Abstract
Purpose
Acute Kidney Injury (AKI) in COVID-19 patients is associated with increased morbidity and mortality. In the present study, we aimed to develop a prognostic score to predict AKI development in these patients.
Materials and methods
This was a retrospective observational study of 2334 COVID 19 patients admitted to 23 different hospitals in Brazil, between January 10th and August 30rd, 2020. The primary outcome of AKI was defined as any increase in serum creatinine (SCr) by 0.3 mg/dL within 48 h or a change in SCr by ≥ 1.5 times of baseline within 1 week, based on Kidney Disease Improving Global Outcomes (KDIGO) guidelines. All patients aged ≥ 18 y/o admitted with confirmed SARS-COV-2 infection were included. Discrimination of variables was calculated by the Receiver Operator Characteristic Curve (ROC curve) utilizing area under curve. Some continuous variables were categorized through ROC curve. The cutoff points were calculated using the value with the best sensitivity and specificity.
Results
A total of 1131 patients with COVID-19 admitted to the ICU were included. Patients mean age was 52 ± 15,8 y/o., with a prevalence of males 60% (n = 678). The risk of AKI was 33% (n = 376), 78% (n = 293) of which did not require dialysis. Overall mortality was 11% (n = 127), while for AKI patients, mortality rate was 21% (n = 80). Variables selected for the logistic regression model and inclusion in the final prognostic score were the following: age, diabetes, ACEis, ARBs, chronic kidney disease and hypertension.
Conclusion
AKI development in COVID 19 patients is accurately predicted by common clinical variables, allowing early interventions to attenuate the impact of AKI in these patients.
Similar content being viewed by others
Introduction
SARS-COV-2 was initially described in December 2019 in Wuhan, China and rapidly escalated to a pandemic in March 2020 [1, 2]. Since this time, our understanding of COVID-19 disease has evolved, but the natural history of this serious disease has not changed. The increasingly recognized systemic involvements such as acute kidney injury (AKI), stroke and myocardial injury contributes to the complexity and poor outcomes in many patients [3,4,5,6].
AKI is of particular interest and is associated with greater incidence, morbidity and mortality in COVID-19 patients, especially in cases with serious pulmonary disease and need for mechanical ventilation, probably as a result of the kidney-lung relationship previously described by other authors [7, 8]. Recently, some authors observed an incidence of AKI in COVID-19 patients varying from 25 to 57%, depending on the population studied and AKI criteria, with higher incidences described in critically ill patients [9, 10]. Numerous studies attempted to characterize the clinical course of renal disease in COVID-19 patients, but the results are variable [10,11,12,13,14].
Based on numerous studies, researchers believe that angiotensin-converting enzyme (ACE2) receptor serves as a co-transporter for SARS-COV-2 to enter the cells. Although the etiology of renal damage in patients with COVID-19 is multifatorial and associated with multiple mechanisms such as hypovolemia, rhabdomyolysis, microthrombosis, inflammation and virus-induced damage to tubular cells [15,16,17], it is believed that ACE2 receptors may play a role, given the high affinity of SARS-COV-2 for ACE2 receptors presented at high concentrations in the brush borders of renal tubular epitelial cells and medications such as angiotensin-converting enzyme (ACE) or angiotensin receptor blocker (ARB) may predispose or protect against AKI development in these patients [18,19,20,21,22,23,24].
Recently some prognostic scores to predict the development of critical illness and need for mechanical ventilation have been proposed and validated for COVID-19 patients. Unfortunately, the natural course of AKI development were not mentioned in these studies, precluding any clinical decision about the profile of AKI patients during hospitalization for COVID-19 [25, 26].
The objective of this study was to describe the risk factors associated with AKI in COVID 19 patients admitted to Intensive Care Unit (ICU) and to develop a specific prognostic score with clinical variables (including ACE and ARB use) that could accurately identify high-risk patients for AKI development during hospitalization for COVID-19 related complications.
Materials and methods
This was a retrospective observational study of 2334 COVID-19 patients admitted to 23 different private hospitals in Brazil, between January 10th and August 30rd, 2020. The study was approved by the National Teaching and Ethical Committee (CONEP) under number: 29496920.8.0000.5262 and informed consent was waived due to the observational nature of the study.
