Abstract
Background
Though bictegravir/emtricitabine/tenofovir (BIC/FTC/TAF) have been regulatory approved and included in the National Reimbursement Drug List in China, due to the affordability concern, generic version of efavirenz + lamivudine + tenofovir (EFV + 3TC + TDF) is still recommended as the first-line therapy in the clinical guideline and widely used in clinical practice. The aim of the study is to assess the persistence with first-line BIC/TAF/TAF and EFV + 3TC + TDF in newly treated HIV-1 patients in the real-world setting in Hunan Province in China.
Methods
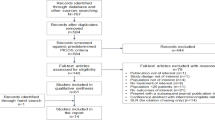
A retrospective analysis of the medical records of HIV patients initiating first-line antiretroviral therapy in the First Hospital of Changsha in January 1st, 2021-July 31st, 2022 was conducted. Persistence was assessed as the number of days on the therapy from the index until treatment discontinuation or end of data availability. Kaplan-Meier Curves and Cox Proportional Hazard models were used to evaluate the discontinuation rates. Subgroup analysis was performed excluding BIC/FTC/TAF patients with treatment discontinuation due to economic reason, and EFV + 3TC + TDF patients with a viral load > 500,000 copies/mL.
Results
A total of 310 eligible patients were included in the study, with 244 and 66 patients in the BIC/FTC/TAF group and EFV + 3TC + TDF group, respectively. Compared with EFV + 3TC + TDF patients, BIC/FTC/TAF patients were older, more living in the capital city currently, and had significantly higher total cholesterol and low-density level (all p < 0.05). No significant difference was shown in the time to discontinuation between BIC/FTC/TAF patients and EFV + 3TC + TDF patients. After excluding BIC/FTC/TAF patients with treatment discontinuation due to economic reason, EFV + 3TC + TDF group were shown to have a significantly higher risk of discontinuation than BIC/FTC/TAF group (hazard ratio [HR] = 11.1, 95% confidence interval [CI] = 1.3–93.2). After further removing the EFV + 3TC + TDF patients with a viral load > 500,000 copies/mL, the analysis showed similar results (HR = 10.1, 95% CI = 1.2–84.1). 79.4% of the EFV + 3TC + TDF patients discontinued treatment due to clinical reasons, while 83.3% of the BIC/FTC/TAF patients discontinued treatment due to economic reasons.
Conclusions
Compared with BIC/FTC/TAF, EFV + TDF + 3TC patients were significantly more likely to discontinue the first-line treatment in Hunan Province in China.
Similar content being viewed by others
Background
In China, HIV imposed a significant disease burden. The China Center for Disease Control and Prevention (CDC) reported 1.05 Million HIV/AIDS cases alive and 351 thousand cumulated death by end of 2020 [1]. From 2005 to 2019, the annually newly reported HIV/AIDS cases increased 15 times from 2,705 cases to 42,406 cases [2]. Specifically, the number of newly diagnosed HIV/AIDS cases among older people increased dramatically. The CDC reported that from 2007 to 2018, the incidence rate of HIV/AIDS among the population aged 60 years and above increased 10.3 times and 10.8 times among males and females, respectively [3]. The aging HIV/AIDS population indicates the needs for treatment regimens with better efficacy and safety profiles. .
Antiretroviral therapy (ART) has been introduced in the HIV treatment and significantly lowered the mortality and morbidity of HIV patients. The Chinese Guidelines for Diagnosis and Treatment of Human Immunodeficiency virus Infection/Acquired Immunodeficiency Syndrome (2021 edition) has recommended initiation ART in treatment-naïve patients with the regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) plus a third drug [4]. The third drug could be either a non-nucleoside reverse transcriptase inhibitor (NNRTI), or a boosted protease inhibitor (PI), or an integrase strand transfer inhibitor (INSTI). The single-table regimen (STR) is recommended. In the guideline, bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF), elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (EVG/c/FTC/TAF), dolutegravir/abacavir/lamivudine (DTG/ABC/3TC) and doravirine/tenofovir/lamivudine (DOR/TDF/3TC) were the four STR regimens recommended, among which, BIC/FTC/TAF and EVG/c/FTC/TAF have been included in the 2021 Edition National Reimbursement Drug List [5]. Nevertheless, mainly due to the affordability concern, multiple tablet regimens (MTRs) with generic drugs, especially the generic version of efavirenz + lamivudine + tenofovir (EFV + 3TC + TDF), are widely used in real-world clinical practice. In China, the national free ART program (NFATP) was launched in 2003 to help HIV patients to obtain the access to ART [6]. The first guideline for HIV/AIDS diagnosis and treatment was published in 2005 and since then, an increasing number of ARV drugs have become available to HIV patients free of charge [7, 8]. Generic version of EFV + 3TC + TDF has been recommended as the first-line therapy since the 2011 China National Guidelines for HIV/AIDS Diagnosis and Treatment, and is widely used in clinical practice in China [9].
