Abstract
Background
This meta-analysis was conducted to assess the relationship between the transforming growth factor-beta 1 (TGF-β1) + 869 T/C gene polymorphism, + 915 G/C gene polymorphism, and the susceptibility of acute rejection in the recipients with renal transplantation.
Methods
Relevant studies were searched and identified from the Cochrane Library and PubMed, and eligible investigations were recruited and data were calculated by meta-analysis.
Results
In this study, we found no relationship between either TGF-β1 + 869 T/C or TGF-β1 + 915 G/C gene polymorphism and acute rejection susceptibility in patients with renal transplantation. No association between either gene polymorphism and acute rejection susceptibility in patients with renal transplantation in Caucasian, Asian, or African populations individually was found.
Conclusion
The TGF-β1 + 869 T/C and + 915 G/C gene polymorphisms are not associated with acute rejection susceptibility in recipients with renal transplantation.
Similar content being viewed by others
Background
End-stage renal disease (ESRD) has been defined as the start of renal replacement therapy or death relegated to renal diseases, its increasing worldwide prevalence represents a major economic and health burden [1, 2]. Renal transplantation is currently the therapy of choice for ESRD in children and adolescents [3, 4]. Approximately 20% of cases of renal disease progress to ESRD, for which the treatment of choice is also a kidney transplant [5]. Acute rejection in patients with renal transplantation can damage the transplanted renal tissue, even to the point of loss of renal function, which can threaten the life of the patient. At present, some studies show that some gene polymorphisms were associated with the risk of acute kidney allograft rejection [6,7,8,9], but some studies indicate that some gene polymorphisms were not associated with the risk of acute rejection susceptibility in recipients with renal transplantation [10,11,12]. Gene polymorphism can affect the expression level and the protein function, and there are some meta-analyses to assess the relationship between some gene polymorphism and the risk of acute rejection of kidney, but the conclusion is conflicting [11, 13,14,15,16]. Early detection and accurate management of acute rejection of kidney are important to the long-term health of each transplant recipient [17]. It is essential and very important to study the risk factors for the acute rejection in patients with renal transplantation.
Transforming-growth factor β1 (TGF-β1) is a multifunctional pro-fibrotic cytokine and involves in the physiological processes associated with growth, differentiation, and fibrosis [18, 19]. In contrast, TGF-β1 is a powerful immunoregulatory cytokine that inhibits T-cell activation, and TGF-β1 gene polymorphisms, especially in the position of + 869 T/C or + 915 G/C, encodes the signaling sequence of the TGF-β1 protein, and modify the production of cytokine [20, 21]. TGF-β1 + 869 T/C gene polymorphism results in the change of codon 10 from leucine (T) to proline (C), and TGF-β1 + 915 G/C gene polymorphism results in the change of codon 25 from arginine (G) to proline (C). In vitro, the presence of leucine or arginine, respectively, has been indicated to lead to a higher production of TGF-β1 [22]. TGF-β1 production might correlate with reduced incidence of acute rejection, since it down-regulates Th1 responses and Th1 cytokine production [21]. Single-nucleotide polymorphisms are associated with the risk of some diseases and drug dose requirement in kidney recipients [23,24,25,26,27]. The current evidence indicates that TGF-β1 involves in the pathogenesis of acute rejection in patients with renal transplantation. TGF-β1 + 869 T/C and + 915 G/C gene polymorphisms, which are important variants of TGF-β1, are reported to be associated with the risk of acute rejection.
In previous, Ge et al. [28] investigated the associations between the TGF-β1 polymorphisms of acute rejection susceptibility. It showed that TGF-β1 + 869 T/C gene polymorphism was not associated with the susceptibility of acute rejection in recipients with renal transplantation. Another meta-analysis [29] including 9 studies had investigated the combined effects of human + 869 T/C and + 915 G/C polymorphisms in the TGF-β1 gene with risk factors of renal transplantation, and indicated that recipient TGF-β1 high producer haplotypes were not significantly associated with an increased risk for acute rejection susceptibility in recipients with renal transplantation. However, the sample size is relatively small that it may be omit a small effect. Different types of ethnicity may also lead to different findings. We performed a meta-analysis of more studies to determine whether the TGF-β1 + 869 T/C and + 915 G/C gene polymorphisms are associated with the risk of acute rejection in renal transplantation.
