Abstract
Background
Exercise has been studied as a prognostic marker for patients with cystic fibrosis (CF), as well as a tool for improving their quality of life and analyzing lung disease. In this context, the aim of the present study was to evaluate and compare variables of lung functioning. Our data included: (i) volumetric capnography (VCAP) parameters: expiratory minute volume (VE), volume of exhaled carbon dioxide (VCO2), VE/VCO2, ratio of dead space to tidal volume (VD/VT), and end-tidal carbon dioxide (PetCO2); (ii) spirometry parameters: forced vital capacity (FVC), percent forced expiratory volume in the first second of the FVC (FEV1%), and FEV1/FVC%; and (iii) cardiorespiratory parameters: heart rate (HR), respiratory rate, oxygen saturation (SpO2), and Borg scale rating at rest and during exercise. The subjects comprised children, adolescents, and young adults aged 6–25 years with CF (CF group [CFG]) and without CF (control group [CG]).
Methods
This was a clinical, prospective, controlled study involving 128 male and female patients (64 with CF) of a university hospital. All patients underwent treadmill exercise tests and provided informed consent after study approval by the institutional ethics committee. Linear regression, Kruskal–Wallis test, and Mann–Whitney test were performed to compare the CFG and CG. The α value was set at 0.05.
Results
Patients in the CFG showed significantly different VCAP values and spirometry variables throughout the exercise test. Before, during, and after exercise, several variables were different between the two groups; statistically significant differences were seen in the spirometry parameters, SpO2, HR, VCO2, VE/VCO2, PetCO2, and Borg scale rating. VCAP variables changed at each time point analyzed during the exercise test in both groups.
Conclusion
VCAP can be used to analyze ventilatory parameters during exercise. All cardiorespiratory, spirometry, and VCAP variables differed between patients in the CFG and CG before, during, and after exercise.
Similar content being viewed by others
Background
The role of exercise as a prognostic indicator and/or therapeutic instrument is of interest in the research of several diseases, particularly respiratory diseases. Over the past three decades, exercise has become well established as an important component in the management of cystic fibrosis (CF) [1].
From birth, patients with CF undergo progressive deterioration of lung structures [1-5]. This deterioration of anatomical structures causes functional changes directly related to respiratory function. Changes include decreases in the peripheral oxygen saturation (SpO2), forced expiratory volume in the first second of the forced vital capacity (FEV1), ratio of dead space to tidal volume (VD/VT) and exercise performance, increases in the respiratory rate (RR) and heart rate (HR), and changes in blood gas parameters.
The benefits of exercise in patients with CF include improved aerobic conditioning, decreased progression of lung disease, and enhanced bronchial hygiene through an increased ciliary beat frequency, activation of immune cells, reduced susceptibility to viral infections, and increased anti-inflammatory activity. Thus, patients experience a better quality of life [1,6-8].
The exercise stress test has been increasingly used to assess the level of exercise intolerance in patients with heart and lung diseases. This test is considered the gold standard for the study of exercise limitations and their causes [9,10].
Volumetric capnography (VCAP) analyzes the pattern of elimination of carbon dioxide (CO2) in the expired air volume and can reveal a link between early structural damage and functional lung abnormalities in patients with CF. Previous studies have evaluated VCAP as a tool for assessing the degree of regional heterogeneity of lung gas exchange [11-13]. Some studies have found a relationship between VCAP parameters and pulmonary involvement indices derived from VCAP [14-16]. The curve of expired CO2 over time, known as a capnograph, can show temporal changes in obstructive lung disease. Besides the volume of exhaled CO2 (VCO2), VCAP measures the expiratory minute volume (VE), HR, RR, SpO2, end-tidal CO2 (PetCO2), and VD/VT ratio, which in normal subjects decreases during exercise and increases when imbalance occurs [17,18].
Ribeiro et al. [19] recently compared VCAP and spirometry in 64 children and adolescents with CF and 94 healthy subjects. Patients with CF showed a higher slope3/VT than did healthy subjects. VCAP also showed a difference in the slope3/VT in patients with CF who had normal spirometry results, indicating an early periphery airway dysfunction. An increased slope3/VT reflects inhomogeneity of ventilation in the distal airways, suggesting the possibility of chronic structural dysfunction as well as reversible acute dysfunction that may be observed, for example, in a bronchoprovocation test. This index may be a useful tool in the evaluation and study of small airway dysfunction in children and adolescents with pulmonary disease.
The positive impact of regular physical activity and the need for information about its effects justify the growing interest in studies on regular exercise as a part of treatment for patients with CF and as a tool to assess pulmonary deterioration. This illustrates the relevance of correlating the exercise stress test results with prognostic indices obtained by VCAP. Moreover, comparison of the results obtained from these tests between patients with CF and healthy individuals demonstrates the importance of physical and pulmonary rehabilitation and justifies the incorporation of exercise in the routine treatment of patients with CF.
