Abstract
Background
Lung recruitment maneuvers (LRMs) improve lung volume at initiation of high-frequency oscillatory ventilation (HFOV), but it is unclear when to repeat LRMs. We evaluated the efficiency of scheduled LRMs.
Methods
In a randomized controlled trial, extremely preterm infants on HFOV received either LRMs at 12-hour intervals and when clinically indicated (intervention) or only when clinically indicated (control). The primary outcome was the cumulative oxygen saturation index (OSI) over HFOV time, limited to 7 days. Additionally, LRMs were analyzed with respect to OSI improvement.
Results
Fifteen infants were included in each group. The mean (SD) postmenstrual age and weight at HFOV start were 23 + 6 (0 + 5) weeks and 650 (115) g in the intervention group and 24 + 4 (0 + 6) weeks (p = 0.03) and 615 (95) g (p = 0.38) in the control group. The mean (SD) cumulative OSI amounted to 4.95 (1.72) in the intervention versus 5.30 (2.08) in the control group (p = 0.61). The mean (SD) number of LRMs in 12 h was 1.3 (0.2) in the intervention versus 1.1 (0.5) in the control group (p = 0.13). Performing LRM when FiO2 > 0.6 resulted in a mean OSI reduction of 3.6.
Conclusion
Regular versus clinically indicated LRMs were performed with equal frequency in preterm infants during HFOV, and consequently, no difference in lung volume was observed. LRMs seem to be most efficient at high FiO2.
Trial registration
ClinicalTrials.gov ID: NCT04289324 (28/02/2020).
Similar content being viewed by others
Background
Lung protective strategies are claimed to be key elements in the treatment of extremely preterm infants [1]. The first-line strategy comprises non-invasive respiratory support, in particular, continuous positive airway pressure and early surfactant administration [2]. Nevertheless, respiratory failure occurs in more than 50% of extremely preterm infants, subsequently requiring mechanical ventilation [3, 4]. Positive pressure ventilation exposes the immature lungs to lung injuries representing a major cause of morbidity in this vulnerable population [5]. Different ventilation modes have been proposed to reduce ventilation-induced lung injuries [6, 7]. Among them, high-frequency oscillatory ventilation (HFOV) has been proven to be an efficient and safe mode of ventilation in preterm infants [8,9,10]. In comparison with volume targeted conventional mechanical ventilation, HFOV might reduce mortality and the incidence of bronchopulmonary dysplasia (BPD) when combined with the open lung principle [11]. The open lung principle aims to recruit the lungs and to keep them open in order to maintain optimal compliance [1, 12]. At present, lung recruitment continues to rely on oxygenation and pressure indices to assess changes in lung volume since no direct bedside tool for monitoring lung volume has been established so far [13, 14]. Stepwise oxygenation-guided lung recruitment maneuvers (LRMs) can efficiently establish and stabilize lung capacity after HFOV initiation [13,14,15]. Studies have further confirmed that such LRMs have a low risk of lung hyperinflation and air leak syndrome, have no adverse cerebral events (e.g., intraventricular hemorrhage) and in addition, do not compromise cardiac function up to a critical level in preterm infants [16]. Therefore, LRMs are recommended when HFOV is initiated [13, 14]. They should be repeated in situations with partial lung collapse to restore lung capacity. Although titrating mean airway pressure (MAP) every 12 h has been discussed, neither the assessment of partial lung collapse nor the time interval when to repeat LRMs are well-defined and the question when to perform LRMs during HVOF has not been addressed so far [1].
The present study explored the question on the efficiency of regularly performed LRMs in terms of cumulative oxygenation and mean airway pressure. We hypothesized that regularly performed LRMs decrease the cumulative oxygenation saturation index (OSI) during HFOV in preterm infants. The study further investigated LRMs with respect to changes in oxygenation indices and mean airway pressure.
Methods
The trial (ClinicalTrials.gov ID: NCT04289324, 28/02/2022) was conducted at a third level neonatal intensive care unit of the Medical University of Vienna. It was approved by the local ethics committee (EK 1161/2019). Written informed consent was obtained from parents or legal guardian of each infant prior to the study participation.