Methods
1. Search strategy
Two investigators (HYL and TBZ) independently searched the Cochrane Library and PubMed databases through October 1, 2018 using the terms ‘(transforming growth factor-beta 1 OR TGF-β1) AND (polymorphism OR genotype OR allele) AND (acute rejection OR early graft rejection OR kidney transplant OR renal transplant OR allograft nephropathy OR rejection graft)’. The references of retrieved reports and association reviews were checked for additional missing data that we failed to identify during the electronic search.
2. Inclusion and exclusion criteria
Inclusion criteria: (1) The study provided detailed genotype data regarding TGF-β1 + 869 T/C and + 915 G/C distribution; (2) the study was given a case-control design; (3) the outcome was risk of acute rejection in the recipients with renal transplantation.
Exclusion criteria
(1) study was unrelated to the association between TGF-β1 + 869 T/C and + 915 G/C gene polymorphism and the risk of acute rejection in the recipients with renal transplantation; (2) review articles, case reports and editorials; (3) study had not provided the data of control group; (4) data was incomplete or missing; (5) animal study; (6) data was duplicated.
Two investigators (HYL and TBZ) independently conducted the literature screening process according to inclusion and exclusion criteria, and disagreements were resolved through discussion (WSL and SJL).
3. Data extraction
The information was extracted by two investigators (HYL and TBZ) independently from each included study: first author, publication years, country/district of study, ethnicity, and the number of case group and control group for TGF-β1 + 869 T/C and + 915 G/C genotypes. The frequencies of T and G alleles in TGF-β1 + 869 T/C and + 915 G/C were counted for the cases and controls. Disagreements were resolved through discussion (WSL and SJL).
4. Quality assessment of the included studies
The quality assessment of the included case-control studies recruited into this study was assessed by 2 investigators (TBZ and HYL) using the method of Newcastle-Ottawa Scale (NOS) [30, 31]. Major aspects to be assessed including selection of study subjects (four scores in total); exposure factors or outcomes (three scores in total); inter-group comparability (two scores in total). A score equal to or higher than 6 was regarded as high-quality studies, otherwise, less than 5 was considered as low-quality studies. Disagreements were resolved through discussion (WSL and SJL).
5. Statistical analysis
The pooled OR (odds ratio) with 95% confidence interval (95% CI) were evaluated to test the strength of associations between the TGF-β1 + 869 T/C or + 915 G/C gene polymorphisms and the risk of acute rejection in the recipients with renal transplantation. The I2 index was used to check heterogeneity assumption. When I2 ≥ 50% and P < 0.05, a Der-Simonian and Laird random-effects model was used to analyze data. Otherwise, a Mantel-Haenszel fixed-effects model was used. The Egger regression asymmetry test [32] and Begg adjusted rank correlation test [33] were used to calculate publication bias (P < 0.1 was considered significant). The available data from each investigation were extracted and calculated using Cochrane Review Manager Version 5.3 [34]. Hardy-Weinberg equilibrium (HWE) teat was assess for the genotype distribution of the control group (HWE; P < 0.05 was considered significant). Sensitivity analysis was performed when studies with controls not in HWE. Sensitivity analysis was also conducted according to omit each individual study and switching from fixed effect to random effect.
Results
Study characteristics
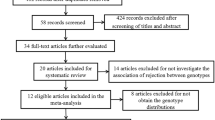
There were 18 studies [20, 35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] about the relationship between TGF-β1 + 869 T/C gene polymorphism and risk of acute rejection in patients with renal transplant (Fig. 1). We extracted the interesting data and calculated the T allele frequencies of TGF-β1 + 869 T/C in the case group and control group. One thousand five hundred eight patients with acute rejection and 2784 controls were included. Basical characteristics of included studies were presented in Table 1. We used the method of NOS to assess the quality of individual studies, and found all the included studies for TGF-β1 + 869 T/C gene polymorphism was regarded as high-quality studies (Table 1).
Eight studies [20, 36, 42 49, 52,53,54,55] about the association of TGF-β1 + 915 G/C gene polymorphism with the susceptibility of acute rejection in patients with renal transplantation were included (Fig. 1). The interesting data were extracted, and the G allele frequencies of TGF-β1 + 915 G/C for the case group and the control group were counted. Four hundred sixty-two patients with acute rejection and 1099 controls were included in the 8 studies. The characteristics of included investigations were showed in Table 2. The method of NOS was used to assess the quality of individual studies, and the results indicated that all the included studies for TGF-β1 + 915 G/C gene polymorphism was regarded as high-quality studies (Table 2).
