Abstract
Background
Sepsis is a common syndrome of multiorgan system dysfunction secondary to the dysregulated inflammatory response to infection. The role of pancreatic stone protein (PSP) in diagnosing sepsis has been investigated in previous studies. The meta-analysis aimed to comprehensively investigate the diagnostic value of PSP in identifying sepsis.
Methods
PubMed, Web of Science, Embase, Cochrane Library, and China National Knowledge Infrastructure (CNKI), were systematically searched. Studies investigating the diagnostic performance of PSP were included. Pooled sensitivity, specificity, positive Likelihood Ratio (+ LR) and negative Likelihood Ratio (-LR), diagnostic odds ratio (DOR), and area under the curve (AUC) of summary receiver operating characteristic (SROC) were calculated.
Results
The sensitivity of PSP was 0.88 (95% CI: 0.77–0.94), and the pooled specificity was 0.78 (95% CI: 0.65–0.87). Pooled + LR, -LR, and DOR were 4.1 (2.3, 7.3), 0.16 (0.07, 0.34), and 26 (7, 98). The AUC value for the SROC of PSP was 0.90 (0.87, 0.92). The pooled sensitivity, specificity, + LR and - LR, and DOR for PSP among neonates were 0.91 (95% CI: 0.84, 0.96), 0.66 (95% CI: 0.58, 0.74), 3.97 (95% CI: 0.53, 29.58), 0.13 (95% CI: 0.02, 1.00), and 31.27 (95% CI: 0.97, 1004.60).
Conclusions
This study indicates that PSP demonstrated favorable diagnostic accuracy in detecting sepsis. Well-designed studies are warranted to ascertain the value of PSP measurement to guide early empirical antibiotic treatment, particularly in neonates.
Similar content being viewed by others
Background
Sepsis is a clinical syndrome characterized by organ dysfunction due to dysregulated host response to infection; it is still one of the most common causes of mortality in critically ill patients [1,2,3]. The mortality rate of sepsis is estimated to be between 20% and 40%, and long-term complications are common, including kidney failure, liver failure, depression, and neurocognitive impairment [4,5,6]. It is estimated that there are over 19 million cases of sepsis and 5 million sepsis-related deaths each year, with the majority occurring in low and middle-income countries [7]. Early identification of sepsis is crucial for improving prognosis and preventing relevant complications [8].
Biochemical markers can help practitioners identify sepsis as early as possible. Nevertheless, commonly used biomarkers, including C-reactive protein (CRP) and procalcitonin, have been proven inaccurate in detecting sepsis in previous meta-analyses [9, 10]. CRP showed promising sensitivity in detecting infection, nevertheless, it is only specific if a high cutoff level is used, which it will in turn reduce sensitivity [11]. Procalcitonin (PCT) has been determined to have the highest performance as a biomarker for the diagnosis and prognosis of sepsis. However, it may be elevated in many diseases other than infection, especially after surgery and trauma [12, 13]. Better biomarkers are warranted to increase the value of sepsis detection. Pancreatic stone protein (PSP), a 144-amino-acid glycoprotein, might be a suitable biomarker for sepsis; however, the physiological role of PSP has not been elucidated yet [14,15,16,17]. PSP is mainly secreted by pancreatic acinar cells and also secreted by intestinal and gastric cell subsets [18]. A study illustrated that PSP is an inflammatory mediator that can bind and activate neutrophils, thereby acting as an acute phase protein in response to injury in the early stages of infection [16].
Several investigations have explored the diagnostic capabilities of PSP in detecting sepsis, demonstrating favorable outcomes [19,20,21,22,23]. However, these studies were limited concerning their sample sizes and the population involved. This meta-analysis aimed to draw a comprehensive knowledge of the diagnostic value of PSP in sepsis identification.
Methods
Search strategy and selection criteria
This meta-analysis was conducted in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) [24]. From the inception date until April 20, 2023, a comprehensive search was executed across multiple electronic databases, encompassing PubMed, Web of Science, Embase, the Cochrane Library, and China National Knowledge Infrastructure (CNKI), considering literature in both English and Chinese languages. The search strategy incorporated an array of keywords and synonyms: pancreatic stone protein, PSP, sepsis, Bloodstream Infection, Bloodstream Pyemia, Pyemias, Pyohemia, Septicemia, Blood Poisoning, Blood Severe Sepsis. A flowchart for search, screening, and eligibility identification was constructed based on the PRISMA guidelines. Inclusion criteria were as follows: (1) subjects suspected of having sepsis; (2) clear documentation of a reference standard; (3) a 2 × 2 contingency table can be formed categorizing participants with true positive (TP), false positive (FP), false negative (FN), true negative (TN) results on PSP test. Exclusions pertained to case reports, reviews, editorials, conference abstracts, animal studies, or studies with unextractable data. The entire process of database querying and study selection was independently executed by two reviewers, with any disagreements resolved through iterative discussions until consensus was achieved.
