Abstract
Background
Trichinellosis, a zoonosis caused by the genus Trichinella, is a widespread foodborne disease. Albendazole, one of the benzimidazole derivatives, is used for treating human trichinellosis, but with limited efficacy in killing the encysted larvae and numerous adverse effects. Cyperus rotundus L. is a herbal plant with a wide range of medicinal uses, including antiparasitic, and is frequently used in traditional medicine to treat various illnesses.
Methods
LC-ESI-MS was used to identify the active phytoconstituents in the methanol extract (MeOH ext.) of the aerial parts of C. rotundus and its derivate fractions ethyl acetate (EtOAc fr.), petroleum ether (pet-ether fr.), and normal butanol (n-BuOH fr.). The in vivo therapeutic effects of C. rotundus fractions of the extracts were evaluated using the fraction that showed the most promising effect after detecting their in vitro anti-Trichinella spiralis potential.
Results
C. rotundus extracts are rich in different phytochemicals, and the LC-ESI-MS of the 90% methanol extract identified 26 phenolic compounds classified as phenolic acids, flavonoids, and organic acids. The in vitro studies showed that C. rotundus extracts had a lethal effect on T. spiralis adults, and the LC50 were 156.12 µg/ml, 294.67 µg/ml, 82.09 µg/ml, and 73.16 µg/ml in 90% MeOH ext., EtOAc fr., pet-ether fr. and n-BuOH fr., respectively. The n-BuOH fr. was shown to have the most promising effects in the in vitro studies, which was confirmed by scanning electron microscopy. The in vivo effects of n-BuOH fr. alone and in combination with albendazole using a mouse model were evaluated by counting adults in the small intestine and larvae in the muscles, in addition to the histopathological changes in the small intestine and the muscles. In the treated groups, there was a significant decrease in the number of adults and larvae compared to the control group. Histopathologically, treated groups showed a remarkable improvement in the small intestine and muscle changes. Remarkably, maximal therapeutic effects were detected in the combination therapy compared to each monotherapy.
Conclusion
Accordingly, C. rotundus extracts may have anti-T. spiralis potential, particularly when combined with albendazole, and they may be used as synergistic to anti-T. spiralis medication therapy.
Similar content being viewed by others
Background
Trichinosis or trichinellosis is an emerging and re-emerging zoonotic parasitic disease with a considerable worldwide distribution [1]. Human infection is caused mainly by Trichinella spiralis, the first species discovered, following ingestion of raw or undercooked pork [2, 3]. Trichinella spiralis has two lifecycle phases. The intestinal phase occurs when adults colonize the small intestine during the first week after infection. In this phase, there is significant pathological damage to the villi, demonstrated as nausea, diarrhea, fever, and vomiting. After that, the muscular phase develops where the larvae invade the muscles and encapsulate. The patient complains of myalgia and muscle weakness during this phase [2, 4].
Benzimidazole derivatives, including albendazole (ABZ) and mebendazole, are the primary anthelminthic drugs used to treat this infection; however, they have a limited effect on encysted larvae [2]. Moreover, they have poor bioavailability and high resistance [5]. Furthermore, most of them are contraindicated in pregnancy and children under three [6]. These drawbacks highlight the necessity for new, effective, safe drugs against trichinellosis. Additionally, natural agents may be a promising option, having proven to be affordable, less toxic, and with no side effects, as observed with synthetic drugs [6].
Around the world, numerous medicinal herbs have been used to treat parasitic infections for hundreds of years [7]. Cyperus contains nearly 600 species belonging to the Cyperaceae family [8]. Cyperus rotundus L. “Nut” grasses are widely distributed in tropical and subtropical regions [9]. It is native to Asia, Africa, the USA, and southern and central Europe [10]. Furthermore, it has been used in folk medicine as a diuretic, aphrodisiac, sedative, carminative, and a remedy for renal colic and dysentery [11, 12]. Also, C. rotundus has many biological and pharmacological activities (in vitro and in vivo) such as cytotoxic [13], antimicrobial [14], anti-inflammatory [15], anti-allergic [16],anti-diarrheal [17], and hepatoprotective [18]. The previous phytochemical studies of C. rotundus have revealed the presence of several types of secondary metabolites, such as sesquiterpenes, flavonoids, iridoids, phenylpropanoids, furochromones, phenolic acids, alkaloids, steroids, and saponins [8, 19,20,21].
Several previous studies documented the anti-parasitic activity of C. rotundus in vitro or in vivo. The in vitro anthelmintic activity of C. rotundus against Pheretima Posthuma was reported by Kasala et al. [22]. In addition, C. rotundus revealed potent activity against Plasmodium falciparum [23] and Entamoeba histolytica trophozoites in vitro and were safe for use [24]. The in vivo anti-cryptosporidiosis and anti-toxoplasmosis effects of C. rotundus extract were reported by Fahmy et al. [25].
This study aimed to carry out qualitative phytochemical screening for the primary constituents of 90% methanol extract and its fractions with the estimation of their phenolic and flavonoid contents, characterization of the active phytoconstituents of the 90% methanol extracts, and evaluation of the anti-trichinellosis effects of C. rotundus extracts (in vitro and in vivo).
Methods
Plant materials
Cyperus rotundus aerial parts were collected from El-Sharkia Governorate, Egypt, in June 2020. The plant sample was characterized by Mrs. Teraza Labib, a taxonomy specialist at Orman Herbarium Garden, and Prof. Mohamed El-Gebaly, Professor of Plant Taxonomy and Botany at the National Research Center, Giza, Egypt, who confirmed the identification of the plant sample. The voucher specimen (No. 20200724) of the plant (Fig. 1) was kept at the Medicinal Chemistry Department of the Theodor Bilharz Research Institute (TBRI). The plant materials were cut into small pieces, dried in the shed, and then powdered using a plant grinding machine.
Extraction and fractionation process
Cyperus rotundus aerial parts dry powder (350 g) was extracted using 90% MeOH (5 × 3 L) in a 5 L conical flask. Then it was carefully closed and kept for 72 h. The supernatant was filtered using Whatman filter paper No.1 and evaporated using a rotary evaporator (Buchi, Switzerland) under a vacuum to obtain the crude extract (50 g). The dried 90% MeOH extract (40 g) was dissolved in 100 ml of water and successively partitioned with pet-ether (7 × 500 ml), EtOAc (7 × 500 ml), and n-BuOH (7 × 500 ml). The solvents were evaporated using a rotary evaporator under pressure to yield a pet-ether fraction (5.3 g), an EtOAc fraction (4.7 g), an n-BuOH fraction (7.2 g), and a residue (20.5 g). The 90% MeOH extract, and its derived fractions were kept in brown vials for further biological and chemical experiments.