Association of TGF-β1 + 869 T/C gene polymorphism with the susceptibility of acute rejection in patients with renal transplantation
In this meta-analysis, there was no statistic association between TGF-β1 + 869 T/C gene polymorphism and the susceptibility of acute rejection in patients with renal transplantation (CC genotype: OR = 1.04, 95% CI: 0.84–1.30, P = 0.70; TT genotype: OR = 1.08, 95% CI: 0.89–1.31, P = 0.44; T allele: OR = 1.01, 95% CI: 0.88–1.15, P = 0.93; Fig. 2 for CC genotype, Fig. 3 for TT genotype and Fig. 4 for T allele; Table 3).
Then we tried a sub-group to control confounding factor, and the results showed no statistic association between TGF-β1 + 869 T/C gene polymorphism and acute rejection in recipients with renal transplantation in Caucasians, Asians, or Africans as well (Table 3).
Relationship between TGF-β1 + 915 G/C gene polymorphism and the susceptibility of acute rejection in patients with renal transplantation
In our meta-analysis, TGF-β1 + 915 G/C gene polymorphism showed no statistic association with the susceptibility of acute rejection in patient with kidney transplantation (CC genotype: OR = 1.67, 95% CI: 0.84–3.31, P = 0.14; GG genotype: OR = 1.24, 95% CI: 0.91–1.69, P = 0.17; G allele: OR = 1.04, 95% CI: 0.79–1.37, P = 0.80; Fig. 5; Table 4).
In the sub-group analysis by ethnicity subsequently, no statistic association was showed between TGF-β1 + 915 G/C gene polymorphism and the susceptibility of acute rejection in patients with kidney transplantation in Asians, Caucasians, and Africans either (Table 4).
Sensitivity analysis
These studies in HWE were included for sensitivity analysis, and the results indicated that TGF-β1 + 869 T/C gene polymorphism was not associated with the susceptibility of acute rejection in patients with renal transplantation (CC genotype: OR = 0.98, 95% CI: 0.77–1.25, P = 0.88; TT genotype: OR = 0.96, 95% CI: 0.77–1.20, P = 0.70; T allele: OR = 0.99, 95% CI: 0.86–1.14, P = 0.88). TGF-β1 + 915 G/C gene polymorphism was also not associated with the susceptibility of acute rejection in patient with kidney transplantation (CC genotype: OR = 1.64, 95% CI: 0.80–3.34, P = 0.17; GG genotype: OR = 1.22, 95% CI: 0.87–1.70, P = 0.25; G allele: OR = 1.09, 95% CI: 0.81–1.45, P = 0.58).
We also conducted the sensitivity analysis by omitting each individual study, and found the results were similar to those non-sensitivity analyses. Sensitivity analysis by switching from fixed effect to random effect was also performed and the results indicated that the results were also similar to those non-sensitivity analyses.
Evaluation of publication bias
There was no significant publication bias for TGF-β1 + T869C gene polymorphism in overall population (Begg P = 0.202, funnel plot was presented in Fig. 6; Egger P = 0.420).
No statistic significant publication bias was detected for TGF-β1 + 915 G/C gene polymorphism in the overall populations (Begg P = 0.711, funnel plot was presented in Fig. 6; Egger P = 0.572).
Discussion
Some reports [56, 57] showed gene polymorphisms were the susceptibility factor of acute rejection in patients with renal transplantation. However, our meta-analysis results indicated that there were no association between TGF-β1 + 869 T/C gene polymorphism, TGF-β1 + 915 G/C gene polymorphism and the susceptibility of acute rejection in patients with kidney transplantation in the overall population; this relationship was somewhat robust. No publication bias was detected for this analysis, and the results might be robust. Sub-group analysis according to ethnicity was conducted to assess the conclusion.
No association was also found between the TGF-β1 + 869 T/C gene polymorphism, the TGF-β1 + 915 G/C gene polymorphism, and the susceptibility of acute rejection in patients with kidney transplantation in Caucasians, Asians, and Africans in subsequent sub-group analysis. Caucasians, Asians, and Africans were included in those studies but all with small sample sizes. The results also should be regarded cautiously, and more association studies were still needed to assess this relationship further.
The sensitivity analyses were conducted according to HWE and by omitting each individual study and switching from fixed effect to random effect, and the results were similar with those from non-sensitivity analyses. All included studies for this meta-analysis were judged as high quality, so we did not conduct the sensitivity analysis according to NOS score. The results might be robust to some extent, but more association studies were also needed to assess this relationship further.