Data extraction and risk of bias assessment
The following study level data were retrieved: name of the first author, publication year, country, sample size, median or mean age, proportion of the female, standard reference, counts of participants with TP, FP, FN, and TN outcomes. The risk of bias assessment of studies was evaluated according to the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) [25]. Two authors independently extracted data from included studies and appraised the risk of bias of these studies; disagreement was solved through consultation with a third investigator.
Statistical analysis
Statistical analyses at the study level were executed using Stata 14.0 software and Meta-DiSc 1.4. The following summary measures were computed, accompanied by their corresponding 95% confidence intervals (CIs): pooled sensitivity, specificity, positive Likelihood Ratio (+ LR), negative Likelihood Ratio (-LR), diagnostic odds ratio (DOR), and the area under the curve (AUC) derived from the summary receiver operating characteristic (SROC) curve. To gauge the heterogeneity among the included studies, Cochran’s Q statistic and the I2 index were employed. According to established thresholds, heterogeneity was categorized as insignificant (I2 = 0–25%), low (I2 = 25–50%), moderate (I2 = 50–75%), or high (I2 = 75–100%) [26]. Publication bias was visually inspected through the construction of funnel plots and further subjected to statistical assessment utilizing Deeks’ method [27]. Additionally, a sensitivity analysis was carried out to gauge the influence of individual studies on the aggregate results. Statistical significance was set at a p-value less than 0.05.
Results
Study selection and characteristics
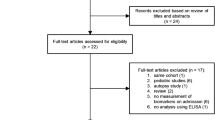
The literature search yielded 265 articles. Among them, 92 duplicated citations were removed, and another 156 studies were excluded through an initial screening of the title and abstract. The full-text reading of the remaining 17 articles identified nine studies with 1364 participants eligible for inclusion in this study [8, 11, 19,20,21,22,23, 28, 29] and five studies in meta-analysis. Figure 1 displays the flowchart of the database search and study selection. Six studies were performed among adults; the other 3 included studies were conducted among newborns. The coverage of countries included the UK, Switzerland, Netherlands, Spain, Egypt, France, and Italy. Characteristics of enrolled studies are depicted in Table 1. The risk of bias for each included study was assessed as low (Fig. 2 and Figure S1).
Diagnostic performance of PSP
The overall sensitivity of PSP was 0.88 (95% CI: 0.77–0.94, I2 = 77.4%, p < 0.01), and the pooled specificity was 0.78 (95% CI: 0.65–0.87, I2 = 90.3%, p < 0.01), respectively (Fig. 3). Pooled + LR, -LR, and DOR were 4.1 (2.3, 7.3), 0.16 (0.07, 0.34), and 26 (7, 98). The AUC value for the SROC of PSP was 0.90 (0.87, 0.92) (Fig. 4). The pooled sensitivity, specificity, + LR and - LR, and DOR for PSP among neonates were 0.91 (95% CI: 0.84, 0.96), 0.66 (95% CI: 0.58, 0.74), 3.97 (95% CI: 0.53, 29.58), 0.13 (95% CI: 0.02, 1.00), and 31.27 (95% CI: 0.97, 1004.60) (Table 2). The pooled sensitivity, specificity, + LR and - LR, and DOR for PSP among adults were 0.85 (95% CI: 0.78, 0.90), 0.72 (95% CI: 0.67, 0.776), 4.09 (95% CI: 1.69, 9.92), 0.19 (95% CI: 0.08, 0.49), and 22.74 (95% CI: 4.25, 121.73) (Table 2).
In the study by Garcia de Guadiana-Romualdo et al., results of the ROC curve analysis revealed an AUC of 0.872 for sepsis identification [11]. Klein et al’s analysis of biomarker kinetics (PSP, routine markers) was performed on 90 burned patients, PSP identified between sepsis, infection and sterile inflammation with an AUC 0.89 [28]. An AUC of 0.69 was reported in de Hond et al’s study including 156 participants [8]. Moreover, in Saleh et al’s study, the ROC revealed that the AUC for PSP reached 0.868 for sepsis diagnosis [29].