In this context, the aim of this study was to evaluate VCAP parameters (VE, VCO2, VD/VT, VE/VCO2, and PetCO2) and cardiorespiratory parameters (HR, RR, and SpO2) at rest and during the 6-minute walk test between patients with CF and healthy subjects and to compare these values with spirometry data and clinical markers.
Methods
This clinical, prospective, controlled study involved male and female patients with CF (CF group [CFG]) from a university hospital and healthy volunteers (control group [CG]).
All patients with CF who agreed to participate were included. All patients aged >18 years and the caregivers of patients aged <18 years provided written informed consent. The inclusion criteria were an age of 6–25 years and the presence of CF diagnosed according to the criteria of the international consensus [20,21] by a sweat test with a chloride level of >60 mEq/L and positive genetic screening for the CF transmembrane regulator (CFTR) mutation [20,22], when possible.
Patients were not in a state of pulmonary exacerbation as verified by the application of two clinical scores: the Cystic Fibrosis Foundation Clinical Score and the Cystic Fibrosis Clinical Score [23,24]. The CG included children, adolescents, and young adults aged 6–25 years who were randomly selected among students from public and private schools in the same university district. All participants answered a questionnaire, and no subjects had other acute or chronic diseases.
The exercise stress test was performed on a treadmill (Pro Cl 5004; Caloi-Electronic, Wallbach, Switzerland). The velocity was increased according to each individual’s tolerance level until a submaximal test level was reached; at no time during the test did any individual exceed 75% of their maximum HR, which was calculated by 220 − the age of the individual [25].
Before starting the exercise test, all patients in the CFG and CG underwent spirometry according to the guidelines of the European Respiratory Society and American Thoracic Society. The test started by measuring cardiorespiratory variables at rest for 3 minutes; this was followed by measurements during exercise testing on the treadmill for 6 minutes and immediately at the end of the test for a further 3 minutes. Patients in both the CG and CFG followed the same test sequence. The same trained professional performed all examinations.
Initial spirometry was performed using a model CPFS/D spirometer (MedGraphics, Saint Paul, MN, USA) with PF BREEZE Software, Version 3.8B for Windows 95/98/NT (MedGraphics).
For VCAP, the CO2SMO Plus DX 8100 monitor (Novametrix Medical Systems, Wallingford, CT, USA) was used. This is a noninvasive monitor with a capnograph, pulse oximeter, and pneumotachograph. The monitor is connected to CO2 flux and pulse oximetry sensors. The CO2 and flow sensors were used in all subjects and discarded after each use according to the specifications in the manual. These flow and CO2 sensors are combined at their distal portions and coupled to a nozzle. The pediatric/adult (#6719) model of the flow sensor was used. Capnography and pneumotachography measures were obtained in real time by analyzing the gases breathed. The CO2SMO Plus was connected to a computer equipped with software to record the flow, volume, pressure, pressure–volume, flow–volume, and capnography measurements and curves.
The sensor monitor was connected to a mouthpiece, and a nose clip was used to prevent air escaping. Participants were seated in a quiet room and breathed for 1 minute to adapt to the equipment. The monitoring was then started with Analysis Plus® software. Participants maintained normal, relaxed breathing for 4 minutes, and the variables were recorded and stored on the computer. At the end of the data collection, an offline sequence of breaths was selected to determine the coefficient of variation for the lowest expired VT of 25% of the average VT; for PetCO2, measured in mmHg, a lower coefficient of variation of 10% was permitted. Breaths that presented a value of zero for the slope3 variable were excluded. The results were obtained through the average of the parameters collected during the 4-minute monitoring.
The analysis of expiratory gases was performed throughout the test, with each patient using his or her own mouthpiece and nose clip from beginning to end. All subjects received prior clarification regarding the test to be performed.
Statistical analysis
For patients in both the CG and CFG, statistical analysis was performed at five different time points during the exercise test: (1) baseline, (2) 1–2 minutes of activity, (3) 3–4 minutes of activity, (4) 5–6 minutes of activity, and (5) immediately postexercise. The data were processed using Statistical Package for Social Sciences, Version 21.0 software (SPSS Inc., Chicago, IL, USA). For numerical data, the mean, confidence interval, standard deviation, median, and minimum and maximum values were used. For categorical data, the absolute number and percentage were used.
Comparisons between the CG and CFG were performed with the Mann–Whitney test for variables with a numeric distribution, and calculations of differences in categorical variables between the CG and CFG were performed with the χ2 test. Correlations between these time points in each group were determined using the Friedman test (non-parametric, repeated measures), and in cases with a positive p-value, the Wilcoxon signed-rank test was performed. Linear regression was performed to compare the FEV1% and the VCAP parameter VCO2 at all time points analyzed. Linear regression was also performed to evaluate VCO2 in relation to the body mass index (BMI) of all subjects.