In previous work, Ge’s et al. meta-analysis of 12 investigations indicated no association in TGF-β1 + 869 T/C gene polymorphism and the susceptibility of acute rejection in patients with renal transplantation in the overall population of China [28]. In our meta-analysis, 18 investigations were included, rendering the sample size much larger, so our results were more robust in some way. Besides, there was no other meta-analysis assessed this relationship, and our results indicated no association between the TGF-β1 + 915 G/C gene polymorphism and the susceptibility of acute rejection in patients with kidney transplantation in Asians, Caucasians, Africans, and the overall population. Nevertheless, these discoveries were considered to be retained, for the reason that many other factors, like objective probability of small sample in the recruited reports, unbalanced cases number between acute rejection group and non-acute rejection group, language bias, limited statistical power, heterogeneity of the enrolled patients, and clinical diversity of different study design and diverse intervention (such as immunosuppressive therapy), are capable of affecting the results. Furthermore, haplotypes analysis might give new information.
Conclusion
In conclusion, our meta-analysis supported no association between TGF-β1 + 869 T/C gene polymorphism and the TGF-β1 + 915 G/C gene polymorphism with the susceptibility of acute rejection in patients with renal transplantation in Asians, Caucasians, Africans, or the overall human population. However, more association investigations are needed to absolutely justify this verdict.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CKD:
-
chronic kidney disease
- ESRD:
-
End-stage renal disease
- HD:
-
hemodialysis
- PD:
-
peritoneal dialysis
- TGF-β1:
-
transforming growth factor-beta 1
References
Zhou H, Chen M, Zhu Y, Wang B, Liu XN, Zuo Z, Tang FY. Polymorphisms in NADPH oxidase CYBA gene modify the risk of ESRD in patients with chronic glomerulonephritis. Ren Fail. 2016;38(2):262–7.
Tan J, Liu S, Segal JB, Alexander GC, McAdams-DeMarco M. Warfarin use and stroke, bleeding and mortality risk in patients with end stage renal disease and atrial fibrillation: a systematic review and meta-analysis. BMC Nephrol. 2016;17(1):157.
Arze Aimaretti L, Arze S. Preemptive renal transplantation-the best treatment option for terminal chronic renal failure. Transplant Proc. 2016;48(2):609–11.
Filler G, Medeiros M. Improving long-term outcomes after pediatric renal transplantation by addressing dyslipidemia. Pediatr Transplant 2017, 21(3).
Naranjo-Escobar J, Manzi E, Posada JG, Mesa L, Echeverri GJ, Duran C, Schweneiberg J, Caicedo LA, Villegas JI, Tobon GJ. Kidney transplantation for end-stage renal disease in lupus nephritis, a very safe procedure: a single Latin American transplant center experience. Lupus. 2017:961203317696591.
Czerewaty M, Tarnowski M, Safranow K, Domanski L, Pawlik A. Mannose binding lectin 2 gene polymorphisms in patients after renal transplantation with acute graft rejection. Transpl Immunol. 2019.
Wang Z, Yang H, Si S, Han Z, Tao J, Chen H, Ge Y, Guo M, Wang K, Tan R, et al. Polymorphisms of nucleotide factor of activated T cells cytoplasmic 2 and 4 and the risk of acute rejection following kidney transplantation. World J Urol. 2018;36(1):111–6.
Jafari D, Nafar M, Yekaninejad MS, Abdolvahabi R, Lesan Pezeshki M, Razaghi E, Amirzargar AA. Investigation of killer immunoglobulin-like receptor (KIR) and HLA genotypes to predict the occurrence of acute allograft rejection after kidney transplantation. Iranian journal of allergy, asthma, and immunology. 2017;16(3):245–55.
Park MS, Kim SK, Park HJ, Seok H, Kang SW, Lee SH, Kim YG, Moon JY, Kim TH, Kim YH, et al. Association studies of bone morphogenetic protein 2 gene polymorphisms with acute rejection in kidney transplantation recipients. Transplant Proc. 2017;49(5):1012–7.
Wu Z, Xu Q, Qiu X, Xu L, Jiao Z, Zhang M, Zhong M. FKBP1A rs6041749 polymorphism is associated with allograft function in renal transplant patients. Eur J Clin Pharmacol. 2019;75(1):33–40.
