Abstract
Background
Exercise and some pre-AKI diets have been shown to improve injury, apoptosis, and lipid profile. In this study, the effect of two different diets along with exercise training on acute kidney injury (AKI) was investigated.
Materials and methods
Laboratory rats were randomly divided into four groups of control, standard diet + exercise, exercise + calorie restriction (CR) and exercise + time restriction (TR). Each group was divided into two subgroups of AKI and no AKI. The animals received endurance training and diet regimens before AKI. Fasting blood glucose, serum creatinine, Bcl-2-associated X protein (Bax), B-cell lymphoma 2 (Bcl2) and histopathological outcome of renal tissue as well as serum lipid profile of animals were assessed 24 h after AKI.
Results
The percentage of changes in renal Bcl2 and Bax after AKI in the group with previous exercise was lower than the group without previous exercise (p < 0.01). After induction of AKI, serum lipid profile changed in non-exercised rats (p < 0.001). Also, after injury, fasting blood glucose levels increased in non-exercised rats (p < 0.05). After injury, the start of both CR and TR diets during exercise caused less change in Bcl2 and Bax of non-exercised rats compared to exercised rats (p < 0.001). CR diet along with exercise improved lipid profile, and also CR diet along exercise decreased fasting blood glucose levels (p < 0.001). Also, both the CR and TR diets during exercise caused fewer changes in histopathological outcome after AKI.
Conclusion
Exercise alone decreased changes in apoptotic and histopathological indexes, fasting blood glucose, as well as lipid profile of rats after AKI. Reduction of apoptosis and improvement of histopathological outcome after AKI appeared more when CR and TR diets were commenced during exercise. The reduction of lipid profile changes was more pronounced in the group that received CR diet during exercise.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is a complex clinical dysfunction that is accompanied with increased serum urea and creatinine and decreased glomerular filtration rate (GFR), [1]. Despite technological progresses in the treatment of renal failure, AKI continues to have unfavorable consequences. People with AKI often develop chronic kidney disease (CKD) [2].
The pathophysiology of AKI is very complex and includes; endothelial dysfunction, microcirculatory dysfunction, formation of microvascular thrombi, inflammation, tubular cell injury, renal venous congestion, tubular obstruction, auto-immune processes, hypersensitivity immune reactions, intra-abdominal hypertension, apoptosis and oxidative stress [3, 4].
Kidney tubular cells have been shown to die from apoptosis as well as necrosis in experimental models of acute ischemic renal failure as well as in humans with acute tubular necrosis [5]. Inside the cells, there is a system that include pro-apoptotic oncogenes including tumor protein with a molecular weight of 53 kDa (p53), cellular myelocytomatosis oncogene (c-myc), fos proto-oncogene (c-fos) with some members of B-cell lymphoma 2 (Bcl2), and Bcl2-associated X protein (Bax) or Bcl2 associated agonist of cell death (Bad) that cause apoptosis as well as anti-apoptotic oncogenes (Bcl2/Bcl-XL), which act as inhibitor of apoptosis. The balance between these oncogenes may determine apoptosis or survival of the cell. Under certain conditions, p53 activates the transcription of effective apoptotic genes, including p53-responding pro-apoptotic genes, factor superfamily 6 (Fas), and other mediators [6,7,8].
Studies have shown that diet manipulation, whether by changing calorie intake level or food intake duration, can lead to delayed onset/progression of diseases or prolongation of life in most organisms [9]. The classic calorie restriction diet (CR), in which daily caloric intake is normally reduced by 15- 40% [10], as well as time restriction diet (TR), in which daily food intake is limited to 4–12 h [11], are among the dietary interventions. Diets that are associated with reduced energy intake exert their positive effects through weight loss and metabolism [12]. The complete superiority of either regime over the other one has not been proven.
CR diet has been shown to improve metabolic health and chronic metabolic diseases, such as type 2 diabetes and cardiovascular disorders, by moderately reducing food intake without malnutrition. Also, dietary restriction initiates a consistent defense program to increase resistance against stress and apoptosis [13]. It is reported that TR diet for 10 h a day improves lipid profile [14]. Athletes who have TR diet have been shown to be able to maintain muscle mass and reduce body fat and inflammation markers [15].
Meanwhile, it has been reported that exercise, like calorie restriction, can affect the survival and recovery of diseases [16, 17]. Physical activity and exercise promote health, help to maintain weight, and prevent health problems, vascular disease, and inflammatory disease [18]. Moderate-intensity aerobic exercise reduces cardiovascular disease such as high blood pressure, and also helps to control cholesterol level and body weight control, and maintain bone mass [19,20,21].
Exercise has been shown to significantly reduce the expression of acetylated P53 and increase the expression ratio of Bcl2 / Bcl2 antagonist / Killer (Bak), suggesting that exercise may inhibit apoptosis by activating the silent information regulator 1 (Sirt1) / AMP-activated protein kinase (AMPK) pathway. Sirt1 can inhibit p53 activity through deacetylation. It can also reduce the induction of apoptosis by reducing the effect of p53 on downstream target gene activation [22].
When regular and progressive aerobic exercise is performed prior to AKI, a reduction in the severity of tubular injury and a decrease in caspase 3 levels within 48 h of reperfusion can be seen in Wistar rats. Regular aerobic exercise and previous adaptation reduce morphological damage to the kidney [23,24,25]. There is little evidence that exercise along with CR can have beneficial effects on several health indicators [26]. Both exercise and CR have been shown to reduce apoptosis and kidney injury [13]. The release of reactive oxygen species (ROS) causes apoptosis and their suppression by CR results in a reduction in apoptosis in the kidney [27]. Attenuation of apoptosis by decreased Bax/ Bcl2 ratio in model of AKI has been proven with CR and TR [28].
TR diet is a form of Islamic fasting [29] and Muslim athletes may fast during Ramadan, CR diet can also be used as an intervention for athletes who want to control their body weight and increase their physical function and energy [26]. On the other hand, these diets in athletes reduce the risk of metabolic diseases and mortality in them [30]. However, it is not clear whether the implementation of these diets in athletes affects their susceptibility to diseases such as AKI. Therefore, in this study, the effects of CR and TR diets on renal apoptosis indexes, lipid profile and histopathological outcome in AKI male rats during exercise were investigated.