The primary outcome of AKI was defined as any increase in serum creatinine (SCr) by 0.3 mg/dL within 48 h or a change in SCr by ≥ 1.5 times of baseline within 1 week, based on Kidney Disease Improving Global Outcomes (KDIGO) guidelines. The lowest SCr reading during hospitalization was used as the baseline for AKI definition. The staging of AKI was also defined according to the KDIGO criteria [27]. We did not use the urine output criteria to define AKI as the documentation of urine output in the electronic health record was unreliable. Chronic Kidney Disease (CKD) was defined as the glomerular filtration rate < 60 mL/min using the CKD-Epidemiology Collaboration equation. Patients on chronic renal replacement therapy were excluded from this analysis.
All patients aged ≥ 18 y/o admitted with confirmed SARS-COV-2 infection were consecutively included. We defined confirmed infection as positive reverse transcriptase-polymerase chain reaction (RT-PCR) from a nasal or throat swab together with clinical symptons or radiological findings suggestive of COVID-19 infection. Data were collected by a highly trained team and included demographic data such as comorbidities and use of medications, in addition to laboratory data at the time of ICU admission and outcomes such as need for mechanical ventilation or vasopressors, hospital discharge and death.
Statistical analysis
The continuous variables were submitted to Kolmogorov–Smirnov and Shapiro–Wilk tests and their values were expressed as median and 25th/75th percentiles or as mean and standard deviation for parametric and non-parametric variables, respectively.
The categorical variables were submitted to Pearson’s chi square or Fisher’s exact test, if applicable, and were presented as absolute values and percentages. Discrimination of was calculated using the ROC (Receiver Operating Characteristic) curve and the categorization of some continuous variables was also performed using the ROC curve. The cut-off scores for each clinical variable were calculated using the p value with the best specificity and sensitivity for that particular variable.
The candidate variables for the multivariable model were included at the p-value of 0.2 in the univariable analysis and a step-by-step method was used to select each significant variable for the final logistic regression model, with the calculations of corresponding adjusted odds ratios (ORs) and 95% confidence intervals (CI).
The AKI Score variables were those that resulted from the COX Regression, being excluded one by one (step by step) until all predictor variables reached significance. The candidate variables for the model were those that reached significance as a value of p < = 0.2 on the Kaplan–Meier Survival Curve using the Log-Rank test. The cutoff points of the continuous variables used in the AKI score were obtained through the ROC curve, corresponding to the highest value of the sum of sensitivity + specificity of all possible cut-off points obtained on the curve.
Variables with missing data up to 10% were: ACEi, ARB, Hypertension and Platelets. Variables with missing data from 10.1% to 20% were: Sodium, Potassium, pH and PCO2. There were no variables with more than 20% missing data of clinical relevance. After this classification, the following criterion was adopted: Variables with up to 10% of missing data were not considered influential and variables with missing data between 10.1% and 20% were performed Canonical Correlation (Canonical Analyses) with Wilk's lambda test to investigate possible biases of association of the missing data with the other variables and with the outcome of the study. Finally, all missing data were submitted to the “Full informationmaximum-likelihood (FIML)”.
Analyses were performed using SPSS 21.0 IBM ® and GraphPad Prism 5.0 GraphPad ® and statistical significance was considered with p ≤ 0,05.
Results
A total of 1131 patients with COVID-19 admitted to the ICU were included. Patients mean age was 52 ± 15,8 y/o., with a prevalence of males 60% (n = 678). Most common comorbidities included hypertension 63.8% (n = 722) and diabetes 23.7% (n = 269). A total of 49% (n = 556) of patients were taking angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) prior to ICU admission. 16% (n = 185) of patients were on vasopressors and 19% (n = 220) were on mechanical ventilation at ICU admission (Table 1).
The risk of AKI was 33% (n = 376), 78% (n = 293) of which did not require dialysis and 22% (n = 83) presented dialytic AKI. Overall mortality was 11% (n = 127), while for AKI patients, mortality rate was 21% (n = 80).
Significant risk factors for AKI development at univariate analysis were the following: comorbidities variables – age, diabetes, hypertension, ACEi or ARB use, chronic obstructive pulmonary disease (COPD) and chronic kidney disease (CKD); ICU admission variables: need for mechanical ventilation or vasopressors, serum potassium > 4.2 mEq/L, serum sodium < 140 mEq/L, pH < 7.35, pCo2 > 48 mmHg, c-reactive protein (CRP) > 8.7 mg/dL and lymphocytes < 720 (Table 2).