For all analyses, we used a value of α = 0.05 with correction for multiple testing by the Bonferroni method (α = 0.05 / 5 = 0.01).
The research ethics committee of the State University of Campinas (#1182/2009) approved this study.
Results
In total, 64 patients with CF and 64 healthy volunteers of both sexes with no statistical differences in age or sex were included in the study (p > 0.05). The complete genotypes of mutations in the CFTR gene are shown in Additional file 1: Table S1. A description of each identified mutation in the CFTR gene is presented in Additional file 2: Table S2.
Weight (kg), height (m2), and BMI (kg/m2) were significantly lower in the CFG (34.80 ± 13.30, 1.42 ± 0.19, and 16.72 ± 3.15, respectively) than in the CG (46.39 ± 14.44, 1.52 ± 0.13, and 19.56 ± 3.33, respectively) (p ≤ 0.001) (Table 1).
The Shwachman–Kulczycki score in the CFG ranged from 60 to 90 (77.93 ± 6.94) (Table 1).
In both groups, assessment of dyspnea using the modified Borg scale (Table 1) showed statistically significant differences before (p ≤ 0.001) and after testing (p ≤ 0.005).
Spirometric values at rest for both groups are shown in Table 1. Patients in the CFG had a significantly lower FVC, FEV1, and FEV1/FVC: (78.61 ± 20.68, 68.98 ± 22.45, and 85.66 ± 11.56, respectively) than did patients in the CG (95.78 ± 11.56, 91.94 ± 16.96, and 98.23 ± 8.95, respectively) (p ≤ 0.001) (Table 1).
The complete list of VCAP values according to the time of analysis between the CG and CFG is shown in Additional file 3: Table S3.
The RR was higher in the CFG than in the CG at all times analyzed, but the differences were not statistically significant (Figure 1).
Comparison of cardiorespiratory variables between the group of patients with cystic fibrosis (CFG) (n=64) and the control group (CG) (n=64). Data are presented as the confidence interval of the median. Time points analyzed: (1) baseline (rest), (2) 1–2 min of activity, (3) 3–4 min of activity, (4) 5–6 min of activity, and (5) postexercise. α=0.01 by Bonferroni correction. The Mann–Whitney test was used to compare the CFG and CG, and correlations between the time points in each group were determined using the Friedman test (non-parametric, repeated measures); in cases with a positive p-value, the Wilcoxon signed-rank test was performed. (A) Respiratory rate. There was no difference between the CG and CFG for the time points analyzed. In the cluster analysis for the CFG, time point 1 differed from 2 to 4; time point 2 differed from 1; time points 3 and 4 differed from 1 and 5; and time point 5 differed from 3 and 4 (p≤0.001). In the cluster analysis for the CG, time points 3 and 4 differed from 5, and time point 5 differed from 3 and 4 (p≤0.001). (B) Heart rate. There was no difference between the CG and CFG for the time points analyzed. In the cluster analysis for the CFG, time point 1 differed from 2 to 4; time point 2 differed from 1, 4, and 5; time point 3 differed from 1 and 5; time point 4 differed from 1, 2, and 5; and time point 5 differed from 2 to 4 (p≤0.001). In the cluster analysis for the CG, time point 1 differed from 2 to 5; time point 2 differed from 1, 3, 4, and 5; time points 3 and 4 differed from 1, 2, and 5; and time point 5 differed from 1 to 4 (p≤0.001). (C) Transcutaneous oxygen saturation. There was no difference between the CG and CFG for time points 4 and 5. For the other time points, the CG exhibited higher values (time points 1 and 2, p≤0.001; time point 3, p=0.002). In the group analysis for the CFG, time point 1 differed from 2 and 4; time point 2 differed from 1 and 5; time point 3 differed from 5; time point 4 differed from 1 and 5; and time point 5 differed from 2 to 4 (p≤0.001). In the CG, time point 1 differed from 2 to 5 (p≤0.001).
The HR was not different between the two groups at any time points analyzed (p > 0.05). The SpO2 was lower in the CFG (93.98 ± 7.22) than in the CG (95.90 ± 5.32), but the difference between time points 4 and 5 was not statistically significant (p ≤ 0.001) (Figure 1B, 1C).
There was an association between the distribution of FEV1(%) and VCO2 at all times during the 6-minute walk test (Figure 2).