Materials and methods
Animals
Male Wistar rats with the weight of 200–250 g (12–14 weeks age) were used in the present study. All laboratory tests and procedures were performed according to the instructions of Animal Care Committee of Kerman University of Medical Sciences (Ethics code: IR.KMU.REC.1398.457 and ID: 98,000,315). Male rats were kept in a cycle of 12 h of darkness and 12 h of light in the animal center of Kerman University of Medical Sciences at 22- 23° C, while having free access to water.
Study groups
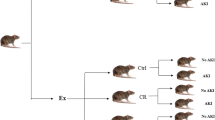
Two groups were used in this study to confirm the induction of AKI, which included AKI and no AKI groups without previous exercise. The rest of them that had previous exercise were divided into three groups of control (Ctrl), calorie restriction diet (CR) and time restriction diet (TR), each of which was divided into two subgroups AKI and no AKI (Fig. 1). The protocol was implemented in all study groups (Fig. 2).
Implementation of calorie restriction and time restriction diets
To calculate the amount of food given to CR group, first the amount of food consumed per week in the group that had free access to food was determined and then the average food consumption in one day was calculated. Then, 70% of the daily food intake in the group that had free access to food was calculated and given to the CR group. This regimen was implemented for 2 months before induction of AKI in the CR group [28]. In the TR group, the animals only had 5 h of free access to food per day [11, 31].
Exercise protocol
Exercise program was implemented for 8 weeks (5 days a week), using a treadmill before induction of AKI. Thus, after 3 days of adaptation at a speed of 3–9 m/min, the treadmill was adjusted at a speed of 15 m/min for 15 min at 0° slop. Then at the end of second week, after ten minutes of warm up, the treadmill speed was gradually increased until it reached to 26 m/min for 60 min and this was maintained until the end of eighth week (Table 1), [32].
Induction of AKI
Two months after the start of study, the animals in the AKI groups were deprived of water for 24 h and then they were injected with only one dose of hypertonic glycerol solution (50% dissolved in sterile saline). The injection was performed in such a way that 10 ml/kg of hypertonic glycerol solution was injected equally into the muscle (i.m) of both lower limbs of the animal [33]. Glycerol generally causes rhabdomyolysis, which in turn causes myoglobinuria, ischemia, and nephrotoxicity in the kidneys. With this method, nephropathy develops quickly about 24 h after the injection [34].
Blood sampling and lipid profile analysis
At the end of the experiment, after 12 h of fasting, the animals underwent deep anesthesia and blood was taken from their heart ventricle and then the serum was prepared. Fasting blood glucose level, creatinine level, and lipid profile including total cholesterol (TC), triglyceride (TG) and high-density lipoprotein (HDL) were measured (Pars Azmoun kit, Iran) in serum. Also, the level of serum low-density lipoprotein (LDL) was calculated using the following formula:
Preparation of homogeneous kidney tissue
After anesthetizing the animals with pentobarbital 50 mg/kg [35, 36], the right kidney was rapidly removed and immediately frozen in liquid nitrogen. The kidney was weighed and homogenized in T-PERTM tissue protein extraction reagent with 0.5% Triton X-100, 150 mM NaCl, 50 Mm tris and a protease inhibitor cocktail (500 mg tissue per 2 ml of reagent). Following homogenization, the samples were shaken by a shaker (Behdad, Iran) for 90 min and then were centrifuged at 4° C and 4000 rpm for 15 min (Routina, Germany), and the supernatant was collected. The protein content of supernatant was estimated using a protein assessment reagent kit and the amount of protein from each sample was used for the assessment [37].
Evaluation of renal apoptosis indexes
Measurement of Bax and Bcl2 levels
The levels of Bax and Bcl2 in homogeneous kidney tissue were measured by ELISA method (Zellbio kit, Germany). In this case, the measurement was done based on the reaction between the antigen and antibody, and finally the adsorption was read at 450 nm. After placing the adsorption and concentration of standard solutions in Excel software, a standard curve was prepared. The concentration of samples was determined based on absorption, using the standard curve line equation. The amount of these proteins was expressed in picograms per milligram of protein [38].
Evaluation of histopathological outcome
Histopathological outcome was evaluated at the end of study and 24 h after AKI induction in terms of inflammation, necrosis, vacuolation, inter-tubular casts, tubular dilatation and vascular congestion. The animal was first anesthetized with 50 mg/kg pentobarbital and then, its left kidney was extracted, washed with saline phosphate buffer and fixed in 10% formalin. Samples were placed in natural 10% formalin for one hour, in 70% alcohol for 90 min, in 80% alcohol for 90 min, in 90% alcohol for 90 min, and finally in 100% alcohol for 90 min. The samples were then placed in xylene twice for 90 min and then were coated with paraffin. After that, 4 micron-thick slides were prepared, which later were stained by hematoxylin and eosin. The slides were washed of paraffin by xylene and 100%, 90% and 70% ethanol (twice each) and finally were washed with water for three minutes before being stained. They were then dehydrated with 70%, 90% and 100% ethanol for three minutes each and finally washed twice with xylene [39]. The changes were semi-quantified and graded as follows:
-
0, unchanged (-)
-
1, very slight change ( ±)
-
2, slight change ( +)
-
3, moderate change (+ +)
-
4, severe change (+ + +)
-
5, very severe change (+ + + +), [40]
Statistical analysis
Data are shown as mean ± SEM. Two-way repeated measures of ANOVA and t-test were used to compare quantitative variables between the test groups after observing the assumptions (normal data distribution). A significant level of 0.05 was considered and statistical analysis was performed by SPSS-22 software.
Results
The changes of serum creatinine levels following AKI
Serum creatinine levels increased after AKI, both in the group with previous exercise and in the group without previous exercise compared to before AKI (p < 0.001) (Fig. 3). The percentage of this increase in the exercised group was less than the group that had no exercise (286.54 compared to 559.21%).