Variables selected for the logistic regression model and inclusion in the final prognostic score were the following: age, diabetes, ACEis, ARBs, chronic kidney disease and hypertension (Table 3). After complete clinical evaluation, each variable is added to one another, divided by 17 and multiplied by 100, indicating the risk of developing AKI during ICU stay (COV-AKI Score) (Table 4).
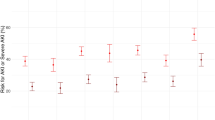
COV-AKI Score presented good performance in the calibration analysis using Hosmer–Lemeshow´s goodness-of-fit test with non-significant difference between the predicted and observed risk for development of AKI in COVID-19 patients during ICU stay (Fig. 1) Discrimination of COV-AKI Score was evaluated by analysis of the area under the receiving operating characteristic (ROC) curve, with an area under the curve (AUC) of 0.78 (0.74–0.81) (Fig. 2).
Discussion
We developed a clinical score to predicit AKI development in COVID-19 patients admitted to the ICU. The variables included in the final model were age, diabetes, chronic kidney disease, hypertension and ARB or ACEi use. This clinical score will enable clinicians to predict the risk of AKI development in COVID-19 patients in order to minimize the risks of renal function decline and further clinical deterioration.
Recently, many clinical scores were developed to predict hospital mortality and ICU transfer or need for mechanical ventilation in COVID-19 patients, but none evaluated the risk for AKI development in these patients [28,29,30,31,32,33]. Wang et al. [34] described a clinical score to predict AKI development in a population of 389 COVID-19 patients, with an AKI incidence of 7.2%, much lower than observed in our study, where 33% of patients developed AKI, probably because we focused on ICU patients with more complex clinical presentations. Our AKI score has a very important feature when compared with other scores developed for AKI prediction in COVID-19 patients. We studied patients admitted to the Intensive Care Unit for the treatment of complications associated with COVID-19. In this way, our score is applied at the time of patient admission to the ICU to predict AKI development during ICU stay, thus selecting a more complex population of patients with worse prognosis and greater chance of developing organ dysfunctions, including kidney failure. Also, other authors did not determine a specific point in time where the score should be applied and combined different populations of patients in the same study, such as patients admitted to the emergency room, ward and the intensive care unit [17, 35,36,37]. Furthermore, Lu et al. [36], describes that the overall prediction performance by Area under Receiver Operating Charactheristic Curve (AUC) was good at day 0, and moderate at day -1 and -2, a finding with doubtful clinical significance, since at that moment (day 0) there is no sufficient time to adopt preventive measures that minimize the risk of developing AKI during hospitalisation.
The presence of advanced age and/or comorbidities, specifically diabetes and hypertension, were frequently included as important variables in many specific scores developed to predict complications in COVID-19 patients [29, 30, 38]. Our study also observed advanced age and diabetes or hypertension as variables associated with AKI development in COVID-19 patients and were included in the final model, probably reflecting a reduced renal reserve associated with the combination of inflammation and microvascular alterations affecting renal fuction and contributing to a greater incidence of AKI in these patients.
Chronic Kidney Disease (CKD) is a well known risk factor for AKI development in different clinical scenarios [39]. In our study, CKD was associated with AKI development in COVID-19 patients and also included in the final model, combining the negative effects of previously damaged kidneys with hyperinflammation, microthrombosis and direct infection of kidney cells seen in these patients.
At the beginning of COVID-19 pandemics, it was unclear if the use of ACEi or ARBs were associated with increased risk of complications and/or severe disease. Indeed, recent studies confirmed that when compared to untreated subjects, those using either ACEi or ARBs showed a similar risk of critical or lethal clinical course associated with COVID-19 infection [19, 21, 40,41,42]. ACE2 (Angiotensin-Converting Enzyme 2) is a extracellular transmembrane enzyme responsible for breaking down angiotensin II into angiotensin heptapeptide and also works as the main receptor for uptake of severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) into the cell. ACEIs and ARBs act on the renin–angiotensin–aldosterone system (RAAS) reducing angiotensin II formation and consequently downregulating ACE2 expression and probably reducing binding of SARS-COV-2 into the kidney cells and protecting against the development of AKI in COVID-19 patients.
The comparison between our score and previous prognostic scores developed to predict complications such as need for mechanical ventilation, ICU transfer or death is not feasible because of different end points. In a recent review, Lombardi e cols [43]. studied the accuracy of 32 scores designed to predict ICU transfer or death and found an area under the receiver operating characteristic curve (AUC ROC curve) > 0.75 in only seven studies. Our score (COV-AKI Score) presented a good discriminative performance to predict AKI development in COVID-19 patients with an AUC ROC curve of 0.78. The COV-AKI Score can be easily calculated at the bedside, without the need for complex laboratory tests or clinical variables hard to compute on a daily basis, making it also appropriate for application in countries with limited resources.