This study was a single-center, open, randomized controlled trial. Preterm infants born below 28 weeks of postmenstrual age (PMA) were screened for eligibility. Infants with congenital anomalies of the heart and/or the lungs as reported in ultrasound and/or fetal MRI were excluded. Infants were enrolled in the study at the time of HFOV initiation (Fabian HFO respirator, Acutronic Medical Systems AG, Hirzel, Switzerland) and received either LRMs at regular 12-hour intervals and upon decision of the care giving team (intervention group), or LRMs only upon decision of the caregiving team (control group). HFOV is used as a primary ventilation mode for extremely preterm infants in our unit. Participants were randomly allocated with a 1:1 ratio, stratified for gender and PMA (less than 26 weeks versus equal or higher than 26 + 0 weeks) using the Randomizer for Clinical Trials tool developed at the Medical University of Graz (http://www.randomizer.at/). Patient data were collected from the medical charts: PMA at birth and at HFOV initiation, weight at birth and at HFOV initiation, day of life at HFOV initiation, the oxygenation saturation index at HFOV initiation, gender, and complete course of antenatal steroids.
Lung recruitment maneuver
LRMs were performed in hemodynamically stable situations only and were conducted as previously described [10, 13, 14, 17, 18]: oscillatory frequency was kept at the clinically set values and the pressure amplitude was adjusted to maintain the transcutaneous pCO2 between 40 and 60 mmHg (SenTec Digital Monitor, Therwil, Switzerland). Starting at the clinically set MAP value, MAP was increased every 5 min (allowing intervals of 2–15 min upon the decision of the caregiving team) by 2 mbar (with steps of 1 mbar when greater than 20 mbar, maximum MAP was set at 25 mbar). FiO2 was reduced stepwise, keeping SpO2 within the predefined target range (88–96% or 90–96% in presence of pulmonary hypertension requiring medication). Increase of MAP was stopped when SpO2 no longer improved or FiO2 was ≤ 0.25 (MAPmax, maximum MAP during the LRM). Next, MAP was gradually decreased every 5 min (allowing intervals of 2–15 min upon the decision of the caregiving team) by 2 mbar until a sustained drop in SpO2 of ≤ 5% of the initial value at MAPmax or an absolute SpO2 value below 88% (minimum MAP 5 mbar) reaching the closing MAP. After a one-step recruitment with the known MAPmax, the final MAP was set 1–2 mbar above the closing MAP (see example in the supplemental material). LRMs were advised after the following situations: change of position (from prone to supine or vice versa), any manipulation with FiO2 increase of 0.1 or SpO2 decrease > 10% for > 5 min (e.g., suctioning, endo-tracheal tube disconnection), surfactant application, and suspected or confirmed atelectasis (e.g., diagnosed on chest X-ray). LRM was compulsory each time HFOV was initiated. LRMs (per protocol) were scheduled for 8AM and 8PM in the intervention group. If a LRM was performed 2 h prior to the schedule or was planned to be performed within 2 h after the schedule (e.g., change of position at 9AM), then the scheduled LRM was omitted. The care giving staff were instructed in how and when to perform LRMs prior to the initiation of the trial.
The primary outcome was defined as the cumulative OSI during HFOV in the study period which started with HFOV initiation and was limited to at most 7 consecutive days. The oxygenation saturation index (OSI) is determined by the product of FiO2 and MAP divided by the peripheral oxygen saturation (SpO2) and is expressed as a percentage. The cumulative OSI is determined by the sum of OSI values divided by the number of OSI values in the study period which is equivalent to the average OSI. The OSI variables were recorded every 15 min in the electronic patient data management system (ICCA, Philips Healthcare, Amsterdam, Netherlands) and structurally obtained. Data was assessed by a clinical expert for plausibility. Outliers as well as LRMs were not considered for analysis.
Secondary outcome variables were the number of LRMs, the duration of mechanical ventilation, oxygenation parameters (SpO2, FiO2, S/F-ratio) and MAP averaged over HFOV time, mortality and morbidity including BPD as defined by the requirement of respiratory support and supplemental oxygen at PMA of 36 weeks, severe intraventricular hemorrhage (IVH > grade II, as seen on cranial ultrasound scans), pneumothorax (PTX, as seen in the chest X-ray), and pulmonary interstitial emphysema (PIE, as described in the radiologic results).
