Abstract
Background
The use of genome-wide sequencing in pediatric medicine and research is growing exponentially. While this has many potential benefits, the normative and empirical literature has highlighted various ethical issues. There have not been, however, any systematic reviews of these issues. The aim of this systematic review is to determine systematically the spectrum of ethical issues that is raised for stakeholders in in pediatric genome-wide sequencing.
Methods
A systematic review in PubMed and Google Books (publications in English or German between 2004 and 2021) was conducted. Further references were identified via reference screening. Data were analyzed and synthesized using qualitative content analysis. Ethical issues were defined as arising when a relevant normative principle is not adequately considered or when two principles come into conflict.
Results
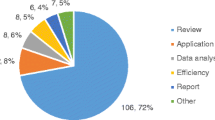
Our literature search retrieved 3175 publications of which 143 were included in the analysis. Together these mentioned 106 ethical issues in pediatric genome-wide sequencing, categorized into five themes along the pediatric genome-wide sequencing lifecycle. Most ethical issues identified in relation to genome-wide sequencing typically reflect ethical issues that arise in general genetic testing, but they are often amplified by the increased quantity of data obtained, and associated uncertainties. The most frequently discussed ethical aspects concern the issue of unsolicited findings.
Conclusion
Concentration of the debate on unsolicited findings risks overlooking other ethical challenges. An overarching difficulty presents the terminological confusion: both with regard to both the test procedure/ the scope of analysis, as well as with the topic of unsolicited findings. It is important that the genetics and ethics communities together with other medical professions involved work jointly on specific case related guidelines to grant the maximum benefit for the care of the children, while preventing patient harm and disproportionate overload of clinicians and the healthcare system by the wealth of available options and economic incentives to increase testing.
Similar content being viewed by others
Background
Genome-wide sequencing, as whole exome or whole genome sequencing (WGS/ WES), can be used to identify variations in a person’s genetic code that might lead to impaired development and disease or disability, that might be ‘otherwise undetectable through clinical history, physical examination, and biochemical or metabolic tests’ [1]. With genome-wide sequencing becoming increasingly faster and more affordable, it is expected that it will have an enormous impact on scientific research, clinical practice, and wider society [2,3,4]. The use of genome-wide sequencing in pediatrics is particularly growing exponentially, and it is hoped that it will help children with undiagnosed genetic diseases to end their diagnostic odyssey sooner and cheaper [5,6,7,8]. The diagnostic and clinical utility of WGS and WES in children with suspected monogenic disorders has been demonstrated in various studies [9,10,11]. Projects such as the Deciphering Developmental Disorders study, offering exome sequencing to children with severe developmental disorders, estimates that if a clinical exome was offered as a first line diagnostic test to children and their parents, over half of these children would instantly receive a diagnosis [12, 13].
In addition to the potential benefits of genome-wide sequencing in pediatrics, however, the empirical and normative literature has also highlighted a number of important regulatory and ethical challenges [14,15,16,17,18,19,20,21,22,23,24]. These ethical issues are often even more challenging in the context of genome-wide sequencing in children, as parents then make decisions for them: complex issues around the child’s future autonomy, parental autonomy, the best interests of the patient, and also the best interests of the wider family have to be considered [21, 25, 26]. Decision-making here needs to integrate not only concern for the long-term welfare of a child or young person, or possible future children, but also for other members of the family. In biomedical ethics, ethical challenges are commonly evaluated using the four Principles of Biomedical Ethics [27]: Beneficence, nonmaleficence, respect for autonomy, and justice.
To date, however, there have not been any systematic reviews of these ethical challenges. Although there have been previous review articles conducted on ethical issues in genome-wide sequencing [2, 28,29,30], these have been either narrative (non-systematic) reviews or limited in scope due to their focus on a few particular issues or on empirical research only.
With the increasing use of genome-wide sequencing, non-genetic medical specialties, such as pediatricians, are also increasingly confronted with it: They are often the first to see and know the affected patient and their families best; they provide pre- and post-test care; they are able to make a referral to a geneticist or, in some countries, can order genome-wide sequencing of their pediatric patients themselves. It is important that these health care practitioners have a comprehensive overview of ethical issues that may arise to guide their decision-making. This systematic qualitative review aims to determine systematically the spectrum of ethical issues that is raised for stakeholders in in pediatric genome-wide sequencing.
Methods
The methods of the study are presented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) as far as they are applicable to qualitative analysis [31].
Inclusion criteria
To be included, publications had to describe and/or assess an ethical issue involved in genome-wide sequencing with children via either conceptual or empirical methods. The definition of ethical issues was based on principlism [27] which has been successfully used in other systematic qualitative ethics reviews [32,33,34,35]. It was assumed that an ethical issue arises when 1) one or more principles have been neglected, or 2) because of conflicts between two or more ethical principles. Genome-wide sequencing was considered to include the terms (whole) genome sequencing, (whole) exome sequencing, genomic sequencing, genome-wide sequencing, genome scale sequencing or complete genome sequencing.
Due to the composition of our research team, only publications in English or German were included. Furthermore, publications needed to be a journal article, book or book chapter, or a national-level report published from April 14, 2003 to May 28, 2021. The date limit was added at the last step of the PubMed search, all other inclusion criteria were applied when screening Google Books and PubMed results. Publications before that date were excluded, because the Human Genome Project had not been completed [36]. The methodological quality, beyond the fact that the paper was identified in scientific databases and published in peer-reviewed journals, did not serve as a criterion of eligibility criteria, as the quality of a publication was irrelevant for the purpose of identifying the spectrum of ethical issues.
