Abstract
Background
Positive associations between the risk of schizophrenia and the level of white blood cells (WBC) count have been suggested by observational studies. However, the causality of this association is still unclear.
Methods
We used a group of bidirectional two-sample Mendelian randomization (MR) analyses to estimate the causal relationship between schizophrenia and WBC count traits (i.e., WBC count, lymphocyte count, neutrophil count, basophil count, eosinophil count, and monocyte count). The threshold of FDR-adjusted P < 0.05 was considered as showing potential evidence of a causal effect. Instrument variables were included based on the genome-wide significance threshold (P < 5 × 10− 8) and linkage disequilibrium (LD) clumping (r2 < 0.01). In total, 81, 95, 85, 87, 76, and 83 schizophrenia-related single nucleotide polymorphisms (SNPs) were used as genetic instruments from Psychiatric Genomics Consortium for six WBC count traits, respectively. And in reverse MR analysis, 458, 206, 408, 468, 473, and 390 variants extracted from six WBC count traits were utilized as genetic instruments, which were obtained from a recent large-scale Genome-Wide Association Study (GWAS).
Results
Genetically predicted schizophrenia was positively associated with the level of WBC count [odds ratio (OR) 1.017, 95% confidence interval (CI) 1.008–1.026; P = 7.53 × 10− 4], basophil count (OR 1.014, 95%CI 1.005–1.022; P = 0.002), eosinophil count (OR 1.021, 95%CI 1.011–1.031; P = 2.77 × 10− 4), monocyte count (OR 1.018, 95%CI 1.009–1.027; P = 4.60 × 10− 4), lymphocyte count (OR 1.021, 95%CI 1.012–1.030; P = 4.51 × 10− 5), and neutrophil count (OR 1.013, 95%CI 1.005–1.022; P = 0.004). WBC count traits are not associated with the risk of schizophrenia in our reverse MR results.
Conclusion
Schizophrenia is associated with elevated levels of WBC count (i.e., higher WBC count, lymphocyte count, neutrophil count, basophil count, eosinophil count, and monocyte count).
Similar content being viewed by others
Background
Schizophrenia is a complex behavioral and cognitive syndrome, which is considered one of the most serious psychiatric disorders. It causes a significant global disease burden and heavy economic burden with a suicide rate of around 5% [1]. Many genetic epidemiological studies have shown that schizophrenia is highly polygenic heritability with the rate ranging from 64 to 81% [2,3,4].
In recent years, the relationship between psychiatric disorders and the inflammatory response has been explored in many studies [5,6,7]. For example, a large cohort study showed that both C-reactive protein (CRP) and leukocytes were significantly associated with both negative and positive symptoms in patients with psychiatric disorders [8]. Numerous avenues of inquiry have suggested the relationship between psychiatric disorders and immune dysfunction (e.g., aberrations in immune cells number and inflammatory markers) [9,10,11]. White blood cells (WBC) are the hallmark cells of the body’s inflammatory response. Several cross-sectional studies and meta-analyses have already suggested that there are increased total and differential WBC counts in patients with schizophrenia, and immune cells might have a significant impact on schizophrenia [12,13,14]. It should be noted that previous studies usually relied on observational studies, which may suffer from confounding factors like reverse causality and selection bias, and meta-analyses have also reported substantial heterogeneity. At the same time, current studies have shown that various antipsychotics have a significant effect on the immune cells count of the body [15, 16]. Therefore, it remains unknown if the alterations of the immune cells number in schizophrenia are causal or potentially compensatory reactions to upstream dysfunction.
Mendelian randomization (MR), a more powerful method for causal inference than the traditional analysis approach (e.g., case-control study) used in observational studies, uses genetic variations as instruments to infer causality, thus overcoming the potential problems of reverse causality and confounding in observational studies [17, 18]. In recent years, thousands of genetic variant loci associated with complex traits have been identified by GWAS, which allowed MR to be widely used in causal inference studies of mental disorders [4, 19,20,21]. Recent studies have used MR analyses to explore the potential causal relationship between inflammatory biomarkers levels and partial blood cell trait with schizophrenia [22,23,24], but the sample size of those studies was not large enough compared with the summary data from new GWAS meta-analysis, and the categories of WBC traits are incomplete. Here, we investigate the causality between schizophrenia susceptibility and WBC count traits via a two-sample bidirectional MR method comprehensively.
