Abstract
Background
Resistance to multiple antibiotics by several pathogens has been widely described in children and has become a global health emergency. This is due to increased use by parents, caregivers, and healthcare providers. This study aims to describe the prevalence rates of antibiotic prescribing, ascertain the impact of antimicrobial stewardship programs, and target improving the quality of antibiotic prescribing in the paediatric population over time in a hospital.
Method
A point prevalence survey of antibiotic use was performed yearly for 4 years to monitor trends in antibiotic prescribing. Data from all patients admitted before 8 a.m. on the day of the PPS were included. A web-based application designed by the University of Antwerp was used for data entry, validation, and analysis (http://www.global-pps.com).
Results
A total of 260 children, including 90 (34.6%) neonates and 170 (65.4%) older children, were admitted during the four surveys. Overall, 179 (68.8%) patients received at least one antibiotic. In neonates, the prevalence of antibiotic use increased from 78.9 to 89.5% but decreased from 100 to 58.8% in older children. There was a reduction in the use of antibiotics for prophylaxis from 45.7 to 24.6%. The most frequently prescribed antibiotic groups were third generation cephalosporins and aminoglycosides. The most common indications for antibiotic prescription were sepsis in neonates and central nervous system infection in older children. The documentation of reason in notes increased from 33 to 100%, while the stop-review date also increased from 19.4 to 70%.
Conclusion
The indicators for appropriate antibiotic prescription improved over time with the introduction of antibiotic stewardship program in the department.
Similar content being viewed by others
Introduction
The inappropriate use of antibiotics has been a source of concern globally, with increased use of last-resort antibiotics recorded globally between 2005 and 2015 [1,2,3,4,5]. The overuse and inappropriate prescription of antibiotics have led to antibiotic resistance in almost all groups of antibiotics [4,5,6]. This is worrisome, as the rate of development of antibiotic resistance is more rapid than the rate of discovery of new antibiotics to treat resistant infections [7]. The burden of antimicrobial resistance (AMR) is now a global issue and emerging threat; leading to an overwhelming increase in economic costs for patients, hospitals, and countries as well as increased mortality, especially in low- and middle-income countries [8, 9]. Antimicrobial resistance may not be eliminated; however, it can be reduced [10].
Resistance to multiple antibiotics, including carbapenems, by various organisms, has been documented in children, and these drug-resistant strains are the cause of morbidity, prolonged hospital stays, and mortality [11,12,13,14,15,16,17,18,19]. Studies have demonstrated patients, caregivers and prescribers are responsible for the misuse of antibiotics in children [6, 20,21,22,23,24,25]. It is therefore imperative to monitor and control antibiotic prescribing to ensure judicious use, prevent resistance, and reduce the cost of healthcare. Strategies to achieve this goal include the development of national antibiotic policies, the formation of antimicrobial stewardship (AMS) committees at hospitals and departmental levels, and adherence to national antibiotic guidelines.
Point prevalence surveys (PPS) have been used to monitor antibiotic use; in addition, they help to evaluate, monitor, and improve the AMS program [26, 27]. Globally, PPS conducted in 41 countries showed high rates of antibiotic prophylaxis, with two-thirds being used for medical prophylaxis [28], while a 15-year PPS in a paediatric hospital in Sweden showed a fourfold increase in total antibiotic prescriptions for prophylactic purposes without an increase in the number of patients [29]. In the United States of America, one in three children is receiving suboptimal therapy, with 50% not being captured in stewardship programs [30].
Point prevalence surveys of antibiotic use in Nigeria have shown a high rate of antibiotic prescribing (> 80%) in children, with more than 90% of antibiotics given intravenously [31,32,33,34]. These PPS were either performed in all age groups or over a short period. However, this study focuses specifically on the paediatric population, over four different periods[35,36].
The first PPS performed in our hospital showed poor prescribing habits, as demonstrated by the low percentage of indicators tested. An antimicrobial stewardship program was started in the hospital; subsequently, three additional PPS were carried out. This study aims to describe the patterns of antibiotic use among the paediatric population in a tertiary hospital in Lagos, Nigeria, over a period of 4 years. The objectives were as follows:
-
1.
To describe the prevalence rates and indications for antibiotics prescription for paediatric patients.
-
2.
To describe the effect of an antibiotic stewardship program on antibiotic prescribing practices in children over a 4-year period and identify areas for improvement.