Previous studies have shown that STRs had higher adherence and persistence compared with MTRs [10,11,12,13]. Nevertheless, limited studies have explored the persistence difference between STR and MTR in the real-world clinical practice in China. Since BIC/FTC/TAF and EFV + 3TC + TDF are the representatives of STR and MTR in China, respectively, the aim of the study is to compared the treatment persistence of BIC/FTC/TAF and EFV + 3TC + TDF in treatment-naïve HIV-1 patients.
Methods
Study design and data source
This is a retrospective database analysis using the electronic medical record data in the Department of Infection in First Hospital of Changsha to evaluate the persistence of ART regimens in treatment-naïve HIV-1 patients. The hospital is a tier 3 A hospital in Hunan Province, covering the majority of the treatment of HIV/AIDS patients in the province. The patients’ data collection started on January 1st, 2021 and ended on July 31st, 2022 with the last hospital visit. The study was conducted in accordance with the Chinese Ethical Guidelines for Medical and Health Research Involving Human Subjects. Ethical approval was required according to the guidelines.
Study population
Adult patients diagnosed with HIV-1 infection between January 1st, 2021 and July 31st, 2022, with the prescription of BIC/FTC/3TC or generic version of EFV + 3TC + TDF as the first-line treatment, and have at least one hospital visit after prescription, were included in the study. The index date was the first time for patients prescribed with BIC/FTC/3TC or EFV + 3TC + TDF. Persistence was measured by the duration on the regimen, calculated from the index date until the last date when the regimen was prescribed. In clinical practice, BIC/FTC/3TC patients could switch to free ARTs because of the economic burden of BIC/FTC/TAF. Therefore, a subgroup analysis was performed excluding BIC/FTC/3TC patients with treatment switch due to affordability reason. Furthermore, since EFV is not recommended for patients with a viral load > 500,000 copies/mL in the 2021 Edition HIV/AIDS diagnosis and treatment guideline, a subgroup patient population was selected with a viral load ≤ 500,000 copies/mL.
Statistical analysis
Continuous variables were reported with mean and standard deviation (SD). Categorical variables were reported with count (frequency) and percentage. T-test and chi-square test were used in between-cohort comparisons for continuous variables and categorical variables, respectively. The time to discontinuation of index regimen was estimated by Kaplan-Meier curves, with log-rank tests to assess the statistically significant difference between the two regimen groups. Cox proportional hazard models were used to assess the risk of discontinuation. Age group, gender, baseline viral load groups, baseline CD4 cell count groups, baseline total cholesterol, baseline low-density lipoprotein, education level, employment status and current residence were included as the covariates in the model. All statistical analysis were performed with SPSS® version 26.0 (IBM Corp., Armonk, NY). The statistical significance of all tests was assessed with a significance level of 0.05.