Phytochemical screening
The qualitative estimation of the major chemical compositions of C. rotundus extracts, such as carbohydrates, flavonoids, alkaloids, terpenoids, phenols, tannins, saponins, and glycosides, was carried out using the standard analytical procedures previously described by Evans [26].
Quantitative determination of total phenolic content (TPC)
The concentration of total phenolics in each plant extract was determined according to the method described by El-Hashash et al. [27]. Briefly, a mixture of 100 µl of plant extract (100 µg ml–1), 500 µl of Folin-Ciocalteu reagent, and 1.5 ml of Na2CO3 (20%) was shaken and diluted up to 10 ml with water. After two hours, the absorbance was measured at 765 nm. All determinations were carried out in triplicate. Gallic acid was standard, and the total phenol contents were expressed as milligrams of gallic acid equivalents per gram dry weight of the extract (mg GAE/g DW).
Quantitative determination of total flavonoid contents (TFC)
The total flavonoid content was determined using the method described by El-Hashash et al. [27]. Briefly, 100 µl of 20% aluminum trichloride (AlCl3) in methanol was mixed with the same volume of extract solution (10 mg/ml) and a drop of acetic acid. The mixture was diluted up to 5 mL with methanol. The absorption was carried out at 415 nm using a spectrophotometer (UV–Vis spectrophotometer, Milton Roy 601), and readings were taken after 40 min against a blank sample. All determinations were carried out in triplicate. The total flavonoid content was expressed as mg rutin equivalents per gram dry weight of extract (mg RE/g DW).
LC–ESI–MS analysis of C. rotundus 90% MeOH extract
In the negative ion mode, the 90% MeOH extract of C. rotundus was chemo-profiled using liquid chromatography coupled with electrospray ionization mass spectrometry (LC–ESI–MS). Experiments were performed on the LC system (Waters Alliance 2695, Waters, USA), using reversed-phase column C18, 250 mm, and 5 μm particle size (Phenomenex, USA). Eluent A was H2O acidified with 0.1% formic acid, and eluent B was CH3CN: MeOH (1:1), acidified with 0.1% formic acid. The injection volume was 20 μl of a 5 mg/ml 90% MeOH extract, and the elution flow was 400 μl/min. The program was the following: 0.0–5.0 min (5% B), 5.0–10 min (5.0%–10% B), 10–55 min (10%–50% B), 55–65 min (50%–95% B), 65–70 min (5% B). The ESI–MS spectra were estimated by scanning in the range of 50–1000 m/z with these parameters: the source temperature was set at 150 C, the cone voltage was 50 eV, the capillary voltage was 3 kV, the desolvation gas flow was 600 L/hour, the desolvation temperature was 350 C, and the cone gas flow was 50 L/hour. The compounds were assigned by retention time, and mass spectroscopic results were compared to standards and literature data.
Animals and parasites
The present study was performed on white albino male mice of the CDI strain (aged 4–6 weeks and weighing 20–25 g) obtained from the TBRI biological unit. The mice were maintained hygienically throughout the study and fed regular commercial pelleted food with water as needed. Following approval from the Research Ethics Committee of TBRI (REC-TBRI), all animal experiments were carried out following guidelines recognized internationally.
The Trichinella spiralis (code: ISS6158) used in this study was provided by the Medical Parasitology Department, Tanta Faculty of Medicine, and was kept in the Parasitology Department, TBRI, by consecutive passages on mice and rats. Mice were orally infected with 200–300 T. spiralis larvae [28, 29].
T. spiralis adult worms and muscle larvae isolation
According to ozkoc et al. [30], T. spiralis adults and muscle larvae were recovered from infected mice. The muscles of white albino mice infected with T. spiralis for 30 days were removed and minced, and the larvae in the muscles were then put in an acid-pepsin solution [31]. An electric stirrer was used to mix the mixture at 37 C for 2 h [32]. The mixture was filtered based on Kapel et al. [33]. The collected larvae were cleaned two to three times using tap water before being suspended in a conical flask for half an hour to permit sedimentation. The adult worms of T. spiralis were recovered from the small intestines of infected mice on the sixth-day post-infection (p.i).
To allow the worms to move out of the tissue, the intestine was cleaned, longitudinally opened along its entire length, sliced into parts measuring 2 cm, and then submerged in normal saline for 3–4 h at 37 C [34].
Design of in vitro and in vivo experiments
On a 24-well tissue culture plate, adult T. spiralis worms (25 parasites per well) were grown using RPMI-1640 media as the incubation medium, which contained 20% fetal bovine serum, 200 U/mL penicillin, and 200 g/ml streptomycin.
The range of concentrations of each C. rotundus extract against adults was 25 to 500 µg/mL [24], while the range of ABZ against adults was 25 to 400 µg/mL [35]. Parasite controls and dimethyl sulfoxide (DMSO) controls were set, and each determination was performed in duplicate. The plates were incubated at 37 C with 5% CO2 for 1, 4, 24, 48, and 72 h. At the end of the incubation periods, the parasites (both dead and living) in the wells were counted by inverted microscopy. The adult motility assay is the method of choice to evaluate the drug sensitivity of different nematode species. Non-motile worms were considered dead, and the survival rate in each well was calculated [36]. Worm mortality % = (the number of dead worms / the total number of worms) × 100%. The criteria for a dead body were that the worm’s body was C-shaped or linear, and there was no movement [37]. Then, adult worms were gathered for scanning electron microscopy examination.
For the in vivo study, mice were distributed into five groups (n = 12):
-
Group I: non-infected and untreated (control-negative).
-
Group II: infected but not treated (control-positive).
-
Group III: infected and treated with ABZ (pure powder was purchased from Sigma-Aldrich, St. Louis, MO, USA) given at a 50 mg/kg dose orally [38].
-
Group IV: infected and treated with the most promising Cyperus rotundus extract in the in vitro studies (given orally at 250 mg/kg) [39].
-
Group V: infected and treated with a combination of ABZ (given at a 50 mg/kg dose orally) and Cyperus rotundus extract (given orally at 250 mg/kg).
To evaluate the effects of the medications administered during the intestinal phase (a) (2–6 days p.i) and the muscular phase (30–34 days p.i) individually, groups II–V were divided into two subgroups (a and b) (n = 6).
Determination of the burden of adult worms and muscle larvae of T. spiralis
In subgroup (a), the mice were sacrificed on day 7 p.i. under light anesthesia by isoflurane inhalation (Forane®, Baxter, UK) to assess the effects of therapy on the intestinal phase, and the small intestine was processed as previously reported [34]. Adults of T. spiralis were collected and counted, and the reduction rate of worms was calculated.
On day 35 after infection, the mice in subgroup (b) were sacrificed under light anesthesia using isoflurane inhalation, and the muscle larvae were recovered using the pepsin digestion procedure. This was carried out to examine the effects of the therapy on the muscular phase.