Yang CH, Chen XX, Chen L, Zheng DH, Liu QS, Xie WF, Zhou TB, Drummen GPC. Relationship between cytotoxic T-lymphocyte antigen 4 -318C/T (rs5742909) gene polymorphism and the risk of acute rejection in renal transplantation. Pediatr Transplant. 2017;21(7).
Dabrowska-Zamojcin E, Dziedziejko V, Safranow K, Domanski L, Sluczanowska-Glabowska S, Pawlik A. STAT4 gene polymorphism in patients after renal allograft transplantation. Central-European journal of immunology. 2016;41(3):255–9.
Yang CH, Chen XX, Chen L, Zheng DH, Liu QS, Xie WF. Association of cytotoxic T-lymphocyte antigen 4 +49A/G gene polymorphism with acute rejection risk in renal transplantation. Pediatr Transplant. 2017;21(4).
Hu Q, Tian H, Wu Q, Li J, Cheng X, Liao P. Interleukin-10-1082 G/a polymorphism and acute renal graft rejection: a meta-analysis. Ren Fail. 2016;38(1):57–64.
Hu Q, Tian H, Wu Q, Li J, Cheng X, Liao P. Association between Interleukin-2 -330 T/G polymorphism and acute renal graft rejection: A meta-analysis. Transplant Proc. 2015;47(6):1746–53.
Ge YZ, Wu R, Jia RP, Liu H, Yu P, Zhao Y, Feng YM. Association between interferon gamma +874 T>A polymorphism and acute renal allograft rejection: evidence from published studies. Mol Biol Rep. 2013;40(10):6043–51.
Lee H, Park YM, We YM, Han DJ, Seo JW, Moon H, Lee YH, Kim YG, Moon JY, Lee SH, et al. Evaluation of digital PCR as a technique for monitoring acute rejection in kidney transplantation. Genomics Inform. 2017;15(1):2–10.
Stojimirovic B, Jovanovic N, Trbojevic-Stankovic J, Nesic DM, Brasanac T, Zunic-Bozinovski S. Levels of transforming growth factor beta1 during first six months of peritoneal dialysis. Ren Fail. 2015;37(4):640–5.
Zhou T, Li HY, Zhong H, Zhong Z. Relationship between transforming growth factor-beta1 and type 2 diabetic nephropathy risk in Chinese population. BMC medical genetics. 2018;19(1):201.
Dhaouadi T, Sfar I, Bardi R, Jendoubi-Ayed S, Abdallah TB, Ayed K, Gorgi Y. Cytokine gene polymorphisms in kidney transplantation. Transplant Proc. 2013;45(6):2152–7.
Bijlsma FJ, van der Horst AA, Tilanus MG, Rozemuller E, de Jonge N, Gmelig-Meyling FH, de Weger RA. No association between transforming growth factor beta gene polymorphism and acute allograft rejection after cardiac transplantation. Transpl Immunol. 2002;10(1):43–7.
Melk A, Henne T, Kollmar T, Strehlau J, Latta K, Offner G, Jhangri GS, Ehrich JH, Von Schnakenburg C. Cytokine single nucleotide polymorphisms and intrarenal gene expression in chronic allograft nephropathy in children. Kidney Int. 2003;64(1):314–20.
Ismail NF, Nik Abdul Malik NM, Mohseni J, Rani AM, Hayati F, Salmi AR, Narazah MY, Zabidi-Hussin ZA, Silawati AR, Keng WT, et al. Two novel gross deletions of TSC2 in Malaysian patients with tuberous sclerosis complex and TSC2/PKD1 contiguous deletion syndrome. Jpn J Clin Oncol. 2014;44(5):506–11.
Sasongko TH, Gunadi ZBA, Zabidi-Hussin Z. Deletion analysis of SMN1 exon 7 alone may be necessary and sufficient for the diagnosis of spinal muscular atrophy. J Neurogenet. 2011;25(1–2):15–6.
Kallel A, Ben Salem T, Hammami MB, Said F, Jemaa R, Houman MH, Feki M. Association of systemic beta-defensin-1 and -20G/A DEFB1 gene polymorphism with Behcet's disease. European journal of internal medicine. 2019.
Qu L, Lu Y, Ying M, Li B, Weng C, Xie Z, Liang L, Lin C, Yang X, Feng S, et al. Tacrolimus dose requirement based on the CYP3A5 genotype in renal transplant patients. Oncotarget. 2017;8(46):81285–94.