Serum creatinine level in different study groups (n = 6 in each group). Data are shown as mean ± SEM. A Serum creatinine level before and after AKI in the non-exercised and exercised groups. @@@p < 0.001, vs. before AKI in the non-exercised group. ^^^p < 0.001 vs. before AKI in the exercised group. AKI: Acute kidney injury; Ctrl: Control; CR: Caloric restriction; TR: Time restriction
The effect of previous exercise and different diets on fasting blood glucose and lipid profile after AKI
Fasting blood glucose level increased after AKI compared to before in the group without previous exercise (p < 0.05). In the exercise group, this level did not change after the AKI (Table 2). The level of blood glucose before AKI in CR group was lower than Ctrl and TR groups (p < 0.001). After AKI, this level was lower in CR group than Ctrl and TR groups (p < 0.001) (Table 3). This level in the CR group was lower than Ctrl group before AKI (p < 0.001), (Table 3).
Serum triglyceride level increased after AKI compared to before in the group without previous exercise (p < 0.001). While in the exercise group, triglyceride level did not change after the AKI (Table 2). The level of triglyceride before AKI in CR group was lower than Ctrl and TR groups (p < 0.01 and p < 0.001; respectively). After AKI, this level was higher in TR group compared to Ctrl and CR groups (p < 0.001), (Table 3). Triglyceride level in the TR group was higher than Ctrl group before AKI (p < 0.001), (Table 3).
Serum cholesterol level increased after AKI in the non-exercise group compared to before injury (p < 0.001), while this change did not occur in the exercise group (Table 2). Pre-AKI serum cholesterol level in the CR group was lower than the TR group (p < 0.05). This level was higher in the TR group after AKI than in the Ctrl and CR groups (p < 0.010 and p < 0.001; respectively), (Table 3).
Following AKI, there was no change in serum HDL level compared to before AKI in both the non-exercise and exercise groups (Table 2). In the exercised rats, HDL level before and after AKI was higher in the TR group than in the Ctrl group (p < 0.05), (Table 3). HDL level before AKI was higher in the CR and TR groups compared to Ctrl group (p < 0.05 and p < 0.01; respectively), (Table 3).
Serum LDL level increased after AKI compared to before in group without previous exercise (p < 0.001), (Table 2), while the exercise prevented the increase in LDL level of rates with previous exercise. In exercised rates, LDL level before and after AKI was lower in the CR group than in the Ctrl group (p < 0.05). Serum LDL level in CR and TR groups was not different from Ctrl group before injury (Table 3).
The effect of exercise and different diets on the level of renal apoptotic indexes after AKI
Level of renal Bax
Renal Bax level in different study groups is shown in Fig. 4. The level of Bax increased after injury compared to before injury (p < 0.001), (Fig. 4A). The percentage of changes in Bax level in the exercised group was less than the non-exercised group (p < 0.01), (Fig. 4B). Bax level before AKI was lower in the TR and CR groups compared to the Ctrl group (p < 0.001). An increase in Bax level after injury appeared in the Ctrl, TR and CR groups compared to before injury (p < 0.001). In CR and TR groups, Bax level was lower than Ctrl group (p < 0.001) and in TR group, it was lower than CR group (p < 0.01). This level was also lower in TR group after injury compared to Ctrl group before injury (p < 0.01), (Fig. 4C).
Renal Bcl2-associated X protein (Bax) level in different study groups (n = 6 in each group). Data are shown as mean ± SEM. A Renal Bax level before and after AKI in the non-exercised and exercised groups. @@@p < 0.001, vs. before AKI in the non-exercised group. ^^^p < 0.001 vs. before AKI in the exercised group. B Percentage of renal Bax changes in the non-exercised and exercised groups after AKI. ¥¥p < 0.01, vs. Ctrl group after injury. C Renal Bax level in the exercised groups. ***p < 0.001, vs. Ctrl group before injury. **p < 0.01, vs. Ctrl group before injury. εεεp < 0.001, vs. Ctrl group after injury. ##p < 0.01, vs. CR group after injury. ^^^p < 0.001, vs. before AKI. AKI: Acute kidney injury; Ctrl: Control; CR: Caloric restriction; TR: Time restriction
Renal Bcl2 level
Renal Bcl2 level in different study groups is shown in Fig. 5. The Bcl2 level decreased after AKI in non-exercised and exercised groups compared to before injury (p < 0.001), (Fig. 5A). The percentage of changes in Bcl2 level in the exercised group was less than the non-exercised group (p < 0.01), (Fig. 5B). During exercise, the level of Bcl2 before AKI in CR and TR groups was higher than Ctrl group (p < 0.001), and this level was higher in TR group than CR group (p < 0.01). This level after AKI decreased in Ctrl, TR and CR groups compared to before injury (p < 0.001), and this decrease was less in CR and TR groups than Ctrl group (p < 0.001). It was also lower in TR group than CR group (p < 0.001). This level was higher in the TR group after injury compared to Ctrl group before injury (p < 0.01). (Fig. 5C).
The level of renal B-cell lymphoma 2 (Bcl2) in different study groups (n = 6 in each group). Data are shown as mean ± SEM. A Renal Bcl2 level before and after AKI in the non-exercised and exercised groups. @@@p < 0.001, vs. before AKI in the non-exercised group. ^^^p < 0.001, vs. before AKI in the exercised group. B Percentage of renal Bcl2 changes in the non-exercised and exercised groups after AKI. ¥¥p < 0.01, vs. Ctrl group after AKI. C) Renal Bcl2 level in the exercised groups. ***p < 0.001, vs. Ctrl group before injury. **p < 0.01, vs. Ctrl group before injury. &&p < 0.01, vs. CR group before injury. εεεp < 0.001, vs Ctrl group after injury. ###p < 0.001, vs. CR group after injury. ^^^p < 0.001, vs. before AKI. AKI: Acute kidney injury; Ctrl: Control; CR: Caloric restriction; TR: Time restriction
Renal Bax to Bcl2 ratio
The ratio of renal Bax to Bcl2 in different study groups is shown in Fig. 6. This ratio increased after the injury compared to before injury (p < 0.001), (Fig. 6A). The percentage of changes in this ratio in the exercised group was lower than the non-exercised group (p < 0.01), (Fig. 6B). Bax to Bcl2 ratio before AKI in CR and TR groups was lower than Ctrl group (p < 0.001) and in TR group was lower than CR group (p < 0.05). This ratio after AKI increased in Ctrl, TR and CR groups compared to before AKI (p < 0.001), but this increase in the TR group was lower than in the CR and Ctrl groups (p < 0.001). This ratio was also lower in the TR group after injury than in the Ctrl group before injury (p < 0.001), (Fig. 6C).