The collected larvae were counted microscopically using a McMaster counting chamber. The number of larvae per gram of digested carcass (Muscle larvae/g) (ML/g) served as a measure of parasite burdens [40, 41].
Scanning electron microscopy
Adult worms were handled using the methods outlined by Abou Rayia et al. [29]. A fresh fixation solution containing 2.5% glutaraldehyde solution buffered with 0.1 M sodium cacodylate at pH 7.2 was used to fix worms from each group immediately and kept overnight at 4 C. After that, the fixed specimens were washed in 0.1 M sodium cacodylate buffer at pH 7.2 for 5 min, post-fixed in 2% osmium tetroxide for 1 h, and rinsed in distilled water.
The samples were dehydrated in ethyl alcohol in increasing concentrations, mounted on adhesive material with a carbon coating, and analyzed by FEI-Philipps scanning electron microscope [42, 43].
Histopathological examination
Skeletal muscle and small intestine segments from the study groups were fixed in 10% formalin for 24 h, cleaned in water for 12 h, dehydrated in increasing grades of alcohol, and cleared in xylene. Pure soft paraffin was used for impregnation for two hours at a temperature of 55 C. Then, stiff paraffin sections were cut using a microtome at 5 μm. Hematoxylin and eosin stains were used to stain the sections [44].
Statistical analysis
The data were analyzed using Microsoft Excel 2016 and a statistical package for social science (IBM SPSS Statistics for Windows, version 26, IBM Corp., Armonk, NY, USA). Continuous normally distributed variables were represented as mean ± SD, with a 95% confidence interval. The Student’s t-test was performed to compare the means of normally distributed variables between groups, besides ANOVA and Dunnett T3 as post hoc tests in multigroups. P-value < 0.05 was significant, and P-value < 0.001 was highly significant [45].
Results
Phytochemical screening and total phenolic and flavonoid contents of C. rotundus extracts
The phytochemical screenings were carried out using standardized lab protocols to investigate the secondary metabolites of C. rotundus 90% MeOH ext. and their derived fractions (EtOAc fr., pet-ether fr., and n-BuOH fr.). The results showed that they contained several groups of active ingredients (Table 1). The 90% MeOH ext., EtOAc fr., and n-BuOH fr. showed a high number of flavonoids and phenols. Moreover, the 90% MeOH ext. and pet-ether fr. demonstrated a high content of terpenoids and sterols. However, the concentrations of the other phytoconstituents, such as alkaloids, tannins, glycosides, and saponins, ranged from none to moderate amounts in the 90% MeOH ext., and their fractions.
The distribution of phenolic and flavonoid compounds in C. rotundus extracts (Table 2) demonstrated that the EtOAc fr. had the highest number of phenols and flavonoids with values of 567.35 ± 7.89 mg GAE/g DW and 316.32 ± 2.59 mg RE/g DW, respectively, followed by n-BuOH fr. (277.40 ± 4.46 mg GAE/g DW and 165.38 ± 2.45 mg RE/g DW, respectively), 90% MeOH ext. (174.66 ± 2.35 mg GAE/g DW, and 121.71 ± 1.67 mg RE/g DW, respectively). The lowest phenolic content was observed in pet-ether fr. with values of 97.03 ± 2.03 mg GAE/g DW and 106.26 ± 0.50 mg RE/g DW, respectively.
LC–ESI–MS characterization of the primary phytochemicals of C. rotundus 90% MeOH ext
The phenolic compounds from C. rotundus 90% MeOH ext. have been obtained using LC–ESI–MS in negative ionization mode. Twenty-six different phenolic compounds were tentatively assigned in C. rotundus 90% MeOH ext., which included five phenolic acids, 18 flavonoids, one organic acid, and two unknown phenolic compounds, as mentioned in Table 3. The total ion chromatogram of the identified compounds is represented in Fig. 2, and the chemical structures of some detected compounds are shown in Fig. 3. Additionally, the chemical components were identified by comparing MS fragmentation patterns and molecular ion peaks with the literature.
In vitro anthelmintic activity
Survival numbers of T. spiralis adult worms incubated with different concentrations of compounds under study by exposure times are shown in Table 4. The effect of different compounds being studied on T. spiralis adult worms’ survival depended on concentration and time.
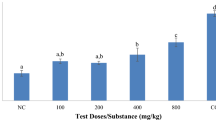
Albendazole caused the death of all adult worms after 48 h at concentrations starting from 100 µg/ml. The statistically significant effect of ABZ was evident from the first hour of incubation at a concentration of 100 µg/ml. The LC50 of ABZ was calculated to be 71.41 µg/ml.
Regarding C. rotundus’ different extracts, the 90% MeOH ext. caused the death of all adult worms at the highest concentration (500 µg/ml) after 72 h. The same activity was recorded with EtOAc fr. at high concentrations (250 µg/ml and 500 µg/ml) after 72 h, pet-ether fr. at the highest concentration (500 µg/ml) after 48 h, and n-BuOH fr. after 48 h at concentrations starting from 125 µg/ml.
A statistically significant difference was recorded initially with 90% MeOH ext. after incubation at 125 µg/ml for 72 h. While in EtOAc fr., it was determined after incubation at 125 µg/ml for 24 h. It was reported with pet-ether fr. after incubation at 50 µg/ml for 72 h. The statistically significant effect of n-BuOH fr. was evident from the first hour of incubation in a 125 µg/ml concentration.
The LC50 was calculated to be 156.12 µg/ml, 294.67 µg/ml, 82.09 µg/ml, and 73.16 µg/ml in 90% MeOH ext., EtOAc fr., pet-ether fr., and n-BuOH fr., respectively.
There was no significant difference between parasite controls and DMSO controls at all test incubation periods.
Mortality rates of T. spiralis adult worms exposed to different concentrations of C. rotundus 90% MeOH ext. and its derived fractions by exposure times
Table 5 shows the mortality rates of T. spiralis adult worms following incubation with different concentrations of the drugs under study by exposure times.
Incubation of adult worms in ABZ drug resulted in increased mortality rates after 4 h to 18% at 50 µg/ml. Increasing the concentration to 100 µg/ml increased the mortality rate to 36% after 4 h. Furthermore, ABZ killed all adult worms after 48 h at 100 µg/ml and after 24 h at 200 µg/ml.
Concerning C. rotundus different extracts, the mortality rates increased after 4 h of incubation at 125 µg/ml in 90% MeOH ext, EtOAc fr., pet-ether fr. and n-BuOH fr. to 8%, 12%, 28%, and 36%, respectively. Additionally, mortality rates increased to 22%, 34%, 42%, and 58% after 24 h at the same concentration in 90% MeOH ext, EtOAc fr., pet-ether fr., and n-BuOH fr., respectively.