Search strategy and data sources
The search terms were developed through an iterative process, where combinations of key words and MeSH terms were piloted in PubMed and the results were assessed for inclusion of a known set of representative literature. This resulting combination of key words and MeSH terms included in the search strategy are presented in Table 1. The search was conducted on May 28, 2021. Google Books was also searched with the search strings “whole genome sequencing” AND ethics as well as “whole exome sequencing” AND ethics. Due to the large number of hits and because Google Books sorts hits by relevance, only the first 100 publications were included. Further publications were identified by screening the reference lists of the included publications.
Study selection
Based on the inclusion criteria, JE along with either SM, DS or BZ independently screened all titles and abstracts in order to assess their eligibility for inclusion for full text screening to insure inter-rater validity. Furthermore, JE and SM screened the back cover descriptions and tables of content of the Google Book’s hits and excluded those not containing any relevant chapters. In case of disagreement, consensus was reached discursively. Full texts of potentially eligible studies were then screened by JE again along with either SM or IK. Excel sheets were used for the entire screening process. Any discrepancies between reviewers during the screening process with regard to the inclusion/exclusion of articles was resolved by consensus.
Data analysis and synthesis
Included full texts were analyzed using conventional qualitative content analysis [37]. Findings were presented as higher- and lower-level categories in a coding frame, which was developed inductively from the data. Only the highest-level codes were generated deductively for a life-cycle perspective; it was assumed that pediatric genome-wide sequencing has five broad phases: (1) the decision regarding when to use genome-wide sequencing, (2) pretest counselling, (3) sequencing, analysis and interpretation, (4) communicating results, and (5) future use of data. JE and SM read and coded five articles purposefully selected to identify inductively as many ethical issues as possible. JE compared the extracted quotes and paraphrases across reviewers and publications and constructed a preliminary coding framework using the qualitative data analysis software MAXQDA. The draft framework was discussed during regular meetings with the research team to increase validity and reliability. For the next five publications, JE and SM again extracted relevant quotes, checking whether the existing coding framework already described the relevant issues, and introduced new categories where necessary. JE integrated the findings and the results were constantly discussed among SM and JE. The remaining publications were analyzed by JE, applying the defined categories and introducing new ones if necessary. Further in-person meetings with co-authors were convened to help resolve any remaining coding problems, and to discuss the framework’s consistency and comprehensibility until all authors agree upon the final matrix of ethical issues.
Results
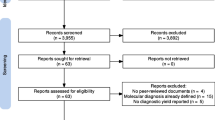
The literature search identified 3175 publications of which 143 were included in the final analysis (see Fig. 1). Of these, 96% (n = 137) were journal articles and the remaining (n = 6) were book chapters. The vast majority of included publications were published after 2014. A list providing full bibliographical information of all 143 publications is provided in Additional file 1.
Ethical issues
A total of 106 distinct ethical issues in the application of genome-wide sequencing in a pediatric population were identified (Table 2). The main findings categorized according to the different phases of the genome-wide sequencing lifecycle include:
-
Issues related to when to use genome-wide sequencing: These ethical issues relate to the questions, if and for which indications pediatric genome-wide sequencing should be used, what the potential risks associated with it are, and the general challenges for the involved clinicians and researchers. For example, the risks of extended newborn screening with WGS/WES, the risks of lacking expertise, training and time especially of non-genetics specialist involved in decision-making, and the risk of injustice due to unequal access to reimbursement by insurances.
-
Issues related to pretest counselling: These ethical issues relate to the general challenges for the informed consent process; including what should be discussed during pretest counselling (e.g. the potential for results to change over time, the potential effects on parent/child bonding), whether there should be different forms of consent and directiveness in counselling depending on the urgency of the situation, the challenges of parental decision-making on behalf of their child, and the challenges to decide how much choice parents should have regarding what types of findings are received.
-
Issues related to sequencing, analysis and interpretation: Here the ethical issues include challenges regarding the infrastructure, such as the risk of inconsistencies and variability due to different bioinformatics pipelines; challenges regarding the interpretation of variants due to the gap between the amount of data which are generated and the knowledge to use them in a clinical context; and the challenge to decide whether it should be actively searched for a certain list of disease-associated genes in every genome-wide sequencing.
-
Issues related to communicating results: The main ethical issues repeatedly raised here relate to the challenge of reporting unsolicited findings (UFs), e.g. the risk of undermining the parents/participants/patients right not to know with an obligatory disclosure of certain UFs; the challenge of balancing the best interests of the child with the best interests of the family regarding the disclosure of UFs; the risk of the diagnosis negatively impacting the parent-child bonding; and the challenges of thoughtfully and effectively framing the results.
-
Issues related to future use of data: These ethical relate mostly to the challenges of data sharing, storage and governance, such as the challenge to determine whose responsibility it is to initiate reanalysis, provide access and recontact patients/parents/participants (especially when pediatric patients reach majority), the risk to privacy and the risk of risk of genetic discrimination (insurance, labor market, access to future medical intervention).
Discussion
To our knowledge, this is the first systematic qualitative review of the full spectrum of ethical issues in pediatric genome-wide sequencing discussed in the literature. Most ethical issues identified in relation to genome-wide sequencing typically reflect ethical issues that arise in general genetic testing in children [80,81,82], but they are often amplified by the increased quantity of data obtained, and associated uncertainties.
