Abstract
Background
The bidirectional causal association between daytime napping frequency and schizophrenia is unclear.
Methods
A bidirectional two-sample Mendelian randomization (MR) analysis was conducted with summary statistics of top genetic variants associated with daytime napping frequency and schizophrenia from genome-wide association studies (GWAS). The single nucleotide polymorphisms (SNPs) data of daytime napping frequency GWAS came from the UK Biobank (n = 452,633) and 23andMe study cohort (n = 541,333), while the schizophrenia GWAS came from the Psychiatric Genomics Consortium (PGC, 36,989 cases and 113,075 controls). The inverse variance weighted (IVW) analysis was the primary method, with the weighted median, MR-Robust Adjusted Profile Score (RAPS), Radial MR and MR-Pleiotropy Residual Sum Outlier (PRESSO) as sensitivity analysis.
Results
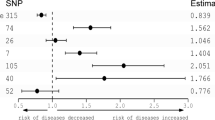
The MR analysis showed a bidirectional causal relationship between more frequent daytime napping and the occurrence of schizophrenia, with the odds ratio (OR) for one-unit increase in napping category (never, sometimes, usually) on schizophrenia was 3.38 (95% confidence interval [CI]: 2.02–5.65, P = 3.58 × 10–6), and the beta for the occurrence of schizophrenia on daytime napping frequency was 0.0112 (95%CI: 0.0060–0.0163, P = 2.04 × 10–5). The sensitivity analysis obtained the same conclusions.
Conclusion
Our findings support the bidirectional causal association between more daytime napping frequency and schizophrenia, implying that daytime napping frequency is a potential intervention for the progression and treatment of schizophrenia.
Similar content being viewed by others
Background
Schizophrenia is a complex and debilitating psychiatric disorder [1] that has been regarded as one of the world's most severe and disabling illnesses [2], with symptoms typically appearing in late adolescence or early adulthood [3]. Approximately 21 million people worldwide suffer from schizophrenia [3]. The lifetime prevalence of schizophrenia is roughly 1% [4], with the most detailed prevalence study conducted in Finland yielding a result of 0.87%, but the variation is up to five times higher around the world [5]. Schizophrenia is characterized by "positive symptoms" of hallucinations, delusions, and verbal confusion, "negative symptoms" of decreased motivation and expressivity, and cognitive deficits involving impaired executive functioning, memory, and mental processing speed [6].
Daytime napping is a cross-cultural phenomenon that occurs throughout human life [7], particularly common in countries with Mediterranean cultures and some non-Mediterranean countries, such as the USA [8]. Daytime napping can reduce fatigue [9], enhance emotional processing[9], facilitate the formation of long-term memory [10], and improve subjective and behavioral measures, as well as mood and subjective sleepiness levels [7], which appears to be a beneficial intervention to facilitate the recovery and mitigate the negative physical and cognitive effects of sleep deprivation [11]. Daytime napping of about 40 min can improve declarative memory performance in patients with schizophrenia [12].
However, frequent napping was associated with a range of adverse outcomes, including hypertension [13], vascular disease [14], depression disorder [15], and diabetes [16], especially cognitive decline in psychiatric disorders[9]. A longer daytime nap was typically associated with more significant cognitive decline and a higher risk of cognitive impairment in older men [17]. Sleep has been identified as a process of re-integrating information in the brain, and cognitive dysfunction is a common feature in patients with schizophrenia [12]. The study by Michael Wainberg et al., showed a significant increase in daytime napping frequency among schizophrenia patients [18], but the bidirectional causality between the more daytime napping frequency and schizophrenia is unclear. The bidirectional causality between more frequent daytime napping and schizophrenia could be biased due to possible confounding factors, which are difficult to confirm by observational epidemiological methods, such as sleep duration, insomnia, daytime sleepiness and wake-up time.
Mendelian randomization (MR) is a statistical approach that uses genetic variation, i.e., single nucleotide polymorphisms (SNPs), as instrumental variables (IVs) to control confounding factors [19]. MR relies on the random assortment of genetic variation during meiosis, leading to a random distribution of genetic variation in the population [20]. MR can overcome the influence of confounding factors and has a superior advantage in the face of causal inference of exposure-outcome due to the genotype being determined before birth [21]. MR uses summary statistics from genome-wide association studies (GWAS) of large amounts of genetic variation to extract SNPs associated with exposure and outcome variables, which can further reveal clear causal relationships between the exposure and outcome [22]. Therefore, this study aims to infer the bidirectional causality between more frequent daytime napping and schizophrenia by using two-sample MR to provide more reliable evidence.