Materials and methods
Study design and setting
This was a serial cross-sectional study conducted over 4 years (2015, 2017, 2018, and 2019 in the Paediatrics Department of the Lagos University Teaching Hospital located in Lagos, Southwest Nigeria. The state is estimated to have a population of more than 20 million people, with more than 50% within the paediatric age group and estimated to have 5 million under-fives [37]. It is a coastal city with a major seaport, metropolitan and cosmopolitan area, consisting of residents from Nigeria and other neighbouring African countries. The hospital is a foremost tertiary centre that is a referral centre for secondary health facilities within and for neighbouring states. There are several secondary health centres in Lagos with 3 tertiary centres providing care for children; however, the study site caters to most of the referrals because it has the largest capacity for the care of children, with a bed capacity of 160, comprising two neonatal wards, three paediatric medical wards, and an emergency room. The neonatal unit has both the inborn (for the neonates delivered in the hospital) and out-born (neonates brought from outside the hospital) wards. Children from birth to 17 years of age are seen and managed. The hospital participated in the global PPS in 2015 and subsequently in 2017, 2018, and 2019, with the paediatrics department involved. There was no global PPS conducted in 2016.
As part of the AMS programme, which started in the hospital following the initial PPS, the Department of Paediatrics AMS team was formed in 2017. The team developed its antibiotic guidelines. These were circulated to all prescribers in the department for review. The final document was adopted after a presentation to the department. The process took over a year, and the guidelines were circulated in late 2018. In addition, an antibiotic policy document was developed by the hospital AMS committee and circulated to all departments. All prescribers were expected to adhere strictly to this protocol and guideline. The Department of Paediatrics also chose prospective audit, with intervention and feedback as an AMS strategy [38], for the department.
Study population
On a predetermined day for the global PPS, all records of paediatric patients admitted before 8 am were reviewed, and relevant information was extracted. All patients admitted to the paediatric wards who were receiving antibiotic (both oral and parenteral) medications were surveyed.
Subsequent PPS was performed to monitor trends in antibiotic prescribing, and in all the surveys, data for all patients admitted before 8 a.m. on the day of the PPS were included. The survey was conducted in all the paediatric wards over 2 weeks in the last quarter of each year. On each survey day, the PPS will be completed in at least one ward. The study population included patients aged 0–17 years and included neonates and older children.
Data collection and analysis
The data were collected from patients’ folders using the global PPS data collection form. The clinical diagnosis was also categorized based on the global PPS. The following data were obtained from the case notes: ward name, patient identifier survey number, age, sex, antibiotic name (generic), indication for antibiotic, dose, duration of the antibiotics given, clinical diagnosis (using diagnostic code), and microbiological data. Community-acquired infection (CAI), was defined as an infection acquired in the community if the patient had not recently been in a healthcare facility or been in contact with someone, who had been recently in a healthcare facility. Hospital care-associated infection, (HAI) was defined as, a fever that started 48 h after hospital admission (1–6). Antibiotic quality indicators such as compliance with antibiotic guidelines, documentation of reasons for antibiotic prescription, route of administration, and stop and review date of prescription were also collected by trained data personnel using a standard PPS form [39]. The initial classification was based on the Anatomic therapeutic chemical classification system (ATC) based on a generic name and anatomic site of function [40]. A web-based application designed by the University of Antwerp was used for data entry, validation, and analysis http://www.global-pps.com.
Data analysis
The data were entered into Microsoft Excel (2017) and analysed using SPSS version 20.0 The data were presented as percentages or proportions. The antibiotics prescribed were further analysed and classified as ‘Access’, ‘Watch’, or ‘Reserve’ using the WHO AWaRe classification list [41, 42]. Compliance with the guidelines and hospital antibiotic policy was also assessed. The chi-square test for trend was used to assess differences in the prevalence of antibiotic prescription and differences in quality indicators across the four PPS. The level of statistical significance was set at p < 0.05.
Results
Hospital and patient characteristics
Two hundred and sixty children, including 90 (34.6%) neonates and 170 (65.4%) older children, were admitted during the four surveys in 2015, 2017, 2018, and 2019. The number of inpatients admitted increased from 39 in 2015 to 49 in 2017, 85 in 2018, and 87 in 2019.