The challenges surrounding UFs are a good example of this and were one of the most frequently discussed ethical issues in the literature. UFs have intensified tremendously in genome-wide sequencing, as the likelihood to generate them and their sheer number have increased a lot. These challenges are even bigger and more in pediatric genomic-sequencing as parents then have to make the decisions regarding the reception of UFs for their child. Issues frequently identified in this review include how much choice parents should be given regarding which findings they want to receive for their children; the risk to undermine the right not to know with an obligatory disclosure of UFs; the challenge of interpreting and balancing the best interests of the child with the best interests of the family regarding the disclosure of UFs (see Table 2). The two ethical principles, which are important to consider when debating the challenges regarding the return of UFs, are beneficence and autonomy. The principle of respect for autonomy includes the right that everybody generally should decide intentionally, with understanding and without substantial external influence. Beneficence implies the idea of ‘doing well’ and acting in someone’s best interests [27]. In pediatric genome-wide sequencing, the application of these principles is complicated as decisions are mostly made on behalf of the children. Parents are usually granted the authority to make decisions believed to be in the best interests of their child. However, children have a developing capacity to make autonomous decisions for themselves, and most will have full capacity for autonomous decision-making in the future as adults. Hence, in decision-making regarding UFs in pediatric genomic sequencing, important ethical concepts that recur, might compete and are weighed against each other are the right not to know, the child’s right to an open future and the best interests principle. Potential conflicts can occur between the parental opinion of what is in the best interests of the child, the healthcare professional’s view of what is in the best interests of the child, the parental authority to make decisions for their children, the child’s future autonomy, and the parent’s view of what is in their own best interests. The right not to know can be endangered, for example, if children/adolescents are not involved in the decision-making process and are suddenly confronted with knowledge about an UF; or also if parents unintentionally learn about their own health status through their children’s findings. The child’s right to an open future captures the idea that a pediatric patient in the future will have the capacity to exercise his/her autonomy and that this right should be preserved for them: when parents make decisions for their children now, they should do it in a way that allows the child the greatest possibility to make a decision for her−/himself in the future as adults [83]. It is particularly cited when it comes to UFs of conditions that do not present in childhood. This is especially challenging when these UFs could at the same time be directly relevant for the parents: a frequently cited example is the detection of a BRCA gene mutation, which does not pose an immediate health risk for children themselves, because it only becomes relevant in adulthood; but which could possibly result in immediate medical measures for the parents and avert danger for them [21, 54, 84]. In these cases, the best interests principle is often brought up and discussed whether the consideration of the child’s best interests includes his/her interests to have healthy parents.
The question can be posed whether we should treat medical information from the genome fundamentally different than we treat other medical information (an idea that is often summarized under the term genetic exceptionalism [85,86,87]) and whether the “right not to know” has special weight and role in the context of pediatric genome-wide sequencing. Several authors in the last years have discussed and criticized an absolute right not to know and argued for a more nuanced application of it, also in genomics [88,89,90,91].
In the discussion following the descriptive presentation of the ethical issues identified in the literature, such as ours, it should not be forgotten that these ethical issues have different qualities, risks and practical relevance and therefore require different solutions: First, there are ethical challenges, such as discrimination or unequal access to reimbursement, which could be solved by legal or policy measures (as, for example, the Genetic Information Nondiscrimination Act of the USA aims to do [92]). Second, there are issues raised that should be examined empirically, such as questions of clinical utility and cost-effectiveness or concerns about parental distress. For example, there have been several empirical studies examining the issues of parental distress recently [93] suggesting that these issues may not be of significant concern to those affected. Finally, there are also genuine ethical dilemmas, such as around the right not to know vs. the right to know in the context of UFs, which can only be approached by weighing up the context-specific risks and benefits. It is important to keep in mind that the ethical risks involved and the ethical issues identified are not of all the same importance in every situation, do not carry the same ethical risk and do not all have the same practical implications.
With the increasing use of genome-wide sequencing, non-genetic medical specialties, such as pediatricians, are also increasingly confronted with it: They are often the first to see and know the affected patient and their families best; they provide pre- and post-test care; they are able to make a referral to a geneticist or, in some countries, can order genome-wide sequencing of their pediatric patients themselves. However, studies show that given the complexity of genome-wide sequencing, pediatricians are often uncomfortable with it [94]. Here, not only further training opportunities are required, but also intense and fruitful interdisciplinary cooperation between the various professionals involved is of great importance in order to ensure high-quality long-term care for patients and their families – and to avoid overburdening the various medical specialists involved.
With the increased complexity and potentially difficult ethical decisions associated with genome-wide sequencing, the difficulty of responsibly designing and conducting the informed consent process also increases enormously. It has even been argued that the traditional idea of informed consent might no longer be feasible [38, 60, 61] given the multitude of possible outcomes and complexities and that, at least in certain clinical situations, more directive patient/parent counselling could be necessary [24, 44, 95]. In any case, this means that the importance of good communication and genetic counselling has increased dramatically to enable patients/parents to make decision as well-informed as possible. Providing the necessary resources here, in terms of finance, personnel and time, is of great importance. These points are also supported by the results of the literature analysis provided by Bertier et al. [28] analyzing ‘unsolved challenges in pediatric whole-exome sequencing’ discussed by technology users. Their analysis also emphasizes that counselling presents a major challenge for health care professionals due to the high complexity of issues and that training for effective communication was needed to best enable the patient and his/her family to make informed decisions. Furthermore, they also stress the particular challenges in pediatric genome-wide sequencing as parents here make the decisions on their children’s behalf and due to the higher likelihood to obtain UFs.
Despite the long list of ethical issues, awareness of some appears to be higher than others, as these are discussed a lot more often, questions and challenges around UFs being the most prominent. This observation is supported by the systematic review of technology users view’s about clinical WES by Bertier et al. [30], which reports UFs to be among the three most raised challenges and a steadily increasing proportion of articles debating these. The fact that the only two other systematic reviews that were among our search results [2, 29] were exclusively dedicated to the topic of UFs also indicates that there is a clear focus of the ethical debate on this topic. On the one hand, this is understandable because, as described above, the topic of UFs poses major challenges, particularly in the pediatric context. On the other hand, this concentration of the debate on a few ethical issues might harbor the danger that ethical challenges in the context of genome-wide sequencing are too quickly equated with the topic of unsolicited findings, and thus other equally important points are neglected. For example, one aspect that is only discussed in three texts of our review is collected in the subcode Risk of irresponsible parental data sharing [41, 42, 66]. For a comprehensive debate and responsible use of genome-wide sequencing it is important though, that users are aware of the full spectrum of ethical challenges. This systematic review is also intended to contribute to this end. Of course, this does not mean that all aspects are always equally relevant for every case, but it always depends on the specific situation (e.g. newborns vs. almost adults; seriously ill in emergency situations vs. a disorder where there is medical emergency, etc.). Furthermore, although awareness of the full spectrum of ethical issues is important, they should be balanced against the enormous potential benefits of pediatric genome-wide sequencing in a context-specific manner.