Methods
Study design
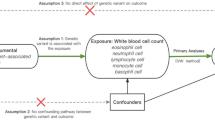
The relationship between schizophrenia and WBC count traits was investigated using 12 MR analyses (Fig. 1). There are three key assumptions that underlie MR. (1) a significant association exists between genetic variants and exposure; (2) there is no correlation between the instrument variables (IVs) and any confounding factors; (3) the exposure is the only way in which genetic variants affect the outcome of interest [25]. We adopted the GWAS summary statistics for schizophrenia and six count traits of WBC (i.e., WBC, lymphocyte, neutrophil, basophil, eosinophil, and monocyte). The six WBC count traits in this study are all the total absolute count. A forward MR analysis was performed to test the causal effect of genetically predicted schizophrenia on WBC count traits. And the reverse analysis was utilized to examine the effects of WBC count traits on schizophrenia risk. Most of the participants in our analyses were of European ancestry. Ethical approval can be obtained from all original studies.
Selection of instrumental variants
The summary statistics of 6 peripheral blood WBC count traits were derived from a meta-analysis of 408,112 participants [26]. Summarized data for schizophrenia were obtained from the Psychiatric Genomics Consortium (PGC) [4]. The detailed sources of the data are shown in Supplementary Table 1. The single nucleotide polymorphisms (SNPs) were retrieved from GWAS summary statistics mentioned above as IVs, which were included based on the genome-wide significance threshold (i.e., P < 5 × 10− 8) and linkage disequilibrium (LD) clumping (r2 < 0.01).
Statistical analyses
In this investigation, F-statistic was calculated as
and F ≥ 10 was typically recommended for strong instruments [27]. The R2 value was calculated to determine how much variance IVs are explained in the exposure factor. We used the inverse-variance-weighted (IVW) random-effects model to generate effect estimates as the major result, and the multiplicative random effect model would be adopted if there was evidence of heterogeneity. The MR-Egger regression and weighted median were used for alternative analyses. The MR-Egger intercept was used to determine directional horizontal pleiotropy. And then the MR-PRESSO test was used to remove detected outliers to correct for horizontal pleiotropy [28]. The heterogeneity between individual SNPs was tested by Cochran’s Q statistics and funnel plots. And the leave-one-out sensitivity analysis was performed to test whether the main results were driven by any individual SNP. The Benjamini-Hochberg false discovery rates (FDR) correction was used to adjust P values in MR analyses. The threshold of FDR-adjusted P < 0.05 was considered as showing potential evidence of a causal effect and all P values are two-tailed [29]. MR analyses mentioned above were conducted by using the MRInstruments, TwoSampleMR, and MRPRESSO packages with R (version 4.1.2).
Results
Forward MR analyses of the effects of schizophrenia on WBC count traits
Firstly, the causal effects of schizophrenia on 6 WBC count traits were investigated. After removing outliners due to horizontal pleiotropic and ambiguous palindrome, we finally included 81, 76, 83, 95, 85, and 87 variants as genetic instruments for WBC count, lymphocyte count, neutrophil count, basophil count, eosinophil count, monocyte count in the forward MR analyses, respectively (Supplementary Tables 5–10). Figure 2a and Supplementary Table 3 present the forward MR results; Supplementary Figs. 1–4 present the scatter plots, forest plots, funnel plots, and leave-one-out analysis results, respectively. Within the six one-way analyses, the smallest F-statistic was equal to 28.86, showing a weak instrumental bias, which was unlikely to have an impact on the IVW estimates (Supplementary Table 2).
The association between schizophrenia and the level of WBCs count in forward MR analyses were all statistically significant via the IVW model [odds ratio (OR) 1.017, 95% confidence interval (CI) 1.008–1.026; P = 7.53 × 10− 4]. The same result arises in the weighted median model (OR 1.015; 95%CI 1.006–1.025; P = 0.009). As to five WBC count traits, in the IVW models, genetically predicted schizophrenia was positively associated with levels of basophil count (OR 1.014, 95%CI 1.005–1.022; P = 0.002), eosinophil count (OR 1.021, 95%CI 1.011–1.031; P = 2.77 × 10− 4), monocyte count (OR 1.018, 95%CI 1.009–1.027; P = 4.60 × 10− 4), lymphocyte count (OR 1.021, 95%CI 1.012–1.030; P = 4.51 × 10− 5), and neutrophil count (OR 1.013, 95%CI 1.005–1.022; P = 0.004) (Supplementary Table 3). The MR-Egger intercepts indicated that no evidence of horizontal pleiotropy was found (all P-Egger intercepts > 0.10). Cochran’s Q statistics suggested the existence of heterogeneity (all P-Q values < 0.001) so the multiplicative random effect model was adopted. But there is no evidence of heterogeneity as shown in the funnel plot (Supplementary Fig. 3). Besides, the results of the leave-one-out analysis suggested the robustness of the observed causality.
