Abstract
The novel coronavirus disease infection (COVID-19) outbreak that was declared a global pandemic in March 2020 had led to an internationally variable but concerning incidence of COVID-associated acute kidney injury (AKI), with prevalence reported as high as 46% in large cohorts of hospitalized patients. Variability in AKI may be explained by differences in traditional risk factors for AKI, heterogeneity among patient cohorts, and differences in racial and ethnic groups. Further, AKI requiring kidney replacement therapies (KRT) has been associated with increased mortality. Proposed mechanisms of kidney injury include direct viral-induced tubular or glomerular injury, sepsis-associated AKI, and thrombotic disease. Kidney pathology include acute tubular injury, glomerular fibrin thrombi, pigmented tubular casts, and collapsing focal segmental glomerulosclerosis. “Viral-like” particles have been observed in renal samples at electron microscopy and viral RNA has been identified in both glomerular and tubular compartments of kidney specimens, but the link between viral presence and injury remain unclear. Though the link between AKI and poor outcomes is clear, prevalence and outcomes of COVID-19 in patients with chronic kidney disease and end stage kidney disease has not yet been reported. In patients on immunosuppression like those with kidney transplants or glomerular disease, COVID-19 has presented a management dilemma. Herein, we review the existing literature on kidney disease in COVID-19 and discuss what remains to be learned.
Similar content being viewed by others
Introduction
The novel coronavirus disease infection (COVID-19) outbreak, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), began in Wuhan, Hubei Province, China in December 2019 and was declared a global pandemic on March 11, 2020 by the World Health Organization (WHO). Though the initial focus of research has been on pulmonary pathophysiology, observed acute kidney injury (AKI) prevalence an associated morality [1] has been high in some reports—particularly AKI requiring kidney replacement therapies (KRT). Herein, we discuss what has been reported about kidney disease in COVID-19, and what remains to be discovered and lies ahead.
The true incidence of acute kidney injury?
Though findings from several patient populations have been reported, the true incidence of AKI in COVID-19 remains unknown. Existing data, while heterogenous with respect to population size, location, severity of illness, and definitions of AKI, show a wide range of rates of AKI occurrence in patients with COVID-19 from 1 to 46% (Fig. 1 [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]). At our institution, the incidence of AKI in 3325 patients with COVID-19 was 46%—with 20% requiring KRT [19]. In the largest cohort of 5449 hospitalized patients with COVID-19 studied so far, the proportion of patients with AKI any stage was 1993 (31.1%) [14]. Few European data are currently available on this topic. In a cohort of 62 patients not on maintenance dialysis in Lombardy, Italy, 40% developed AKI and 10% required KRT [18]. In this cohort, a mortality rate of 88% was reported in patients with advanced chronic kidney disease (CKD). In a large tertiary care hospital (Parma University Hospital, Parma, Italy) with 800 medicine beds and 60 COVID-19-dedicated ICU beds, 120 COVID-19 patients were admitted to the ICU between February 23rd to May 5th. AKI (as defined by the 2019 Kidney Diseases: Improving Global Outcomes Consensus Conference [20]) was observed in 26/120 (21.7%) before or at ICU admission, and in 29/126 (24.2%) during ICU stay; four patients (4/120, 3.3%) underwent KRT, performed as sustained low-efficiency dialysis (SLED) with regional citrate anticoagulation (personal communication).
Not surprisingly, there are noticeable differences in AKI rates between not only different countries, but also among those studies that included all hospitalized patients compared to those which analyzed only patients required intensive care. Similarly, the percentage of those requiring KRT widely varied from 0 to 23.2% [21]. Potential explanations for these differences include the prevalence of co-morbid conditions and heterogeneity along racial and ethnic lines, local institutional policies about KRT timing, the use of extracorporeal KRT beyond classical “nephrological” indications. One study reported that of the five New York City boroughs, the Bronx had not only higher rates of mortality, but also the highest proportion of racial and ethnic minoritized groups, most persons living in poverty, and lowest levels of educational attainment [22].