The limitations of the present study are related to the lack of external validity to evaluate model performance in different settings and also limited to ICU patients, precluding any conclusion about the risk profile to AKI development in distinct scenarios such as wards or emergency departments.
Conclusions
Our study identified the main risk factors for the development of AKI in patients with COVID-19 admitted to the Intensive Care Unit and also developed a prognostic score capable of identifying patients at high risk for AKI, facilitating the adoption of preventive measures that minimize the risk of this complication in COVID-19 patients.
Availability of data and materials
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
Ranzani OT, Bastos LSL, Gelli JGM, Marchesi JF, Baião F, Hamacher S, et al. Characterisation of the first 250 000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2021;9(4):407–18. https://doi.org/10.1016/S2213-2600(20)30560-9.
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA - J Am Med Assoc. 2020;323(20):2052–9. https://doi.org/10.1001/jama.2020.6775.
Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020;383(23):2255–73. https://doi.org/10.1056/nejmra2026131.
Goligher EC, Ranieri VM, Slutsky AS. Is severe COVID-19 pneumonia a typical or atypical form of ARDS? And does it matter? Intensive Care Med. 2021;47(1):83–5. https://doi.org/10.1007/s00134-020-06320.
Schmidt M, Hajage D, Dres M, Kimmoun A, Prevost M. Intensive Care Medicine Clinical Characteristics and Day-90 Outcomes of 4,244 critically ill adults with COVID-19: a prospective cohort study COVID-ICU group, for the REVA network and the COVID-ICU investigators*. Intensive Care Med. 2021;47(1):60–73. https://doi.org/10.1007/s00134-020-06294-x.
Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with COVID-19 in New York: retrospective case series. BMJ. 2020;369:1996. https://doi.org/10.1101/2020.04.20.20072116.
Teixeira JP, Ambruso S, Griffin BR, Faubel S. Pulmonary Consequences of Acute Kidney Injury. Semin Nephrol. 2019;39(1):3–16. https://doi.org/10.1016/j.semnephrol.2018.10.001.
Domenech P, Perez T, Saldarini A, Uad P, Musso CG. Kidney–lung pathophysiological crosstalk: its characteristics and importance. Int Urol Nephrol. 2017;49(7):1211–5. https://doi.org/10.1007/s11255-017-1585-z.
Chan L, Chaudhary K, Saha A, et al. AKI in Hospitalized Patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151–60. https://doi.org/10.1681/ASN.2020050615.
Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns T, et al. AKI in Hospitalized Patients with and without COVID-19: A Comparison Study. J Am Soc Nephrol. 2020;31(9):2145–57. https://doi.org/10.1681/ASN.2020040509.
Gagliardi I, Patella G, Michael A, Serra R, Provenzano M, Andreucci M. COVID-19 and the Kidney: From Epidemiology to Clinical Practice. J Clin Med. 2020;9(8):2506. https://doi.org/10.3390/jcm9082506.
Robbins-Juarez SY, Qian L, King KL, Stevens JS, Husain SA, Radhakrishnan J, et al. Outcomes for Patients With COVID-19 and Acute Kidney Injury: A Systematic Review and Meta-Analysis. Kidney Int Reports. 2020;5(8):1149–60. https://doi.org/10.1016/j.ekir.2020.06.013.
Qian J-Y, Wang B, Liu B-C. Acute Kidney Injury in the 2019 Novel Coronavirus Disease. Kidney Dis. 2020;323:1–6. https://doi.org/10.1159/000509086.
Perico L, Benigni A, Remuzzi G. Should COVID-19 Concern Nephrologists? Why and to What Extent? the Emerging Impasse of Angiotensin Blockade. Nephron. 2020;144(5):213–21. https://doi.org/10.1159/000507305.
De Almeida DC, Franco MDCP, Dos Santos DRP, Santos MC, Maltoni IS, Mascotte F, et al. Acute kidney injury: Incidence, risk factors, and outcomes in severe COVID-19 patients. PLoS One. 2021;16(5):e0251048. https://doi.org/10.1371/journal.pone.0251048.
Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–18. https://doi.org/10.1016/j.kint.2020.05.006.