Reverse MR analyses of the effects of WBC count traits on schizophrenia
After removing the unavailable and the palindromic variants, we utilized 458, 473, 390, 206, 408, and 468 variants for WBC count, lymphocyte count, neutrophil count, basophil count, eosinophil count, and monocyte count as genetic instruments, respectively, where weak instrumental bias have been ruled out (Supplementary Tables 2, Supplementary Tables 11–16). The results of MR analysis by using MR Egger, weighted median, and IVW method all indicated that neither WBC nor the other five traits were causally related to schizophrenia (Fig. 2b and Supplementary Table 4). There was no horizontal pleiotropy in the reverse analyses (all MR-Egger intercept P values > 0.1). And the funnel plots (Supplementary Fig. 6) suggested no significant asymmetry of the MR analyses. However, Cochran’s Q statistics indicated the existence of heterogeneity (Q ≥ 357.50, all P values < 0.001).
Discussion
To the best of our knowledge, this is the first bidirectional two-sample MR research that, examines the causal effects of six WBC characteristics in schizophrenia susceptibility. This MR study indicated that schizophrenia was positively associated with levels of WBC, lymphocyte, neutrophil, basophil, eosinophil, and monocyte. No evidence in the reverse MR analyses was observed that six WBC count traits were associated with schizophrenia.
Leukocytes are an essential component of the body’s immunological defense mechanism. In total, there are five types of leukocytes in the blood, namely neutrophils, eosinophils, basophils, lymphocytes, and monocytes, each of which has a specific physiological function. A recent meta-analysis showed that peripheral blood WBC count, neutrophil count, and monocyte count were significantly elevated in both patients with first-episode of psychosis (FEP) and chronic psychosis [30], which is consistent with the results of this study. However, possibly limited by heterogeneity between studies and small sample size, no correlation was found between several other categorical counts and schizophrenia in this meta-analysis. Newly published findings showed consistent results of elevated WBC and neutrophil counts in drug-naive FEP patients [31]. Several studies have pointed out that impaired inflammatory regulation represented by elevated WBC may be a manifestation of more severe neuropathological changes and associated with a higher risk of metabolic syndrome in patients with schizophrenia [31,32,33]. And it is worth noting that schizophrenia patients with increased WBC and neutrophil counts may show a tendency for increased self-aggression [34]. Neutrophils, a complex of transcriptionally active cells, have multiple essential roles such as secreting cytokines, regulating the activity of neighboring cells, and regulating macrophages for a long-term immune response [35]. As for studies on schizophrenia, in FEP patients, elevated neutrophil counts may serve as an important monitoring indicator and indirect evidence of reduced gray matter tissue, increased cerebrospinal fluid volume, higher PANSS scores, and more severe clinical presentation in patients [36]. The results of studies on lymphocyte count in patients with schizophrenia are not yet consistent. Some studies suggested that patients with schizophrenia exhibit a blood cell pattern of increased neutrophils, decreased lymphocytes, and increased neutrophil-to-lymphocyte ratio [37, 38]. However, there is evidence indicating that pro-inflammatory-prone monocytes were significantly overrepresented and the T-lymphocyte network was significantly activated in patients with recurrent-onset schizophrenia [14]. In addition, a study of brain tissue from patients with schizophrenia and mood disorder found that high-density lymphocyte infiltration may predict the onset of neuroinflammation associated with blood-brain barrier damage [39]. The correlation between eosinophil and basophil counts and schizophrenia was less well studied. No differences in eosinophil count between FEP patients and healthy controls have been observed [30]. However, some studies have indicated that eosinophil levels were reduced in patients with FEP and tended to rebound with the course of treatment [40].