A proposed pathogenesis of kidney injury
In a relatively short period of time, several case reports and two series of post-mortem tissue sample analysis from China have begun to shed some light on potential mechanisms of kidney injury associated with COVID-19, even though many open issues remain (Table 1 [23,24,25,26]).
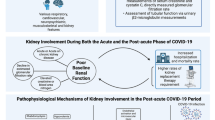
It is still not clearly ascertained whether SARS-CoV-2 directly damages the kidney injury or if kidney injury results from complications arising in the course of the viral infection outside the kidney. Reports of urinary abnormalities in COVID-19 patients, positive staining of tubules with viral antigens and complement components in one autoptic series, visualization of viral particles in tubular epithelial cells and podocytes on ultrastructural examination in other kidney biopsies and isolation of SARS-CoV-2 in urine, raised the possibility of a “SARS-CoV-2 nephropathy” (Table 1). In a kidney histological analysis of the largest autopsy series so far on 26 patients with COVID-19 from China [27], acute tubular injury ranging from mild to severe was almost always observed. Three biopsy specimens revealed segmental glomerular fibrin thrombi, 3 had pigmented tubular casts, and 7 were thought to have viral-like particles identified under electron microscopy (EM). What was perhaps most interesting of this study was that only 9/26 (34.6%) showed clinical signs of AKI and 5/26 (19.2%) required KRT. Acute tubular necrosis may be related to a similar presentation as seen with sepsis-induced AKI, mediated by the inflammatory milieu [28, 29]. Sixteen patients out of 26 (61.5%) of patients were hypotensive or required vasopressor support, thus making poor perfusion another likely mechanism of tubular injury. Could SARS-CoV2 itself be playing a role in tubular injury? Viral entry via angiotensin converting enzyme 2 (ACE2), expressed on proximal tubular cells, seems to support such a hypothesis [30, 31]. Additional support of this theory comes from the observation of spherical viral particles characteristic of viral inclusions on EM [27, 32, 33] as well as viral ribonucleic acid (RNA) detection [6] in the kidney. Of note, the presence of presumed viral particles and RNA have not been consistently reported throughout the literature (Table 1), and many cell structures could mimic the virus itself (Table 1). Whether or not the presence of virus is an incidental finding or indeed contributing to pathology has not been determined. One recent study detected SARS-CoV-2 in the kidneys of autopsy specimens using in situ hybridization and indirect immunofluorescence [34]. They noted preferential targeting of glomerular cells in the kidney, with viral detection also in liver, heart, brain, blood and most highly in the respiratory tract. It is likely, as suggested by the authors, that multiorgan tropism influences the course of COVID-19.
The presence of fibrin thrombi typically points towards a diagnosis of thrombotic microangiopathy (TMA), though the hypercoagulable state in patients with COVID-19 raises the likelihood of thrombi both in pulmonary blood vessels [35] and within microvasculature of the kidney. It is also possible that thrombotic occlusion of large kidney blood vessels (e.g. renal artery, renal vein) may contribute to kidney injury. A kidney biopsy from a critically ill patient with COVID-19 showed a classical pattern of acute tubular injury (ATI) with marked regenerative changes and focal acute tubular necrosis (ATN) [36].
Evidence of glomerular injury in patients with COVID-19 also exist, as documented by the presence of proteinuria or hematuria in up to 60% of the cases in some series [37]. Though not reported in the autopsy series, several reports of collapsing focal segmental glomerulosclerosis (FSGS) have been published [32, 38, 39]. Two out of 3 reported the presence of 2 high-risk APOL1 alleles in these patients. Similar to other viral-associated collapsing FSGS (e.g. human immunodeficiency virus (HIV), parvovirus 19) tubuloreticular inclusions suggesting systemic interferon activity [40] were reported.
Like the majority of kidney diseases, the mechanisms are most likely to be multifactorial and data thus far point to contributions from direct viral infection, inflammatory syndrome-mediated injury, hemodynamic instability, and perhaps the hypercoagulable state.