Regarding C. rotundus different extracts, 90% MeOH extract killed all adult worms at the highest concentration (500 µg/ml) after 72 h. The same action was recorded with EtOAc fr. at high concentrations (250 µg/ml and 500 µg/ml) after 72 h, pet-ether fr. at the highest concentration (500 µg/ml) after 48 h, and n-BuOH fr. after 48 h at concentrations starting from 125 µg/ml.
Scanning electron microscope (SEM) findings
Regarding the adult worm’s morphology in the control group (incubated in culture media or DMSO without drugs), the cuticle retained the typical structure as annulations, ridges, transverse creases, and hypodermal gland openings (Fig. 4A, B).
SEM results of adult T. spiralis in culture: A, B showing typical worm, C, D demonstrating group that received albendazole with severe destruction of the adult worm, normal crease loss, and appearance of cauliflower masses (red arrows), and E, F groups that received n-BuOH fraction with remarkable adult worm destruction, annulation loss, and appearance of blebs and vesicles (red arrows)
In the ABZ-treated group, marked destruction of the adult worms was obvious. There was a loss of annulations, loss of the normal creases, and appearance of cauliflower masses (Fig. 4C, D).
Regarding C. rotundus, different extracts, including n-BuOH fr. proved to have the most promising effects in in vitro studies. As a result, the n-BuOH fr. treated group was subjected to SEM examination. The loss of the normal morphology was observed in the n-BuOH fr., and the treated group lost the typical creases, annulations, and formation of areas containing blebs and vesicles (Fig. 4E, F).
In vivo results
Count of the adult worms in the small intestine
When compared to the control group (105.8 ± 7.3), the mean number of adult worms significantly (P < 0.001) decreased in all treatment groups. The lowest mean adult worm count was witnessed in GVa, which received combination therapy (9.8 ± 1.9) and showed the highest elimination of T. spiralis adult worms with (91%) efficacy, followed by GIIIa, which received ABZ (12.8 ± 3.2) with 88% efficacy. While in GIVa, which received n-BuOH fr., the mean adult worm count was 32.6 ± 3.487 with a reasonable percentage of reduction (70%) (Table 6).
Count of encysted larvae in muscles
Regarding the effects of a drug on muscle phase, there was a significant decrease (P < 0.001) in the mean larval count per gram muscle in all treated groups when compared with the control group (3520.0 ± 432.4).
The highest mean larval count reduction was detected in group GVb, which received the combination therapy (682.0 ± 34.2) with 81% efficacy, followed by the ABZ-treated group (GIIIb) (839.6 ± 49.8) with 76% efficacy. In the n-BuOH fr., treated group (GIVb), the mean larval count was 1236.0 ± 68.8 with a 65% reduction percentage (Table 7).
Histopathological finding
Small intestine examination
Histopathological examination of small intestine sections from the standard control group (GI) demonstrated typical architecture with healthy mucosa and average crypts/villous ratio. Goblet cells were moderate in number with a well-defined brush border and lamina propria (Fig. 5A).
Histopathological examination of sections from the small intestine: A GI, normal control mice (uninfected), showing a normal villous pattern (H&E, X200). B GII: positive control mice (infected), showing an irregular villous pattern, with villous expansion by inflammatory cells (red arrow) and scattered fragments of the worms (yellow arrows) (H&E, X200). C Infected mice treated with ABZ showed mostly regular villous patterns, with focal villous tip erosion (yellow arrow) and mild inflammation (red arrow) (H&E, X200). D Infected mice treated with n-BuOH fr. showed a focally irregular villous pattern, with villous tip erosion (black arrow) and mild inflammation (yellow arrow) (H&E, X200). E Infected mice treated with combination therapy showed a nearly restored regular villous pattern with fewer inflammatory cells (H&E, X200)
The intestinal wall of the infected control group (GII) showed abundant intervillous inflammatory cellular infiltration that was primarily composed of lymphocytes and plasma cells, which are mononuclear cells. The intestinal villi showed broadening and atrophy. Additionally, adult worm fragments were found within the intestinal lumen (Fig. 5B).
According to the results of the sections from the examined treated groups (Fig. 5C, D, and E), an obvious reduction in the severity of the inflammatory cellular infiltration was witnessed, along with a striking improvement in the other histopathological changes of the intestine and the return of the regular villous pattern in GVa (Fig. 5E).
Skeletal muscle examination
Histopathological examination of sections from muscles of the normal control group (GI) showed a normal skeletal muscle pattern (Fig. 6A). The infected control group (GI) demonstrated the presence of numerous encysted T. spiralis larvae dispersed throughout the muscle sarcoplasm and several chronic inflammatory cells in the form of lymphocytes, plasma cells, and histiocytes, infiltrating muscle bundles and surrounding the encysted larvae (Fig. 6B).
Histopathological examination of muscular sections: A GI, normal control mice (uninfected) showed normal skeletal muscle pattern (H&E, X200). B GII: positive control mice (infected) showed several cysts and a moderate inflammatory cellular reaction (yellow arrows) (H&E, X200). c Infected mice treated with ABZ showed remnants of cysts, with degenerated capsules (black arrow) and contents surrounded by many macrophages (yellow arrow) (H&E, X400). D Infected mice treated with n-BuOH fr. demonstrated many cysts with degenerated capsules (black arrow) and contents surrounded by many macrophages (yellow arrow) (H&E, X400). E Infected mice treated with combination therapy demonstrated a single cyst with degenerated capsule and content (black arrow) surrounded and infiltrated by many macrophages (yellow arrow) (H&E, X400)
Examination of muscular sections from the treated groups (Fig. 6 C, D, and E) showed a remarkable improvement in the histopathological findings compared to GII, the infected control group. GIIIb and GIVb revealed a reduced number of cysts with degenerated capsules and localized pericapsular plasma-lymphocytic inflammatory cellular infiltration (Fig. 6C & D). While GVb demonstrated the best improvement with the least number of cysts and degenerated larvae capsules. Additionally, the size of the larvae was reduced, and their internal structure was destroyed (Fig. 6E).
Discussion
Since ancient times, medicinal plants have been crucial in developing powerful therapeutic substances. According to current estimates, 80% of people in developing nations still rely on folk medicine to treat various common health issues. Furthermore, herbal medications are in more significant demand than ever, and their acceptance has grown over time [46, 47].
The pharmacological treatment of trichinellosis is debatable. Albendazole is one of the benzimidazoles and is the drug of choice in trichinellosis treatment. However, albendazole has been linked to various adverse medication responses, including fatalities, encephalitis, seizures, and severe drug eruptions [6, 48].
Additionally, it exhibits a weak susceptibility to migrating and encapsulated muscle larvae [49]. That might explain the urgent need for a new, secure, potent therapy to eradicate Trichinella spp. infection.