Current research has demonstrated that psychiatric disorders are closely linked to inflammatory responses, and the inflammatory immune mechanisms of schizophrenia have received extensive attention. On the one hand, lots of observational studies suggested that patients with schizophrenia exhibit many characteristics of low-grade inflammation, for example, higher plasma levels of inflammatory cytokines, chemokines [31, 41, 42], and inducers [43]. Interestingly, however, several MR studies have successively reported that genetically predicted higher level of CRP may be a protective factor of schizophrenia [22, 44, 45]. On the other hand, studies have also suggested that higher levels of inflammatory mediators are associated with the pathogenesis and disease progression of psychiatric disorders [41, 46]. Cytokines may enter the brain tissue associated with neurological disorders to drive neuroinflammation and activate the neuroendocrine axis, thereby triggering depressive behavior and cognitive impairment [47,48,49]. It is interesting to note that we reviewed the results of previous GWAS studies and found that the loci significantly associated with schizophrenia and leukocyte count all contained SNPs associated with Y RNAs. And human Y RNAs have the primary function of mediating the initiation of chromosomal DNA replication and regulating the autoimmune protein Ro60 [50, 51]. In addition, previous studies have identified a common genetic link between lymphocyte count and schizophrenia in the MHC region [23]. And numerous studies have also identified the association between immunomodulatory genes and the increased risk of several psychiatric disorders, such as schizophrenia and major depressive disorder [4, 52]. All this evidence suggests that the underlying cause of elevated leukocytes in schizophrenia patients may be closely related to immune inflammatory responses and immune dysfunction. The results of the present study point to a significant unidirectional causal relationship between schizophrenia and WBC count traits, which may provide new evidence to support early inflammatory mechanisms in schizophrenic patients, suggesting the need for monitoring of WBC count traits and the management of low-grade inflammatory responses in SCZ patients. Besides, this study for the first time suggests that schizophrenia may be potentially causally related to elevated levels of eosinophils and basophils, and further studies are needed to verify the causal relationship found in this study.
The main advantage of the present study is that we used a bidirectional IVs analysis to avoid reverse causality and confounding factors as much as possible. To address the inability of cross-sectional studies to determine causality, Astle’s group carried out a series of one-way MR analyses in 2016 (n = 173,480), and only the causal relationship between lymphocyte count and schizophrenia was detected in the result [23]. Then, the potential causal association of neutrophils, and lymphocytes on susceptibility to three psychiatric disorders (seminal schizophrenia, major depression, bipolar disorder) was explored again in a bidirectional MR analysis published in 2021 using summary data published in 2016 [23], however, all the results were not significant, and vice versa [24]. In contrast, the results of the present study comprehensively showed a unidirectional positive correlation between schizophrenia and the levels of all six WBC count traits. This may be because the sample size of GWAS data used in this study was 2.3 times larger than the data used in the previous MR study [23, 24], and therefore this study had a higher power for causal association testing and more accurate effect estimates. Also, this study used different methods to estimate the causal effects, and the mutually corroborating results made the causal effects more convincing. However, several limitations cannot be ignored in this study. First, although the funnel plots all showed symmetrical distributions of IVs and the results of the leave-one-out analysis demonstrated the robustness of the results, Cochran’s Q values still showed the presence of heterogeneity. Unfortunately, subgroup analyses could not be performed for this study because neither primary data nor subgroup summary statistics data were available. Second, although this study showed no horizontal pleiotropy by the MR-Egger intercepts, some potentially pleiotropic IVs could not be completely excluded. What’s more, the practical effects of IVs on outcome variables cannot be fully explained yet. Finally, it is important to note, that the present evidence can only be limited to the European population because potential heterogeneity among different ethnic groups. Therefore, the present evidence can only be limited to the European population.
Therefore, we highlight that the results of this study should be interpreted cautiously and more studies are needed in the future to elucidate and validate the leukocyte count changes in schizophrenia and their inflammatory mechanisms.
Conclusions
In conclusion, this bidirectional MR study indicated a positive causal effect of schizophrenia on the elevated levels of WBCs (including five white blood cells classification count traits). No causal effects of WBC count traits on the susceptibility of schizophrenia were found. Our findings provided new insights into the immune-inflammatory hypothesis of schizophrenia, suggesting the need for monitoring WBC count traits and the management of low-grade inflammatory responses in SCZ patients.