What makes the discussion of the ethical aspects even more difficult, especially for other specialists/non-geneticists, is the confusing terminology. With regard to both the test procedure/ the scope of analysis, as well as with the topic of UFs [24, 96, 97], there is a multitude of terms, some of which are used synonymously, while other authors clearly distinguish them from each other. Thus, for example, for this review it was decided to use the term genome-wide sequencing instead of the term whole genome/exome sequencing used in most articles, since the technology does not even cover the whole exome or genome and furthermore, in most cases of clinical application only a part of the sequenced data is actually looked at. In addition, the term ‘whole’ runs the risk of obscuring the fact that a large part of the data collected cannot yet be meaningfully clinically interpreted and of raising unrealistic expectations not only for patient’s parents but also for non-geneticist medical physicians.
In light of the increased complexity of genome-wide sequencing, including of the ethical challenges, it is of great importance that the necessary resources, financially, in terms of personnel and also in terms of time, are available. This is the only way to ensure that genome-wide sequencing is used responsibly, that despite the described complexity the decisions of patients and parents are made as informed as possible, and that they are also well cared for in the long term. This also includes good cooperation between all professionals involved and sufficient further training opportunities, e.g. for pediatricians, as they are becoming increasingly involved in testing and will play a key role in providing information, support and follow-up for patients and their families. The comprehensive overview of ethical issues, provided in this review, can inform educational material and raise awareness among practitioners and serve as a check-list helping parents and their pediatricians to obtain more information.
Limitations
One limitation of this review might be seen in the fact that the searches were restricted to PubMed and Google Books with relevancy ranking. It is true that although the review is systematic, not all the existing literature dealing with ethical issues concerning genome-wide sequencing might have been included. However, this is not to be considered an overly disadvantageous factor and the approach was considered to be appropriate for various reasons: the search strategy allowed thematic saturation, and the publications that were finally analysed covered journals from all relevant fields (medicine, public health, nursing, social science and philosophy); additionally, former systematic reviews [98, 99] in the bioethics field, which based their research on additional databanks such as EMBASE, CINAHL or Euroethics, found few additional references. Furthermore, as the articles relevant to this review’s pediatric focus were extracted at a later step of our literature search, hence our search algorithm was not specific to the pediatric context. However, it is believed that this made our search more comprehensive. One could further note that our spectrum will not comprehensively give guidance on how to deal with the issues addressed. There are two main reasons for the restriction to the descriptive presentation of the ethical issues. First, the aim is to provide an evidence base for the further assessment of ethical issues. Hence, neither the relevance of single ethical issue is evaluated, nor the best solutions for each issue determined. Second, there are currently no best practice standards for the development of practice recommendation for ethical issues [33]. This includes the lack of well-established methods for the critical appraisal of ethical issues themselves or the corresponding sources/literature. Additionally, because of the heterogeneous use of terms for genome-wide sequencing technologies, it might be possible that some publications were not identified.
Conclusion
This review gives a comprehensive overview of ethical issues in pediatric genome-wide sequencing which are discussed in the literature. It can inform educational material and raise awareness among practitioners. Ethical issues related to the analysis of human DNA in the context of clinical care and research have been discussed continually for the past 50 years. Most issues are not new as such but multiplied and amplified by genome-wide sequencing. This review is a first step to map the huge variety of issues. This is particularly important as awareness of the possibilities, but also the challenges, of genome-wide sequencing for children is becoming increasingly urgent also for other medical fields, i.e. non-geneticists. It highlights the importance that the medical genetics and ethics communities together with other medical professions involved work jointly on specific case related guidelines to grant the maximum benefit for the care of the children, while preventing patient harm and disproportionate overload of clinicians and the healthcare system by the wealth of available options and economic incentives to increase testing.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- HCP:
-
Health care professional
- UF:
-
Unsolicited Findings
- WES:
-
Whole exome sequencing
- WGS:
-
Whole genome sequencing
References
Ontario Health (Quality). Genome-Wide Sequencing for Unexplained Developmental Disabilities or Multiple Congenital Anomalies: A Health Technology Assessment. Ont Health Technol Assess Ser. 2020;20(11):1–178.
Delanne J, Nambot S, Chassagne A, Putois O, Pelissier A, Peyron C, et al. Secondary findings from whole-exome/genome sequencing evaluating stakeholder perspectives. A review of the literature. Eur J Med Genet. 2019;62(6):103529.
Angers A, Bohacova A, Kaye A, Gardner R, Petrillo M, Querci M, et al. JRC science for policy report. Overview of EU National Legislation on Genomicse. 2018. https://ec.europa.eu/jrc/en/publication/overview-eu-national-legislation-genomics. Accessed 2 Dec 2020.
National Human Genome Research Institute. The cost of sequencing a human genome. 2019. https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost. Accessed 2 Dec 2020.
Wu AC, McMahon P, Lu C. Ending the diagnostic odyssey-is whole-genome sequencing the answer? JAMA Pediatr. 2020;174(9):821–2. https://doi.org/10.1001/jamapediatrics.2020.1522.
National Health Service. National Genomic Test Directory FAQ. 2018. https://www.england.nhs.uk/wp-content/uploads/2018/08/national-genomic-test-directory-faqs.pdf. Accessed 2 Dec 2020.
