–302.
Kuglerova M, Tesarova H, Grade JT, Halamova K, Maganyi OW, Van Damme P, et al. Antimicrobial and antioxidative effects of Ugandan medicinal barks. Afr J Biotechnol. 2011;10(18):3628–32.
Lulekal E, Rondevaldova J, Bernaskova E, Cepkova J, Asfaw Z, Kelbessa E, et al. Antimicrobial activity of traditional medicinal plants from Ankober District, North Shewa Zone, Amhara Region, Ethiopia. Pharm Biol. 2014;52(5):614–20.
Abouzeed YM, Elfahem A, Zgheel F, Ahmed MO. Antibacterial in-vitro activities of selected medicinal plants against methicillin resistant staphylococcus aureus from Libyan environment. J Environ Anal Toxicol. 2013;3(6):1–10.
Togan T, Evren E, Çiftci Ö, Narci H, Özcan MM, Arslan H. The antibacterial effect of Propolis against clinical isolates. J Sci Issues Res Essay. 2015;2(12):551–3.
Geleta M, Bryngelsson T, Bekele E, Dagne K. Comparative analysis of genetic relationship and diagnostic markers of several taxa of Guizotia Cass. (Asteraceae) as revealed by AFLPs and RAPDs. Plant Syst Evol. 2007;265:221–39.
Fujimoto Y, Kakinuma K, Eguchi T, Ikekawa N, Hirayama N, Mbarushimana A, et al. 12,15-Dihydroxylabda-8(17),13-dien-19-oic acid from Guizotia scabra. Phytochemistry. 1990;29:319–21.
Mukazayire MJ, Allaeys V, Buc Calderon P, Stévigny C, Bigendako MJ, Duez P. Evaluation of the hepatotoxic and hepatoprotective effect of Rwandese herbal drugs on in vivo (guinea pigs barbiturate-induced sleeping time) and in vitro (rat precision-cut liver slices, PCLS) models. Exp Toxicol Pathol. 2010;62(3):289–99.
Noge K, Becerra JX. Germacrene D, a common sesquiterpene in the genus Bursera (Burseraceae). Molecules. 2009;14:5289–97.
Motamedi H, Darabpour E, Gholipour M, Seyyed Nejad SM. In-vitro assay for the anti-brucella activity of medicinal plants against tetracycline-resistant Brucella melitensis. J Zhejiang Univ (Sci). 2010;11(7):506–11.
El Astal ZY, Ashour AERA, Kerrit AAM. Antimicrobial activity of some medicinal plant extracts in Palestine. Pak J Med Sci. 2005;21(2):187–93.
Negi JS, Singh P, Joshi GP, Rawat MS, Bisht VK. Chemical constituents of Asparagus. Pharmacogn Rev. 2010;4:215–20.
Madikizela B, Ndhlala AR, Finnie JF, Van Staden J. In-vitro antimicrobial activity of extracts from plants used traditionally in South Africa to treat tuberculosis and related symptoms. Evid Based Complement Altern Med. 2013;2013:840719.
Pavithra PS, Janani VS, Charumathi KH, Indumathy R, Potala S, Verma RS. Antibacterial activity of plants used in Indian herbal medicine. Int J Green Pharmacol. 2010;4(1):22–8.
Alamri SA, Moustafa MF. Antimicrobial properties of 3 medicinal plants from Saudi Arabia against some clinical isolates of bacteria. Saudi Med J. 2012;33(3):272–7.
Parker ME, Chabot S, Ward BJ, Johns T. Traditional dietary additives of the Maasai are antiviral against the measles virus. J Ethnopharmacol. 2007;114:146–52.
Ramesha BT, Suma HK, Senthilkumar U, Priti V, Ravikanth G, Vasudeva R, et al. New plant sources of the anti-cancer alkaloid, camptothecine from the Icacinaceae taxa, India. Phytomedicine. 2013;20:521–7.
Hailemariam T, Demissew S, Asfaw Z. An ethnobotanical study of medicinal plants used by local people in the lowlands of Konta Special Woreda, southern nations, nationalities and peoples regional state, Ethiopia. J Ethnobiol Ethnomed. 2009;5:26.
Cowan MM. Plant product as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564–82.
Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. Wayne: CLSI approved standard M100-S17; 2013.
Acknowledgements
We are grateful to University of Gondar (UoG), Ethiopia, for sponsoring the study. The Research and Graduate Programs Office, Addis Ababa University (AAU) is acknowledged for funding costs of field work. We would also like to thank the local communities and informants for their support and for sharing their knowledge on medicinal plant use. We are grateful to the staff of the National Herbarium, Addis Ababa University, for their kind cooperation in allowing us use of herbarium facilities. We are also indebted to the Bijzonder Onderzoeksfonds (BOF), Ghent University (UGent), for providing the travel grant for the laboratory work in Prague, Czech Republic. Czech University of Life Sciences (CULS), Laboratory of Ethnobotany and Ethnopharmacology, is deeply acknowledged for providing laboratory facilities. Last but not least, we would like to thank Prof. Townsend Peterson (University of Kansas Biodiversity Institute) for comments that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AT performed field data collection, carried out the main experimental work and prepared first draft of the paper. JR and LK designed the experiment. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Teka, A., Rondevaldova, J., Asfaw, Z. et al. In vitro antimicrobial activity of plants used in traditional medicine in Gurage and Silti Zones, south central Ethiopia. BMC Complement Altern Med 15, 286 (2015). https://doi.org/10.1186/s12906-015-0822-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-015-0822-1