Distribution points for linear regression of FEV1% and VCO2. A. Time point 1 - [Combined: y = 0.599 x + 134.183 (p = 0.011); R2 = 0.0495], [CF: y = 0.547 x + 132.642 (p = 0.091); R2 = 0.045], [Control: y = 197.146 – 0.0195 x (p = 0.973); R2 = 0.000018]. B. Time point 2 - [Combined: y = 1.290 x + 196.511 (p = 0.0006); R2 = 0.09095], [CF: y = 1.408 x + 184.347 (p = 0.005); R2 = 0.1198], [Control: y = 291.749 + 0.313 x (p = 0.736); R2 = 0.001876]. C. Time point 3 - [Combined: y = 1.695 x + 234.349 (p = 0.001); R2 = 0.079], [CF: y = 1.256 x + 254.893 (p = 0.063); R2 = 0.05544], [Control: y = 254.560 + 1.584 x (p = 0.253); R2 = 0.02173]. D. Time point 4 - [Combined: y = 1.805 x + 269.757 (p = 0.005); R2 = 0.06034], [CF: y = 1.219 x + 300.252 (p = 0.164); R2 = 0.03098], [Control: y = 2.114 x + 250.909 (p = 0.189); R2 = 0.02815]. E. Time point 5 - [Combined: y = 237.724 + 1.548 x (p = 0.002); R2 = 0.0740], [CF: y = 251.939 + 1.248 x (p = 0.058); R2 = 0.05680], [Control: y = 249.636 + 1.492 x (p = 0.244); R2 = 0.02256].
The VE/VCO2 increased throughout the test and decreased at the end in both groups, but without statistical significance (Figure 3A).
Variables from volumetric capnography between the group of patients with cystic fibrosis (CFG) (n=64) and the control group (CG) (n=64). Data are presented as the confidence interval of the median. Time points analyzed: (1) baseline (rest), (2) 1–2 min of activity, (3) 3–4 min of activity, (4) 5–6 min of activity, and (5) postexercise. α=0.01 by Bonferroni correction. The Mann–Whitney test (data not normally distributed) was used to compare the CFG and CG, and correlations between the time points in each group were determined using the Friedman test (non-parametric, repeated measures); in cases with a positive p-value, the Wilcoxon signed-rank test was performed. (A) Expired minute volume (VE). There was no difference between the CF and CFG for the different time points analyzed. In the cluster analysis, for the CFG, time point 1 differed from 2 to 5; time point 2 differed from 1, 4, and 5; time point 3 differed from 1 and 5; time point 4 differed from 1, 2, and 5; and time point 5 differed from 1 to 4 (p≤0.001). For the CG, time point 1 differed from 2 to 5; time point 2 differed from 1, 3, 4, and 5; time point 3 differed from 1, 2, and 5; and time point 5 differed from 1 to 4 (p≤0.001). (B) VCO2. Higher values were observed in the CG than CFG (time point: (1) p=0.003; (2) p=0.005; (3) p=0.001; (4) p=0.002; (5) p≤0.001). In the cluster analysis, for the CFG, time point 1 differed from 2 to 5; time point 2 differed from 1, 3, 4, and 5; time point 3 differed from 1, 2, and 5; and time point 5 differed from 1 to 4 (p≤0.001). For the CG, time point 1 differed from 2 to 5; time point 2 differed from 1, 3, and 4; time points 3 and 4 differed from 1, 2, and 5; and time point 5 differs from 1, 3, and 4 (p≤0.001). (C) PetCO2. Higher values were observed in the CG than CFG (time point 1, p = 0.002; other time points, p≤0.001). In the cluster analysis for the CFG, time point 1 differed from 2 to 4; time points 2 to 4 differed from 1 and 5; and time point 5 differed from 2 to 4 (p≤0.001). For the CG, time point 1 differed from 2 to 4; time point 2 differed from 1, 4, and 5; time point 3 differed from 1 and 5; time point 4 differed from 1, 2, and 5; and time point 5 differed from 2 to 4 (p≤0.001). (D) VD/VT. There was no difference between the CG and CFG for the different time points analyzed. In the cluster analysis for the CFG, time point 1 differed from 2 to 4; time point 2 differed from 1; time points 3 and 4 differed from 1 and 5; and time point 5 differed from 3 and 4 (p≤0.001). For the CG, time point 1 differed from 2 to 5; time point 2 differed from 1; time points 3 and 4 differed from 1 and 5; and time point 5 differed from 1, 3, and 4 (p≤0.001).
The Exhaled VCO2 increased in both groups and decreased at the end of the test, with statistical differences at all time points analyzed; higher values were observed in the CG (391.16 ± 128.98) than in the CFG (338.03 ± 117.55) (p ≤ 0.001) (Figure 3B).
The PetCO2 increased over time; higher values were observed in the CG (42.00 ± 3.82) than in the CFG (38.41 ± 5.73) (p ≤ 0.001) (Figure 3C).