Data Availability
The datasets supporting the conclusions of this article are included within the article and its additional files (Supplementary Information and Supplementary Tables 5–16). Publicly available summary-level datasets which were analyzed in this study could be found in Psychiatric Genomics Consortium (https://www.med.unc.edu/pgc) and GWAS Catalog (https://www.ebi.ac.uk/gwas/ home; White blood cells count: GCST90002407; Eosinophil count: GCST90002481; Basophil count: GCST90002379; Neutrophil count: GCST90002398; Monocyte count: GCST90002393; Lymphocyte count: GCST90002388). The details of the data are shown in Supplementary Table 1.
Abbreviations
- WBC:
-
White blood cells
- MR:
-
Mendelian randomization
- SNPs:
-
Single nucleotide polymorphisms
- GWAS:
-
Genome-Wide Association Study
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- CRP:
-
C-reactive protein
- IVs:
-
Instrument variables
- PGC:
-
Psychiatric Genomics Consortium
- LD:
-
Linkage disequilibrium
- FDR:
-
False discovery rates
- FEP:
-
First-episode of psychosis
References
Hor K, Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24(4 Suppl):81–90.
Jauhar S, Johnstone M, McKenna PJ, Schizophrenia. Lancet. 2022;399(10323):473–86.
Owen MJ, Sawa A, Mortensen PB, Schizophrenia. Lancet. 2016;388(10039):86–97.
Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–7.
Vallee A. Neuroinflammation in Schizophrenia: the key role of the WNT/β-Catenin pathway. Int J Mol Sci. 2022;23(5):2810.
Chaudhry IB, Husain MO, Khoso AB, Husain MI, Buch MH, Kiran T, Fu B, Bassett P, Qurashi I, Ur Rahman R, et al. A randomised clinical trial of methotrexate points to possible efficacy and adaptive immune dysfunction in psychosis. Transl Psychiatry. 2020;10(1):415.
Anderson G, Maes M, Berk M. Schizophrenia is primed for an increased expression of depression through activation of immuno-inflammatory, oxidative and nitrosative stress, and tryptophan catabolite pathways. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:101–14.
Liemburg EJ, Nolte IM, Klein HC, Knegtering H. Relation of inflammatory markers with symptoms of psychotic disorders: a large cohort study. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:89–94.
Kappelmann N, Arloth J, Georgakis MK, Czamara D, Rost N, Ligthart S, Khandaker GM, Binder EB. Dissecting the Association between inflammation, metabolic dysregulation, and specific depressive symptoms: a genetic correlation and 2-Sample mendelian randomization study. JAMA Psychiatry. 2021;78(2):161–70.
Pearlman DM, Najjar S. Meta-analysis of the association between N-methyl-d-aspartate receptor antibodies and schizophrenia, schizoaffective disorder, bipolar disorder, and major depressive disorder. Schizophr Res. 2014;157(1–3):249–58.
Gibney SM, Drexhage HA. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J Neuroimmune Pharmacol. 2013;8(4):900–20.
Mazza MG, Lucchi S, Rossetti A, Clerici M. Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio and platelet-lymphocyte ratio in non-affective psychosis: a meta-analysis and systematic review. World J Biol Psychiatry. 2020;21(5):326–38.
Nikkilä HV, Müller K, Ahokas A, Rimón R, Andersson LC. Increased frequency of activated lymphocytes in the cerebrospinal fluid of patients with acute schizophrenia. Schizophr Res. 2001;49(1–2):99–105.
Drexhage RC, Hoogenboezem TA, Cohen D, Versnel MA, Nolen WA, van Beveren NJ, Drexhage HA. An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int J Neuropsychopharmacol. 2011;14(6):746–55.
Myles N, Myles H, Xia S, Large M, Bird R, Galletly C, Kisely S, Siskind D. A meta-analysis of controlled studies comparing the association between clozapine and other antipsychotic medications and the development of neutropenia. Aust N Z J Psychiatry. 2019;53(5):403–12.
Cohen D, Monden M. White blood cell monitoring during long-term clozapine treatment. Am J Psychiatry. 2013;170(4):366–9.
Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–63.
Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, Hartwig FP, Holmes MV, Minelli C, Relton CL, et al. Guidelines for performing mendelian randomization investigations. Wellcome Open Res. 2019;4:186.
Williams JA, Burgess S, Suckling J, Lalousis PA, Batool F, Griffiths SL, Palmer E, Karwath A, Barsky A, Gkoutos GV, et al. Inflammation and brain structure in Schizophrenia and other Neuropsychiatric Disorders: a mendelian randomization study. JAMA Psychiatry. 2022;79(5):498–507.