Waldrop MA, Pastore M, Schrader R, Sites E, Bartholomew D, Tsao CY, et al. Diagnostic utility of whole exome sequencing in the neuromuscular clinic. Neuropediatrics. 2019;50(2):96–102. https://doi.org/10.1055/s-0039-1677734.
Levenson D. Whole-exome sequencing emerges as clinical diagnostic tool: testing method proves useful for diagnosing wide range of genetic disorders. Am J Med Genet A. 2014;164a(1):ix–x.
Iglesias A, Anyane-Yeboa K, Wynn J, Wilson A, Truitt Cho M, Guzman E, et al. The usefulness of whole-exome sequencing in routine clinical practice. Genet Med. 2014;16(12):922–31. https://doi.org/10.1038/gim.2014.58.
Kingsmore SF, Cakici JA, Clark MM, Gaughran M, Feddock M, Batalov S, et al. A randomized, controlled trial of the analytic and diagnostic performance of singleton and trio, rapid genome and exome sequencing in ill infants. Am J Hum Genet. 2019;105(4):719–33. https://doi.org/10.1016/j.ajhg.2019.08.009.
Bertoli-Avella AM, Beetz C, Ameziane N, Rocha ME, Guatibonza P, Pereira C, et al. Successful application of genome sequencing in a diagnostic setting: 1007 index cases from a clinically heterogeneous cohort. Eur J Human Genet. 2021;29(1):141–53. https://doi.org/10.1038/s41431-020-00713-9.
Wright CF, McRae JF, Clayton S, Gallone G, Aitken S, FitzGerald TW, et al. Making new genetic diagnoses with old data: iterative reanalysis and reporting from genome-wide data in 1,133 families with developmental disorders. Genet Med. 2018;20(10):1216–23. https://doi.org/10.1038/gim.2017.246.
Ceyhan-Birsoy O, Murry JB, Machini K, Lebo MS, Yu TW, Fayer S, et al. Interpretation of genomic sequencing results in healthy and ill newborns: results from the BabySeq project. Am J Hum Genet. 2019;104(1):76–93. https://doi.org/10.1016/j.ajhg.2018.11.016.
American College of Medical Genetics and Genomics. Incidental findings in clinical genomics: a clarification. Genet Med. 2013;15(8):664–6.
American society of human genetics updates guidance on genetic testing in children: Group addresses predictive genetic testing, use of secondary findings from genomic sequencing tests. Am J Med Genet A. 2015;167a(10):viii–x.
Abdul-Karim R, Berkman BE, Wendler D, Rid A, Khan J, Badgett T, et al. Disclosure of incidental findings from next-generation sequencing in pediatric genomic research. Pediatrics. 2013;131(3):564–71. https://doi.org/10.1542/peds.2012-0084.
Anderson JA, Hayeems RZ, Shuman C, Szego MJ, Monfared N, Bowdin S, et al. Predictive genetic testing for adult-onset disorders in minors: a critical analysis of the arguments for and against the 2013 ACMG guidelines. Clin Genet. 2015;87(4):301–10. https://doi.org/10.1111/cge.12460.
Ayuso C, Millan JM, Dal-Re R. Management and return of incidental genomic findings in clinical trials. Pharmacogenomics J. 2015;15(1):1–5. https://doi.org/10.1038/tpj.2014.62.
Blackburn HL, Schroeder B, Turner C, Shriver CD, Ellsworth DL, Ellsworth RE. Management of Incidental Findings in the era of next-generation sequencing. Curr Genomics. 2015;16(3):159–74. https://doi.org/10.2174/1389202916666150317232930.
Eno C, Bayrak-Toydemir P, Bean L, Braxton A, Chao EC, El-Khechen D, et al. Misattributed parentage as an unanticipated finding during exome/genome sequencing: current clinical laboratory practices and an opportunity for standardization. Genet Med. 2019;21(4):861–6. https://doi.org/10.1038/s41436-018-0265-4.
Holm IA, McGuire A, Pereira S, Rehm H, Green RC, Beggs AH. Returning a genomic result for an adult-onset condition to the parents of a newborn: insights from the BabySeq project. Pediatrics. 2019;143(Suppl 1):37–43.
Hufnagel SB, Martin LJ, Cassedy A, Hopkin RJ, Antommaria AH. Adolescents' preferences regarding disclosure of incidental findings in genomic sequencing that are not medically actionable in childhood. Am J Med Genet A. 2016;170(8):2083–8. https://doi.org/10.1002/ajmg.a.37730.
Senecal K, Rahimzadeh V, Knoppers BM, Fernandez CV, Avard D, Sinnett D. Statement of principles on the return of research results and incidental findings in paediatric research: a multi-site consultative process. Genome. 2015;58(12):541–8. https://doi.org/10.1139/gen-2015-0092.
Wouters RHP, Cornelis C, Newson AJ, Bunnik EM, Bredenoord AL. Scanning the body, sequencing the genome: dealing with unsolicited findings. Bioethics. 2017;31(9):648–56. https://doi.org/10.1111/bioe.12375.
Anderson JA, Meyn MS, Shuman C, Zlotnik Shaul R, Mantella LE, Szego MJ, et al. Parents perspectives on whole genome sequencing for their children: qualified enthusiasm? J Med Ethics. 2017;43(8):535–9. https://doi.org/10.1136/medethics-2016-103564.
Newson AJ. Whole genome sequencing in children: ethics, choice and deliberation. J Med Ethics. 2017;43(8):540–2. https://doi.org/10.1136/medethics-2016-103943.
Beauchamp TLC, J.F. Principles of biomedical ethics. New York: Oxford University Press; 2009.
Bertier G, Senecal K, Borry P, Vears DF. Unsolved challenges in pediatric whole-exome sequencing: a literature analysis. Crit Rev Clin Lab Sci. 2017;54(2):134–42. https://doi.org/10.1080/10408363.2016.1275516.