Prevalence of antibiotic use
Overall, 179 (68.8%) patients received at least one antibiotic on the day of the survey. A total of 72.2% (65/90) of the patients were neonates, and 67.1% (114/170) were older. Table 1 provides the general patient characteristics and antibiotic use for the survey periods. The prevalence of antibiotic use generally decreased from 89.7% in 2015 to 65.5% in 2019 (p = 0.01). In neonates, the prevalence of antibiotic use decreased from 78.9% in 2015 to 36.8% in 2017 and then started to increase until 2019 (p = 0.001). In older children, the prevalence of antibiotic use significantly decreased from 100% in 2015 to 58.8% in 2019 (p = 0.004).
Most of the patients on antibiotics were prescribed more than one antibiotic, but there was no significant difference between 2015 and 2019 (Table 1). Most of the antibiotics were administered intravenously, with an average of 91.6%. There was no significant difference in the proportion of patients who received intravenous antibiotics over the 4 years. All the neonates were administered antibiotics intravenously.
Antibiotic use among the age groups
A total of 314 antibiotics were prescribed during the four survey periods, including 135 antibiotics for neonates and 179 antibiotics for older children. The five most common antibiotics prescribed for neonates were amikacin 51 (37.8%), cefotaxime 44 (32.6%), levofloxacin 15 (11.1%), meropenem 9 (6.7%) and metronidazole 7 (5.2%), while in older children, the five most common antibiotics prescribed were ceftriaxone 47 (26.3%), cefotaxime 26 (14.5%), amikacin 26 (14.5%), metronidazole 22 (12.3%) and cefuroxime 18 (10.1%).
Indications for antibiotic use
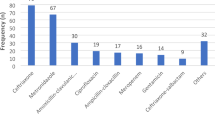
Among the 135 antibiotics prescribed for neonates over the 4 years, 42 (31.1%) were prescribed for community-acquired infections, 41 (30.4%) for healthcare-associated infections, and 52 (38.5%) for prophylaxis (Table 2). Among the older children, 90 (50.3%) were prescribed for community-acquired infections, 26 (14.5%) for healthcare-associated infections, 59 (33%) for prophylaxis, and 4 (2.2%) for unknown reasons (Table 2). In neonates, the top three antibiotics prescribed for CAI and HAI combined were amikacin (33.7%), cefotaxime (27.7%), and levofloxacin (15.7%), while for medical prophylaxis, the top three were amikacin (44.2%), cefotaxime (40.4%) and metronidazole (5.8%) (Fig. 1a and b). In older children, the top three antibiotics prescribed for CAI and HAI combined were ceftriaxone (19.8%), cefotaxime (15.5%), and amikacin (15.4%), while for surgical prophylaxis, the top three were ceftriaxone (41.2%), metronidazole (23.5%) and amikacin (11.8%) (Fig. 2a and b).
Based on the diagnosis of infections, in neonates, antibiotics were mainly prescribed for neonatal sepsis and medical prophylaxis, while in older children, antibiotics were mainly prescribed for sepsis, central nervous system infections, bone and joint infections, and urinary tract infections (Table 3).
Use of quinolones
A total of 24 prescriptions of quinolones were made over the 4 years, 15 (62.5%) in neonates. The most common indication for quinolone prescription in neonates was healthcare-associated infection (66.7%), but for older children, it was community-acquired infection (77.8%). The most common diagnosis for the prescription was sepsis in neonates. There was a high use of antibiotics in the watch group (Figs. 3 and 4).
Quality indicators
There was a general trend in favour of improvement in the quality indicators for paediatric and neonatal wards. The prevalence of antibiotic prescriptions increased from 36.4% in 2015 to 100% in 2019 (p < 0.0001), and the percentage of reviews increased from 19.7% in 2015 to 92.3% in 2018 and then decreased to 67.4% in 2019 (p < 0.0001) (Table 4). Compliance with guidelines decreased significantly from 97.1% in 2015 to 67.2% in 2019 (p < 0.0001). Surgical antibiotic prophylaxis was given for more than 24 h for all patients who underwent surgery. Targeted therapy increased significantly from 3% in 2015 to 14.1% in 2019, and the increase was mainly among neonates (p = 0.004) (Table 4).
Discussion
The study showed the overall prevalence of antibiotic use in children (both neonates and older children) was greater than that recommended; more so even greater than that reported in European countries [28, 43] however, it is similar to reports of other studies in Nigeria and Africa [32, 44] Therefore, if we are to gain meaningful control of antibiotic use, we must focus on children and neonates, especially in Africa. The significantly greater decrease in the prevalence of antibiotic use in older children is attributed to the implementation of the AMS program in the hospital with the development of antibiotic guidelines and compliance with its use in the Department of Paediatrics.