Gunadi, Iskandar K, Makhmudi A, Kapoor A. Combined genetic effects of RET and NRG1 susceptibility variants on multifactorial Hirschsprung disease in Indonesia. J Surg Res. 2019;233:96–9.
Ge YZ, Yu P, Jia RP, Wu R, Ding AX, Li LP, Zhao Y, Feng YM, Gui ZL, Liao S. Association between transforming growth factor beta-1 +869T/C polymorphism and acute rejection of solid organ allograft: A meta-analysis and systematic review. Transpl Immunol. 2014;30(2–3):76–83.
Ge YZ, Wu R, Lu TZ, Jia RP, Li MH, Gao XF, Jiang XM, Zhu XB, Li LP, Tan SJ, et al. Combined effects of TGFB1 +869 T/C and +915 G/C polymorphisms on acute rejection risk in solid organ transplant recipients: a systematic review and meta-analysis. PLoS One. 2014;9(4):e93938.
Zhang ZL, Zhang CZ, Li Y, Zhao ZH, Yang SE. Association between ERalpha gene Pvu II polymorphism and breast cancer susceptibility: A meta-analysis. Medicine. 2018;97(17):e0317.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
The Cochrane Collaboration (2014). Review manager (Revman) [computer program]. Version 5.3. Copenhagen: the Nordic Cochrane Centre.
Marshall SE, McLaren AJ, Haldar NA, Bunce M, Morris PJ, Welsh KI. The impact of recipient cytokine genotype on acute rejection after renal transplantation. Transplantation. 2000;70(10):1485–91.
Alakulppi NS, Kyllonen LE, Jantti VT, Matinlauri IH, Partanen J, Salmela KT, Laine JT. Cytokine gene polymorphisms and risks of acute rejection and delayed graft function after kidney transplantation. Transplantation. 2004;78(10):1422–8.
Ligeiro D, Sancho MR, Papoila A, Barradinhas AM, Almeida A, Calao S, Machado D, Nolasco F, Guerra J, Sampaio MJ, et al. Impact of donor and recipient cytokine genotypes on renal allograft outcome. Transplant Proc. 2004;36(4):827–9.
Chow KM, Szeto CC, Poon P, Lau WY, Lai FM, Li PK. Transforming growth factor-beta1 gene polymorphism in renal transplant recipients. Ren Fail. 2005;27(6):671–5.
Guo YF, Tan JM, Li RY, Liu SZ, Li Y, Ying K, Xie Y, Mao YM. Impacts of donor and recipient's SNP of cytokine and cytokine receptor on early acute renal allograft rejection. Zhonghua Yi Xue Za Zhi. 2005;85(44):3126–33.
Gendzekhadze K, Rivas-Vetencourt P, Montano RF. Risk of adverse post-transplant events after kidney allograft transplantation as predicted by CTLA-4 +49 and TNF-alpha −308 single nucleotide polymorphisms: a preliminary study. Transpl Immunol. 2006;16(3–4):194–9.
Hueso M, Navarro E, Moreso F, Beltran-Sastre V, Ventura F, Grinyo JM, Seron D. Relationship between subclinical rejection and genotype, renal messenger RNA, and plasma protein transforming growth factor-beta1 levels. Transplantation. 2006;81(10):1463–6.
Dmitrienko S, Hoar DI, Balshaw R, Keown PA. Immune response gene polymorphisms in renal transplant recipients. Transplantation. 2005;80(12):1773–82.
Canossi A, Piazza A, Poggi E, Ozzella G, Di Rocco M, Papola F, Iaria G, Adorno D. Renal allograft immune response is influenced by patient and donor cytokine genotypes. Transplant Proc. 2007;39(6):1805–12.
Brabcova I, Petrasek J, Hribova P, Hyklova K, Bartosova K, Lacha J, Viklicky O. Genetic variability of major inflammatory mediators has no impact on the outcome of kidney transplantation. Transplantation. 2007;84(8):1037–44.
Manchanda PK, Mittal RD. Analysis of cytokine gene polymorphisms in recipient's matched with living donors on acute rejection after renal transplantation. Mol Cell Biochem. 2008;311(1–2):57–65.
Mendoza-Carrera F, Ojeda-Duran S, Angulo E, Rivas F, Macias-Lopez G, Buen EP, Leal C. Influence of cytokine and intercellular adhesion molecule-1 gene polymorphisms on acute rejection in pediatric renal transplantation. Pediatr Transplant. 2008;12(7):755–61.