Methods
Study design
The MR approach is based on three main assumptions (Fig. 1). Firstly, genetic variants used as IVs are strongly associated with exposure (Assumption 1). Secondly, genetic variants are independent of confounding factors (Assumption 2). Thirdly, horizontal pleiotropy should be avoided (Assumption 3). Horizontal pleiotropy means that genetic variation affects multiple traits through independent pathways, it occurs when the genetic variant influences the outcome outside of its effect on the exposure in Mendelian randomization [22].
Genetic associations with daytime napping frequency
The daytime napping frequency summary statistics came from the GWAS study of the UK Biobank (n = 452, 633) and was replicated and validated in the 23andMe cohort (n = 541,333) [23]. The UK Biobank is a population-based cohort that recruited 500,000 40–69 years old participants during 2006–2010 and obtained a wide range of phenotypes and health information, such as biometric measurements, biomarkers in blood and urine, and lifestyle indicators [24]. In this study, a total of 123 SNPs reached genome-wide significance (P < 5 × 10–8) and explained 1.1% of the variance in daytime napping frequency. 23andMe is a direct-to-consumer genetic testing company. These 123 SNPs were replicated and validated in the 23andMe study cohort (n = 541,333), of which 94 SNPs were still present in the 23andMe cohort. For the genetic variant replication of daytime napping frequency in the 23andMe cohort, participants were asked the question "How many days per week do you take a daytime nap? (15 min or more)” and chose one from the following four options: "never/rarely (0 or 1)", "sometimes (2 to 5)", "usually (6 or 7)", "Tend not to answer". Since the population of the subsequent GWAS study on schizophrenia may partially overlap with that of the UK Biobank study, the 94 SNPs that were replicated and validated in the 23andMe study cohort were used as IVs in this study for subsequent analyses to avoid the bias of winner's curse caused by the overlapping samples [25].
Genetic associations with schizophrenia
The schizophrenia summary statistics came from a GWAS study by the Psychiatric Genomics Consortium (PGC) that constructed the genome-wide genotype data of 49 ancestry-matched, non-overlapping case–control samples (34,241 cases and 45,604 controls) and three family-based European ancestry samples (1,235 parent-affected-offspring trios) [26]. The GWAS study identified 128 independent SNPs associated with schizophrenia, of which 108 met genome-wide significance(P < 5 × 10–8). The summary statistics is available in the IEU OpenGWAS project database (https://gwas.mrcieu.ac.uk/) with the GWAS ID "ieu-b-42".
Instrumental variables selection
We extracted the SNPs that met genome-wide significance from the GWAS summary statistics of daytime napping frequency and schizophrenia, respectively. To ensure the reliability of the findings, we performed the PLINK clumping method with a stringent clumping threshold (r2 < 0.001, kb = 10,000) to ensure that SNPs in residual linkage-disequilibrium (LD) within a particular window were pruned to assess the bias caused by residual LD of genetic variants. We calculated the F-statistic for IVs, and an F > 10 means sufficient for MR analysis in general [22]. In addition, we selected appropriate proxy SNPs (r2 > 0.8) when exposure-associated SNPs were not present in the outcome summary statistics and removed the SNPs with palindromic structure during the analysis.
MR analyses
WE applied the inverse-variance weighted (IVW) method as the primary MR analysis to estimate the bidirectional causal relationship between daytime napping frequency and schizophrenia. To assess the robustness of the IVW analysis results, we performed additional tests for horizontal pleiotropy to detect heterogeneous outcomes, including the Cochran’s Q statistic test and the MR Egger intercept test. We also performed (i) the weighted median method, which allows SNPs with the more significant beta to contribute more to the estimate and can be derived by weighting the contribution of each SNP by the inverse variance [27]; (ii) MR robust adjusted profile score (MR-RAPS), which can give a robust inference for MR analysis with weak instrumental variables, especially when both exposure and outcome are complex traits [28]; (iii) MR Pleiotropy Residual Sum and Outlier (MR-PRESSO) test, which detects and corrects for horizontal pleiotropic outliers [29] and (iv) Radial MR, which improves detection of outliers in IVW and MR-Egger analysis by radial plots [30] as sensitivity analyses afterward.