All LRMs performed in the study period were analyzed with respect to the change of OSI prior and after LRM in dependence of the oxygen requirement and MAP at the start of the LRM, of the duration of the LRM, and of the time difference between two adjacent LRMs.
Sample size calculation
Zannin et al. observed that LRMs after HFOV initiation reduced the OSI of approximately 25%, from 6.15 to 4.55 (SD approximately 1.5) on average [13]. We assumed that this reduction extends to the cumulative OSI during HFOV when performing LRM at regular intervals (null-hypothesis: average OSI between intervention and control group are not different). Based thereupon, 15 infants need to be enrolled in each group to attain 80% power for detecting a difference of 25% in cumulative OSI between the groups (at a two-sided alpha level of 5%). The sample size is based on calculations using G*Power 3.1.9.2 (University of Kiel, Germany) with the following inputs (t-tests): difference between two independent means (two groups); tails = 2; effect size d = 1.067; alpha = 0.05, power (1-β error probability) = 0.8, allocation ratio N2/N1 = 1.
Statistical analysis
For hypothesis testing of the primary outcome (cumulative OSI), the unpaired t-test was used. For evaluating differences of continuous parametric and continuous non-parametric variables between the intervention and the control group the unpaired t-test and the Mann-Whitney U-test were used, respectively. The difference of the dichotomy variables between the groups was tested using the Fisher’s exact test. MAP, S/F ratio, and OSI before and after LRM for all LRMs were compared using the paired t-test. Differences were considered statistically significant for p < 0.05. Multivariable logistic regression analysis to determine independent risk factors for BPD was performed using cumulative OSI over HFOV time, number of LRMs, PMA at birth, birth weight, ventilation days, and antenatal steroids as risk factors for death or moderate to severe BPD (Grade II-III(A)) [19]. Linear regressions between change in OSI and FiO2 levels were performed to assess differences between LRM indications. Analyses of the secondary outcome variables were considered as hypothesis generating and therefore no adjustment for multiple testing was performed. Statistical analysis was performed with R version 4.0.2 (The R Foundation for Statistical Computing, Vienna, Austria) using RStudio version 1.3.1056 (RStudio Team 2020, RStudio, Inc., Boston, MA).
Results
Study participants
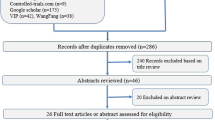
The study was conducted from March 2020 to June 2021. Thirty-nine extremely preterm infants were screened for eligibility prior to HFOV initiation. In 2 cases, parents refused trial participation. Five patients did not receive HFOV. Thirty-two preterm infants were randomized at HFOV initiation, 16 allocated in each group. Two participants were excluded during the trial, one in each group. The infant excluded from the control group was on HFOV less than 12 h and no LRM did take place violating the trial protocol. The infant in the intervention group was excluded due to technical problems with data monitoring. After exclusion, 15 participants were analyzed in each group as presented in the flow diagram (Fig. 1).
The baseline characteristics of the study participants are presented in Table 1. The PMA at the time of study initiation was slightly different between the intervention and the control group (p = 0.03). In each group, only 1 participant was born at a PMA greater than 26 weeks. All participants received surfactant in the first hour of life.
Primary outcome
The cumulative OSI value during HFOV was lower in the intervention group, however, no statistical significance could be demonstrated (4.95 versus 5.30, p = 0.61). The percentage of OSI values that were excluded (outliers or recordings during LRMs) from the calculation of the cumulative OSI amounted to 1.9 (SD 2.4) and were similar between both groups, 2.0 (SD 2.1) in the intervention group and 1.8 (SD 2.7) in the control group (p = 0.83). Considering the excluded OSI values for analysis did not change the primary outcome: 4.99 (SD 1.75) in the intervention group and 5.40 (SD 2.24) in the control group (p = 0.58). We point out that the average number of performed LRMs within 12 h in the intervention group was only slightly greater than in the control group (1.33 versus 1.11, p = 0.13), see Table 2.