Publication bias
Deek’s tests for publication bias yielded p-values of 0.42 for the meta-analysis, indicating no statistically significant publication bias (Fig. 5).
Sensitivity analysis
Results of sensitivity analysis demonstrated that the two included studies investigating the diagnostic value of PSP in neonatal sepsis had a significant impact on the overall effect size, and thus subgroup analysis based on the age of the study population was performed (Figure S2).
Discussion
Results of this study demonstrated an overall sensitivity of 0.88 (95% CI: 0.77–0.94) and a pooled specificity of 0.78 (95% CI: 0.65–0.87) with an overall AUC value of 0.90 (0.87, 0.92). Compared to other biomarkers, the pooled sensitivity in this meta-analysis was higher than the pooled sensitivity (0.79) in Chen et al’s meta-analysis investigating neutrophil to lymphocyte ratio (NLR) in the diagnosis of sepsis, while the specificity was lower than that (0.91) in Chen et al’s meta-analysis [30]; the comparison between PSP and calprotectin (sensitivity: 0.88 vs. 0.77, specificity: 0.78 vs.0.85) was similar to NLR [31]; Poggi’s meta-analysis assessing the accuracy of presepsin for the sepsis diagnosis showed that the pooled sensitivity and specificity were 0.93 (95% CI, 0.86–0.95) and 0.91 (95% CI, 0.85–0.95), respectively, which were higher than PSP [32]; the overall sensitivity and specificity were higher than those of CRP and PCT [33].
Several research findings suggest that PSP is involved in the early defense mechanism of sepsis [16, 19, 28]. In these studies, PSP has been shown to be associated with the severity of inflammation and can activate neutrophils by upregulating activation markers CD11b and CD62L [16]. In addition to the activation of neutrophils, PSP possesses antibacterial functions; it can induce bacterial aggregation, which may help prevent bacteria from penetrating the intestinal barrier [34]. Moreover, PSP levels rise 72 h before the onset of clinical symptoms of sepsis [8]. The results of this meta-analysis verified that PSP displayed favorable diagnostic performance in detecting sepsis. The results were consistent with previous studies regarding the diagnostic value of PSP in sepsis [8, 11, 29]. Notably, neonatal sepsis is difficult to diagnose due to the nonspecific clinical signs in response to sepsis [35]. Schlapbach et al.‘s study are the first to investigate PSP in neonatal sepsis; results demonstrated that the level of PSP in infected infants was significantly higher than in uninfected ones with an AUC of 0.69 [20]. Subgroup analysis of this meta-analysis showed favorable pooled sensitivity but low specificity for PSP alone in the diagnosis of neonatal sepsis, suggesting the necessity for combining different biomarkers to detect sepsis in this specific population. More well-designed prospective studies are required to clarify this opinion.
Interestingly, the pooled sensitivity for adults was lower than that in neonates, while the difference in specificity is the opposite. The underlying reason needed to be clarified based on the current meta-analysis. However, it was reported that maximum PSP levels in neonates were lower than those in adults with sepsis, which may be one of the reasons for the different diagnostic values for PSP in newborns and adults. Relevant studies are warranted to investigate this difference.
Strengths and limitations
To our knowledge, this is the first meta-analysis to evaluate the diagnostic value of PSP in the context of sepsis in general. Data from previously published citations were synthesized to enhance the statistical power of the diagnostic value of PSP. Results of this study were favorable and promising, which may serve as both advanced level of evidence and reference for practitioners to make decisions on the diagnosis of sepsis in their clinical practice.
Like other meta-analyses, there are several limitations of this meta-analysis. The study protocol was not registered on PROSPERO. Significant heterogeneity existed between included studies; it may be attributed to differences in the study population, standard reference, and cutoffs of PSP in component studies. Although Deek’s funnel plots asymmetry test revealed no statistically significant publication bias in the meta-analysis, bias caused by published and unpublished studies inherently existed because this study is only focused on published articles. The number of included studies was limited owing to the inclusion criteria of this meta-analysis; aside from subgroup analysis based on the study population, subgroup analysis on other covariates was not performed. The interpretation of findings from this study ought to be with caution; more similar studies are needed to specify the diagnostic value of PSP in detecting sepsis.
Conclusion
In this meta-analysis, evidence suggests that PSP was a promising biomarker for diagnosing patients suspected of sepsis. According to the findings presented in this meta-analysis, specifically designed studies on different populations are needed to ascertain the validity of PSP measurement to guide early empirical antibiotic treatment, particularly in neonates.