Mackley MP, Fletcher B, Parker M, Watkins H, Ormondroyd E. Stakeholder views on secondary findings in whole-genome and whole-exome sequencing: a systematic review of quantitative and qualitative studies. Genet Med. 2017;19(3):283–93. https://doi.org/10.1038/gim.2016.109.
Bertier G, Hetu M, Joly Y. Unsolved challenges of clinical whole-exome sequencing: a systematic literature review of end-users' views. BMC Med Genet. 2016;9(1):52. https://doi.org/10.1186/s12920-016-0213-6.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. https://doi.org/10.1371/journal.pmed.1003583.
Kahrass H, Strech D, Mertz M. The Full Spectrum of Clinical Ethical Issues in Kidney Failure. Findings of a Systematic Qualitative Review. PloS one. 2016;11(3):e0149357.
Mertz M, Kahrass H, Strech D. Current state of ethics literature synthesis: a systematic review of reviews. BMC Med. 2016;14(1):152. https://doi.org/10.1186/s12916-016-0688-1.
Seitzer F, Kahrass H, Neitzke G, Strech D. The full spectrum of ethical issues in the care of patients with ALS: a systematic qualitative review. J Neurol. 2016;263(2):201–9. https://doi.org/10.1007/s00415-015-7867-4.
Strech D, Mertz M, Knüppel H, Neitzke G, Schmidhuber M. The full spectrum of ethical issues in dementia care: systematic qualitative review. Br J Psychiatry. 2013;202(6):400–6. https://doi.org/10.1192/bjp.bp.112.116335.
National Human Genome Research Institute. International Consortium Completes Human Genome Project. 2003. https://www.genome.gov/11006929/2003-release-international-consortium-completes-hgp. Accessed 2 Dec 2020.
Schreier M. Qualitative content analysis in practice. London: Sage; 2012.
Bunnik EM, de Jong A, Nijsingh N, de Wert GM. The new genetics and informed consent: differentiating choice to preserve autonomy. Bioethics. 2013;27(6):348–55. https://doi.org/10.1111/bioe.12030.
Sequenced from the start. Nature. 2013;501(7466):135. https://doi.org/10.1038/501135a.
Landau YE, Lichter-Konecki U, Levy HL. Genomics in newborn screening. J Pediatr. 2014;164(1):14–9. https://doi.org/10.1016/j.jpeds.2013.07.028.
Sabatello M, Appelbaum PS. Raising genomic citizens: adolescents and the return of secondary genomic findings. J Law Med Ethics. 2016;44(2):292–308. https://doi.org/10.1177/1073110516654123.
Clayton EW. How much control do children and adolescents have over genomic testing, parental access to their results, and parental communication of those results to others? J Law Med Ethics. 2015;43(3):538–44. https://doi.org/10.1111/jlme.12296.
Cornelis C, Wouters RHP. Genome Sequencing in Pediatrics: Ethical Issues. In: Biesecker BB, Tibben A, editors. Clinical Genome Sequencing Psychological considerations: Academic Press; 2019. p. 143–56. https://doi.org/10.1016/B978-0-12-813335-4.00009-X.
Gyngell C, Newson AJ, Wilkinson D, Stark Z, Savulescu J. Rapid challenges: ethics and genomic neonatal intensive care. Pediatrics. 2019;143(Suppl 1):S14–21. https://doi.org/10.1542/peds.2018-1099D.
Botkin JR, Belmont JW, Berg JS, Berkman BE, Bombard Y, Holm IA, et al. Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. Am J Hum Genet. 2015;97(1):6–21. https://doi.org/10.1016/j.ajhg.2015.05.022.
Beale S, Sanderson D, Sanniti A, Dundar Y, Boland A. A scoping study to explore the cost-effectiveness of next-generation sequencing compared with traditional genetic testing for the diagnosis of learning disabilities in children. Health Technol Assess. 2015;19(46):1–90. https://doi.org/10.3310/hta19460.
Burke LW. Disclosure of genome sequencing results: are pediatricians ready? Pediatrics. 2015;136(4):e1005–6. https://doi.org/10.1542/peds.2015-1740.
Friedman JM, Bombard Y, Cornel MC, Fernandez CV, Junker AK, Plon SE, et al. Genome-wide sequencing in acutely ill infants: genomic medicine’s critical application? Genet Med. 2019;21(2):498–504. https://doi.org/10.1038/s41436-018-0055-z.
Lantos JD. The false-negative phenotype. Pediatrics. 2019;143(Suppl 1):S33–6. https://doi.org/10.1542/peds.2018-1099G.
Knoppers BM, Avard D, Senecal K, Zawati MH. Return of whole-genome sequencing results in paediatric research: a statement of the P3G international paediatrics platform. Eur J Human Genet. 2014;22(1):3–5. https://doi.org/10.1038/ejhg.2013.176.
Howard HC, Knoppers BM, Cornel MC, Wright Clayton E, Senecal K, Borry P. Whole-genome sequencing in newborn screening? A statement on the continued importance of targeted approaches in newborn screening programmes. Eur J Human Genet. 2015;23(12):1593–600. https://doi.org/10.1038/ejhg.2014.289.
May T, Zusevics KL, Strong KA. On the ethics of clinical whole genome sequencing of children. Pediatrics. 2013;132(2):207–9. https://doi.org/10.1542/peds.2012-3788.
Bowdin SC, Hayeems RZ, Monfared N, Cohn RD, Meyn MS. The SickKids genome clinic: developing and evaluating a pediatric model for individualized genomic medicine. Clin Genet. 2016;89(1):10–9. https://doi.org/10.1111/cge.12579.