Cyperus rotundus, a worldwide herb used in conventional medicine to treat several diseases, is regarded as a plant with infinite medicinal properties validated by the scientific committee [12, 50, 51]. Moreover, C. rotundus has a wide range of safety features. The researchers documented that administering C. rotundus extract orally in rats did not induce acute toxicity, and there was no mortality or behavior changes for subacute toxicity [52].
In the current study, the in vitro anti-trichinellosis potential of the active phytoconstituents of C. rotundus aerial part 90% methanol extract and its derived fractions (EtOAc fr., pet-ether fr., and n-BuOH fr.) were determined. The fraction with the most promising effects was then used to evaluate the in vivo therapeutic effects of C. rotundus.
The preliminary phytochemical screening tests are valuable for investigating the bioactive plant secondary metabolites [53]. C. rotundus 90% MeOH extract, and its derived fractions included high quantities of flavonoids, phenols, sterols, and triterpenoids, which had different pharmacological properties. The previous phytochemical surveys on the different parts of C. rotundus documented the presence of sesquiterpenes, phenylpropanoids, phenolics, alkaloids, flavonoids, and iridoids in rich amounts [12, 19]. These secondary metabolites played an insignificant role in the growth of the plant. However, they were essential for various defense mechanisms against the harmful effects of UV radiation, herbivore, and microbial attack [54].
Phenolics and flavonoids are the major groups of secondary metabolites, especially in plants, and have been considered responsible for various pharmacological activities [55]. Phenolic and flavonoids represent one of the most diverse groups of natural compounds. Therefore, the 90% MeOH ext. of C. rotundus and its derived fractions were analyzed for total phenolic and flavonoid contents in this study. The findings showed that C. rotundus' EtOAc fr. had a higher concentration of phenols and flavonoids than its n-BuOH fr., 90% MeOH ext., and pet-ether fr. It was reported that the concentration of phenolic compounds in plants depended on environmental factors such as light, temperature, and soil salinity. Furthermore, the solubility of phenolic compounds is governed by the kind of extraction and solvent polarity [56]. The previous reports stated that the 70% ethanol extract of dried rhizomes of C. rotundus had a total phenolic content value of 73.27 ± 4.26 mg catechin equivalents/g of dried rhizome extract [57]. In addition, the different extracts of C. rotundus (hexane, petroleum ether, ethyl acetate, chloroform, 70% acetone, 70% ethanol, 70% methanol, and water) were quantitatively analyzed for TPC and TFC. The TPC of the different extracts ranged from 0.0358 ± 0.002 to 118.924 ± 5.946 μg GAE/mg dry extract, and the TFC ranged from 7.196 ± 0.359 to 200.654 ± 10.032 μg quercetin equivalent (QE)/mg dry extract [58]. Other findings showed that the TPC of C. rotundus extracts (70% ethanol, MeOH, and water) ranged from 70.75 ± 4.48 to 254.50 ± 5.26 μg GAE/mg extract. In comparison, the TFC ranged from 51.23 ± 2.62 to 164.34 ± 3.75 μg catechin equivalents (CE)/mg extract [59]. Thus, our investigation proved that the Egyptian C. rotundus extracts had a significant number of phenolics and flavonoids, which could contribute to its promising medicinal properties.
The LC–ESI–MS analysis of the 90% MeOH extract of C. rotundus in negative ion mode revealed the presence of polyphenolic compounds, including phenolic acids, flavonoids (C-glycosyl and O-glycosyl), and organic acids. A detailed description of these identified compounds can be found in the Supplementary data file.
Moreover, to the best of our knowledge, some of these compounds were detected in C. rotundus for the first time, for example, compounds 3, 4, 9, 11, 12, 14, 16, 20, and 24, which encourages us to do further chromatographic isolation for these bioactive ingredients.
To save time and money, it is common practice to assess an agent’s potential anti-parasitic activity in vitro before trying in vivo research. It is not always the case that an agent’s in vivo activity will follow from its in vitro performance. This variance results from various factors, including the pharmacology and bioavailability of these drugs in the host [60].
An effective in vitro agent must be tested additionally in vivo. Therefore, we tested C. rotundus 90% MeOH ext. and its derived fractions (pet-ether fr. EtOAc fr., and n-BuOH fr.) in vitro. The in vitro studies proved that n-BuOH fr. had the most promising effects; as a result, the n-BuOH fr. treated group was subjected to SEM examination. Besides, in vivo testing was carried out for n-BuOH fr. using the murine model.
Regarding the in vitro studies for C. rotundus different extracts, the LC50 was calculated to be 156.12 µg/ml, 294.67 µg/ml, 82.09 µg/ml, and 73.16 µg/ml for 90% MeOH ext., EtOAc fr., pet-ether fr. and n-BuOH fr., respectively.
The in vitro or in vivo anti-parasitic activity of C. rotundus was documented in previous studies. The in vitro anthelmintic activity of C. rotundus against Pheretima posthuma was reported by Kasala et al. [22]. In addition, C. rotundus revealed potent activity against E. histolytica trophozoites in vitro and showed verified safety evidence for use [24].
In Trichinella spp., the cell wall includes the cuticle, hypodermis, and somatic musculature. Cuticle integrity is essential for parasite shape, protection, and nutrition and is necessary for osmoregulation [49].
In this study, electron microscopy scans demonstrated substantial adult worm destruction, loss of the normal morphology in groups treated with n-BuOH fr. and albendazole. It kept its typical appearance when incubated in the culture medium.
Transcuticular passive diffusion is the primary route by which drugs enter nematodes, followed by the worm’s surface being destroyed. Surface blebs indicated effective anti-parasitic activity because they were thought to be the worm’s replacement for its destroyed surface membrane [41, 61].
In the present research, we explored the therapeutic effect of n-BuOH fr. by administering n-BuOH fr., ABZ, and combined treatment (n-BuOH fr. and ABZ). All treated groups notably decreased the adult worm total count compared to the control-infected group. The best results were demonstrated by the group GVa, which received combined therapy and showed the best reduction of adult worms of T. spiralis with an efficacy of 91%, followed by GIIIa, which was administered albendazole with an efficacy of 88%, and GIVa that received n-BuOH fr. with a satisfactory percentage of reduction of 70%.
Concerning how drugs affect the muscle phase, a significant decrease in the mean larval count per gram muscle was reported in all treated groups. The best reduction was found in group GVb, which received combination therapy with an efficacy of 81%, followed by the mice group that received ABZ (GIIIb) with an efficacy of (76%), and the mice group that received n-BuOH fr. (GIVb), with a 65% reduction.
These findings were consistent with the results of Fahmy et al. [25], as they reported that the combined therapies of C. rotundus extract with the standard drugs (nitazoxanide and spiramycin) had the highest effectiveness against murine cryptosporidiosis and toxoplasmosis, respectively, followed by standard medications.