The increased use of antibiotics, especially in inborn neonatal wards, could be primarily due to recent advancements and improvements in facilities with the acquisition of more modern equipment as well as the increased capacity of the newborn unit to care for more preterm low weight babies, resulting in more referrals of preterm infants from other centres. It is known that a good number of preterm babies develop sepsis, especially in low and medium-income countries (LMIC) where there are poor antenatal and delivery services, with home delivery in an unhygienic environment and poor supervision during delivery. Moreso, preterm neonates tend to stay longer in the hospital and hence are more prone to HAI.
The antibiotic recommended in our guidelines for the treatment of sepsis in neonates was combination therapy with a third-generation cephalosporin and an aminoglycoside; based on a local antibiogram conducted before the start of PPS. However, it differs from the WHO recommendation for the treatment of neonatal sepsis, which involves the use of ampicillin and gentamicin, based on surveys of 56 paediatric hospitals worldwide [45, 46]. These antibiotics are also the most commonly prescribed for neonates in African hospitals [45, 47], and it reflects the resistance profile of relevant pathogens in our hospitals [48]. Continuous surveillance and antibiograms should be conducted because the current trend may not be what was previously obtained.
The decreased rate of overall use of prophylactic antibiotics is attributed to increased awareness and adherence to guidelines [49, 50] In children, suspected central nervous system infection and sepsis are very common life-threatening infections that requires antibiotics prescriptions [32, 45, 47]. Lack of access to affordable and rapid diagnosis may be the driving force as patients need to pay out of pocket for blood cultures.
High rates of medical prophylaxis, occurred in neonates, such as in other parts of Africa [32, 44]. One possible explanation for this prophylaxis is that it was used for a diagnosis of suspected sepsis in full-term newborns with major risk factors for sepsis. The antibiotic prophylaxis in neonates was combination therapies (except in 2017), including a third-generation cephalosporin and an aminoglycoside, which contrasts with our local guideline’s recommendation of second-generation cephalosporin as well as the WHO recommendation for penicillin and an aminoglycoside [45, 51, 52]. Absence of documented indications for use may be the reason for assigning them as prophylaxes, since a follow-up was not part of the PPS, the final reason cannot be ascertained. There needs to be more engagement with prescribers for newborns specifically to adhere to documented guidelines. Research on the needs and outcomes of antibiotic prophylaxis in children, especially neonates, is needed to improve rational antibiotic prescribing.
The gold standard for identifying sepsis is blood culture, which may not be affordable and may take more than 24 h to obtain a result in the LMIC. Due to ethical concerns, in centres where patients pay out of pocket for a service, and where there is a very high probability of severe and life-threatening complications from infections, an antibiotic delay is usually avoided. This calls for more affordable modern, rapid, and accurate diagnostic tests to quickly distinguish babies who have sepsis from those who do not. Thus, conserve and avoid the unnecessary use of antibiotics.
In older children, even though the rate of antibiotic use for prophylaxis decreased significantly; the use of third-generation cephalosporins is still a challenge due to its variance to both local and international guidelines. Ceftriaxone and other third-generation cephalosporins, often in combination with metronidazole for more than 24 h, are commonly observed in Nigeria and East Africa [34, 53, 54]. Prolonged surgical prophylaxis with broad-spectrum antibiotics can increase the risk of antibiotic resistance [53]. Appropriate surgical antibiotic prophylaxis is potentially a low-hanging fruit for improving the quality of antimicrobial prescribing in children. The AMS team presently includes surgeons. There is currently a departmental drive, where the team engages the surgeons regularly to inform and educate them on appropriate surgical prophylaxis and liaise with the pharmacy to make it available. The pharmacy department is at the forefront of our stewardship programs, recommended surgical prophylaxis would be made available and dispensed in surgical packs. More enforcement is needed for strict adherence to pre-authorisation. The AMS member in surgery also ensures compliance by constant reminders.
Parenteral antimicrobial therapy in all periods was similar to rates previously documented in studies in Nigeria, other African countries, East and South Asia, and North America from the Global –PPS network [34, 55,56,57]. An AMS intervention using an intravenous (IV) to oral antibiotic switch is a low-hanging fruit to consider in our hospitals to reduce the high rate of parenteral therapy, especially in older children. The IV-to-oral switch for older children as a supplementary strategy can help to reduce the length of hospital stay, the cost of healthcare, staff workload, and the risk of catheter-associated infections [26,27,28]. We currently advocate for early discharge and ambulatory care for children to reduce HAI.