COVID-19 and special populations
Chronic kidney disease (CKD) and end-stage kidney disease (ESKD)
It is not yet known whether CKD or ESKD are a significant risk factors for COVID-19 infection and associated hospitalizations. While the development of AKI is associated with increased in-hospital mortality, hospitalized patients with a prior history of ESKD do not appear to require the same rate of intensive unit level care anecdotally. Though immune dysfunction (both immunosuppression and overactivation) have been described in this population [41], it is possible that ESKD patients do not mount the same cytokine storm response implicated in multiorgan dysfunction noted in critically ill patients. Mortality data on this patient populations remains limited, though a 30% mortality rate was reported in a cohort of 20 hemodialysis patients with COVID-19 [18]. In a large cohort of patients in New York City, patients with CKD and either atrial fibrillation, heart failure, or ischemic heart disease had a higher mortality rate. [42]. In an Italian cohort, 94 patients on hemodialysis with COVID-19 who were managed as either inpatient or outpatient had mortality rates of 42% and 8%, respectively [43]. At our outpatient hemodialysis center, we found an incidence and mortality rate of 17.5% and 3.6%, respectively.
Transplant
Kidney transplant recipients appear to be at higher risk for hospitalization and death from COVID-19. Similar to incidence rates of AKI, reported mortality rates in this population have varied and as high as 28% [22, 44]. There is an absence of consensus about how to adjust immunosuppression in Covid-19 kidney transplant recipients. While immunosuppression may prevent an effective anti-SARS-CoV-2 T cell response, it may also allow controlling inflammatory response that is, at least in part, responsible for COVID-19 related mortality. Calcineurin inhibitors, the backbone of the majority of immunosuppression regimens, have shown in vitro activity against coronaviruses—though clinical data are lacking [45]. At our center, our approach has been to maintain both corticosteroids and anti-metabolite immunosuppressive therapy while lowering calcineurin trough targets. While most centers reduce immunosuppression, approaches vary significantly from institution to institution [46].
Glomerular disease
The impact of COVID-19 on glomerular disease patients remains unclear. An individualized risk–benefit analysis to reduce, defer, or maintain immunosuppression seems appropriate. Some centers, including our own, have noted a marked reduction in rapidly progressive glomerulonephritis cases and urgent kidney biopsies during widespread home quarantining—supporting an environmental component in exacerbating glomerular disease [47].
A look to the future
Several months into the COVID-19 pandemic, there is much that we do not understand. The relationship between acute kidney injury and multiorgan failure is unclear. Details on the contribution of hypercoagulability, complement activation and endothelial injury to organ dysfunction are lacking. Presentation of a catabolic state in patients with COVID-19 [48] raises the possibly of acquired metabolic or mitochondrial disorders, in addition to the impact of cytokines and the inflammatory state on these pathways. Though this catabolic states seems to mimic rhabdomyolysis, creatine phosphokinase (CPK) levels have not been found to be dramatically elevated. While ACE2 plays an important role in viral cellular entry, proposed angiotensin dysregulation, innate and adaptive immune pathway activation, and hypercoagulation have emerged as proposed mechanisms of kidney and other organ injury [49]. Most concerning is the high level of residual kidney dysfunction in patients who have recovered from COVID-19. We are yet to fully appreciate their long-term risk for progression to end-stage kidney disease or whether the virus like HIV, could use kidney epithelial cells as a reservoir. A better understanding of kidney disease pathophysiology may aid in the development of effective therapies. As we move forward, it is plausible that computational biology will be important in understanding COVID-19 pathophysiology in identifying both therapies and innovative strategies to design a vaccine [50].