Data availability
All data generated or analyzed during this study are included in this article and supplementary information files.
Abbreviations
- PSP:
-
pancreatic stone protein
- CNKI:
-
China National Knowledge Infrastructure
- LR:
-
Likelihood Ratio
- DOR:
-
diagnostic odds ratio
- AUC:
-
area under the curve
- SROC:
-
summary receiver operating characteristic
- CRP:
-
C-reactive protein
- PCT:
-
Procalcitonin
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- TP:
-
true positive
- FP:
-
false positive
- FN:
-
false negative
- TN:
-
true negative
- QUADAS-2:
-
Quality Assessment of Diagnostic Accuracy Studies-2
- CIs:
-
confidence intervals
References
Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392:75–87.
Dugar S, Choudhary C, Duggal A. Sepsis and septic shock: Guideline-based management. Cleve Clin J Med. 2020;87:53–64.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10.
Woźnica EA, Inglot M, Woźnica RK, Łysenko L. Liver dysfunction in sepsis. Adv Clin Exp Med. 2018;27:547–51.
Mostel Z, Perl A, Marck M, Mehdi SF, Lowell B, Bathija S, et al. Post-sepsis syndrome - an evolving entity that afflicts survivors of sepsis. Mol Med. 2019;26:6.
Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891.
Rudd KE, Kissoon N, Limmathurotsakul D, Bory S, Mutahunga B, Seymour CW, et al. The global burden of sepsis: barriers and potential solutions. Crit Care. 2018;22:232.
de Hond TAP, Oosterheert JJ, van Hemert-Glaubitz SJM, Musson REA, Kaasjager KAH. Pancreatic stone protein as a Biomarker for Sepsis at the Emergency Department of a large Tertiary Hospital. Pathogens. 2022;11.
Pontrelli G, De Crescenzo F, Buzzetti R, Jenkner A, Balduzzi S, Calò Carducci F, et al. Accuracy of serum procalcitonin for the diagnosis of sepsis in neonates and children with systemic inflammatory syndrome: a meta-analysis. BMC Infect Dis. 2017;17:302.
Shabuj KH, Hossain J, Moni SC, Dey SK. C-reactive protein (CRP) as a single biomarker for diagnosis of neonatal Sepsis: a Comprehensive Meta-analysis. Mymensingh Med J. 2017;26:364–71.
García de Guadiana-Romualdo L, Berger M, Jiménez-Santos E, Rebollo-Acebes S, Jiménez-Sánchez R, Esteban-Torrella P, et al. Pancreatic stone protein and soluble CD25 for infection and sepsis in an emergency department. Eur J Clin Invest. 2017;47:297–304.
Riedel S. Procalcitonin and the role of biomarkers in the diagnosis and management of sepsis. Diagn Microbiol Infect Dis. 2012;73:221–7.
Clec’h C, Fosse JP, Karoubi P, Vincent F, Chouahi I, Hamza L, et al. Differential diagnostic value of procalcitonin in surgical and medical patients with septic shock. Crit Care Med. 2006;34:102–7.
De Caro A, Lohse J, Sarles H. Characterization of a protein isolated from pancreatic calculi of men suffering from chronic calcifying pancreatitis. Biochem Biophys Res Commun. 1979;87:1176–82.
Terazono K, Yamamoto H, Takasawa S, Shiga K, Yonemura Y, Tochino Y, et al. A novel gene activated in regenerating islets. J Biol Chem. 1988;263:2111–4.
Keel M, Härter L, Reding T, Sun LK, Hersberger M, Seifert B, et al. Pancreatic stone protein is highly increased during posttraumatic sepsis and activates neutrophil granulocytes. Crit Care Med. 2009;37:1642–8.
Eggimann P, Que YA, Rebeaud F. Measurement of pancreatic stone protein in the identification and management of sepsis. Biomark Med. 2019;13:135–45.
Graf R, Schiesser M, Reding T, Appenzeller P, Sun LK, Fortunato F, et al. Exocrine meets endocrine: pancreatic stone protein and regenerating protein–two sides of the same coin. J Surg Res. 2006;133:113–20.
Llewelyn MJ, Berger M, Gregory M, Ramaiah R, Taylor AL, Curdt I, et al. Sepsis biomarkers in unselected patients on admission to intensive or high-dependency care. Crit Care. 2013;17:R60.
Schlapbach LJ, Graf R, Woerner A, Fontana M, Zimmermann-Baer U, Glauser D, et al. Pancreatic stone protein as a novel marker for neonatal sepsis. Intensive Care Med. 2013;39:754–63.