AWaRe antibiotics
The high use of “Watch” antibiotics may reflect high resistance rates to antibiotics, as shown by our antibiotic guidelines. Setting a target of 60% for access group antibiotics as recommended by the WHO would require strengthening our AMS program to reverse the trend of irrational antibiotic use [41, 58].
Notably, quinolones were frequently used in the neonates. Quinolones are contraindicated in children except for those with shigellosis or salmonellosis, immunocompromised children with multidrug-resistant organisms, and those for whom there are no other safe or effective antibiotics [59,60,61,62]. However, none of these diagnoses were made in the children. Quinolone has been used extensively in Asia and Europe even beyond its licensed indications for use [60, 62,63,64,65,66], especially in Belgium, where it was found to be prescribed off-label and did not follow bacteriological findings [60]. Although contraindicated, reasons for use in children include local antibiogram results and poor response to other conventional antibiotics, as studies have shown less toxicity in humans [62, 67]. This is even more worrisome, especially as organisms will become resistant to quinolones. Intervention programs targeted at minimizing the use of quinolone as they are medications in the W.H.O reserve group are urgently needed. There is a current target of reducing this in children as AMS time is allocated every week in the paediatrics department to educate and inform prescribers on AMS and the importance of using the antibiotic guidelines.
The HAI rates in neonates doubled, which was attributed to more admissions of preterm neonates with prolonged hospital stays and prolonged use of invasive procedures such as the insertion of central catheters and ventilatory support. These HAI rates are similar to the rates found in northern Nigeria (14.3%) [68], implying that a sustained infection prevention and control (IPC) program is desirable in Nigeria to attain lower rates, as recorded in Ghana (8.2%) [69] and the USA (2.98–3.13%) [70]. We suggested and activated more robust and sustained IPC within the hospital AMS program, emphasizing HAIs.
Conclusion
Antimicrobial prescription rates were generally reduced with the hospital AMS program. However, there is still extensive use of antibiotics in our children, with the use of multiple antibiotics and reserve antibiotics, with the major diagnosis being sepsis. Cephalosporin was the antibiotic most often prescribed. There should be urgent and intensive interventions to reduce this risk.
Recommendation
AMS programs should be enforced to curtail and reduce the unnecessary use of antibiotics. There should be continuous education and training of healthcare professionals on the use of the guidelines and diagnostic tests before the commencement of antibiotics to avoid the irrational use of antibiotics. The AMS team is currently involved in continuing professional education activities by training and giving talks on stewardship to all cadres of health workers. At each weekly departmental meetings, a five-minute talk on the rational use of antibiotics and at every other opportunity is given. Posters on AMS are also pasted at strategic places within the hospital to remind prescribers. Other AMS strategies, such as preauthorization and prospective audit feedback and intervention, have been included, as these strategies could have synergistic effects on reducing antibiotic use. The AMS team has also embarked on AMS in the outpatient and is currently mentoring peripheral hospitals.
Limitations of the study
A point prevalence survey is a one-point analysis and may not provide a true picture of the actual situation. Other details of the use of antibiotics cannot be accessed by this method; however, multiple PPS may also be a good indication of the prevailing situation. A study on the prospective audit intervention and feedback would complement PPS to give more information on the effectiveness of AMS activities within the hospital. The study was based on in-patients only, an outpatient prescription survey would expand the scope of knowledge on antibiotic use.
Data availability
The datasets used and/or analysed during the current study will be available from the corresponding author upon reasonable request.
Abbreviations
- A.T.C:
-
Anatomic therapeutic chemical classification system
- AMR:
-
Antimicrobial resistance
- AMS:
-
Antimicrobial stewardship
- AWaRe:
-
‘Access’, ‘Watch’, and ‘Reserve’
- CAI:
-
Community-acquired infection
- CNS:
-
Central nervous system
- GI inf:
-
GI infections
- HAI:
-
Healthcare associated infection
- IPC:
-
Infection prevention and control program
- IUGR:
-
Intrauterine Growth Restriction
- MDRO:
-
Multidrug-resistant Organisms
- PPS:
-
Point Prevalence Survey
- SAP:
-
Surgical antibiotic prophylaxis
- SSTBJ:
-
Skin & Soft Tissue, Bone Joint Infection
- UTI:
-
Urinary Tract Infection
- VLBW:
-
Very Weight low birth weight
- W.H.O:
-
World Health Organization
References
Perry J, Waglechner N, Wright G. The prehistory of antibiotic resistance. Cold Spring Harb Perspect Med. 2016;6(6).