Secondary outcome
None of the secondary outcome parameters showed any statistically significant difference between groups, see Table 2. Logistic regression analysis showed cumulative OSI over HFOV time (p = 0.016) to be a significant independent risk factor for death or moderate to severe BPD while number of LRMs, PMA, birth weight, ventilation days, and antenatal steroids were not. The mean (SD) of cumulative OSI between respiratory failure due to severe RDS (n = 18) and non-RDS (n = 12) amounted 4.9 (2.1) and 5.4 (1.5), respectively (p = 0.46).
Lung recruitment maneuver
The number of LRMs performed during the study period amounted to 338. We observed at most a slight and transient decrease in blood pressure during the inflation limb of LRMs. FiO2 clearly improved, whereas the change in MAP was less pronounced. The mean (SD) FiO2 improvement for all LRMs amounted to 11% (16%). No significant difference in FiO2 improvement was found among groups of different LRM indication. The largest improvement in FiO2 occurred when LRM took place after surfactant application and after changing the patient’s position with an average FiO2 reduction of 14% and 13%, respectively, see Table 3.
When considering FiO2 and MAP prior to LRM, we observed that FiO2 values above 0.6 exhibit the highest OSI reduction of 3.6 (SD 2.6) on average (Fig. 2, a). For MAP levels below 10 mbar, the OSI reduction amounted only to 0.5 (SD 0.5) on average (Fig. 2, b).
Change of oxygenation saturation index (mean difference between start and end of LRM) in dependence of (a) different FiO2 levels at the start of LRM, (b) MAP levels at the start of LRM, (c) the duration of LRM, and (d) of the time difference between two adjacent LRM for all (bold), clinically indicated (A), and clinically not indicated (B, intervention only) LRMs. The number of LRMs is displayed under each bar. The unpaired t-test was applied to calculate p-values for all recruitment maneuvers in the intervals specified below each figure. OSI, oxygenation saturation index; FiO2, fraction of inspired oxygen; LRM, lung recruitment maneuver; MAP, mean airway pressure
LRMs with a duration of less than 45 min resulted in a mean OSI reduction of 1.1 (SD 1.4) whereas a duration of more than 90 min resulted in significantly higher OSI reduction of 2.3 (SD 2.5, p < 0.001), (Fig. 2, c). ). In comparison with LRMs with a duration of more than 90 min, those with a duration of less than 45 min had a significantly lower number of recruitment steps (median of 8 versus 15, p < 0.001) and a significantly shorter duration of recruitment steps (mean of 4.0 versus 7.6 min, p < 0.001). LRMs performed after 3 to 9 h of the previous LRM showed a mean OSI reduction of 2.1 (SD 2.2), whereas LRMs after 9 to 15 h of the previous LRM showed a mean OSI reduction of 1.6 (SD 2.1). However, this finding did not reach statistical significance (p = 0.09), (Fig. 2, d). No remarkable difference in OSI reduction between LRM indications could be observed (Fig. 3).
Boxplot displaying changes of the oxygenation saturation index after lung recruitment for different groups of lung recruitment indication. On each box, the central line indicates the median, and the bottom and top edges represent the 25th and 75th percentiles, respectively. The whiskers extend to the most extreme data points within two times the interquartile range. The ‘+’ symbol indicates the outliers. No statistically significant difference could be found among the groups
Discussion
Primary outcome
This randomized controlled trial is the first study that analyzed repeatedly performed LRMs during HFOV in extremely preterm infants. The primary cause for respiratory failure in this cohort was severe RDS. The study was targeting 12-hourly scheduled versus clinically indicated lung recruitment maneuvers. The findings did not result in a significant change in the number of lung recruitment maneuvers, and as a consequence, no difference in oxygenation was observed between the study groups. Our observation suggests that the course of HFOV lead to situations of reduced lung volume that require LRMs at least once every 12 h. As a result, the policy of performing LRM based solely on the clinical judgement seems not to be inferior as compared to a fixed 12-hour recruitment regime. We conclude that imposing scheduled lung recruitment maneuvers twice a day during HFOV is not needed in a lung protective setting using lung recruitment maneuvers for preventing prolonged lung collapse in preterm infants. The study was part of a quality improvement process in which we aimed to standardize LRMs during HFOV. Physicians received appropriate training regarding LRMs prior to the study initiation. Before standardization, LRMs were performed occasionally and only in situations of severe respiratory deterioration and not in the sense of a lung protective strategy. Standardized indication of LRMs could have led to an increased awareness for lung protective strategies among the caregiving team. Both, the awareness for lung protective strategies and the implementation of guidelines for LRMs might account for the lack of difference in both study groups.