Palmiere C, Augsburger M. Pancreatic stone protein as a postmortem biochemical marker for the diagnosis of sepsis. Leg Med (Tokyo). 2015;17:9–13.
Rass AA, Talat MA, Arafa MA, El-Saadany HF, Amin EK, Abdelsalam MM, et al. The role of Pancreatic Stone protein in diagnosis of early onset neonatal Sepsis. Biomed Res Int. 2016;2016:1035856.
Pugin J, Daix T, Pagani JL, Morri D, Giacomucci A, Dequin PF et al. Serial measurement of pancreatic stone protein for the early detection of sepsis in intensive care unit patients: a prospective multicentric study. Crit Care. 2021;25.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142.
Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–93.
Klein HJ, Niggemann P, Buehler PK, Lehner F, Schweizer R, Rittirsch D, et al. Pancreatic stone protein predicts Sepsis in severely burned patients irrespective of Trauma Severity: a Monocentric Observational Study. Ann Surg. 2021;274:e1179–86.
Saleh NY, Aboelghar HM, Garib MI, Rizk MS, Mahmoud AA. Pediatric sepsis diagnostic and prognostic biomarkers: pancreatic stone protein, copeptin, and apolipoprotein A-V. Pediatr Res. 2023.
Chen J, Yasrebinia S, Ghaedi A, Khanzadeh M, Quintin S, Dagra A, et al. Meta-analysis of the role of neutrophil to lymphocyte ratio in neonatal sepsis. BMC Infect Dis. 2023;23:837.
Gao RY, Jia HM, Han YZ, Qian BS, You P, Zhang XK, et al. Calprotectin as a diagnostic marker for sepsis: a meta-analysis. Front Cell Infect Microbiol. 2022;12:1045636.
Poggi C, Lucenteforte E, Petri D, De Masi S, Dani C. Presepsin for the diagnosis of neonatal early-onset Sepsis: a systematic review and Meta-analysis. JAMA Pediatr. 2022;176:750–58.
Tan M, Lu Y, Jiang H, Zhang L. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: a systematic review and meta-analysis. J Cell Biochem. 2019;120:5852–59.
Iovanna J, Frigerio JM, Dusetti N, Ramare F, Raibaud P, Dagorn JC. Lithostathine, an inhibitor of CaCO3 crystal growth in pancreatic juice, induces bacterial aggregation. Pancreas. 1993;8:597–601.
Cantey JB, Lee JH. Biomarkers for the diagnosis of neonatal Sepsis. Clin Perinatol. 2021;48:215–27.
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250-6.
Stocker M, Hop WC, van Rossum AM. Neonatal procalcitonin intervention study (NeoPInS): Effect of Procalcitonin-guided decision making on duration of antibiotic therapy in suspected neonatal early-onset sepsis: a multi-centre randomized superiority and non-inferiority intervention study. BMC Pediatr. 2010;10:89.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644-55.
Graf R, Schiesser M, Lüssi A, Went P, Scheele GA, Bimmler D. Coordinate regulation of secretory stress proteins (PSP/reg, PAP I, PAP II, and PAP III) in the rat exocrine pancreas during experimental acute pancreatitis. J Surg Res. 2002;105:136–44.
Bimmler D, Schiesser M, Perren A, Scheele G, Angst E, Meili S, et al. Coordinate regulation of PSP/reg and PAP isoforms as a family of secretory stress proteins in an animal model of chronic pancreatitis. J Surg Res. 2004;118:122–35.
Calandra T, Cohen J, Conference, ftISFDoIitIC. The International Sepsis Forum Consensus Conference on Definitions of Infection in the Intensive Care Unit. Critical Care Medicine. 2005;33:1538-48.
Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Biwei Mai, Lirong Zhou and Zhixian Lei carried out the studies, participated in collecting data, and drafted the manuscript. Qi Wang, Bo Ding and Naiyun Zhu performed the statistical analysis and participated in its design. Yifeng Zhan, Shanqing Qin and Zhuangxing Li participated in acquisition, analysis, or interpretation of data and draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article is a meta-analysis. The data comes from published articles and does not require ethical approval and written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.


Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mai, B., Zhou, L., Wang, Q. et al. Diagnostic accuracy of pancreatic stone protein in patients with sepsis: a systematic review and meta-analysis. BMC Infect Dis 24, 472 (2024). https://doi.org/10.1186/s12879-024-09347-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09347-4