References
Cheng Y, Luo R, Wang K et al (2020) Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97:829–838. https://doi.org/10.1016/j.kint.2020.03.005
Yang X, Yu Y, Xu J et al (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. https://doi.org/10.1016/S2213-2600(20)30079-5
Zhou F, Yu T, Du R et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3
Wang L, Li X, Chen H et al (2020) Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. https://doi.org/10.1159/000507471
Wang D, Hu B, Hu C et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323:1061–1069. https://doi.org/10.1001/jama.2020.1585
Diao B, Wang C, Wang R et al (2020) human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. https://doi.org/10.1101/2020.03.04.20031120
Xiao G, Hu H, Wu F et al (2020) Acute kidney injury in patients hospitalized with COVID-19 in Wuhan, China: a single-center retrospective observational study. medRxiv. https://doi.org/10.1101/2020.04.06.20055194
Guan W, Ni Z, Hu Y et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720. https://doi.org/10.1056/NEJMoa2002032
Cao M, Zhang D, Wang Y et al (2020) Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. medRxiv. https://doi.org/10.1101/2020.03.04.20030395
Liu Y, Yang Y, Zhang C et al (2020) Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63:364–374. https://doi.org/10.1007/s11427-020-1643-8
Wu J, Liu J, Zhao X et al (2020) Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis Off Publ Infect Dis Soc Am. https://doi.org/10.1093/cid/ciaa199
Arentz M, Yim E, Klaff L et al (2020) Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 323:1612–1614. https://doi.org/10.1001/jama.2020.4326
ICNARC—Intensive Care National Audit & Research Centre. https://www.icnarc.org/. Accessed 20 May 2020
Hirsch JS, Ng JH, Ross DW et al (2020) Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. https://doi.org/10.1016/j.kint.2020.05.006
Mohamed MM, Lukitsch I, Torres-Ortiz AE et al (2020) Acute kidney injury associated with coronavirus disease 2019 in urban New Orleans. Kidney. https://doi.org/10.34067/KID.0002652020
Pei G, Zhang Z, Peng J et al (2020) Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. https://doi.org/10.1681/ASN.2020030276
Chen N, Zhou M, Dong X et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513. https://doi.org/10.1016/S0140-6736(20)30211-7
Malberti F, Pecchini P, Marchi G, Foramitti M (2020) When a nephrology ward becomes a COVID-19 ward: the Cremona experience. J Nephrol. https://doi.org/10.1007/s40620-020-00743-y
Chan L, Chaudhary K, Saha A et al (2020) Acute kidney injury in hospitalized patients with COVID-19. medRxiv. https://doi.org/10.1101/2020.05.04.20090944
Levey AS, Eckardt K-U, Dorman NM et al (2020) Nomenclature for kidney function and disease: report of a kidney disease: improving global outcomes (KDIGO) consensus conference. Kidney Int 97:1117–1129. https://doi.org/10.1016/j.kint.2020.02.010
Acute Kidney Injury. In: NephJC. http://www.nephjc.com/news/covidaki. Accessed 4 May 2020
Akalin E, Azzi Y, Bartash R et al (2020) Covid-19 and kidney transplantation. N Engl J Med. https://doi.org/10.1056/NEJMc2011117
Calomeni E, Satoskar A, Ayoub I et al (2020) Multivesicular bodies mimicking SARS-CoV-2 in patients without COVID-19. Kidney Int. https://doi.org/10.1016/j.kint.2020.05.003
Miller SE, Brealey JK (2020) Visualization of putative coronavirus in kidney. Kidney Int. https://doi.org/10.1016/j.kint.2020.05.004
Aiamkitsumrit B, Sullivan NT, Nonnemacher MR et al (2015) Human immunodeficiency virus type 1 cellular entry and exit in the T lymphocytic and monocytic compartments: mechanisms and target opportunities during viral disease. Adv Virus Res 93:257–311. https://doi.org/10.1016/bs.aivir.2015.04.001
Pacciarini F, Ghezzi S, Canducci F et al (2008) Persistent replication of severe acute respiratory syndrome coronavirus in human tubular kidney cells selects for adaptive mutations in the membrane protein. J Virol 82:5137–5144. https://doi.org/10.1128/JVI.00096-08
Su H, Yang M, Wan C et al (2020) Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. https://doi.org/10.1016/j.kint.2020.04.003
Lerolle N, Nochy D, Guérot E et al (2010) Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med 36:471–478. https://doi.org/10.1007/s00134-009-1723-x