The VD/VT ratio decreased over time and then returned to baseline; higher values were observed in the CFG, but without statistical significance (p > 0.05) (Figure 3D).
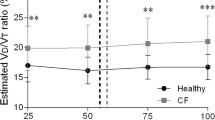
The VE/VCO2 ratio decreased during the test and increased at the end. A statistically significant difference was observed at all time points analyzed, and patients in the CFG had higher ratios at the end of the test (38.81 ± 8.83) than did patients in the CG (32.72 ± 4.74) (p ≤ 0.001) (Figure 4A). The distribution of FEV1(%) showed an inverse association with the VE/VCO2 at time points 1 and 5 in both the CFG and CG (Figure 4B and C).
VE/VCO 2 by confidence intervals in relation to different time points and linear regression for time points 1 and 5 with respect to FEV1%. (A) Confidence interval of VE/VCO2 for the cystic fibrosis group (CFG) (n=64) and control group (CG) (n=64). Data are presented as the confidence interval of the median. Time points analyzed: (1) baseline (rest), (2) 1–2 min of activity, (3) 3–4 min of activity, (4) 5–6 min of activity, and (5) immediately postexercise. α=0.01 by Bonferroni correction. The Mann–Whitney test was used to compare the CFG and CG, and correlations between the time points in each group were determined using the Friedman test (non-parametric, repeated measures); in cases with a positive p-value, the Wilcoxon signed-rank test was performed. Higher values were observed in the CG at all time points (p≤0.001). In the cluster analysis for the CFG, time point 1 differed from 2 to 5; time point 2 differed from 1 and 4; time point 3 differed from 1 and 5; time point 4 differed from 1, 2, and 5; and time point 5 differed from 1, 3, and 4 (p≤0.001). For the CG, time point 1 differed from 2 to 5; time point 2 differed from 1, 3, and 4; time point 3 differed from 1, 2, and 4; time point 4 differed from 1, 2, 3, and 5; and time point 5 differed from 1 and 4 (p≤0.001). (B) Linear regression of the VE/VCO2 and FEV1%. Time point 1 - [(Combined: y= 52.5615 – 0.1343x) (p≤0.0001); R2=0.01522; (CFG: y= 51.8380 – 0.1139x) (p=0.0056); R2=0.1122; (CG: y = 48.0635 – 0.0935x) (p=0.1756); R2=0.02986]. (C) Linear regression of the EV/VCO2 and FEV1%. Moment 5 - [(Combined: y= 47.2563 – 0.1411x) (p≤0.001); R2=0.1622; (CFG: y= 46.6691 – 0.1139x) (p=0.024); R2=0.08373; (CG: y= 35.5437 – 0.02961x) (p=0.5310); R2=0.006467].
Linear regression of VCO2 in relation to BMI is shown in Additional file 4: Figure S4
All children completed the test without exhibiting cardiorespiratory symptoms requiring cessation of the test.
Discussion
Exercise has been studied as a tool with which to assess the improvement or worsening of cardiorespiratory function [26-29]. To the best of our knowledge, this is the first study to evaluate ventilatory efficiency through the use of VCAP before, during, and after exercise between patients with and without CF. Furthermore, it reinforces the positive effects of the association between exercise and therapy in these patients. Few studies have been conducted using VCAP for evaluation of CF [19,30,31].
Our results reinforce the importance of exercise as a useful tool in cardiorespiratory evaluation of patients with CF. All cardiorespiratory, spirometry, and VCAP markers differed between the CFG and CG before and after exercise.
It is well known that CF causes progressive deterioration in the ability to perform physical exercise. However, regardless of severity, both children and adults may develop increased ability to tolerate exercise and benefit from it in the long term [28].
Various authors have studied transverse or longitudinal models and the benefits of exercise on lung function in patients with severe CF [27,29]. However, our population of patients with CF had higher FEV1 values than those in other studies [26,27]. This reflects a population with minor deterioration in lung function. There are several potential explanations for this difference; for example, the patients in our center received medications at no cost and underwent care by multidisciplinary teams, the CFTR mutations belonged to different classes, early diagnosis was achieved, interdisciplinary monitoring was performed, and the patients included in our study were relatively young.
Various markers of the deterioration of lung function in patients with CF have been investigated and include clinical and tomographic scores, spirometry values, VCAP values, the lung clearance index, and others [19]. One confirmed paradigm is that high-resolution computed tomography detects changes in lung function earlier than does spirometry, which illustrates the need for new tools with which to investigate lung function. The use of VCAP has not been extensively studied in patients with CF. Hopefully, it will become the focus of new studies to enable more accurate diagnosis of early pulmonary disease [19,30,31]. Thus, in the present study, VCAP was analyzed as a practical, inexpensive, and easy-to-perform method that can give important information about ventilatory function.