Results
A total of 310 eligible patients were included in the study, with 244 and 66 patients in the BIC/FTC/TAF group and EFV + 3TC + TDF group, respectively. The demographic and clinical characteristics are described in Table 1. The mean ± standard deviation ages of patients with BIC/FTC/TAF and EFV + 3TC + TDF were 35.8 ± 13.6 and 31.4 ± 12.5 years, respectively (P = 0.015). There were more male patients than female patients (BIC/FTC/3TC group: 97.0%; EFV + 3TC + TDF group 94.2%) and nearly half of the patients (BIC/FTC/3TC: 45.3%; EFV + 3TC + TDF: 48.0%) have a viral load ≤ 50,000 copies/mL. There were significantly more patients in BIC/FTC/3TC (95.5%) group living in Changsha, the capital city of Hunan Province, than those in the EFV + 3TC + TDF (85.2%) group (p = 0.027). Patients in BIC/FTC/TAF group (35.9%) reported a higher proportion with a CD4 counts less than 200 cells per µL, compared with patients in EFV + 3TC + TDF (23.8%) group (p = 0.054). BIC/FTC/FAF group had significantly higher total cholesterol and low-density level, compared with EFV + 3TC + TDF group (both p < 0.05). There were no significant differences between BIC/FTC/TAF group and EFV + 3TC + TDF group in gender, education level, employment status, HIV-1 RNA concentration, CD4 counts, creatinine clearance, triglyceride, and high-density level (all p > 0.05).
The time to discontinuation for BIC/FTC/TAF vs. EFV + 3TC + TDF in all patients was shown in Fig. 1. No significant difference was shown in the time to discontinuation between BIC/FTC/TAF and EFV + 3TC + TDF (p = 0.800). Nevertheless, after excluding BIC/FTC/TAF patients with treatment discontinuation due to economic reason, the Kaplan-Meier estimates showed that BIC/FTC/TAF group had a significantly longer time to treatment discontinuation than EFV + 3TC + TDF group (p = 0.036). After the adjustment of age group, gender, baseline CD4 count group and baseline viral load group, EFV + 3TC + TDF patients had a significantly higher risk of discontinuation than BIC/FTC/TAF patients (hazard ratio [HR] = 11.1, 95% CI = 1.3–93.2). After further removing the patients with a viral load > 500, 000 copies/mL in EFV + 3TC + TDF group, the subgroup analysis showed similar results in both Kaplan-Meier estimates (p = 0.042) and cox model (HR = 10.1, 95% CI = 1.2–84.1).
Time to discontinuation for EFV + 3TC + TDF and BIC/FTC/TAF. (A) All patients. (B) BIC/FTC/TAF patients with treatment discontinuation due to economic reason excluded. (C) Both BIC/FTC/TAF patients with treatment discontinuation due to economic reason and EFV + 3TC + TDF patients with a viral load > 500, 000 copies/mL excluded.
The reasons for the treatment discontinuation cases in BIC/FTC/TAF group and EFV + 3TC + TDF group were shown in Table 2. Among the 6 treatment discontinuation cases in BIC/FTC/TAF group, only 1 treatment discontinuation case was due to dyslipidemia, while majority (79.4%) of the treatment discontinuation cases were due to clinical reasons in EFV + 3TC + TDF group. Dizziness and/or sleep disturbance (40.9%), rash (22.7%), and hepatic impairment (rash and hepatic impairment: 13.6%, hepatic impairment alone: 9.1%) were the main adverse event for treatment discontinuation in EFV + 3TC + TDF group. The economic reason of the treatment discontinuation in BIC/FTC/TAF group and EFV + 3TC + TDF group was different. In EFV + 3TC + TDF group, the economic reason for treatment discontinuation was the affordable economic cost of patent drugs with better safety and efficacy profile after included in NRDL. Nevertheless, even partially reimbursed in NRDL, BIC/FTC/TAF might still imposed unaffordable economic burden to some patients, who switched from BIC/FTC/TAF to cheaper or free ART.