Johnson LM, Hamilton KV, Valdez JM, Knapp E, Baker JN, Nichols KE. Ethical considerations surrounding germline next-generation sequencing of children with cancer. Expert Rev Mol Diagn. 2017;17(5):523–34. https://doi.org/10.1080/14737159.2017.1316665.
Char D. Preventive genomic sequencing and Care of the Individual Patient. Am J Bioethics. 2015;15(7):32–3. https://doi.org/10.1080/15265161.2015.1039725.
Deem MJ. Whole-genome sequencing and disability in the NICU: exploring practical and ethical challenges. Pediatrics. 2016;137(Suppl 1):S47–55. https://doi.org/10.1542/peds.2015-3731I.
Thornock BS. A strategic stakeholder approach for addressing further analysis requests in whole genome sequencing research. Life Sci Soc Policy. 2016;12(1):4. https://doi.org/10.1186/s40504-016-0037-3.
Lunshof JE. Whole genomes, small children, big questions. Per Med. 2012;9(7):667–9. https://doi.org/10.2217/pme.12.75.
Rotz SJ, Kodish E. Ethical conundrums in pediatric genomics. Hematol Am Soc Hematol Educ Program. 2018;2018(1):301–6. https://doi.org/10.1182/asheducation-2018.1.301.
Burke K, Clarke A. The challenge of consent in clinical genome-wide testing. Arch Dis Child. 2016;101(11):1048–52. https://doi.org/10.1136/archdischild-2013-304109.
Wilfond BS, Diekema DS. Engaging children in genomics research: decoding the meaning of assent in research. Genet Med. 2012;14(4):437–43. https://doi.org/10.1038/gim.2012.9.
Li KC, Birch PH, Garrett BM, MacPhee M, Adam S, Friedman JM. Parents' perspectives on supporting their decision making in genome-wide sequencing. J Nurs Scholarsh. 2016;48(3):265–75. https://doi.org/10.1111/jnu.12207.
Oberg JA, Glade Bender JL, Cohn EG, Morris M, Ruiz J, Chung WK, et al. Overcoming challenges to meaningful informed consent for whole genome sequencing in pediatric cancer research. Pediatr Blood Cancer. 2015;62(8):1374–80. https://doi.org/10.1002/pbc.25520.
Berg JS, Powell CM. Potential Uses and Inherent Challenges of Using Genome-Scale Sequencing to Augment Current Newborn Screening. Cold Spring Harb Perspect Med. 2015;5(12):a023150.
Hens K, Dierickx K. Double trouble: preventive genomic sequencing and the case of minors. Am J Bioethics. 2015;15(7):30–1. https://doi.org/10.1080/15265161.2015.1039723.
Sabatello M, Appelbaum PS. Honey, I sequenced the kids: preventive genomics and the complexities of adolescence. Am J Bioeth. 2015;15(7):19–21. https://doi.org/10.1080/15265161.2015.1039722.
McCullough LB, Brothers KB, Chung WK, Joffe S, Koenig BA, Wilfond B, et al. Professionally responsible disclosure of genomic sequencing results in pediatric practice. Pediatrics. 2015;136(4):e974–82. https://doi.org/10.1542/peds.2015-0624.
Rosell AM, Pena LD, Schoch K, Spillmann R, Sullivan J, Hooper SR, et al. Not the end of the odyssey: parental perceptions of whole exome sequencing (WES) in pediatric undiagnosed disorders. J Genet Couns. 2016;25(5):1019–31. https://doi.org/10.1007/s10897-016-9933-1.
Dimmock D. Whole genome sequencing: a considered approach to clinical implementation. Curr Protoc Hum Genet. 2013;9:9.22.
McGowan ML, Prows CA, DeJonckheere M, Brinkman WB, Vaughn L, Myers MF. Adolescent and parental attitudes about return of genomic research results: focus group findings regarding decisional preferences. J Empir Res Hum Res Ethics. 2018;13(4):371–82. https://doi.org/10.1177/1556264618776613.
Bell SG. Ethical implications of rapid whole-genome sequencing in neonates. Neonatal Netw. 2018;37(1):42–4. https://doi.org/10.1891/0730-0832.37.1.42.
Werner-Lin A, Zaspel L, Carlson M, Mueller R, Walser SA, Desai R, et al. Gratitude, protective buffering, and cognitive dissonance: how families respond to pediatric whole exome sequencing in the absence of actionable results. Am J Med Genet A. 2018;176(3):578–88. https://doi.org/10.1002/ajmg.a.38613.
Reinstein E. Challenges of using next generation sequencing in newborn screening. Genet Res (Camb). 2015;97:e21.
Lantos JD. Ethical and psychosocial issues in whole genome sequencing (WGS) for newborns. Pediatrics. 2019;143(Suppl 1):S1–5. https://doi.org/10.1542/peds.2018-1099B.
Vears DF, Senecal K, Clarke AJ, Jackson L, Laberge AM, Lovrecic L, et al. Points to consider for laboratories reporting results from diagnostic genomic sequencing. Eur J Human Genet. 2018;26(1):36–43. https://doi.org/10.1038/s41431-017-0043-9.
Tarini BA, Goldenberg AJ. Ethical issues with newborn screening in the genomics era. Annu Rev Genomics Hum Genet. 2012;13(1):381–93. https://doi.org/10.1146/annurev-genom-090711-163741.
Holm IA, Savage SK, Green RC, Juengst E, McGuire A, Kornetsky S, et al. Guidelines for return of research results from pediatric genomic studies: deliberations of the Boston Children’s Hospital Gene Partnership informed cohort oversight board. Genet Med. 2014;16(7):547–52. https://doi.org/10.1038/gim.2013.190.
Green RC, Goddard KAB, Jarvik GP, Amendola LM, Appelbaum PS, Berg JS, et al. Clinical sequencing exploratory research consortium: accelerating evidence-based practice of genomic medicine. Am J Hum Genet. 2016;98(6):1051–66. https://doi.org/10.1016/j.ajhg.2016.04.011.