Throughout the exercise test, we observed that the RR and HR were higher in the CFG than in the CG at all time points, and the HR was higher in the CG only immediately after exercise. This finding is in agreement with a recent study by Pereira et al. [8] in which 55 patients with CF and 185 healthy individuals were evaluated during the 6-minute walk test. During the test, patients with CF exhibited a lower SpO2 than did healthy individuals. The same result was observed by Holland et al. [32] in a study of 101 adults with moderate to severe CF performing the 3-minute step test. The authors reported that desaturation during the test was associated with long-term pulmonary deterioration and longer hospitalization periods in adults with CF. In a study by Pereira et al. [8], SpO2 remained stable during the 6-minute walk test.
In the present study, evaluation of dyspnea using the modified Borg scale showed that the highest dyspnea ratings were found in the CFG before and after the test; this is in agreement with the data reported by Pereira et al. [8].
With progressive deterioration of the lungs, the amount of VD increases, requiring changes in ventilation to maintain adequate alveolar ventilation during exercise. Thus, changes in lung function over time have been correlated with changes in exercise capacity. CO2 retention during the maximal exercise stress test also reportedly contributed to a faster rate of decline in FEV1% in a study of children with CF aged 11–15 years [26].
Throughout our analysis, patients in the CFG showed significantly greater retention of Exhaled VCO2 than did patients in the CG, who exhaled larger amounts of VCO2. This finding can be explained by dysfunction that causes difficultly in gas exchange, such as disruption of the lung architecture, destruction of parts of the capillary bed, increased DS, and secretion retention [33-35]. In the present study, we chose not to correct the VCAP values by the BMI because the lower value of BMI in the CFG was associated with the patients’ clinical disease status, was a common factor in the population tested, and represented the clinical picture at each time point analyzed. If such a correction were made, the obtained data would mask the true severity of the disease.
Assessment of the cardiorespiratory responses of healthy children during the maximal exercise test revealed that the responses were higher for HR, RR, VCO2, VD/VT, and PetCO2, while VT and PetCO2 showed a smaller increase in children than in adults. At peak exercise, larger differences were observed in HR and VD/VT in children than in adults, suggesting there is increased ventilation in the anatomical VD [36].
Leroy et al. [27] studied the impact of dyspnea and alveolar hypoventilation during exercise in 18 patients with CF through a maximal exercise test on a cycle. They found that during exercise, patients exhibited progressive increases in their VT, reaching a peak VT at 48% of FVC [27]. The VD/VT ratio also increased during exercise and represented 34% of the VT [27].
A main limitation of our study is the exclusion of a curve of normal VCAP values. Although our sample has statistical power for analysis, future studies with larger numbers of patients of the same age and CFTR mutation class should be performed.
Conclusions
We conclude that VCAP can be used as a tool for analysis of ventilatory efficiency during exercise. All cardiorespiratory, spirometry, and VCAP variables differed between patients with CF and healthy subjects before and after exercise. These results indicate imbalanced ventilation/perfusion characterized by both hypoxemia and CO2 retention, which compromises exercise tolerance in patients with CF. Continued research of cardiorespiratory markers in transverse and longitudinal models at rest and after exercise is needed to better understand the deterioration of lung function in patients with CF.
Abbreviations
- VCAP:
-
Volumetric capnography
- CFTR:
-
Cystic fibrosis transmembrane regulator
- CO2:
-
Carbon dioxide
- FVC:
-
Forced vital capacity
- CF:
-
Cystic fibrosis
- HR:
-
Heart rate
- RR:
-
Respiratory rate
- CG:
-
Control group
- CFG:
-
Cystic fibrosis group
- BMI:
-
Body mass index
- PetCO2:
-
End-tidal carbon dioxide
- SpO2:
-
Oxygen saturation
- VCO2:
-
Volume of exhaled carbon dioxide
- VD:
-
Dead space
- VE:
-
Expiratory minute volume
- FEV1:
-
Forced expiratory volume in the first second of the forced vital capacity
- VT:
-
Tidal volume
- VE/VCO2:
-
Ventilatory efficiency index
References
Rand S, Prasad A. Exercise as part of a cystic fibrosis therapeutic routine. Expert Rev Respir Med. 2012;6 Suppl 3:341–52.
Flume PA, Van Devanter DR. State of progress in treating cystic fibrosis respiratory disease. BMC Med. 2012;10:88.
Bernardi DM, Ribeiro AF, Mazzola TN, Vilela MMS, Sgarbieri VC. The impact of cystic fibrosis on the immunologic profile of pediatric patients. J Pediatr. 2013;89 Suppl 1:40–7.
Chaves CRMM, Oliveira CQ, Britto JAA, Elsas MICG. Exercise testing, aerobic and strength training for adolescents with cystic fibrosis: a literature review. Rev Bras Saúde Matern Infant. 2007;7 Suppl 3:245–50.