Discussion
To our knowledge, this is the first study to evaluate the persistence of BIC/FTC/TAF and EFV + 3TC + TDF in real-world setting in China. Our study showed that after excluding the economic burden between ARTs, BIC/FTC/TAF had significant better persistence compared with EFV + 3TC + TDF, which is consistent with previous real-world studies in western countries [10, 11]. The treatment discontinuation of EFV + 3TC + TDF group is mainly due to safety reason, which is also shown in other studies reporting the neuropsychiatric side effects related with EFV [14,15,16]. Our study result is consistent with previous study findings showing EFV-based regimen to increase the treatment discontinuation risk due to neuropsychiatric side effects [17,18,19]. HIV patients in China has a high prevalence rate of depression (> 60%) and anxiety (> 40%), which could be explained by the psychological burden at diagnosis, the aversive symptoms, the stigma and guilt of the infection, and the prejudice and misconceptions about HIV [20, 21]. Therefore, ART with better safety profile should be recommended. Nevertheless, the study result is different from a prospective cohort study result published recently to evaluate the EFV discontinuation due to neuropsychiatric adverse events in China [22]. The China study showed EFV-based regimens (mainly EFV + 3TC + TDF) associated with a low risk of discontinuation due to neuropsychiatric adverse events in treatment-naïve HIV patients. One possible reason could be the higher baseline viral load in the EFV group in our study (mean HIV-1 RNA level: 50,119 [10^4.7] copies/mL) than that in the published study (mean HIV-1 RNA level: 19,175 copies/mL).
TDF related side effect is another contributor of treatment discontinuation due to safety concerns in the EFV + 3TC + TDF group. The finding is inconsistent with previous studies. Moreover, though there is a large number of HIV patients with TDF-based regimens in China, the benefits in renal functions of switching from TDF to TAF are supported by previous studies in both Western and Asian HIV patient population [23,24,25,26,27]. In the Swiss HIV cohort study, switching from TDF to TAF showed an improvement in eGFR and proteinuria in patients with renal dysfunction [23]. In the Japan studies, TDF patients with poor renal function could benefits from switching to TAF [24, 25].
Our study finding about the treatment discontinuation due to drug resistance in EFV + 3TC + TDF group is consistent with the previous studies about the drug resistance to free NRTI and NNRTI in China [28,29,30,31,32,33,34]. Drug resistance is a big threat to HIV patients in China, especially in HIV patients not taking drug resistance test before treatment initiation. Since drug resistance test is not mandatory before ART initiation and the expenditure is out-of-pocket, HIV patients may choose to initiate ART without taking drug resistance test. Although the overall prevalence of transmitted drug resistance (TDR) is low in China, high TDR rates to NNRTI in certain areas and an increasing TDR rate since 2010 have been reported [29,30,31]. Meanwhile, acquired drug resistance (ADR) is the main driver for treatment failure and ADR rate to NNRTI or NRTI is reported to be > 50% among treatment-failure HIV patients [32, 33]. Another study conducted in Hunan Province showed that the primary drug resistance rate of EFV and 3TC was 5.6% and 3.3%, respectively, which is higher than that reported in the EFV + 3TC + TDF group in this study [35]. The underestimated drug resistance rate could be explained by the delayed drug resistance test. In clinical practice, HIV patients would be recommended to take the drug resistance test if their viral load is not under control after taking the treatment regimens for at least half a year. Since no routine drug resistance tests performed after taking the treatment regimens, HIV patients treated with EFV + 3TC + TDF could already have the drug resistance to the treatment without knowing it. Therefore, considering the threat of drug resistance to the free NRTI and NNRTI, HIV drugs with higher resistance barrier needs to be prioritized.
The study shows BIC/FTC/TAF has significantly longer persistence compared with EFV + 3TC + TDF; however, the affordability of BIC/FTC/TAF is still a concern for HIV patients in China. After government reimbursement (≥ 70%), the out-of-pocket expenditure of BIC/FTC/TAF could still be a barrier for HIV patients, especially in HIV patients with low socio-economics status or in rural areas. Previous studies have shown that even in HIV/AIDS patient with the free ART, the out-of-pocket expenditure is still high because of the other costs related with laboratory tests, examinations, medical service and drugs for opportunistic infections, which are not covered by the government [36, 37]. A study conducted in Nantong in 2017 shows the annual hospitalization expense per HIV patient with free ART is CNY 5,454, which is slightly higher than the annual out-of-pocket expenditure of BIC/FTC/TAF, indicating a potential heavy economic burden to HIV patients taking BIC/FTC/TAF [36]. Considering the large number of HIV patients with low socio-economic status or in rural area, additional financial support needs to be prioritized to help HIV patients have access to drugs with better safety and efficacy profile.