The ratio of renal Bax to Bcl2 in different study groups (n = 6 in each group). Data are represented as mean ± SEM. A The ratio of renal Bax to Bcl2 before and after AKI in the exercised and non-exercised groups. @@@p < 0.001, vs. before AKI in the non-exercised group. ^^^p < 0.001, vs. before AKI in the exercised group. B Percentage of changes in renal Bax to Bcl2 ratio in the non-exercised and exercised groups after AKI. ¥¥p < 0.01, vs. Ctrl group after AKI. C Renal Bax to Bcl2 ratio in exercise groups. ***p < 0.001, vs. Ctrl group before injury. &p < 0.05, vs. CR group before injury. εεεp < 0.001, vs. Ctrl group after injury. ###p < 0.001, vs. CR group after injury. ^^^p < 0.001, vs. before AKI. AKI: Acute kidney injury; Ctrl: Control; CR: Caloric restriction; TR: Time restriction
The effect of exercise and different diets on histopathological outcome of kidney following AKI
In the non-exercised and exercised groups, cell vacuolation, congestion, intra-tubular cast, tubular dilatation, inflammation, and necrosis increased after AKI compared to before AKI (Fig. 7). In the exercised group, these changes were moderate (+ +) and lower compared to the non-exercised group in which, the changes were severe (+ + +) (Table 4). During the exercise, changes in cell vacuolation, congestion, intra-tubular cast, tubular dilatation, inflammation and necrosis in the CR and TR groups were mild ( +) after AKI compared to the control group (Table 5, Fig. 7).
Renal histopathological changes after Acute kidney injury (AKI) in experimental groups (n = 6 in each group) (Findings are reported based on H&E × 100). A Non-exercised group before AKI. B Non-exercised group after AKI. C Exercised group after AKI. D Exercised + Caloric restriction group after AKI. E Exercised + Time restriction group after AKI. Histopathological changes are presented: intra-tubular cast (bluish arrows), tubular dilatation (red arrows), cellular vacuolization (green arrows), congestion (purple arrows) and necrosis (black arrows)
Discussion
In this study, for the first time, the combined effects of exercise and different diets on apoptotic indexes, lipid profile and histopathological outcome were investigated in rats with AKI. In this study, following 8 weeks of moderate-intensity endurance exercise in combination with different diets, the following findings were obtained: 1) Exercise along with CR and TR diets caused less changes in the renal Bax, Bcl2 and renal Bax to Bcl2 ratio of rats following AKI, and these changes were greater in the TR group than in the CR group. 2) The CR diet during exercise decreased fasting blood glucose level and prevented an increase in cholesterol, triglycerides, and LDL levels following AKI. It also increased HDL levels. 3) Both TR and CR diets during exercise improved histopathological outcome following AKI.
AKI is rare in athletes, but kidney dysfunction during endurance exercise is thought to be caused by a combination of several factors, including dehydration, muscle breakdown, heat stress, and the use of non-steroidal anti-inflammatory drugs (NSAIDs), [41]. Intense, prolonged, and repetitive exercise can cause rhabdomyolysis. Although most cases are asymptomatic and resolve without complications, rhabdomyolysis is the most common cause of AKI in athletes, and many functional kidney biomarkers will change when they reach their baseline, about 1 to 10 h after injury. Athletes with rhabdomyolysis and AKI need 2–10 and 1–17 days to recover, respectively. Athletics’ readiness and recovery strategies (medical, therapeutic, nutritional, and other life-supportive strategies) should be considered for AKI to be effective [42,43,44]. Therefore, in this study, the effect of TR and CR diets on exercised rats with AKI was investigated.
In the present study, after AKI, an increase in Bax and Bax to Bcl2 ratio, a decrease in Bcl2, and a pathological damage were observed. In one study, an increase in Bax and a decrease in Bcl2 in the kidney were reported following aging [27]. Shu et al. (2019) also reported an increase in Bax, a decrease in Bcl2, and an increase in the Bax to Bcl2 ratio in the cisplatin-induced AKI model [45]. P53 activity in renal tubular cells has been reported as a transcriptional regulator of Bax expression in a glycerol-induced AKI model [46]. Excessive endoplasmic reticulum stress and oxidative stress can contribute to renal apoptosis [47].
In this study, we found that rats with previous exercise experienced fewer changes in the Bax, Bcl2, and histopathology of their kidney tissue after injury than rats without exercise. In other words, exercise was able to reduce the rate of apoptosis after AKI. Swimming for 8 weeks in rats with hypertension-induced renal failure decreased Bax and increased Bcl2 [48]. Exercise before induction of renal ischemia reperfusion improved histopathological outcomes such as reduced tubular cast and dilatation post-injury [49]. Better physical condition of exercised animals may lead to better hemodynamic and metabolic conditions and ultimately activation of molecular mechanisms of cell survival in kidney tissue [49]. SIRT1 expression was reduced in the AKI model [1], and it was shown that exercise reduces apoptosis by increasing SIRT1 expression [50]. SIRT1 is an evolutionary biological stress response that is associated with increased long-term function and survival of vital cell types [51]. Further research is needed to determine that exercise through which pathways reduces apoptosis in AKI.
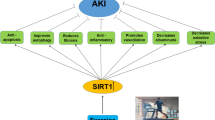
In the present study, we found that CR and TR diets along with exercise caused fewer changes in Bax, Bcl-2, Bax / Bcl-2 ratio and histopathology of kidney following AKI compared to exercise alone. TR and CR diets have been shown to reduce atrophy and dilatation of renal tubules in diabetic animals [52]. CR diet reduces aging-induced kidney damage by reducing cast formation and inflammation in the kidney [53, 54]. TR reduces the symptoms of necrosis in histopathological observations in AKI [55]. Although fasting has not been reported to reduce the symptoms of necrosis after renal ischemic reperfusion [56], the reasons for the difference between results could be due to short fasting period or calorie restriction, short-term follow-up and AKI model. Cohen et al. (2004) found that restricted food intake probably prevents the excessive increase of Bax by inducing SIRT1 [51]. Therefore, in this study, CR diet during exercise probably reduced apoptosis by increasing SIRT1, but this hypothesis needs to be confirmed. Ren et al. (2019) found that TR diet may reduce apoptosis in hepatic reperfusion ischemia by reducing caspase-3 [57]. It has been reported that Bcl-2 and Bcl-XL are both anti-apoptotic proteins and have higher expression in the TR group. TLR9 is a membrane protein whose activation leads to signaling of inflammatory cytokines and promotes apoptosis in the AKI model by increasing caspase-3 [58]. Animals on the TR diet have lower levels of TLR4, which is thought to protect the liver from I/R damage [57]. These results suggest that the TR diet may reduce apoptosis by reducing inflammation and injury, which requires further research to confirm. The possible effect mechanism of diet and exercise on apoptosis has been shown according to the results of this study (Fig. 8). However, more research is required for consolidation.