The bidirectional two-sample MR analysis, horizontal pleiotropy tests, and sensitivity analyses were performed with the TwoSampleMR package (version 0.5.6), mr. raps (version 0.2), Radial MR (version 1.0), and MR-PRESSO (version 1.0) in R program (R Foundation for Statistical Computing, version 4.1.2).
Results
Instrumental variables selection and mendelian randomization analysis
The flow chart for the selection of IVs and MR analysis is shown in Fig. 2. We extracted 94 and 108 SNPs as the initial IVs from the GWAS summary statistics of daytime napping frequency and schizophrenia, respectively. A total of 64 and 77 SNPs associated with daytime napping frequency and schizophrenia were included in the follow-up MR analysis after PLINK clumping progress, selection of appropriate proxy SNPs and exclusion of SNPs with palindromic structure, respectively. The Cochran’s Q statistic test suggested significant heterogeneity, and MR Egger intercept test suggested no significant horizontal pleiotropy (Table 1 and Table 2). After the detection of outliers by Radial MR, we performed MR analysis again with 47 and 51 SNPs (Supplementary Table S1 and Table S2) associated with daytime napping frequency and schizophrenia as the final IVs, respectively.
Causality of daytime napping frequency and schizophrenia
The F-statistic for all IVs associated with daytime napping frequency and schizophrenia were > 10, which effectively avoided weak IVs bias. The IVW method showed a bidirectional causal relationship between daytime napping frequency and the occurrence of schizophrenia (Table 1 and Table2), with the odds ratio (OR) for one-unit increase in napping category (never, sometimes, usually) on schizophrenia was 3.48 (95% confidence interval [CI]: 1.54–7.85, P = 2.65 × 10–3; without outliers: OR = 3.38, 95% CI: 2.02–5.65, P = 3.58 × 10–6) and the beta for the occurrence of schizophrenia on daytime napping frequency was 0.0095 (95%CI: 0.0012–0.0178, P = 2.50 × 10–2; without outliers: beta = 0.0112, 95%CI: 0.0060–0.0163, P = 2.04 × 10–5). The weighted median, MR-RAPS, Radial MR, MR-PRESSO methods obtained consistent conclusions (Tables 1 and 2).
Discussion
Schizophrenia is frequently regarded as one of the most serious mental illnesses[2], with patients having a life expectancy approximately 15 years shorter than the general population and a 5% to 10% lifetime risk of suicide[4]. Schizophrenia is one of the most disabling of all disorders in both developing and developed countries and is associated with reduced social connectedness, lower employment rates, and impaired independent living[1]. Therefore, the development of effective strategies to prevent schizophrenia and reduce or avoid risk factors for schizophrenia is important to improve population health. We validated IVs from the GWAS study and performed the MR method to strengthen the bidirectional causal inference between the daytime napping frequency and schizophrenia. Results suggested a potential bidirectional causal association between more frequent daytime napping and the occurrence of schizophrenia.
Schizophrenia is accompanied by sleep abnormalities and is associated with more serious psychotic symptoms and worse clinical outcomes [31]. Sleep abnormalities in patients with schizophrenia include insomnia, nightmares, and poor sleep quality, etc. and can lead to exacerbation of psychiatric symptoms, such as hallucinations, delusions, and cognitive impairment [32]. Recent studies suggested that frequent napping was linked with multiple adverse outcomes, including depression [33], increased mortality [9] and cognitive decline [33], implying the directionality of daytime napping and adverse outcomes is important. In a study of the sleep habits of non-hospitalized middle-aged men and women with schizophrenia, it was demonstrated that daytime napping was more frequent in schizophrenia patients compared to healthy controls, and reflected a circadian rhythm disturbance in patients with schizophrenia [34]. Studies indicated that schizophrenia patients have defects in the sleep spindle, while daytime napping can efficiently estimate nocturnal sleep spindle density in schizophrenia patients [35, 36]. Our findings can provide a basis for daytime napping in the intervention and treatment of schizophrenia.
The MR approach can effectively reduce the bias due to confounding factors and unknown reverse causal associations, which were difficult to eliminate in traditional observational studies. Sensitivity analysis also provided consistent results, increasing the robustness and credibility of the conclusions. The current study still has limitations. Firstly, even though the MR method was able to avoid confounder influences and control horizontal pleiotropy through multiple sensitivity analyses, there are still potential and unknown confounding factors and horizontal pleiotropy that could affect the study's results. Secondly, the more frequent daytime napping was a binary exposure, so we were not able to precisely calculate the GWAS data on frequent napping to specific hours or minutes. Thirdly, our study may not be effectively extended to other populations as the exposure GWAS and outcome GWAS were selected from European populations and cannot be extended to Asian populations or other populations at this time. Fourthly, participants in the UK Biobank and 23andMe cohorts were healthier, so our conclusions cannot be generalized to patients with other diseases for now. Finally, there are currently few independent randomized controlled trials on more frequent daytime napping and schizophrenia; the results need to be validated in future clinical trials.