Jones HJ, Borges MC, Carnegie R, Mongan D, Rogers PJ, Lewis SJ, Thompson AD, Zammit S. Associations between plasma fatty acid concentrations and schizophrenia: a two-sample mendelian randomisation study. Lancet Psychiatry. 2021;8(12):1062–70.
Ma J, Jin C, Yang Y, Li H, Wang Y. Association of daytime napping frequency and schizophrenia: a bidirectional two-sample mendelian randomization study. BMC Psychiatry. 2022;22(1):786.
Hartwig FP, Borges MC, Horta BL, Bowden J, Davey Smith G. Inflammatory biomarkers and risk of Schizophrenia: a 2-Sample mendelian randomization study. JAMA Psychiatry. 2017;74(12):1226–33.
Astle WJ, Elding H, Jiang T, Allen D, Ruklisa D, Mann AL, Mead D, Bouman H, Riveros-Mckay F, Kostadima MA, et al. The allelic Landscape of Human Blood Cell Trait Variation and Links to Common Complex Disease. Cell. 2016;167(5):1415–1429e19.
Perry BI, Upthegrove R, Kappelmann N, Jones PB, Burgess S, Khandaker GM. Associations of immunological proteins/traits with schizophrenia, major depression and bipolar disorder: a bi-directional two-sample mendelian randomization study. Brain Behav Immun. 2021;97:176–85.
Emdin CA, Khera AV, Kathiresan S, Mendelian Randomization. JAMA. 2017;318(19):1925–6.
Vuckovic D, Bao EL, Akbari P, Lareau CA, Mousas A, Jiang T, Chen MH, Raffield LM, Tardaguila M, Huffman JE, et al. The polygenic and monogenic basis of Blood Traits and Diseases. Cell. 2020;182(5):1214–1231e11.
Burgess S, Thompson SG. Avoiding bias from weak instruments in mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–64.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Korthauer K, Kimes PK, Duvallet C, Reyes A, Subramanian A, Teng M, Shukla C, Alm EJ, Hicks SC. A practical guide to methods controlling false discoveries in computational biology. Genome Biol. 2019;20(1):118.
Jackson AJ, Miller BJ. Meta-analysis of total and differential white blood cell counts in schizophrenia. Acta Psychiatr Scand. 2020;142(1):18–26.
Yan J, Chen Y, Ju P, Gao J, Zhang L, Li J, Wang K, Zhang J, Li C, Xia Q, et al. Network Association of biochemical and inflammatory abnormalities with Psychiatric symptoms in First-Episode Schizophrenia Patients. Front Psychiatry. 2022;13:834539.
Sneller MH, de Boer N, Everaars S, Schuurmans M, Guloksuz S, Cahn W, Luykx JJ. Clinical, biochemical and genetic variables Associated with metabolic syndrome in patients with Schizophrenia Spectrum Disorders using second-generation antipsychotics: a systematic review. Front Psychiatry. 2021;12:625935.
Miller BJ, Kandhal P, Rapaport MH, Mellor A, Buckley P. Total and differential white blood cell counts, high-sensitivity C-reactive protein, and cardiovascular risk in non-affective psychoses. Brain Behav Immun. 2015;45:28–35.
Barzilay R, Lobel T, Krivoy A, Shlosberg D, Weizman A, Katz N. Elevated C-reactive protein levels in schizophrenia inpatients is associated with aggressive behavior. Eur Psychiatry. 2016;31:8–12.
Rosales C, Neutrophil. A cell with many roles in inflammation or several cell types? Front Physiol. 2018;9:113.
Núñez C, Stephan-Otto C, Usall J, Bioque M, Lobo A, González-Pinto A, Pina-Camacho L, Vieta E, Castro-Fornieles J, Rodriguez-Jimenez R, et al. Neutrophil Count is Associated with reduced Gray Matter and Enlarged ventricles in First-Episode Psychosis. Schizophr Bull. 2019;45(4):846–58.
Bhikram T, Sandor P. Neutrophil-lymphocyte ratios as inflammatory biomarkers in psychiatric patients. Brain Behav Immun. 2022;105:237–46.
Semiz M, Yildirim O, Canan F, Demir S, Hasbek E, Tuman TC, Kayka N, Tosun M. Elevated neutrophil/lymphocyte ratio in patients with schizophrenia. Psychiatr Danub. 2014;26(3):220–5.