Grinyo J, Vanrenterghem Y, Nashan B, Vincenti F, Ekberg H, Lindpaintner K, Rashford M, Nasmyth-Miller C, Voulgari A, Spleiss O, et al. Association of four DNA polymorphisms with acute rejection after kidney transplantation. Transpl Int. 2008;21(9):879–91.
Karimi MH, Daneshmandi S, Pourfathollah AA, Geramizadeh B, Yaghobi R, Rais-Jalali GA, Roozbeh J, Bolandparvaz S. A study of the impact of cytokine gene polymorphism in acute rejection of renal transplant recipients. Mol Biol Rep. 2012;39(1):509–15.
Seyhun Y, Mytilineos J, Turkmen A, Oguz F, Kekik C, Ozdilli K, Nane I, Aydin F, Carin M. Influence of cytokine gene polymorphisms on graft rejection in Turkish patients with renal transplants from living related donors. Transplant Proc. 2012;44(6):1670–8.
Saigo K, Akutsu N, Maruyama M, Otsuki K, Hasegawa M, Aoyama H, Matsumoto I, Asano T, Kenmochi T. Study of transforming growth factor-beta1 gene, mRNA, and protein in Japanese renal transplant recipients. Transplant Proc. 2014;46(2):372–5.
Seyhun Y, Ciftci HS, Kekik C, Karadeniz MS, Tefik T, Nane I, Turkmen A, Oguz FS, Aydin F. Genetic association of interleukin-2, interleukin-4, interleukin-6, transforming growth factor-beta, tumour necrosis factor-alpha and blood concentrations of calcineurin inhibitors in Turkish renal transplant patients. Int J Immunogenet. 2015;42(3):147–60.
Park J, Park M, Park H, Ha J, Kim S, A C. TNF-alpha and TGF-beta1 gene polymorphisms and renal allograft rejection in Koreans. Tissue Antigens. 2004;64(6):660–6.
Li C, Yu L, Xu J, Fu S, Deng W, Du C, Wang Y. Association between transforming growth factor beta-1 gene polymorphism and chronic allograft nephropathy. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27(4):535–7.
Chen Z, Bouamar R, Van Schaik R, De Fijter J, Hartmann A, Zeier M, Budde K, Kuypers D, Weimar W, Hesselink D, et al. Genetic polymorphisms in IL-2, IL-10, TGF-β1, and IL-2RB and acute rejection in renal transplant patients. Clin Transpl. 2014;28(6):649–55.
Seyhun Y, Ciftci H, Kekik C, Karadeniz M, Tefik T, Nane I, Turkmen A, Oguz F, Aydin F. Genetic association of interleukin-2, interleukin-4, interleukin-6, transforming growth factor-β, tumour necrosis factor-α and blood concentrations of calcineurin inhibitors in Turkish renal transplant patients. Int J Immunogenet. 2015;42(3):147–60.
Kloda K, Mierzecki A, Domanski L, Borowiecka E, Safranow K, Ciechanowicz A, Ciechanowski K. Joint assessment of donor and recipient hTERT gene polymorphism provides additional information for early kidney transplantation outcomes. Med Sci Monit. 2017;23:1812–8.
Sanchez-Fructuoso AI, Perez-Flores I, Valero R, Moreno MA, Fernandez-Arquero M, Urcelay E, Fernandez-Perez C, Santiago JL. The polymorphism -308G/A of tumor necrosis factor-alpha gene modulates the effect of immunosuppressive treatment in first kidney transplant subjects who suffer an acute rejection. J Immunol Res. 2016;2016:2197595.
Acknowledgements
The authors would like to gratefully acknowledge the most helpful comments on this paper received from Zhiqing Zhong and Hongzhen Zhong, Department of Nephrology, the Second Affiliated Hospital of Shantou University Medical College, Shantou, China.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
TBZ was in charge of conceived and designed the study. TBZ, and HYL were responsible for collection of data and performing the statistical analysis and manuscript preparation. WSL and SJL were responsible for checking the data. All authors were responsible for drafting the manuscript, read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, HY., Zhou, T., Lin, S. et al. Relationship between TGF-β1 + 869 T/C and + 915 G/C gene polymorphism and risk of acute rejection in renal transplantation recipients. BMC Med Genet 20, 113 (2019). https://doi.org/10.1186/s12881-019-0847-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12881-019-0847-2