Possible mechanisms of action for the effects of diet and exercise on apoptosis. AKI: Acute kidney injury; Bak: Bcl2 Antagonist/Killer; Bax: Bcl-2-associated X protein; Bcl2: B-cell lymphoma 2; Bid: BH3 interacting-domain death agonist; CR: Calorie restriction; Ctrl: Control; Ex: Exercise; P53: Tumor protein with a molecular weight of 53 kDa; ROS: Reactive oxygen species; SIRT1: Silent information regulator 1; TR: Time restriction; TLR: Toll like receptor
Caspases are types of cysteinyl proteases that belong to C14 family. The caspases gene family contains 11 human members that can be divided into three groups. Caspases 2, 3 and 7 and caspases 6, 8, 9 and 10 are involved in regulation of apoptosis [59]. Mitochondrial dysfunction is a change in the mitochondrial membrane potential, production of ROS, permeability transition (PT), and the release of inter-membrane space protein cytochrome c (Cyt c). The cytochrome c activates apoptotic protease activating factor-1 (Apaf-1), which activates a downstream caspase [60]. Caspases activate and affect mitochondria. Caspases are activated via Apaf-1/cytochrome c or they can be activated directly by activation of cell surface death receptors [61]. Caspases ultimately lead to cell death. BH3 interacting-domain death agonist (Bid) is a pro-apoptotic member and binds with either Bcl-2 or Bax. It also promotes cell death. Bak is needed for the activation of Bid to promote apoptosis [62]. Importantly, p53 can also regulate the expression of extrinsic apoptotic pathway [62].
In the present research, we found that the level of fasting blood glucose increased after the induction of AKI in the non-exercised group, which is in agreement with the study of Qin et al. [63]. In the exercised group, no changes in fasting blood glucose level were observed after injury. Leehey and colleagues found that aerobic exercise for 24 weeks in patients with CKD improved their fasting blood glucose level [64].
In the aerobic exercised group with calorie restriction, fasting blood glucose level reduced following AKI, which is consistent with the findings of a study by Jashni and colleagues [65]. Negative correlation between adiponectin and fasting blood glucose concentration probably has a role in the increase of insulin sensitivity. It seems that when calorie restriction and exercise are combined, it has a stronger effect on fasting blood glucose and adiponectin [65].
In the present study, we observed that the levels of triglycerides, cholesterol and LDL increased after induction of AKI in the non-exercised group, but the level of HDL did not change. These results are in agreement with the findings of a study by Abdel and colleagues [66]. It has been shown that changes in HDL level caused by interventions are likely to occur over a long period of time [67]. However, in the exercised group, no changes in lipid profile were observed after injury. In a study, regular exercise for 12 weeks improved lipid profile following diabetes nephropathy [68]. Boor et al. (2009) reported that exercise for 5 weeks has no effect on the lipid profile following diabetic nephropathy, which is inconsistent with the results of present study [69]. Perhaps the reason for the difference between the above study and our study is the difference in the type of kidney injury, the type of animal, the weight of animal and the duration of exercise in the two studies. The reason for lack of change in the lipid profile after injury could be due to the positive effects of pre-injury exercise on plasma lipid levels. Exercise blocks hepatic HDL clearance increases cholesterol flow, and potentially enhances the uptake of cholesterol by liver cells. The clearance of TG-rich lipoproteins by lipoprotein lipase along with a possible reduction in exercise-related liver synthesis is another possible mechanism in this regard [70, 71].
In the present study, total cholesterol, triglyceride and LDL levels in the aerobic exercised group with calorie restriction were reduced following AKI. This finding is consistent with some other studies [72, 73]. Before AKI, since CR reduced total cholesterol, triglycerides and LDL level during exercise, this effect appears to be retained after injury. In regard to the reaction in above indexes following AKI, which was caused by exercise and also calorie restriction during exercise, it can be said that the effect of exercise has increased the effects of calorie restriction. Postprandial lipaemia has been shown to be important for lipid metabolism and plasma HDL levels [74]. Increased level and length of lipaemia during food intake followed by longer absorption time during food restriction would lead to a net balance that is more effective in the metabolism of triglyceride-rich lipoproteins [75]. Therefore, the increase in HDL level after injury in the exercised group can be due to a decrease in the amount of other fats caused by exercise. Another possibility is that, the reduction of these fats is due to decreased adipose tissue and increased lipoprotein lipase activity following calorie restriction [76].
However, TR with exercise increased total cholesterol, triglycerides, and HDL after injury, while HDL levels were high in the TR group before injury, so we can argue that this increase continued after injury. A study by Rahman and colleagues also showed that TR diet increased HDL level in the absence of exercise [77]. Since the TR diet is similar to Islamic fasting, the reason for the increase in HDL level may be due to changes in postprandial lipaemia that occur during fasting in Ramadan [75]. Therefore, the increase in HDL level in the present study cannot be due to exercise, so the TR diet alone has caused this increase in HDL level. The metabolic changes associated with fasting during Ramadan may affect all or some of these processes, leading to lipid changes. Elnasri and colleagues reported that consuming large amount of food after several hours of fasting may lead to increased endogenous cholesterol synthesis [78].
It seems the changes of lipid profile after AKI have been caused by CR along with exercise, which resulted in alleviating apoptosis and improving histopathology. Apoptosis along with changes in lipid profile have also been reported in breast cancer and cerebral ischemia [79, 80]. The evidence shows the stress oxidative is induced by increased blood lipids in breast cancer and polycystic ovary syndrome [81, 82].