Damas C, Amorim A, Gomes I. Cystic fibrosis: review. Rev Port Pneumol. 2008;14 Suppl 1:89–112.
Leeuwen PBVW, Arets HGM, Ent CKV, Beekman JM. Infection, inflammation and exercise in cystic fibrosis. Respir Res. 2013;14:32.
Elbasan B, Tunali N, Duzgun I, Ozcelik U. Effects of chest physiotherapy and aerobic exercise training on physical fitness in Young children with cystic fibrosis. Ital J Pediatr. 2012;38:2.
Pereira FM, Ribeiro MAGO, Ribeiro AF, Toro AADC, Hessel G, Ribeiro JD. Functional performance on the six-minute walk test in patients with cystic fibrosis. J Bras Pneumol. 2011;37 Suppl 6:735–44.
Silva OB, Filho DCS. A new proposal to guide velocity and inclination in the ramp protocol for the treadmill ergometer. Arq Bras Cardiol. 2003;81 Suppl 1:42–7.
Ferrazza AM, Martolini D, Valli G, Palange P. Cardiopulmonary exercise testing in the functional and prognostic evaluation of patients with pulmonary diseases. Respiration. 2009;77 Suppl 1:3–17.
Hoffbrand BL. The expiratory capnogram: a measure of ventilation-perfusion inequalities. Thorax. 1966;21:518–23.
Wolff G, Brunner JK. Series dead space volume assessed as the mean value of a distribution function. Int J Clin Monit Comp. 1984;1:177–81.
Fletcher R, Jonson B. Dead space and the single breath test carbon dioxide during anaesthesia and artificial ventilation. Br J Anasth. 1984;6:109–76.
Toulou PP. Distribution of ventilation: clinical evaluation by rapid CO2 analysis. Chest. 1996;49:139–45.
Smidt U. Emphysema as possible explanation for the alteration of PO2 and PCO2 curves. Bull Eur Physiopathol Respir. 1976;12:607–24.
Worth H. Expiratory partial pressure curves in the diagnostic of emphysema. Bull Eur Physiopathol Respir. 1986;22:191–9.
Davis JA, Sorrentino KM, Ninness EM, Pham PH, Dorado S, Costello KB. Test-retest reliability for two indices of ventilatory efficiency measured during cardiopulmonary exercise testing in healthy men and women. Clin Physiol Funct Imaging. 2006;26 Suppl 3:191–6.
Tumminello G, Guazzi M, Lancellotti P, Pie’rard L. Exercise ventilation inefficiency in heart failure: pathophysiological and clinical significance. Eur Heart J. 2007;28:673–8.
Ribeiro MA, Silva MT, Ribeiro JD, Moreira MM, Almeida CC, Almeida-Junior AA, et al. Volumetric capnography as a tool to detect early peripheric lung obstruction in cystic fibrosis patients. J Pediatr. 2012;88 Suppl 6:509–17.
Ribeiro JD, Ribeiro MAGO, Ribeiro AF. Controversies in cystic fibrosis – from pediatrician to specialist. J Pediatr (Rio J). 2002;78 Suppl 2:S171–86.
Döring G, Conway SP, Heijerman HG, Hodson ME, Høiby N, Smyth A, et al. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur Respir J. 2000;16(4):749–67.
Coelho CC, Aquino Eda S, de Almeida DC, Oliveira GC, Pinto RC, Rezende IM, et al. Comparative analysis and reproducibility of the modified shuttle walk test in normal children and in children with cystic fibrosis. J Bras Pneumol. 2007;33(2):168–74.
CFF. Cystic Fibrosis Foundation, Patient Registry 2002 Annual Data Report. Bethesda, Maryland: Patient Registry; 2002.
Kanga J, Kuhn R, Craigmyle, Haverstock D, Church D. Cystic Fibrosis clinical score: a new scoring system to evalute acute pulmonary exacerbation. Clin Ther. 1999;21:1343–56.
ATS – American Thoracic Society. ATS Statement: guidelines for the sixminute walk test. Am J Respir Crit Care Med. 2002;166:111–7.
Schneiderman-Walker J, Coates AL. Does carbon dioxide retention during exercise predict a more rapid decline in FEV1 in cystic fibrosis? Arch Dis Child. 2005;90 Suppl 8:792–5.
Leroy S, Perez T, Neviere R, Aquilanju B, Wallaert B. Determinants of dyspnea and alveolar hypoventilation during exercise in cystic fibrosis: Impact of inspiratory muscle endurance. J Cyst Fibros. 2011;10 Suppl 3:159–65.