Lung recruitment maneuver
LRMs aim to (re)-open collapsed lung areas that are poorly aerated avoiding overdistention with the ultimate goal to prevent ventilation induced lung injury [20, 21]. The important question in this context refers to the recruitability of lung units. Assessing recruitability of the lung is difficult in clinical practice. We have observed that performing LRMs at high FiO2 levels was most beneficial in terms of OSI reduction which is indicative of an improvement in lung volume. Hence, FiO2 might serve as an predictor for the recruitability of the lung. However, we acknowledge that oxygenation varies with systemic hemodynamics in respiratory failure, and therefore does not always reflect alveolar recruitment [22]. Reduction in OSI for higher MAP levels prior to the LRM was less pronounced. High MAP levels bear the risk of overdistention and regularly performed LRMs might overcome this problem by eventually de-recruiting overdistended lung areas. LRMs with a duration longer than 90 min showed a significantly larger OSI reduction than short LRMs. This observation is in accordance with the findings by Thome et al., namely that alveolar recruitment may take up to 25 min after MAP changes [23]. No clear benefit in OSI reduction has been found when repeating LRMs in less than 9 h versus more than 15 h (Fig. 2, d). It seems that clinical indication for performing LRMs is more effective than LRMs per protocol (Fig. 2). This might be explained by the fact that FiO2 at LRM initiation was lower for LRMs per protocol than for clinical indication (Table 3). However, a difference in OSI reduction between LRM indications could not be detected (Fig. 3). We further observed, that LRMs were most often performed after positional change (Table 3). Changing the patient’s position, in particular from supine to prone, is a common procedure in ventilated infants and a key element in lung protective strategies in patients with acute respiratory distress syndrome [24,25,26]. Recruiting the lung after positional change seems to be more beneficial in terms of OSI reduction than performing LRMs for other reasons (Table 3). This might be explained by the fact that LRMs are thought to potentiate the recruiting effect induced by changing the patient’s position [27]. Overall, we observed that OSI was markedly reduced by LRM when FiO2 was high regardless of the indication to perform the LRM. However, we need to mention that it remains unclear to what extent an episodic reduction in OSI has a lung protective effect. Our observations of the effect on OSI by LRMs remain hypothetical and further research is needed for confirmation.
This study has some limitations. Firstly, changes in lung volume during the recruitment maneuvers were not measured directly and the cumulative OSI might not be an adequate parameter to assess lung volume. However, to date, no bedside parameter other than oxygenation is established to guide LRMs. Moreover, data suggest that a reduction in cumulative FiO2 may reduce the incidence of BPD [28]. In addition, the average product of FiO2 and MAP over ventilation time has been identified as an independent predictor of BPD and necrotizing enterocolitis [29]. OSI serves as an alternative to the product of FiO2 and MAP, taking into account different SpO2 targets [30]. Secondly, the assumption that the 25% reduction in OSI after a single LRM as reported by Zanin et al. would expand to the cumulative OSI during HFOV was speculative [13]. We found an average reduction of OSI for all LRMs of 22% confirming the results by Zanin et al. but could not find any difference in the cumulative OSI between the groups since the average number of LRMs did not differ. However, the cumulative OSI appeared to be an independent predictor for BPD which supports its use for sample size estimation. Thirdly, we chose to perform LRMs by clinical indicators for lung collapse only. Therefore, we were not able to report the main cause of lung collapse or increasing FiO2 prior to every LRM which could have been helpful to classify the respiratory condition.
Conclusion
During HFOV, stepwise oxygenation-guided LRMs were performed at least once in 12 h when using clinical indicators for collapsed lung units in extremely preterm infants. Imposing scheduled LRMs twice a day did not improve oxygenation. LRMs seem to be most effective at high oxygen requirement and should be considered particularly after changing the patient’s position facilitating the increase of lung compliance.