Lobanovska M, Pilla G. Penicillin’s discovery and antibiotic resistance: lessons for the future? Yale J Biol Med. 2017;90(1):135–45.
Choudhury R, Panda S, Singh DV. Emergence and dissemination of antibiotic resistance: a global problem. Indian J Med Microbiol. 2012;30(4):384–90.
Xu Y, et al. A cross-sectional study of antibiotic misuse among Chinese children in developed and less developed provinces. J Infect Dev Ctries. 2020;14(2):129–37.
Klein EY, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115(15):E3463–70.
Xu J, et al. Parental self-medication with antibiotics for children promotes antibiotic over-prescribing in clinical settings in China. Antimicrob Resist Infect Control. 2020;9(1):150.
de Ponce S, Arredondo-Hernández R, López-Vidal Y. Resistance to antibiotic: a serious global problem. Gac Med Mex. 2015;151(5):681–9.
Rocha C, Reynolds ND, Simons MP. Emerging antibiotic resistance: a global threat and critical healthcare problem]. Rev Peru Med Exp Salud Publica. 2015;32(1):139–45.
Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS ONE. 2017;12(12):e0189621.
Bush K, et al. Tackling antibiotic resistance. Nat Rev Microbiol. 2011;9(12):894–6.
Megged O, et al. Gastrointestinal carriage of Pseudomonas aeruginosa in children residing in chronic care institutes in Jerusalem: high prevalence and high rates of antibiotic resistance. Harefuah. 2009;148(11):766–8.
Seidman JC, et al. Risk factors for antibiotic-resistant E. Coli in children in a rural area. Epidemiol Infect. 2009;137(6):879–88.
Seidman JC, et al. Longitudinal comparison of antibiotic resistance in diarrheagenic and non-pathogenic Escherichia coli from young Tanzanian children. Front Microbiol. 2016;7:1420.
Vazouras K, et al. Antibiotic treatment and antimicrobial resistance in children with urinary tract infections. J Glob Antimicrob Resist. 2020;20:4–10.
Oundo JO, et al. Antibiotic susceptibility and genotypes of non-typhi Salmonella isolates from children in Kilifi on the Kenya coast. Trans R Soc Trop Med Hyg. 2000;94(2):212–5.
Yue M, et al. Serotypes, antibiotic resistance, and virulence genes of Salmonella in children with diarrhea. J Clin Lab Anal. 2020;34(12):e23525.
Wu X, et al. A multicentric study on clinical characteristics and antibiotic sensitivity in children with methicillin-resistant Staphylococcus aureus infection. Zhonghua Er Ke Za Zhi. 2020;58(8):628–34.
Udo SM, Eja ME. Prevalence and antibiotic resistant shigellae among primary school children in urban Calabar, Nigeria. Asia Pac J Public Health. 2004;16(1):41–4.
Tyrstrup M, et al. Children with respiratory tract infections in Swedish primary care; prevalence of antibiotic resistance in common respiratory tract pathogens and relation to antibiotic consumption. BMC Infect Dis. 2017;17(1):603.
Xu JJ, et al. Analysis of antibiotic treatment of children in a Shanghai tertiary hospital based on point prevalence surveys. BMC Infect Dis. 2020;20(1):804.
Zheng C, et al. Determinants and patterns of antibiotic consumption for children under five in Nepal: analysis and modelling of demographic health survey data from 2006 to 2016. Trop Med Int Health. 2021;26(4):397–409.
Zamunu A, et al. Antibiotic use in the management of children with the common cold at a provincial hospital in Papua New Guinea: a point-prevalence study. Paediatr Int Child Health. 2018;38(4):261–5.
Zhang JS, et al. Antibiotic usage in Chinese children: a point prevalence survey. World J Pediatr. 2018;14(4):335–43.
Zhang M, et al. Survey of antibiotic use among hospitalised children in a hospital in Northeast China over a 4-year period. J Spec Pediatr Nurs. 2020;25(2):e12282.
Costenaro P, et al. Antibiotic prescriptions for children with community-acquired pneumonia: findings from Italy. Pediatr Infect Dis J. 2021;40(2):130–6.
Versporten A, et al. The antibiotic resistance and prescribing in European children project: a neonatal and pediatric antimicrobial web-based point prevalence survey in 73 hospitals worldwide. Pediatr Infect Dis J. 2013;32(6):e242–53.