Schlaaff K, Dobrowolny H, Frodl T, Mawrin C, Gos T, Steiner J, Bogerts B. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497–506.
Memic-Serdarevic A, Burnazovic-Ristic L, Sulejmanpasic G, Tahirovic A, Valjevac A, Lazovic E. Review of Standard Laboratory Blood Parameters in patients with Schizophrenia and Bipolar Disorder. Med Arch. 2020;74(5):374–80.
Yuan N, Chen Y, Xia Y, Dai J, Liu C. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl Psychiatry. 2019;9(1):233.
Zozulya SA, Golubev SA, Tikhonov DV, Kaleda VG, Klyushnik TP. Immunological and clinical aspects of the long-term stages of youth schizophrenia. Zh Nevrol Psikhiatr Im S S Korsakova. 2022;122(1–2):5–12.
Cox SS, Speaker KJ, Beninson LA, Craig WC, Paton MM, Fleshner M. Adrenergic and glucocorticoid modulation of the sterile inflammatory response. Brain Behav Immun. 2014;36:183–92.
Perry BI, Burgess S, Jones HJ, Zammit S, Upthegrove R, Mason AM, Day FR, Langenberg C, Wareham NJ, Jones PB, et al. The potential shared role of inflammation in insulin resistance and schizophrenia: a bidirectional two-sample mendelian randomization study. PLoS Med. 2021;18(3):e1003455.
Lin BD, Alkema A, Peters T, Zinkstok J, Libuda L, Hebebrand J, Antel J, Hinney A, Cahn W, Adan R, et al. Assessing causal links between metabolic traits, inflammation and schizophrenia: a univariable and multivariable, bidirectional mendelian-randomization study. Int J Epidemiol. 2019;48(5):1505–14.
Osimo EF, Perry BI, Cardinal RN, Lynall ME, Lewis J, Kudchadkar A, Murray GK, Perez J, Jones PB, Khandaker GM. Inflammatory and cardiometabolic markers at presentation with first episode psychosis and long-term clinical outcomes: a longitudinal study using electronic health records. Brain Behav Immun. 2021;91:117–27.
Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5(5):405–14.
Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M, Lundberg K, Postolache TT, Träskman-Bendz L, Guillemin GJ, et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology. 2013;38(5):743–52.
Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–41.
Sim S, Hughes K, Chen X, Wolin SL. The bacterial Ro60 protein and its noncoding Y RNA regulators. Annu Rev Microbiol. 2020;74:387–407.
Hall AE, Turnbull C, Dalmay T. Y RNAs: recent developments. BioMol Concepts. 2013;4(2):103–10.
Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–52.
Acknowledgements
The authors would like to thank participants from the UKB and MRC-IEU UK Biobank for sharing genetic association summary-level data used in this study. And we also thank all investigators contributing to these GWAS summary statistics publicly available.
Funding
This research was supported by the National Natural Science Foundation of China (No. 82073644).
Author information
Authors and Affiliations
Contributions
Changgui Kou designed the study. Zibo Gao contributed to the data analysis and wrote the first draft. Biao Li contributed to the data analysis. Xinru Guo and Wei Bai contributed to the critical revision of the manuscript. All authors read and approved the final draft of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All analyses were based on summary-level GWAS statistics, which can be freely used without restriction, and all subjects are de-identified. Participants of the original GWAS gave informed consent as per the procedures reported in the relevant study papers. Each method was carried out in accordance with relevant guidelines and regulations. And all experimental protocols were approved by relevant licensing committees. The study of PGC received approval from more than 35 relevant licensing committees, which include the regional Ethical Review Board at the University of Umea, the Norwegian Scientific-Ethical Committee, the Scotland A Research Ethics Committee, and so on. And the blood cells count GWAS was approved by the UKB Committee. Ethical approval can be obtained from all original studies.
Consent for publication
Not applicable.
Competing interests
There are no competing interests to declare.
All authors have read and agreed with the submission of the manuscript. This manuscript has not been published or presented elsewhere in part or entirety. This manuscript was not pre-registered.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gao, Z., Li, B., Guo, X. et al. The association between schizophrenia and white blood cells count: a bidirectional two-sample Mendelian randomization study. BMC Psychiatry 23, 271 (2023). https://doi.org/10.1186/s12888-023-04760-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-023-04760-6