Availability of data and materials
The data will be made available from the corresponding author on reasonable request.
Abbreviations
- BPD:
-
Bronchopulmonary dysplasia
- FiO2:
-
Fraction of inspired oxygen
- HFOV:
-
High-frequency oscillatory ventilation
- IVH:
-
Intraventricular hemorrhage
- LRM:
-
Lung recruitment maneuver
- MAP:
-
Mean airway pressure
- OSI:
-
Oxygen saturation index
- PMA:
-
Postmenstrual age
- PTX:
-
Pneumothorax
- PIE:
-
Pulmonary interstitial emphysema
- S/F:
-
Oxygen saturation to fraction of inspired oxygen ratio
- SpO2:
-
Peripheral oxygen saturation
References
Dargaville PA, Tingay DG. Lung protective ventilation in extremely preterm infants. J Paediatr Child Health. 2012;48(9):740–6.
Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Te Pas A, Plavka R, Roehr CC, Saugstad OD, Simeoni U, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome – 2019 Update. Neonatology. 2019;115(4):432–50.
Kribs A, Roll C, Gopel W, Wieg C, Groneck P, Laux R, Teig N, Hoehn T, Bohm W, Welzing L, et al. Nonintubated surfactant application vs conventional therapy in extremely Preterm Infants: a Randomized Clinical Trial. JAMA Pediatr. 2015;169(8):723–30.
Schmolzer GM, Kumar M, Pichler G, Aziz K, O’Reilly M, Cheung PY. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ. 2013;347:f5980.
Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157(1):294–323.
Berger TM, Fontana M, Stocker M. The journey towards lung protective respiratory support in preterm neonates. Neonatology. 2013;104(4):265–74.
van Kaam AH, Rimensberger PC. Lung-protective ventilation strategies in neonatology: what do we know–what do we need to know? Crit Care Med. 2007;35(3):925–31.
Cools F, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2015;(3):CD000104. https://doi.org/10.1002/14651858.CD000104.pub4.
Rimensberger PC, Beghetti M, Hanquinet S, Berner M. First intention high-frequency oscillation with early lung volume optimization improves pulmonary outcome in very low birth weight infants with respiratory distress syndrome. Pediatrics. 2000;105(6):1202–8.
Salvo V, Zimmermann LJ, Gavilanes AW, Barberi I, Ricotti A, Abella R, Frigiola A, Giamberti A, Florio P, Tagliabue P, et al. First intention high-frequency oscillatory and conventional mechanical ventilation in premature infants without antenatal glucocorticoid prophylaxis. Pediatr Crit Care Med. 2012;13(1):72–9.
Sun H, Cheng R, Kang W, Xiong H, Zhou C, Zhang Y, Wang X, Zhu C. High-frequency oscillatory ventilation versus synchronized intermittent mandatory ventilation plus pressure support in preterm infants with severe respiratory distress syndrome. Respir Care. 2014;59(2):159–69.
Bond DM, McAloon J, Froese AB. Sustained inflations improve respiratory compliance during high-frequency oscillatory ventilation but not during large tidal volume positive-pressure ventilation in rabbits. Crit Care Med. 1994;22(8):1269–77.
Zannin E, Doni D, Ventura ML, Fedeli T, Rigotti C, Dellaca RL, Tagliabue PE. Relationship between Mean Airways pressure, lung mechanics, and right ventricular output during high-frequency Oscillatory Ventilation in Infants. J Pediatr. 2017;180:110–5.
De Jaegere A, van Veenendaal MB, Michiels A, van Kaam AH. Lung recruitment using oxygenation during open lung high-frequency ventilation in preterm infants. Am J Respir Crit Care Med. 2006;174(6):639–45.
Miedema M, de Jongh FH, Frerichs I, van Veenendaal MB, van Kaam AH. Changes in lung volume and ventilation during lung recruitment in high-frequency ventilated preterm infants with respiratory distress syndrome. J Pediatr. 2011;159(2):199–205 e192.