Frenette C, et al. The 2017 global point prevalence survey of antimicrobial consumption and resistance in Canadian hospitals. Antimicrob Resist Infect Control. 2020;9(1):104.
Hufnagel M, et al. High rates of prescribing antimicrobials for prophylaxis in children and neonates: results from the antibiotic resistance and prescribing in European children point prevalence survey. J Pediatr Infect Dis Soc. 2019;8(2):143–51.
Luthander J, et al. Antimicrobial use in a Swedish pediatric hospital: results from eight point-prevalence surveys over a 15-year period (2003–2017). Pediatr Infect Dis J. 2019;38(9):929–33.
Tribble AC, et al. Appropriateness of antibiotic prescribing in United States children’s hospitals: a national point prevalence survey. Clin Infect Dis. 2020;71(8):e226–34.
Umar LW, et al. Prescribing pattern and antibiotic use for hospitalized children in a northern Nigerian teaching hospital. Ann Afr Med. 2018;17(1):26–32.
Chioma O. Rational use of antibiotics – a point prevalence study carried out at a tertiary hospital in South-South Nigeria. International Journal of Tropical Disease & Health. 2020:39–47.
Ejembi J et al. Antimicrobial prescription pattern in a tertiary hospital. Sahel Med J. 2020;23(2).
Umeokonkwo CD, et al. Point prevalence survey of antimicrobial prescription in a tertiary hospital in South East Nigeria: a call for improved antibiotic stewardship. J Glob Antimicrob Resist. 2019;17:291–5.
Magsarili HK, et al. Making a case for pediatric antimicrobial stewardship programs. Pharmacotherapy. 2015;35(11):1026–36.
Godbout EJ, et al. Pediatric antimicrobial stewardship: state of the art. Curr Infect Dis Rep. 2018;20(10):39.
STATISTICS NBO. and M. 2019, Final_2018 Statistical Report on Women and Men in Nigeria_Publication_SG OFFICE_29052019 < NIgeria-State-Profiles-Lagos-Zamfara.pdf>
Patel SJ, Saiman L. Principles and strategies of antimicrobial stewardship in the neonatal intensive care unit. Semin Perinatol. 2012;36(6):431–6.
Data-collection-forms. -Global-PPS-with-optional-HAI-module_April2023-final and P. (www.global-pps.com), -collection-form-Global-PPS-2019.pdf>
WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment, 2024. Oslo, 2024
Mudenda S, Daka V, Matafwali SK. World Health Organization AWaRe framework for antibiotic stewardship: where are we now and where do we need to go? An expert viewpoint. Antimicrob Steward Healthc Epidemiol. 2023;3(1):e84.
Sharland M, et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use—the new 2019 essential medicines list becomes a global antibiotic stewardship tool. Lancet Infect Dis. 2019;19(12):1278–80.
Gharbi M, et al. Using a simple point-prevalence survey to define appropriate antibiotic prescribing in hospitalised children across the UK. BMJ Open. 2016;6(11):e012675.
Labi AK, et al. Antibiotic prescribing in paediatric inpatients in Ghana: a multi-centre point prevalence survey. BMC Pediatr. 2018;18(1):391.
Hsia Y, et al. Use of the WHO access, watch, and reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. Lancet Glob Health. 2019;7(7):e861–71.
W.H.O, World Health Organization NEONATAL SEPSIS.pdf>
Hsia Y, et al. Consumption of oral antibiotic formulations for young children according to the WHO access, watch, reserve (AWaRe) antibiotic groups: an analysis of sales data from 70 middle-income and high-income countries. Lancet Infect Dis. 2019;19(1):67–75.
Ekwochi U, Onah SK, Ndu IK. Bacterial profile and antibiogram of neonatal sepsis in Nigeria: literature review. Anatol J Family Med. 2020;3(1):2–9.
Gandra S, et al. Point prevalence surveys of antimicrobial use among eight neonatal intensive care units in India: 2016. Int J Infect Dis. 2018;71:20–4.
Korinteli IG et al. The global point prevalence survey (Pps) of antimicrobial use and antimicrobial resistance among hospitalized children in Georgia. Georgian Med News. 2019(292–3):72–75.
Du Pont-Thibodeau G, Joyal JS, Lacroix J. Management of neonatal sepsis in term newborns. F1000Prime Rep. 2014;6:p67.