Conclusion
Previous exercise reduced injury and apoptosis without changing fasting blood glucose and lipid profile after AKI. When there were two CR and TR diets along with exercise, the reduction in injury and renal apoptosis followed by AKI was greater. The reduction in lipid profile changes post-AKI in the CR group was more pronounced during exercise. It seems that a decrease in lipid profile changes following AKI due to CR in athletes could reduce apoptosis and injury of kidney tissue. To evaluate the effectiveness of different diets in athletes, more research is needed according to the type, intensity, and duration of exercise.
Availability of data and materials
The datasets used and analyzed during the current study are available via corresponding author on reasonable request.
Abbreviations
- AKI:
-
Acute kidney injury
- AMPK:
-
Activation of AMP-activated protein kinase
- Apaf-1:
-
Apoptotic protease activating factor-1
- Bad:
-
BCL2 associated agonist of cell death
- Bak:
-
Bcl2 Antagonist/Killer
- Bax:
-
Bcl-2-associated X protein
- Bcl2:
-
B-cell lymphoma 2
- Bid:
-
BH3 interacting-domain death agonist
- CR:
-
Calorie restriction
- CKD:
-
Chronic kidney disease
- c-fos:
-
Fos proto-oncogene
- c-myc:
-
Cellular myelocytomatosis oncogene
- Ctrl:
-
Control
- ESKD:
-
End-stage kidney disease
- Fas:
-
Apo 1 / CD 95 / tumor necrosis factor superfamily 6
- GFR:
-
Glomerular filtration rate
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- p53:
-
Tumor protein with a molecular weight of 53 kDa
- ROS:
-
Reactive oxygen species
- Sirt1:
-
Silent information regulator 1
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- TLR:
-
Toll-like receptors
- TR:
-
Time restriction
References
Sabet N, Soltani Z, Khaksari M, Raji-Amirhasani A. The effects of two different dietary regimens during exercise on outcome of experimental acute kidney injury. J Inflamm. 2022;19(1):1–14.
Vaghefi SSE, Mousavi F, Khaksari M, Asadikaram G, Soltani Z. Sex-related changes in circadian rhythm of inflammatory and oxidative stress markers in CKD. Iran J Kidney Dis. 2021;15(5):351–63.
Makris K, Spanou L. Acute kidney injury: definition, pathophysiology and clinical phenotypes. Clin Biochem Rev. 2016;37(2):85.
Ostermann M, Liu K. Pathophysiology of AKI. Best Pract Res Clin Anaesthesiol. 2017;31(3):305–14.
Bonegio R, Lieberthal W. Role of apoptosis in the pathogenesis of acute renal failure. Curr Opin Nephrol Hypertens. 2002;11(3):301–8.
Agarwal ML, Taylor WR, Chernov MV, Chernova OB, Stark GR. The p53 network. J Biol Chem. 1998;273(1):1–4.
Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88(3):323–31.
Amundson SA, Myers TG, Fornace AJ. Roles for p53 in growth arrest and apoptosis: putting on the brakes after genotoxic stress. Oncogene. 1998;17(25):3287–99.
Hall IE, Coca SG, Perazella MA, Eko UU, Luciano RL, Peter PR, et al. Risk of poor outcomes with novel and traditional biomarkers at clinical AKI diagnosis. Clin J Am Soc Nephrol. 2011;6(12):2740–9.
Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17(4):1143–50.
Cheng C-W, Villani V, Buono R, Wei M, Kumar S, Yilmaz OH, et al. Fasting-mimicking diet promotes Ngn3-driven β-cell regeneration to reverse diabetes. Cell. 2017;168(5):775-88.e12.
Raji-Amirhasani A, Khaksari M, Shahrokhi N, Soltani Z, Nazari-Robati M, Mahani FD, et al. Comparison of the effects of different dietary regimens on susceptibility to experimental acute kidney injury: The roles of SIRT1 and TGF-β1. Nutrition. 2022;96:111588.
Estrela GR, Wasinski F, Batista RO, Hiyane MI, Felizardo RJ, Cunha F, et al. Caloric restriction is more efficient than physical exercise to protect from cisplatin nephrotoxicity via PPAR-alpha activation. Front Physiol. 2017;8:116.
Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848–60.
Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14(1):1–10.
Soltani N, Soltani Z, Khaksari M, Ebrahimi G, Hajmohammmadi M, Iranpour M. The changes of brain edema and neurological outcome, and the probable mechanisms in diffuse traumatic brain injury induced in rats with the history of exercise. Cell Mol Neurobiol. 2020;40(4):555–67.
Sadeghinejad M, Soltani Z, Afzalpour ME, Khaksari M, Pourranjbar M. What is the combined effect of intense intermittent exercise and Ginkgo biloba plant on the brain neurotrophic factors levels, and learning and memory in young rats? Pharmacol Rep. 2019;71(3):503–8.
Moghetti P, Bacchi E, Brangani C, Donà S, Negri C. Metabolic effects of exercise. Sports. Endocrinology. 2016;47:44–57.
Guimarães GV, Ciolac EG, Carvalho VO, D’Avila VM, Bortolotto LA, Bocchi EA. Effects of continuous vs. interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens Res. 2010;33(6):627–32.
Tsapras P, Nezis IP. Caspase involvement in autophagy. Cell Death Differ. 2017;24(8):1369–79.
Zhu Y, Zhao L, Liu L, Gao P, Tian W, Wang X, et al. Beclin 1 cleavage by caspase-3 inactivates autophagy and promotes apoptosis. Protein Cell. 2010;1(5):468–77.
Sabet N, Soltani Z, Khaksari M. The effects of exercise on kidney injury: the role of SIRT1. Mol Biol Rep. 2022;49(5):4025–38.
Kroshian VM, Sheridan AM, Lieberthal W. Functional and cytoskeletal changes induced by sublethal injury in proximal tubular epithelial cells. Am J Physiol-Renal Physiol. 1994;266(1):F21–30.
Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z. Regulated cell death in AKI. J Am Soc Nephrol. 2014;25(12):2689–701.
Haase VH. Mechanisms of hypoxia responses in renal tissue. J Am Soc Nephrol. 2013;24(4):537–41.
Pons V, Riera J, Capó X, Martorell M, Sureda A, Tur JA, et al. Calorie restriction regime enhances physical performance of trained athletes. J Int Soc Sports Nutr. 2018;15(1):1–10.