The albendazole effect on T. spiralis was reported in many previous studies, with a variance in efficacy [37, 47, 62, 63]. The variation in the efficacy of albendazole on intestinal and muscular phases was attributed to variance in treatment dose, time, and duration [63].
Albendazole acts mainly by inhibiting microtubule polymerization through selective binding to the parasite beta-tubulin monomer, besides having a small effect on host tubulin binding [64]. However, a study by Siriyasatien et al. [63] concluded that for the early stage of T. spiralis infection, 20 mg/kg albendazole given for 15 days was effective in treating infection in mice, while the late stage of infection was witnessed to be tolerant of albendazole. However, the duration of treatment was longer.
The result was similar to a study by McCracken [65], who documented that the Trichinella population became less susceptible to treatment when the worms matured. Comparing previous results with the results of the current study might explain the promising results found in the groups treated with the combined therapy.
In this study, the infected control group’s small intestinal sections underwent histological evaluation and revealed significant intravillous inflammatory cellular infiltration, primarily composed of lymphocytes and plasma cells. The intestinal villi showed broadening and atrophy. Besides, adult worm fragments were found in the intestinal lumen. The infected control group’s muscle tissue samples showed a massive amount of encysted T. spiralis larvae widely distributed in the sarcoplasm of muscle cells and several chronic inflammatory cells. These results agreed with that of El-Wakil et al. [41] and Dyab et al. [66]. There was an obvious reduction in damaging and inflammatory alterations in the treated groups. The group that received combination therapy had the most promising results in regaining typical architecture, having the fewest cysts with degenerating capsules, and focal pericapsular plasma-lymphocytic inflammatory cellular infiltration.
Conclusion
The present study concluded that C. rotundus 90% MeOH extract, and its derived fractions had anti-trichinellosis activity. Their lethal effects were evident in in vitro studies on adult worms with the n-BuOH fr. proved to have the most promising effects. Moreover, the n-BuOH fr. demonstrated therapeutic effects, especially when co-administrated with albendazole. The anti-trichinellosis effect of n-BuOH and pet-ether fractions might be due to other secondary metabolites such as terpenoids, saponins, steroids, and alkaloids.
Additionally, 26 phenolic compounds were successfully characterized in 90% MeOH extract by the LC–ESI–MS technique in the negative ion mode. The identified compounds were classified into phenolic acids, flavonoids, and organic acids. The major compounds in this investigation are relative to apigenin and luteolin O or C-glycosides, which have a therapeutic effect. Therefore, these findings encourage us to further biological and chemical investigations of the active fractions, delineate their mechanisms of action, and support their use in pharmaceutical formulations.
Availability of data and materials
The datasets generated or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ABZ:
-
Albendazole
- C. rotundus:
-
Cyprus rotundus
- DMSO:
-
Dimethyl sulfoxide
- DW:
-
Dry weight
- EtOAc:
-
Ethyl acetate
- g:
-
gram
- GAE:
-
Gallic acid equivalent
- Kg:
-
Kilogram
- LC-ESI-MS:
-
Liquid chromatography electrospray ionization mass spectrometry
- MeOH:
-
Methanol
- mg:
-
Milligram
- n-BuOH:
-
Normal butanol
- pet-ether:
-
Petroleum ether
- RE:
-
Rutin equivalent
References
Bai X, Hu X, Liu X, Tang B, Liu M. Current research of trichinellosis in China. Front J Microbiol. 2017;8:1472–147.
Gottstein B, Pozio E, Nöckler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev. 2009;22:127–45.
Pozio E. Trichinella and trichinellosis in Europe. Veterinarski Glasnik. 2019;73:65–84.
Ren HJ, Liu RD, Wang ZQ, Cui J. Construction and use of a Trichinella spiralis phage display library to identify the interactions between parasite and host enterocytes. Parasitol Res. 2013;112:1857–63. https://doi.org/10.1007/s00436-013-3339-x.
Caner A, Döşkaya M, Değirmenci A, Can H, Baykan S, Uner A, et al. Comparison of the effects of Artemisia vulgaris and Artemisia absinthium growing in western Anatolia against trichinellosis (Trichinella spiralis) in rats. Exp Parasitol. 2008;119:173–9.
Yadav AK, Temjenmongla N. Efficacy of Lasia spinosa leaf extract in treating mice infected with Trichinella spiralis. Parasitol Res. 2012;110:493–8.
Behnke JM, Buttle DJ, Stepek G, Lowe A, Duce IR. Developing novel anthelmintics from plant cysteine proteinases. Parasit Vectors. 2008;1:29. https://doi.org/10.1186/1756-3305-1-29.
Elshamy AI, Farrag AH, Ayoub IM, Mahdy KA, Taher RF, Gendy EI. UPLC-qTOF-MS phytochemical profile and antiulcer potential of cyperus conglomeratus rottb alcoholic extract. Molecules. 2020;25:4234. https://doi.org/10.3390/molecules25184234.
Zhang LL, Zhang LF, Hu QP, Hao DL, Xu JG. Chemical composition, antibacterial activity of Cyperus rotundus rhizomes essential oil against Staphylococcus aureus via membrane disruption and apoptosis pathway. Food Control. 2017;80:290–6.
Samra RM, Soliman AF, Zaki AA, Ashour A, Al-Karmalawy AA, Hassan MA, Afr, et al. Bioassay-guided isolation of a new cytotoxic ceramide from Cyperus rotundus L. S. J Bot. 2021;139:210–6. https://doi.org/10.1016/j.sajb.2021.02.007.
Mohamed GA. Iridoids and other constituents from Cyperus rotundus L. rhizomes. Bull Fac Pharm Cairo Univ. 2015;53:5–9.
Peerzada AM, Ali HH, Naeem M, Latif M, Bukhari AH, Tanveer A. Cyperus rotundus L. Traditional uses, phytochemistry, and pharmacological activities. J Ethnopharmacol. 2015;174:540–60. https://doi.org/10.1016/j.jep.2015.08.012.
Lin CH, Peng SF, Chueh FS, Cheng ZY, Kuo CL, Chung JG. The ethanol crude extraction of Cyperus rotundus regulates apoptosis-associated gene expression in HeLa human cervical carcinoma cells in vitro. Anticancer Res. 2019;39:3697–709. https://doi.org/10.21873/anticanres.13518.
Sasidharan S, Pottail L. Antimicrobial activity of metal and non-metallic nanoparticles from Cyperus rotundus root extract on infectious disease causing pathogens. J Plant Biochem Biotechnol. 2020;29:134–43. https://doi.org/10.1007/s13562-019-00523-1.
Tsoyi K, Jang HJ, Lee YS, Kim YM, Kim HJ, Seo HG, et al. (+)-Nootkatone and (+)-valencene from rhizomes of Cyperus rotundus increase survival rates in septic mice due to heme oxygenase-1 induction. J Ethnopharmacol. 2011;137:1311–7. https://doi.org/10.1016/j.jep.2011.07.062.