De Jaegere AP, Deurloo EE, van Rijn RR, Offringa M, van Kaam AH. Individualized lung recruitment during high-frequency ventilation in preterm infants is not associated with lung hyperinflation and air leaks. Eur J Pediatr. 2016;175(8):1085–90.
McCulloch PR, Forkert PG, Froese AB. Lung volume maintenance prevents lung injury during high frequency oscillatory ventilation in surfactant-deficient rabbits. Am Rev Respir Dis. 1988;137(5):1185–92.
de Waal K, Evans N, van der Lee J, van Kaam A. Effect of lung recruitment on pulmonary, systemic, and ductal blood flow in preterm infants. J Pediatr. 2009;154(5):651–5.
Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, Ryan RM, Kallapur SG, Steinhorn RH, Konduri GG, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. 2018;197:300–8.
Mols G, Priebe HJ, Guttmann J. Alveolar recruitment in acute lung injury. Br J Anaesth. 2006;96(2):156–66.
Lachmann B. Open up the lung and keep the lung open. Intensive Care Med. 1992;18(6):319–21.
Dantzker DR, Lynch JP, Weg JG. Depression of cardiac output is a mechanism of shunt reduction in the therapy of acute respiratory failure. Chest. 1980;77(5):636–42.
Thome U, Topfer A, Schaller P, Pohlandt F. Effects of mean airway pressure on lung volume during high-frequency oscillatory ventilation of preterm infants. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1213–8.
Curley MA, Hibberd PL, Fineman LD, Wypij D, Shih MC, Thompson JE, Grant MJ, Barr FE, Cvijanovich NZ, Sorce L, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA. 2005;294(2):229–37.
Fineman LD, LaBrecque MA, Shih MC, Curley MA. Prone positioning can be safely performed in critically ill infants and children. Pediatr Crit Care Med. 2006;7(5):413–22.
Allareddy V, Cheifetz IM. Clinical trials and future directions in pediatric acute respiratory distress syndrome. Ann Transl Med. 2019;7(19):514.
Lupton-Smith A, Argent A, Rimensberger P, Frerichs I, Morrow B. Prone positioning improves Ventilation Homogeneity in Children with Acute Respiratory Distress Syndrome. Pediatr Crit Care Med. 2017;18(5):e229–34.
Wai KC, Kohn MA, Ballard RA, Truog WE, Black DM, Asselin JM, Ballard PL, Rogers EE, Keller RL. Trial of late surfactant study G: early cumulative Supplemental Oxygen predicts bronchopulmonary dysplasia in high risk extremely low gestational age newborns. J Pediatr. 2016;177:97–102 e102.
Thome UH, Dreyhaupt J, Genzel-Boroviczeny O, Bohnhorst B, Schmid M, Fuchs H, Rohde O, Avenarius S, Topf HG, Zimmermann A, et al. Influence of PCO2 Control on Clinical and Neurodevelopmental Outcomes of Extremely Low Birth Weight Infants. Neonatology. 2018;113(3):221–30.
Rawat M, Chandrasekharan PK, Williams A, Gugino S, Koenigsknecht C, Swartz D, Ma CX, Mathew B, Nair J, Lakshminrusimha S. Oxygen saturation index and severity of hypoxic respiratory failure. Neonatology. 2015;107(3):161–6.
Acknowledgements
The authors deeply thank all parents and their children who participated in the present study.
Funding
This study was partially funded by the Medical Scientific Fund of the Mayor of the City of Vienna (Project number 19103).
Author information
Authors and Affiliations
Contributions
T.W., L.A., and E.K. conceptualized and designed the study, collected data, carried out the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript. L.P., K.G., and A.B. designed the data collection instruments, critically reviewed the manuscript for important intellectual content, and revised the manuscript. M.H. and K.K.-S. conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of the Medical University of Vienna (EK 1161/2019). All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from parents or legal guardian of each infant prior to the study participation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Werther, T., Kueng, E., Aichhorn, L. et al. Regular lung recruitment maneuvers during high-frequency oscillatory ventilation in extremely preterm infants: a randomized controlled trial. BMC Pediatr 22, 710 (2022). https://doi.org/10.1186/s12887-022-03780-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03780-7