McAdams MC, Xu P, Saleh SN, Li M, Ostrosky-Frid M, Gregg LP, et al. Risk Prediction for Acute Kidney Injury in Patients Hospitalized With COVID-19. KidneyMed. 2022;4(6):100463. https://doi.org/10.1016/j.xkme.2022.100463.
Legrand M, Bell S, Forni L, Joannidis M, Koyner JL, Liu K, et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021;17(11):751–64. https://doi.org/10.1038/s41581-021-00452-0.
Simon Rico-Mesa J, White A, Anderson AS. HOT TOPIC Outcomes in Patients with COVID-19 Infection Taking ACEI/ARB. Curr Cardiol Rep. 2020;22(5):31. https://doi.org/10.1007/s11886-020-01291-4.
Patoulias D, Katsimardou A, Stavropoulos K, Imprialos K, Kalogirou M-S, Doumas M. Renin-Angiotensin System Inhibitors and COVID-19: a Systematic Review and Meta-Analysis. Evidence for Significant Geographical Disparities. Curr Hypertens Rep. 2020;22(11):90. https://doi.org/10.1007/s11906-020-01101.
Flacco ME, Acuti Martellucci C, Bravi F, Parruti G, Cappadona R, Mascitelli A, et al. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID-19: A meta-analysis. Heart. 2020;106(19):1519–24. https://doi.org/10.1136/heartjnl-2020-317336.
Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, et al. COVID-19–Associated Kidney Injury: A Case Series of Kidney Biopsy Findings. J Am Soc Nephrol. 2020;31(9):1948–58. https://doi.org/10.1681/ASN.2020050699.
Farouk SS, Fiaccadori E, Cravedi P, Campbell KN. COVID-19 and the kidney: what we think we know so far and what we don’t. J Nephrol [Internet]. 2020;33(6):1213–8. https://doi.org/10.1007/s40620-020-00789-y.
Farkash EA, Wilson AM, Jentzen JM. Ultrastructural Evidence for Direct Renal Infection with SARS-CoV-2. J Am Soc Nephrol. 2020;31(8):1683–7. https://doi.org/10.1681/ASN.2020040432.
Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–27. https://doi.org/10.1016/j.kint.2020.04.003.
Yadaw AS, Li Y-C, Bose S, Iyengar R, Bunyavanich S, Pandey G. Clinical predictors of COVID-19 mortality. medRxiv Prepr Serv Heal Sci. 2020;2020.05.19.20103036. https://doi.org/10.1101/2020.05.19.20103036. Preprint
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron - Clin Pract. 2012;120(4):179–84. https://doi.org/10.1159/000339789.
Haimovich A, Ravindra NG, Stoytchev S, Young HP, PerryWilson F, van Dijk D, et al. Development and validation of the quick COVID-19 severity index (qCSI): a prognostic tool for early clinical decompensation. Ann EmergMed. 2020;76(4):442–53. https://doi.org/10.1016/j.annemergmed.2020.07.022.
Mejía-Vilet JM, Córdova-Sánchez BM, Fernández-Camargo DA, Méndez-Pérez RA, Morales-Buenrostro LE, Hernández-Gilsoul T. A risk score to predict admission to the intensive care unit in patients with Covid-19: the ABC-GOALS score. Salud Publica Mex. 2020;63(1):1–11. https://doi.org/10.21149/11684.
Tanboğa IH, Canpolat U, Çetin EHÖ, Kundi H, Çelik O, Çağlayan M, et al. Development and validation of clinical prediction model to estimate the probability of death in hospitalized patients with COVID-19: Insights from a nationwide database. J Med Virol. 2021;93(5):3015–22. https://doi.org/10.1002/jmv.26844.
Sun H, Jain A, Leone MJ, Alabsi HS, Brenner LN, Ye E, et al. CoVA: An Acuity Score for Outpatient Screening that Predicts COVID-19 Prognosis. J Infect Dis. 2021;223(1):38–46. https://doi.org/10.1093/infdis/jiaa663/5938525.
Gupta RK, Harrison EM, Ho A, Docherty AB, Knight SR, van Smeden M, et al. Development and validation of the ISARIC 4C Deterioration model for adults hospitalised with COVID-19: a prospective cohort study. Lancet Respir Med. 2021;9(4):349–59. https://doi.org/10.1016/S2213-2600(20)30559-2.