Jin JH, Lee DU, Kim YS, Kim HP. Anti-allergic activity of sesquiterpenes from the rhizomes of Cyperus rotundus. Arch Pharm Res. 2011;34:223–8.
Daswani PG, Brijesh S, Tetali P, Birdi TJ. Studies on the activity of Cyperus rotundus Linn. Tubers against infectious diarrhea. Indian J Pharmacol. 2011;43(3):340.
Kumar SS, Mishra S. Hepatoprotective activity of rhizomes of Cyperus rotundus Linn against carbon tetrachloride-induced hepatotoxicity. Indian J Pharm Sci. 2005;6:84–8.
Bezerra JJL, Pinheiro AAV. Traditional uses, phytochemistry, and anticancer potential of Cyperus rotundus L. (Cyperaceae): A systematic review. S Afr J Bot. 2022;144:175–86.
Park YJ, Zheng H, Kwak JH, Chung KH. Sesquiterpenes from Cyperus rotundus and 4α,5α-oxidoeudesm-11-en-3-one as a potential selective estrogen receptor modulator. Biomed Pharmacother. 2019;109:1313–8. https://doi.org/10.1016/j.biopha.2018.10.186.
Sayed HM, Mohamed MH, Farag SF, Mohamed GA, Proksch P. A new steroid glycoside and furochromones from Cyperus rotundus L. Nat Prod Res. 2007;21(4):343–50.
Kasala S, Ramanjaneyulu K, Himabindhu J, Alluri R, Babu RR. Preliminary phytochemical screening and in vitro anthelmintic activity of Cyperus rotundus (L). J Pharmacogn Phytochem. 2016;5:407.
Thebtaranonth C, Thebtaranonth Y, Wanauppathamkul S, Yuthavong Y. Antimalarial sesquiterpenes from tubers of Cyperus rotundus: structure of 10, 12-peroxycalamenene, a sesquiterpene endoperoxide. Phytochemistry. 1995;40:125–8.
Kabbashi AS, Osman EE, Abdrabo AM, Abuzeid N, Garbi MI, Koko WS, et al. Antiamoebic activity and cytotoxicity of ethanolic extract of Cyperus rotundus L. Adv Med Plant Res. 2015;3:155–61.
Fahmy AM, Alshenawy AM, El-Wakil EA, Hegab AM. Efficacy of Cyperus rotundus extract against cryptosporidiosis and toxoplasmosis in murine infections. Egypt Pharm J. 2021;20:242.
Evans WC. Trease and evans’ pharmacognosy E-book. Amsterdam: Elsevier Health Sciences; 2009:135-148.135-148.
El-Hashash MM, Abdel-Gawad MM, El-Sayed MM, Sabry WA, Abdel-Hameed ES, Abdel-Lateef EE. Antioxidant properties of methanolic extracts of the leaves of seven egyptian Cassia species. Acta Pharm. 2010;60:361–7.
Wassom DL, Debra A, Dick TA. Trichinella spiralis infections of inbred mice: immunologically specific responses Induced by different Trichinella isolates. J Parasitol. 1988;74(2):283–7.
Abou Raya DM, Saad AE, Ashour DS, Oreiby RM. Implication of artemisinin nematocidal activity on experimental trichinellosis: in vitro and in vivo studies. Parasitol Int. 2017;66(2):56–63. https://doi.org/10.1016/j.parint.2016.11.012.
Ozkoc S, Tuncay S, Delibas SB, Akisu C. Vitro effects of resveratrol on Trichinella spiralis. Parasitol Res. 2009;105:1139–43. https://doi.org/10.1007/s00436-009-1533-7.
Dennis D, Despommier D, Davis N. Infectivity of the newborn larva of Trichinella spiralis in the rat. J Parasitol. 1970;56(5):974–7. https://doi.org/10.2307/3277516.
Dunn IJ, Wright KA. Cell injury caused by Trichinella spiralis in the mucosal epithelium of B10A mice. J Parasitol. 1985;71(6):757–66.
Kapel CMO, Webster P, Gamble R. Muscle distribution of sylvatic and domestic Trichinella larvae in production animals and wildlife. Vet Parasitol. 2005;132:101–5. https://doi.org/10.1016/j.vetpar.2005.05.036.
Wakelin D, Margaret MW. Immunity to Trichinella spiralis in irradiated mice. Int J Parasitol. 1980;10(1):37–41. https://doi.org/10.1016/0020-7519(80)90062-4.
Keiser J, Tritten L, Adelfio R, Vargas M. Effect of combinations of marketed human anthelmintic drugs against Trichuris muris in vitro and in vivo. Parasit Vectors. 2012;5(1):1–7.
Tritten L, Nwosu U, Vargas M, Keiser J. In vitro and in vivo efficacy of tribendimidine and its metabolites alone and in combination against the hookworms Heligmosomoides bakeri and Ancylostoma ceylanicum. Acta Trop. 2012;122(1):101–7.
Huang H, Yao J, Liu K, Yang W, Wang G, Shi C, et al. Sanguinarine has anthelmintic activity against the enteral and parenteral phases of Trichinella infection in experimentally infected mice. Acta Trop. 2020;201:105226.
Attia RA, Mahmoud AE, Farrag HM, Makboul R, Mohamed ME, Ibraheim Z. Effect of myrrh and thyme on Trichinella spiralis enteral and parenteral phases with inducible nitric oxide expression in mice. Mem Inst Oswaldo Cruz. 2015;110(8):1035–41. https://doi.org/10.1590/0074-02760150295.
Uddin SJ, Mondal K, Shilpi JA, Rahman MT. Antidiarrhoeal activity of Cyperus rotundus. Fitoterapia. 2006;77(2):134–6.
Nuñez G, Gentile T, Costantino S, Sarchi M, Venturiello S. In vitro and in vivo effects of progesterone on Trichinella spiralis newborn larvae. Parasitology. 2005;131(2):255–9. https://doi.org/10.1017/S0031182005007468.
El-Wakil ES, Abdelmaksoud HF, AbouShousha TS, Ghallab MMI. Evaluation of Annona muricata (Graviola) leaves activity against experimental trichinellosis: in vitro and in vivo studies. J Helminthol. 2021;95:e53.
Kim CW, Myron CL. Surface morphology of Trichinella spiralis by scanning electron microscopy. J Parasitol. 1980;66(1):75–81.
Bughdadi FA. Ultrastractural studies on the parasitic worm Trichinella spiralis. J Taibah Univ Sci. 2010;3:3–38. https://doi.org/10.1016/S1658-3655(12)60018-1.
Drury R, Wallington E. Carltonʹs Histological Technique, 5th Edn. Oxford, New York: Oxford University Press,1980.