Clotilde TS, Motara F, Laher AE. Prevalence and presentation of neonatal sepsis at a paediatric emergency department in Johannesburg, South Africa. Afr J Emerg Med. 2022;12(4):362–5.
Alemkere G. Antibiotic usage in surgical prophylaxis: a prospective observational study in the surgical ward of Nekemte referral hospital. PLoS ONE. 2018;13(9):e0203523.
Abubakar U. Antibiotic use among hospitalized patients in northern Nigeria: a multicenter point-prevalence survey. BMC Infect Dis. 2020;20(1):86.
<205638-Article Text-512920-1-10-20210408 (1).pdf>
Oduyebo O, et al. A point prevalence survey of antimicrobial prescribing in four Nigerian tertiary hospitals. Annals Trop Pathol. 2017;8(1):42–6.
Versporten A, et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6(6):e619–29.
Abdelsalam Elshenawy R, Umaru N, Aslanpour Z. WHO AWaRe classification for antibiotic stewardship: tackling antimicrobial resistance – a descriptive study from an English NHS Foundation Trust prior to and during the COVID-19 pandemic. Front Microbiol. 2023;14.
Jackson MA, Schutze GE. The use of systemic and topical fluoroquinolones. Pediatrics. 2016;138(5).
Meesters K, et al. Systemic fluoroquinolone prescriptions for hospitalized children in Belgium, results of a multicenter retrospective drug utilization study. BMC Infect Dis. 2018;18(1):89.
Schaad UB. Use of the new quinolones in pediatrics. Isr J Med Sci. 1994;30(5–6):463–8.
Yang ZT, et al. Current ciprofloxacin usage in children hospitalized in a referral hospital in Paris. BMC Infect Dis. 2013;13:245.
Velissariou IM. The use of fluoroquinolones in children: recent advances. Expert Rev Anti Infect Ther. 2006;4(5):853–60.
[Investigation on the rational use of antibacterial agents by Chinese pediatricians in 2016] Zhonghua Er Ke Za Zhi. 2018;56(12):897–906.
Bozic B, Bajcetic M. Use of antibiotics in paediatric primary care settings in Serbia. Arch Dis Child. 2015;100(10):966–9.
Genuini M, et al. Fluoroquinolones in pediatrics: review of hospital prescription use over 2 years. Int J Clin Pharmacol Ther. 2014;52(11):940–7.
Uwe NO, et al. Antimicrobial susceptibility and neonatal sepsis in a tertiary care facility in Nigeria: a changing trend? JAC-Antimicrobial Resistance. 2022;4;5.
Abubakar U. Point-prevalence survey of hospital acquired infections in three acute care hospitals in Northern Nigeria. Antimicrob Resist Infect Control. 2020;9(1):63.
Labi AK, et al. Multi-centre point-prevalence survey of hospital-acquired infections in Ghana. J Hosp Infect. 2019;101(1):60–8.
Spaulding AB, et al. Epidemiology of bloodstream infections in hospitalized children in the United States, 2009–2016. Clin Infect Dis. 2019;69(6):995–1002.
Acknowledgements
The authors wish to acknowledge all staff of the paediatricians, doctors, nurses, and pharmacists for their cooperation during the data collection.
Funding
The Global Point Prevalence Survey is coordinated by the University of Antwerp Belgium and sponsored through an unrestricted grant annually by bioMerieux. However, the participating hospitals did not receive any funding support directly.
Author information
Authors and Affiliations
Contributions
O.O, P.O C. O, designed the initial concept. while O.O.I, O.O, P.O, C, O. A.R, collected the data. P. A, and P.O, O.O, wrote up the introduction, tables, and discussion .P.A , P.O , A.R, I.F, and E.T did the manuscript review. All authors were given equal participation in joint manuscript writing of the final after the initial draft. All authors equally contributed to the review and editing of the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Ethical approval was obtained from the Lagos University Teaching Hospital (LUTH) Human Research and Ethics Committee (HREC). (ADM/DST/HREC/APP/045). This is an improvement project of the hospital and not an experimental study. Informed consent was waived from the LUTH HREC, and all methods were carried out under relevant guidance and regulations.
The authors declare that this work has not been submitted to another journal.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Akintan, P., Oshun, P., Osuagwu, C. et al. Point prevalence surveys of antibiotic prescribing in children at a tertiary hospital in a resource constraint, low-income sub-Saharan African country—the impact of an antimicrobial stewardship program. BMC Pediatr 24, 383 (2024). https://doi.org/10.1186/s12887-024-04847-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-024-04847-3