Paranjape N, Staples LL, Stradwick CY, Ray HG, Saldanha IJ. Development and validation of a predictive model for critical illness in adult patients requiring hospitalization for COVID-19. PLoS One. 2021;16(3):e0248891. https://doi.org/10.1371/journal.pone.0248891.
Wang RR, He M, Kang Y. A risk score based on procalcitonin for predicting acute kidney injury in COVID-19 patients. J Clin Lab Anal. 2021;35(6):1–9. https://doi.org/10.1002/jcla.23805.
Lu JY, Hou W, Duong TQ. Longitudinal prediction of hospital-acquired acute kidney injury in COVID-19: a two-center study. Infection. 2022;50(1):109–19. https://doi.org/10.1007/s15010-021-01646-1.
Lu JY, Zhu J, Zhu J, Duong TQ. Long-short-term memory machine learning of longitudinal clinical data accurately predicts acute kidney injury onset in COVID-19: a two-center study. Int J Infect Dis. 2022;122:802–10. https://doi.org/10.1016/j.ijid.2022.07.034.
Ponce D, de Andrade LGM, Del GRC, Ferreiro-Fuentes A, Lombardi R. Development of a prediction score for in-hospital mortality in COVID-19 patients with acute kidney injury: a machine learning approach. Sci Rep. 2021;11(1):24439. https://doi.org/10.1038/s41598-021-03894-5.
Jehi L, Ji X, Milinovich A, Erzurum S, Merlino A, Gordon S, et al. Development and validation of a model for individualized prediction of hospitalization risk in 4,536 patients with COVID-19. PLoS ONE. 2020;15(8):1–15. https://doi.org/10.1371/journal.pone.0237419.
Singh P, Rifkin DE, Blantz RC. Chronic kidney disease: An inherent risk factor for acute kidney injury? Clin J Am Soc Nephrol. 2010;5(9):1690–5. https://doi.org/10.2215/CJN.00830110.
Sarzani R, Giulietti F, Di PC, Giordano P, Spannella F. Disequilibrium between the classic renin-angiotensin system and its opposing arm in SARS-CoV-2-related lung injury. Am J Physiol - Lung Cell Mol Physiol. 2020;319(2):325–36. https://doi.org/10.1152/AJPLUNG.00189.2020.
Elena Flacco M, Acuti Martellucci C, Bravi F, Parruti G, Cappadona R, Mascitelli A, et al. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID-19: a meta-analysis Cardiac risk factors and prevention. Heart. 2020;0:1–6. https://doi.org/10.1136/heartjnl-2020-317336
Onweni CL, Zhang YS, Caulfield T, Hopkins CE, Fairweather DL, Freeman WD. ACEI/ARB therapy in COVID-19: the double-edged sword of ACE2 and SARS-CoV-2 viral docking. Crit Care. 2020;24(1):475. https://doi.org/10.1186/s13054-020-03195-9.
Lombardi Y, Azoyan L, Szychowiak P, Bellamine A, Lemaitre G, Bernaux M, et al. External validation of prognostic scores for COVID-19: a multicenter cohort study of patients hospitalized in Greater Paris University Hospitals. Intensive Care Med. 2021;47(12):1426–39. https://doi.org/10.1007/s00134-021-06524-w.
Acknowledgements
Not applicable.
Funding
The authors have no funding sources to declare.
Author information
Authors and Affiliations
Contributions
Henrique Palomba Designed the work; the acquisition, the analysis and interpretation of the data, wrote the manuscript, revised the paper, approved the final version and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Daniel Cubos Designed the work; the acquisition, the analysis and interpretation of the data Fernando Bozza, Designed the work, revised the paper, approved the final version and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Fernando Godinho Zampieri, Designed the work, revised the paper, approved the final version and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Thiago Gomes Romano. Designed the work; the acquisition, the analysis and interpretation of the data, wrote the manuscript, revised the paper, approved the final version and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the National Teaching and Ethical Committee (CONEP) in São Paulo / Brazil, number: 29496920.8.0000.5262 and informed consent was waived due to the observational nature of the study. The study was performed in accordance with the Declaration of Helsinki, the International Council for Harmonization and Good Clinical Practice.
Consent for publication
Informed Consent to Publish – Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Palomba, H., Cubos, D., Bozza, F. et al. Development of a Risk Score for AKI onset in COVID-19 Patients: COV-AKI Score. BMC Nephrol 24, 46 (2023). https://doi.org/10.1186/s12882-023-03095-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-023-03095-4