Aslan A, van den Heuvel MC, Stegeman CA et al (2018) Kidney histopathology in lethal human sepsis. Crit Care 22:359. https://doi.org/10.1186/s13054-018-2287-3
Bourgonje AR, Abdulle AE, Timens W et al (2020) Angiotensin-converting enzyme-2 (ACE2), SARS-CoV-2 and pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. https://doi.org/10.1002/path.5471
Brojakowska A, Narula J, Shimony R, Bander J (2020) Clinical implications of SARS-COV2 interaction with renin angiotensin system. J Am Coll Cardiol. https://doi.org/10.1016/j.jacc.2020.04.028
Kissling S, Rotman S, Gerber C et al (2020) Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. https://doi.org/10.1016/j.kint.2020.04.006
Varga Z, Flammer AJ, Steiger P et al (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395:1417–1418. https://doi.org/10.1016/S0140-6736(20)30937-5
Puelles VG, Lütgehetmann M, Lindenmeyer MT et al (2020) Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. https://doi.org/10.1056/NEJMc2011400
Connors JM, Levy JH (2020) Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost JTH. https://doi.org/10.1111/jth.14849
Rossi GM, Delsante M, Pilato FP et al (2020) Kidney biopsy findings in a critically ill COVID-19 patient with dialysis-dependent acute kidney injury: a case against “SARS-CoV-2 nephropathy”. Kidney Int Rep. https://doi.org/10.1016/j.ekir.2020.05.005
Li Z, Wu M, Volunteers A-2019-nCoV et al (2020) Caution on kidney dysfunctions of COVID-19 patients. medRxiv. https://doi.org/10.1101/2020.02.08.20021212
Larsen CP, Bourne TD, Wilson JD et al (2020) Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19). Kidney Int Rep. https://doi.org/10.1016/j.ekir.2020.04.002
Peleg Y, Kudose S, D’Agati V et al (2020) Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep. https://doi.org/10.1016/j.ekir.2020.04.017
Bromfield M, McQuillan R, John R, Avila-Casado C (2014) The significance of tubuloreticular inclusions as a marker of systemic stimulation by interferons in a case of focal and segmental glomerulosclerosis associated with cytomegalovirus (CMV) infection. Clin Kidney J 7:174–178. https://doi.org/10.1093/ckj/sft156
Kato S, Chmielewski M, Honda H et al (2008) Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol CJASN 3:1526–1533. https://doi.org/10.2215/CJN.00950208
Yamada T, Mikami T, Chopra N et al (2020) Patients with chronic kidney disease have a poorer prognosis of coronavirus disease 2019 (COVID-19): an experience in New York City. Int Urol Nephrol. https://doi.org/10.1007/s11255-020-02494-y
Alberici F, Delbarba E, Manenti C et al (2020) A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. https://doi.org/10.1016/j.kint.2020.04.030
Alberici F, Delbarba E, Manenti C et al (2020) A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int 97:1083–1088. https://doi.org/10.1016/j.kint.2020.04.002
Willicombe M, Thomas D, McAdoo S (2020) COVID-19 and calcineurin inhibitors: should they get left out in the storm? J Am Soc Nephrol 31:1145–1146. https://doi.org/10.1681/ASN.2020030348
Fishman JA, Grossi PA (2020) Novel coronavirus-19 (COVID-19) in the immunocompromised transplant recipient: #Flatteningthecurve. Am J Transplant 20:1765–1767. https://doi.org/10.1111/ajt.15890
Bomback AS, Canetta PA, Ahn W et al (2020) How COVID-19 has changed the management of glomerular diseases. Clin J Am Soc Nephrol. https://doi.org/10.2215/CJN.04530420
Billah M, Santeusanio A, Delaney V et al (2020) A catabolic state in a kidney transplant recipient with COVID-19. Transpl Int Off J Eur Soc Organ Transpl. https://doi.org/10.1111/tri.13635
Batlle D, Soler MJ, Sparks MA et al (2020) Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. https://doi.org/10.1681/ASN.2020040419
Oberg AL, Kennedy RB, Li P et al (2011) Systems biology approaches to new vaccine development. Curr Opin Immunol 23:436–443. https://doi.org/10.1016/j.coi.2011.04.005
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This manuscript has not been published previously, in whole or part. The authors have no conflicts of interest to disclose.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farouk, S.S., Fiaccadori, E., Cravedi, P. et al. COVID-19 and the kidney: what we think we know so far and what we don’t. J Nephrol 33, 1213–1218 (2020). https://doi.org/10.1007/s40620-020-00789-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-020-00789-y