Conclusion
The findings of this bidirectional two-sample Mendelian randomization study suggested a bidirectional causal association between more frequent daytime napping the occurrence of schizophrenia, implying that daytime napping frequency is a potential intervention for the progression and treatment of schizophrenia.
Availability of data and materials
The datasets generated and analyzed during the current study are included in Genetic determinants of daytime napping and effects on cardiometabolic health [including its supplementary information files, https://doi.org/10.1038/s41467-020-20585-3], and are available in the IEU OpenGWAS project database [https://gwas.mrcieu.ac.uk/] and Psychiatric Genomics Consortium [https://www.med.unc.edu/pgc/].
Abbreviations
- MR:
-
Mendelian randomization
- GWAS:
-
Genome-wide association studies
- SNP:
-
Single nucleotide polymorphism
- IV:
-
Instrumental variable
- PGC:
-
Psychiatric genomics consortium
- LD:
-
Linkage-disequilibrium
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- IVW:
-
Inverse variance weighted
- MR-RAPS:
-
Mendelian randomization robust adjusted profile score
- MR-PRESSO:
-
Mendelian randomization pleiotropy residual sum and outlier
References
Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. 2015;16(10):620–31.
Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol. 2014;10:425–48.
Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, McGrath JJ, Whiteford HA. Global Epidemiology and Burden of Schizophrenia: Findings From the Global Burden of Disease Study 2016. Schizophr Bull. 2018;44(6):1195–203.
McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-An Overview. JAMA Psychiat. 2020;77(2):201–10.
Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, O’Donovan M, Correll CU, Kane JM, van Os J, et al. Schizophrenia Nat Rev Dis Primers. 2015;1:15067.
Marder SR, Cannon TD. Schizophrenia. N Engl J Med. 2019;381(18):1753–61.
Milner CE, Cote KA. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res. 2009;18(2):272–81.
Gradisar M, Wolfson AR, Harvey AG, Hale L, Rosenberg R, Czeisler CA. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9(12):1291–9.
Mantua J, Spencer RMC. Exploring the nap paradox: are mid-day sleep bouts a friend or foe? Sleep Med. 2017;37:88–97.
McDevitt EA, Sattari N, Duggan KA, Cellini N, Whitehurst LN, Perera C, Reihanabad N, Granados S, Hernandez L, Mednick SC. The impact of frequent napping and nap practice on sleep-dependent memory in humans. Sci Rep. 2018;8(1):15053.
Souabni M, Hammouda O, Romdhani M, Trabelsi K, Ammar A, Driss T. Benefits of Daytime Napping Opportunity on Physical and Cognitive Performances in Physically Active Participants: A Systematic Review. Sports Med 2021;51(10):2115-46.
Seeck-Hirschner M, Baier PC, Sever S, Buschbacher A, Aldenhoff JB, Göder R. Effects of daytime naps on procedural and declarative memory in patients with schizophrenia. J Psychiatr Res. 2010;44(1):42–7.
Cao Z, Shen L, Wu J, Yang H, Fang W, Chen W, Yuan J, Wang Y, Liang Y, Wu T. The effects of midday nap duration on the risk of hypertension in a middle-aged and older Chinese population: a preliminary evidence from the Tongji-Dongfeng Cohort Study China. J Hypertens. 2014;32(10):1993–8 (discussion 1998).
Zonoozi S, Ramsay SE, Papacosta O, Lennon L, Ellins EA, Halcox JPJ, Whincup PH, Goya Wannamethee S. Self-reported sleep duration and napping, cardiac risk factors and markers of subclinical vascular disease: cross-sectional study in older men. BMJ Open. 2017;7(6):e016396.
Liu Y, Peng T, Zhang S, Tang K. The relationship between depression, daytime napping, daytime dysfunction, and snoring in 0.5 million Chinese populations: exploring the effects of socio-economic status and age. BMC Public Health. 2018;18(1):759.