Friedman JM, Cornel MC, Goldenberg AJ, Lister KJ, Senecal K, Vears DF. Genomic newborn screening: public health policy considerations and recommendations. BMC Med Genet. 2017;10(1):9. https://doi.org/10.1186/s12920-017-0247-4.
Borry P, Stultiens L, Nys H, Cassiman JJ, Dierickx K. Presymptomatic and predictive genetic testing in minors: a systematic review of guidelines and position papers. Clin Genet. 2006;70(5):374–81. https://doi.org/10.1111/j.1399-0004.2006.00692.x.
Wertz DC, Fanos JH, Reilly PR. Genetic testing for children and adolescents. Who decides? Jama. 1994;272(11):875–81. https://doi.org/10.1001/jama.1994.03520110055029.
Lantos JD. Ethical and psychosocial issues in whole-genome sequencing for newborns. In: Demkow U, Płoski R, editors. Clinical Applications for Next-Generation Sequencing: Academic Press; 2016. p. 295–300.
Feinberg J. Freedom and fulfillment: philosophical essays. New York: Princeton University Press; 1994.
Biesecker BB. Predictive genetic testing of minors: evidence and experience with families. Genet Med. 2016;18(8):763–4. https://doi.org/10.1038/gim.2015.191.
Rothstein MA. Genetic exceptionalism and legislative pragmatism. J Law, Med Ethics. 2007;35(2 Suppl):59–65. https://doi.org/10.1111/j.1748-720X.2007.00154.x.
Evans JP, Burke W. Genetic exceptionalism. Too much of a good thing? Genet Med. 2008;10(7):500–1. https://doi.org/10.1097/GIM.0b013e31817f280a.
Garrison NA, Brothers KB, Goldenberg AJ, Lynch JA. Genomic Contextualism: shifting the rhetoric of genetic exceptionalism. Am J Bioethics. 2019;19(1):51–63. https://doi.org/10.1080/15265161.2018.1544304.
Davies B. The right not to know and the obligation to know. J Med Ethics. 2020;46(5):300–3. https://doi.org/10.1136/medethics-2019-106009.
Berkman B. Commentary on ‘The right not to know and the obligation not to know’. J Med Ethics. 2020;46(5):304–5. https://doi.org/10.1136/medethics-2020-106082.
Davies B, Savulescu J. The right not to know: some steps towards a compromise. Ethical Theory Moral Pract. 2021;24(1):137–50. https://doi.org/10.1007/s10677-020-10133-9.
Vears DF. Should we respect parents’ views about which results to return from genomic sequencing? Human genetics; 2021.
Genetic Information Nondiscrimination Act of 2008. United States of America, 2008: 122 STAT. 881. PUBL233.PS (govinfo.gov) (assessed 24.8.2021).
Hart MR, Biesecker BB, Blout CL, Christensen KD, Amendola LM, Bergstrom KL, et al. Secondary findings from clinical genomic sequencing: prevalence, patient perspectives, family history assessment, and health-care costs from a multisite study. Genet Med. 2019;21(5):1100–10. https://doi.org/10.1038/s41436-018-0308-x.
Szego MJ, Meyn MS, Shuman C, Zlotnik Shaul R, Anderson JA, Bowdin S, et al. Views from the clinic: healthcare provider perspectives on whole genome sequencing in paediatrics. Eur J Med Genet. 2019;62(5):350–6. https://doi.org/10.1016/j.ejmg.2018.11.029.
De Wert GM, Dondorp WJ, Knoppers BM. Preconception care and genetic risk: ethical issues. J Commu Genet. 2012;3(3):221–8. https://doi.org/10.1007/s12687-011-0074-9.
Saelaert M, Mertes H, De Baere E, Devisch I. Incidental or secondary findings: an integrative and patient-inclusive approach to the current debate. Eur J Human Genet. 2018;26(10):1424–31. https://doi.org/10.1038/s41431-018-0200-9.
Vears DF, Niemiec E, Howard HC, Borry P. How do consent forms for diagnostic high-throughput sequencing address unsolicited and secondary findings? A content analysis. Clin Genet. 2018;94(3–4):321–9. https://doi.org/10.1111/cge.13391.
Sofaer N, Strech D. Reasons why post-trial access to trial drugs should, or need not be ensured to research participants: a systematic review. Public health ethics. 2011;4(2):160–84. https://doi.org/10.1093/phe/phr013.
Strech D, Persad G, Marckmann G, Danis M. Are physicians willing to ration health care? Conflicting findings in a systematic review of survey research. Health Policy (Amsterdam, Netherlands). 2009;90(2–3):113–24.
Acknowledgements
Not applicable.
Funding
This study was funded internally.
Author information
Authors and Affiliations
Contributions
JE drafted the protocol, collected, analyzed and interpreted the data and wrote the manuscript. BE contributed to the development of the selection criteria and data extraction criteria, interpreted the data and critically revised the manuscript. IK collected data and critically revised the manuscript. IF provided feedback on the collected data and critically revised the manuscript. DS collected data and critically revised the manuscript. BZ collected data and critically revised the manuscript. SM supported JE in designing the study, contributed to the development of the selection criteria and data extraction criteria, collected, analyzed and interpreted data and critically revised the manuscript. All authors read, provided feedback and approved the final protocol.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Bibliographical Information of Analysed Publications. A list providing full bibliographical information of all 87 publications included in the analysis of this systematic review.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Eichinger, J., Elger, B.S., Koné, I. et al. The full spectrum of ethical issues in pediatric genome-wide sequencing: a systematic qualitative review. BMC Pediatr 21, 387 (2021). https://doi.org/10.1186/s12887-021-02830-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-021-02830-w