Peat J, Barton B. Medical statistics. A guide to data analysis and critical appraisal. First edition. Wiley-Blackwell. 2005;113–119.
Pathak K, Das R. Herbal medicine-a rational approach in health care system. Int J Herb Med. 2013;1:86–9.
El-Wakil ES, El-Shazly MA, El-Ashkar AM, Aboushousha T, Ghareeb MA. Chemical profiling of Verbena officinalis and assessment of its anti-cryptosporidial activity in experimentally infected immunocompromised mice. Arab J Chem. 2022;103945:1–13.
Shalaby MA, Moghazy FM, Shalaby HA, Nasr SM. Effect of methanolic extract of Balanites aegyptiaca fruits on enteral and parenteral stages of Trichinella spiralis in rats. Parasitol Res. 2010;107(1):17–25.
Djurković-Djaković O, Bobić B, Nikolić A, Klun I, Dupouy-Camet J. Pork as a source of human parasitic infection. Clin Microbiol Infect. 2013;19(7):586–94.
Singh P, Khosa RL, Mishra G, Jha KK. Antidiabetic activity of ethanolic extract of Cyperus rotundus rhizomes in streptozotocin-induced diabetic mice. J Pharm Bioallied Sci. 2015;7:289–92. https://doi.org/10.4103/0975-7406.168028.
Lydia J, Sudarsanam D. Docking of a Cyperus rotundus compound ‘15-Hydroxy-4-oxo-10-pentadecynoic acid lactone’with antidiabetic drug targets: a comparative study. Int J Fund Appl Sci. 2014;3(2):17–21.
Sivapalan SR. Medicinal uses and pharmacological activities of Cyperus rotundus Linn – a review. Int J Sci Res Publ. 2013;3:1–8.
Farag RS, Abdel-Latif MS, Abd El Baky HH, Tawfeek LS. Phytochemical screening and antioxidant activity of some medicinal plants’ crude juices. Biotechnol Rep. 2020;28:e00536.
Rana A, Negi PB, Sahoo NG. Phytochemical screening and characterization of bioactive compounds from Juniperus squamata root extract. Materials Today: Proceedings. 2022;48:672–5. https://doi.org/10.1016/j.matpr.2021.07.305.
Guo H, Saravanakumar K, Wang M-H. Total phenolic, flavonoid contents and free radical scavenging capacity of extracts from tubers of Stachys affinis. Biocatal Agric Biotechnol. 2018;15:235–9.
Medini F, Fellah H, Ksouri R, Abdelly C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. JTaibahUniv Sci. 2014;8(3):216–24. https://doi.org/10.1016/j.jtusci.2014.01.003.
Nagulendran KR, Velavan S, Mahesh R, Hazeena Begum V. In vitro antioxidant activity and total polyphenolic content of Cyperus rotundus Rhizomes. E-J Chem. 2007;4(3):440–9.
Kamala A, Middha SK, Gopinath C, Sindhura HS, Karigar CS. In vitro antioxidant potentials of Cyperus rotundus L. rhizome extracts and their phytochemical analysis. Pharmacogn Mag. 2018;14:261–7.
Kumar KH, Razack S, Nallamuthu I, Khanum F. Phytochemical analysis and biological properties of Cyperus rotundus L. Ind Crops Prod. 2014;52:815–26.
Boyom FF, Madiesse EK, Bankeu JJ, Tsouh VP, Lenta BN, Mbacham WF, et al. Falcipain 2 inhibitors and antiplasmodial compounds from a bio-guided fractionation of the fruits of Sorindeia juglandifolia A. Rich. (Anacardiaceae) growing in Cameroon. Malar J. 2010;9(2):1–2.
Abdul Wahab SM, Jantan I, Haque MA, Arshad L. Exploring the leaves of Annona muricata L. as a source of potential anti-inflammatory and anticancer agents. Front Pharmacol. 2018;9:661.
Chung MS, Joo KH, Quan FS, Kwon HS, Cho SW. Efficacy of flubendazole and albendazole against Trichinella spiralis in mice. Parasite. 2001;8:195-S198.
Siriyasatien P, Yingyourd P, Nuchprayoon S. Efficacy of albendazole against early and late stage of Trichinella spiralis infection in mice. J Med Assoc Thail. 2003;86:257-S262.
Aguayo-Ortiz R, Méndez-Lucio O, Medina-Franco JL, Castillo R, Yépez-Mulia L, Hernández-Luis F, et al. Towards the identification of the binding site of benzimidazoles to β-tubulin of Trichinella spiralis: insights from computational and experimental data. J Mol Graph Model. 2013;41:12–9.
McCracken RO. Efficacy of mebendazole and albendazole against Trichinella spiralis in mice. J Parasitol. 1978;64:214–9.
Dyab AK, Ahmed MA, Abdelazeem AG. Prevalence and histopathology of Trichinella spiralis larvae of slaughtered pigs in Cairo governorate, Egypt. J Egypt Soc Parasitol. 2019;49(2):439–42.
Acknowledgements
The authors would like to thank Dr. Trease Labib (National Gene Bank and Orman Botanical Garden Consultant) and Prof. Mohamed El-Gebaly, Professor of Plant Taxonomy and Botany, National Research Center, Giza, Egypt, for their help in identifying and authenticating the Cyprus rotundus plant. We want to acknowledge the Department of Medical Parasitology, Faculty of Medicine, Tanta University, for providing the strain of T. spiralis used in this study. We would like to acknowledge the staff of the biological unit at TBRI for their sincere guidance and help during the in vivo study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research received no specific grant from any funding agency, commercial or not-for-profit sectors. The Science, Technology & Innovation Funding Authority (STDF) will provide open-access funding in cooperation with the Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Eman S. El-Wakil: Designed the plan of anti-parasitic work, performed the parasitological practical part, and shared in writing and revising the manuscript. Shimaa Shaker: Shared in designing the plan of anti-parasitic work, analyzed the data, wrote and revised the manuscript. Tarek Aboushousha: Performed the histopathological study. El-Sayed S. Abdel-Hameed, Ezzat E.A. Osman: Designed the phytochemical study, performed extraction and fractionation processes, performed the phytochemical profiling of the plant, and wrote and revised the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the animal experiments were carried out after approval by the Research Ethical Committee of the Theodor Bilharz Research Institute (TBRI-REC), with the protocol serial number: PT (716). TBRI-REC was operated according to the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals (eighth edition) and adhered to the ARRIVE guidelines.
The TBRI-REC approved the experimental research and field studies on plants, including the Cyprus rotundus collection, and they adhered to the relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
El-Wakil, E.S., Shaker, S., Aboushousha, T. et al. In vitro and in vivo anthelmintic and chemical studies of Cyperus rotundus L. extracts . BMC Complement Med Ther 23, 15 (2023). https://doi.org/10.1186/s12906-023-03839-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-023-03839-7