Hublin C, Lehtovirta M, Partinen M, Koskenvuo M, Kaprio J. Napping and the risk of type 2 diabetes: a population-based prospective study. Sleep Med. 2016;17:144–8.
Leng Y, Redline S, Stone KL, Ancoli-Israel S, Yaffe K. Objective napping, cognitive decline, and risk of cognitive impairment in older men. Alzheimers Dement. 2019;15(8):1039–47.
Wainberg M, Jones SE, Beaupre LM, Hill SL, Felsky D, Rivas MA, Lim ASP, Ollila HM, Tripathy SJ. Association of accelerometer-derived sleep measures with lifetime psychiatric diagnoses: A cross-sectional study of 89,205 participants from the UK Biobank. PLoS Med. 2021;18(10):e1003782.
Morrison J, Knoblauch N, Marcus JH, Stephens M, He X. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat Genet. 2020;52(7):740–7.
Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA. 2017;318(19):1925–6.
Grover S, Del Greco MF, Stein CM, Ziegler A. Mendelian Randomization. Methods Mol Biol. 2017;1666:581–628.
Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J Am Soc Nephrol. 2016;27(11):3253–65.
Dashti HS, Daghlas I, Lane JM, Huang Y, Udler MS, Wang H, Ollila HM, Jones SE, Kim J, Wood AR, et al. Genetic determinants of daytime napping and effects on cardiometabolic health. Nat Commun. 2021;12(1):900.
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–9.
Shi J, Park JH, Duan J, Berndt ST, Moy W, Yu K, Song L, Wheeler W, Hua X, Silverman D, et al. Winner’s Curse Correction and Variable Thresholding Improve Performance of Polygenic Risk Modeling Based on Genome-Wide Association Study Summary-Level Data. PLoS Genet. 2016;12(12):e1006493.
Ripke S, Neale BM, Corvin A, Walters JTR, Farh KH, Holmans PA, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–27.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408.
Wu F, Huang Y, Hu J, Shao Z. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med. 2020;18(1):312.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Bowden J, Spiller W, Del Greco MF, Sheehan N, Thompson J, Minelli C, Davey Smith G. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int J Epidemiol. 2018;47(4):1264–78.
Kaskie RE, Ferrarelli F. Sleep disturbances in schizophrenia: what we know, what still needs to be done. Curr Opin Psychol. 2020;34:68–71.
Ferrarelli F. Sleep Abnormalities in Schizophrenia: State of the Art and Next Steps. Am J Psychiatry. 2021;178(10):903–13.
Cross N, Terpening Z, Rogers NL, Duffy SL, Hickie IB, Lewis SJ, Naismith SL. Napping in older people “at risk” of dementia: relationships with depression, cognition, medical burden and sleep quality. J Sleep Res. 2015;24(5):494–502.
Poulin J, Chouinard S, Pampoulova T, Lecomte Y, Stip E, Godbout R. Sleep habits in middle-aged, non-hospitalized men and women with schizophrenia: a comparison with healthy controls. Psychiatry Res. 2010;179(3):274–8.
Manoach DS, Stickgold R. Abnormal Sleep Spindles, Memory Consolidation, and Schizophrenia. Annu Rev Clin Psychol. 2019;15:451–79.
Mylonas D, Tocci C, Coon WG, Baran B, Kohnke EJ, Zhu L, Vangel MG, Stickgold R, Manoach DS. Naps reliably estimate nocturnal sleep spindle density in health and schizophrenia. J Sleep Res. 2020;29(5):e12968.
Acknowledgements
Special thanks to Dr. Hassan S. Dashti et al. for their contributions to the raw data and analyses, to the IEU OpenGWAS project database [https://gwas.mrcieu.ac.uk/] , and to the Psychiatric Genomics Consortium [https://www.med.unc.edu/pgc/] for providing the summarized data.
Funding
Not appliable.
Author information
Authors and Affiliations
Contributions
JM and YW conceptualized and designed the study, JM was a major contributor in writing the manuscript, CJ, YY and LH collected the data and performed the analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the data sources used in this study are summarized data from relevant GWAS studies and the IEU OpenGWAS project database, which are publicly available for free download and do not require approval from the review agency.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1 and Table S2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, J., Jin, C., Yang, Y. et al. Association of daytime napping frequency and schizophrenia: a bidirectional two-sample Mendelian randomization study. BMC Psychiatry 22, 786 (2022). https://doi.org/10.1186/s12888-022-04431-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-022-04431-y