Lee JH, Jung KJ, Kim JW, Kim HJ, Yu BP, Chung HY. Suppression of apoptosis by calorie restriction in aged kidney. Exp Gerontol. 2004;39(9):1361–8.
Mahani FD, Khaksari M, Iranpour M, Soltani Z, Raji-Amirhasani A, Hajializadeh Z. Effects of caloric and time restriction diets on kidney health in rat model of postmenopausal acute kidney injury: An apoptosis and histopathological study. Iran J Basic Med Sci. 2022;25(3):390.
Osman F, Haldar S, Henry CJ. Effects of time-restricted feeding during Ramadan on dietary intake, body composition and metabolic outcomes. Nutrients. 2020;12(8):2478.
Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11(3):390–8.
Di Francesco A, Di Germanio C, Bernier M, de Cabo R. A time to fast. Science. 2018;362(6416):770–5.
Arrick DM, Yang S, Li C, Cananzi S, Mayhan WG. Vigorous exercise training improves reactivity of cerebral arterioles and reduces brain injury following transient focal ischemia. Microcirculation. 2014;21(6):516–23.
Zhang Y, Du Y, Yu H, Zhou Y, Ge F. Protective effects of ophiocordyceps lanpingensis on glycerol-induced acute renal failure in mice. J Immunol Res. 2017;2017:2012585.
Sun X, Luan Q, Qiu S. Valsartan prevents glycerol-induced acute kidney injury in male albino rats by downregulating TLR4 and NF-κB expression. Int J Biol Macromol. 2018;119:565–71.
Shahrokhi N, Khaksari M, Nourizad S, Shahrokhi N, Soltani Z, Gholamhosseinian A. Protective effects of an interaction between vagus nerve and melatonin on gastric ischemia/reperfusion: the role of oxidative stress. Iran J Basic Med Sci. 2016;19(1):72.
Dehghan F, Shahrokhi N, Khaksari M, Soltani Z, Asadikorom G, Najafi A, et al. Does the administration of melatonin during post-traumatic brain injury affect cytokine levels? Inflammopharmacology. 2018;26(4):1017–23.
Shahrokhi N, Khaksari M, AsadiKaram G, Soltani Z, Shahrokhi N. Role of melatonin receptors in the effect of estrogen on brain edema, intracranial pressure and expression of aquaporin 4 after traumatic brain injury. Iran J Basic Med Sci. 2018;21(3):301.
Sarkaki AR, Khaksari Haddad M, Soltani Z, Shahrokhi N, Mahmoodi M. Time-and dose-dependent neuroprotective effects of sex steroid hormones on inflammatory cytokines after a traumatic brain injury. J Neurotrauma. 2013;30(1):47–54.
Atwa A, Hegazy R, Shaffie N, Yassin N, Kenawy S. Protective effects of vasodilatory βeta-blockers carvedilol and nebivolol against glycerol model of rhabdomyolysis-induced acute renal failure in rats. Open Access Maced J Med Sci. 2016;4(3):329.
Kocoglu H, Ozturk H, Ozturk H, Yilmaz F, Gulcu N. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney: a histopathologic study. Ren Fail. 2009;31(1):70–4.
Clarkson PM. Exertional rhabdomyolysis and acute renal failure in marathon runners. Sports Med. 2007;37(4):361–3.
Patel DR, Gyamfi R, Torres A. Exertional rhabdomyolysis and acute kidney injury. Phys Sportsmed. 2009;37(1):71–9.
Rojas-Valverde D, Sánchez-Ureña B, Crowe J, Timón R, Olcina GJ. Exertional rhabdomyolysis and acute kidney injury in endurance sports: A systematic review. Eur J Sport Sci. 2021;21(2):261–74.
Betts JA, Williams C. Short-term recovery from prolonged exercise. Sports Med. 2010;40(11):941–59.
Shu Y, Yang Y, Zhao Y, Ma L, Fu P, Wei T, et al. Melittin Inducing the Apoptosis of Renal Tubule Epithelial Cells through Upregulation of Bax/Bcl-2 Expression and Activation of TNF-α Signaling Pathway. BioMed Res Int. 2019;2019:9450368.
Homsi E, Brito SMd, Janino P. Silymarin exacerbates p53-mediated tubular apoptosis in glycerol-induced acute kidney injury in rats. Ren Fail. 2010;32(5):623–32.
Chen K-C, Peng C-C, Hsieh C-L, Peng RY. Exercise ameliorates renal cell apoptosis in chronic kidney disease by intervening in the intrinsic and the extrinsic apoptotic pathways in a rat model. Evidence-Based Complement Alternat Med. 2013;2013:368450.
Duan Y-C, Shi L, Jin Z, Hu M, Huang H, Yan T, et al. Swimming exercise ameliorates hypertension-induced kidney dysfunction via alleviating renal interstitial fibrosis and apoptosis. Kidney Blood Press Res. 2021;46(2):219–28.
de Lima WV, Visona I, Schor N, Almeida WS. Preconditioning by aerobic exercise reduces acute ischemic renal injury in rats. Physiol Rep. 2019;7(14):e14176.
Yang L, Li DX, Cao BQ, Liu SJ, Xu DH, Zhu XY, et al. Exercise training ameliorates early diabetic kidney injury by regulating the H2S/SIRT1/p53 pathway. FASEB J. 2021;35(9):e21823.
Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305(5682):390–2.
Belkacemi L, Selselet-Attou G, Antoine M-H, Nortier J, Hupkens E, Sener A, et al. Intermittent fasting modulation of the diabetic syndrome in streptozotocin-injected rats: post-mortem investigations. The Open Physiology Journal. 2013;5:1-6.
Loupal G, Url A, Skalicky M, Viidik A. Physical exercise retards the development of chronic nephropathy in the ageing rat as efficiently as food restriction does. Gerontology. 2005;51(2):83–93.
Keenan KP, Coleman JB, Mccoy CL, Hoe C-M, Soper KA, Laroque P. Chronic nephropathy in ad libitum overfed Sprague-Dawley rats and its early attenuation by increasing degrees of dietary (caloric) restriction to control growth. Toxicol Pathol. 2000;28(6):788–98.
Beshyah SA, Badi A, El-Ghul AM, Gabroun A, Dougman KK, Eledrisi MS. The year in “Ramadan Fasting and Health”(2018): a narrative review. IJMBS. 2019;11(4):151.