Pianosi P, Hochman J. End-tidal estimates of arterial PCO2 for cardiac output measurement by CO2 rebreathing: a study in patients with cystic fibrosis and healthy controls. Pediatr Pulmonol. 1996;22 Suppl 3:154–60.
Paranjape SM, Barnes LA, Carson KA, Von Berg K, Loosen H, Mogayzel Jr PJ. Exercise improves lung function and habitual activity in children with cystic fibrosis. J Cyst Fibros. 2012;11 Suppl 1:18–23.
Fuchs SI, Junge S, Ellemunter H, Ballmann M, Gappa M. Calculation of the capnographic index based on expiratory molar mass-volume-curves-a suitable tool to screen for cystic fibrosis lung disease. J Cyst Fibrosis. 2013;12 Suppl 3:277–83.
Veronez L, Moreira MM, Soares ST, Pereira MC, Ribeiro MA, Ribeiro JD, et al. Volumetric capnography for the evaluation of pulmonary disease in adult patients with cystic fibrosis and noncystic fibrosis bronchiectasis. Lung. 2010;188 Suppl 3:263–8.
Holland AE, Rasekaba T, Wilson JW, Button BM. Desaturation during the 3-minute step test predicts impaired 12-month outcomes in adult patients wh cystic fibrosis. Respir Care. 2011;56 Suppl 8:1137–42.
Marcus BH, Williams DM, Dubbert PM, Sallis JF, King AC, Yancey AK, et al. Physical activity intervention studies: what we know and what we need to know. Circulation. 2006;114(24):2739–52.
Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364 Suppl 9435:709–21.
Calverley PMA, Koulouris NG. Flow limitation and dynamic hyperinsuflation: key concepts in modern respiratory physiology. Eur Respir J. 2005;25(1):186–99.
Hibbert A, Lanning A, Raven J, Landau L, Phelan P. Gender differences in lung growth. Pediatr Pulmonol. 1995;19:129–34.
Acknowledgments
The authors acknowledge Silvana Dalge Severino for his assistance in performing the spirometry test; the laboratory team of the Faculty of Medical Sciences, Unicamp (http://www.laboratoriomultiusuario.com.br/) for helping with the statistical analysis; the Brazilian agency FAPESP for the PhD scholarship granted to FALM [process 2011/12939-4]; and Professor Carmen Silvia Bertuzzo for the screening of CFTR mutations.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PLFP, FALM, MAGOR, CISS, and JDR made substantial contributions to the study conception and design, acquisition of data, or analysis and interpretation of data; were involved in drafting the manuscript or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CCBA, IAP, and AADCT made substantial contributions to the study conception and design, acquisition of data, or analysis and interpretation of data. LCM was involved in drafting the manuscript and revising it critically for important intellectual content. All authors read and approved the final manuscript.
Additional files
Additional file 1: Table S1.
Genotypes for the CFTR mutations of patients with cystic fibrosis enrolled in the present study (n = 64).
Additional file 2: Table S2.
CFTR mutations found in individuals enrolled in the study. Gene and protein localization. Mutation classification and frequency from the present study are designated. Traditional and Human Genome Variation Society standard nomenclature for CFTR mutations are also indicated.
Additional file 3: Table S3.
Volumetric capnograph markers taking into account groups of patients with cystic fibrosis and healthy subjects. Time points analyzed by groups.
Additional file 4: Figure S4.
Linear regression of VCO2 and FEV1% by body mass index (BMI). A. Time point 1 - VCO2 by BMI - [Combined: y = 12.382 + 0.031 x (p ≤ 0.001)], [CF: y = 12.381 + 0.025 x (p = 0.001)], [Control: y = 13.810 + 0.029 x (p ≤ 0.001)]. B. Time point 3 - VCO2 by BMI - [Combined: y = 12.573 x + 0.015 (p ≤ 0.001)], [CF: y = 12.278 + 0.013 x (p < 0.001)], [Control: y = 14.244 + 0.013 x (p ≤ 0.001)]. C. Time point 5 - VCO2 by BMI - [Combined: y = 11.854 x + 0.017 (p ≤ 0.001)], [CF: y = 11.712 + 0.015 x (p ≤ 0.001)], [Control: y = 13.266 + 0.016 x (p ≤ 0.001)]. D. Time point 1 - FEV1% by BMI - [Combined: y = 13.566 + 0.056 x (p = 0.001)], [CF: y = 15.127 + 0.023 x (p = 0.196)], [Control: y = 14.919 + 0.049 x (p = 0.133)].
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Parazzi, P.L.F., Marson, F.A.d.L., Ribeiro, M.A.G.d.O. et al. Ventilatory abnormalities in patients with cystic fibrosis undergoing the submaximal treadmill exercise test. BMC Pulm Med 15, 63 (2015). https://doi.org/10.1186/s12890-015-0056-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-015-0056-5