Klooster A. Thiamine, fasting and the kidney. 2013.
Ren J, Hu D, Mao Y, Yang H, Liao W, Xu W, et al. Alteration in gut microbiota caused by time-restricted feeding alleviate hepatic ischaemia reperfusion injury in mice. J Cell Mol Med. 2019;23(3):1714–22.
Han SJ, Li H, Kim M, Shlomchik MJ, Lee HT. Kidney proximal tubular TLR9 exacerbates ischemic acute kidney injury. J Immunol. 2018;201(3):1073–85.
Schmitt E, Steyaert A, Cimoli G, Bertrand R. Bax-α promotes apoptosis induced by cancer chemotherapy and accelerates the activation of caspase 3-like cysteine proteases in p53 double mutant B lymphoma Namalwa cells. Cell Death Differ. 1998;5(6):506–16.
Cai J, Yang J, Jones D. Mitochondrial control of apoptosis: the role of cytochrome c. (BBA)-Bioenergetics. 1998;1366(1–2):139–49.
Cain K, Bratton SB, Cohen GM. The Apaf-1 apoptosome: a large caspase-activating complex. Biochimie. 2002;84(2–3):203–14.
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–98.
Qin Y, Tang H, Yan G, Wang D, Qiao Y, Luo E, et al. A high triglyceride-glucose index is associated with contrast-induced acute kidney injury in Chinese patients with type 2 diabetes mellitus. Front Endocrinol. 2021;11:522883.
Leehey DJ, Moinuddin I, Bast JP, Qureshi S, Jelinek CS, Cooper C, et al. Aerobic exercise in obese diabetic patients with chronic kidney disease: a randomized and controlled pilot study. Cardiovasc Diabetol. 2009;8(1):1–8.
Jashni HK, Mohebbi H, Delpasand A, Jahromy HK. Caloric restriction and exercise training, combined, not solely improve total plasma adiponectin and glucose homeostasis in streptozocin-induced diabetic rats. Sport Sci Health. 2015;11(1):81–6.
Abdel-Gayoum A, Ali B, Ghawarsha K, Bashir A. Plasma lipid profile in rats with gentamicin-induced nephrotoxicity. Hum Exp Toxicol. 1993;12(5):371–5.
Saremi A, Asghari M, Ghorbani A. Effects of aerobic training on serum omentin-1 and cardiometabolic risk factors in overweight and obese men. J Sports Sci. 2010;28(9):993–8.
Nuhu JM, Maharaj SS. Influence of a mini-trampoline rebound exercise program on insulin resistance, lipid profile and central obesity in individuals with type 2 diabetes. J Sports Med Phys Fitness. 2017;58(4):503–9.
Boor P, Celec P, Behuliak M, Grančič P, Kebis A, Kukan M, et al. Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats. Metabolism. 2009;58(11):1669–77.
Jafari M, Leaf DA, MacRae H, Kasem J, O’conner P, Pullinger C, et al. The effects of physical exercise on plasma prebeta-1 high-density lipoprotein. Metabolism. 2003;52(4):437–42.
Jin F-Y, Kamanna VS, Kashyap ML. Niacin decreases removal of high-density lipoprotein apolipoprotein AI but not cholesterol ester by Hep G2 cells: implication for reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 1997;17(10):2020–8.
Wegge JK, Roberts CK, Ngo TH, Barnard RJ. Effect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery disease. Metabolism. 2004;53(3):377–81.
Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults—a randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2009;64(1):90–5.
Patsch JR, Karlin JB, Scott LW, Smith LC, Gotto AM. Inverse relationship between blood levels of high density lipoprotein subfraction 2 and magnitude of postprandial lipemia. Proc Natl Acad Sci. 1983;80(5):1449–53.
Maislos M, Abou-Rabiah Y, Zuili I, Iordash S, Shany S. Gorging and plasma HDL-cholesterol—the Ramadan model. Eur J Clin Nutr. 1998;52(2):127–30.
MacKay H, Khazall R, Patterson ZR, Wellman M, Abizaid A. Rats perinatally exposed to food restriction and high-fat diet show differences in adipose tissue gene expression under chronic caloric restriction. Adipocyte. 2013;2(4):237–45.
Rahman M, Rashid M, Basher S, Sultana S, Nomani M. Improved serum HDL cholesterol profile among Bangladeshi male students during Ramadan fasting. EMHJ-Eastern Mediterr Health J. 2004;10(12):131–7.
Elnasri H, Ahmed A. Effects of Ramadan fasting on blood levels of glucose, triglyceride and cholesterol among type II diabetic patients. Sudan J Public Health. 2006;1(3):203–6.
Cao X-L, Du J, Zhang Y, Yan J-T, Hu X-M. Hyperlipidemia exacerbates cerebral injury through oxidative stress, inflammation and neuronal apoptosis in MCAO/reperfusion rats. Exp Brain Res. 2015;233(10):2753–65.
Semenza GL. Cellular and molecular dissection of reperfusion injury: ROS within and without. Circ Res. 2000;86(2):117–8.
Macut D, Bjekić-Macut J, Savić-Radojević A. Dyslipidemia and oxidative stress in PCOS. Polycystic Ovary Syndrome. 2013;40:51–63.
Gupta RK, Patel AK, Kumari R, Chugh S, Shrivastav C, Mehra S, et al. Interactions between oxidative stress, lipid profile and antioxidants in breast cancer: a case control study. Asian Pac J Cancer Prev. 2012;13(12):6295–8.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NS: Collection of data and manuscript writing. ZS and MK: Supervised and directed the project, contributed to the data analyses and interpretations, and prepared the manuscript. FM: Design the study. AR, MI, and RMA: Collection of data. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The animal in this study were treated in accordance with the standard ethical guidelines. All methods are reported in accordance with ARRIVE guidelines. The study was approved by the Ethical Committee of Kerman University of Medical Sciences with code: IR.KMU.REC.1398.457.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sabet, N., Soltani, Z., Khaksari, M. et al. The effect of two types of diet on apoptosis indexes, lipid profile and histopathological outcome in acute kidney injury during exercise. BMC Nephrol 23, 315 (2022). https://doi.org/10.1186/s12882-022-02938-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-022-02938-w