The strength of the study includes the most recent clinical outcomes of treatment options in China. There are several limitations in our study. Firstly, since the study period is from January 1st, 2021 to July 31st, 2022, the follow-up period was relatively short and the sample size was small. The long-term persistence of BIC/FTC/3TC may need to be further assessed with a larger sample size. Secondly, data of the HIV patients in the study were collected from a single hospital in Hunan Province and therefore the study findings might not be generalized to HIV patients in cities/provinces in China. Thirdly, some variables were adjusted in the cox model, confounding effects or selection bias could not be completely ruled out.
Conclusions
In summary, our study demonstrated the longer persistence of BIC/FTC/TAF compared with EFV + 3TC + TDF in HIV patients in Hunan Province in China. Given the 95-95-95 goals, the high burden of HIV/AIDS in China highlight the important to select treatment regimens with better safety and efficacy profiles.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request. Participant data without names and identifiers will be made available after approval from the corresponding author. After publication of study findings, the data will be available for others to request. The research team will provide an email address for communication once the data are approved to be shared with others.
Abbreviations
- ADR:
-
acquired drug resistance
- ART:
-
antiretroviral therapy
- BIC/TAF/TAF:
-
bictegravir/emtricitabine/tenofovir
- CI:
-
Confidence interval
- CDC:
-
Center for Disease Control and Prevention
- DTG/ABC/3TC:
-
dolutegravir/abacavir/lamivudine
- DOR/TDF/3TC:
-
doravirine/tenofovir/lamivudine
- EFV + 3TC + TDF:
-
efavirenz + lamivudine + tenofovir
- EVG/c/FTC/TAF:
-
elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide
- HR:
-
hazard ratio
- INSTI:
-
integrase strand transfer inhibitor
- MTR:
-
multiple tablet regimens
- NFATP:
-
the national free ART program
- NRTI:
-
nucleoside reverse transcriptase inhibitors
- NNRTI:
-
non-nucleoside reverse transcriptase inhibitor
- PI:
-
protease inhibitor
- SD:
-
standard deviation
- STR:
-
single-table regimen
- TDR:
-
transmitted drug resistance
References
He N. Research Progress in the epidemiology of HIV/AIDS in China. China CDC Wkly. 2021;3(48):1022–30.
Xu JJ, et al. Prevention and control of HIV/AIDS in China: lessons from the past three decades. Chin Med J (Engl). 2021;134(23):2799–809.
Zhang Y, et al. Disproportionate increase of new diagnosis of HIV/AIDS infection by sex and age - China, 2007–2018. China CDC Wkly. 2020;2(5):69–74.
[Chinese guidelines for diagnosis and treatment of HIV/AIDS (2021 edition)] Zhonghua nei ke za zhi, 2021. 60(12): p. 1106–28.
The National Healthcare Security Administration. A renewed catalog of drugs covered by its national medical insurance systemhttp://www.gov.cn/zhengce/zhengceku/2021-12/03/content_5655651.htm Accessed on Oct 1st, 2022, 2021.
Zhang FJ, et al. Current progress of China’s free ART program. Cell Res. 2005;15(11–12):877–82.
Guidelines for diagnosis and treatment of HIV/AIDS in China (2005). Chin Med J (Engl), 2006. 119(19): p. 1589–608.
Cao W, Hsieh E, Li T. Optimizing treatment for adults with HIV/AIDS in China: successes over two decades and remaining Challenges. Curr HIV/AIDS Rep. 2020;17(1):26–34.
AIDS Professional Group. Society of Infectious Diseases Chinese Medical Association: guidelines for diagnosis and treatment of HIV/AIDS in China (2011). ZHONGHUA CHUAN RAN BING ZA ZHI. 2011;29(10):629–40.
Ambrosioni J, et al. Real-life experience with bictegravir/emtricitabine/tenofovir alafenamide in a large reference clinical centre. J Antimicrob Chemother. 2022;77(4):1133–9.
Mounzer K, et al. Advanced HIV infection in Treatment-Naïve individuals: effectiveness and persistence of recommended 3-Drug regimens. Open Forum Infect Dis. 2022;9(3):ofac018.
Cohen J, et al. Real-world adherence and persistence for newly-prescribed HIV treatment: single versus multiple tablet regimen comparison among US medicaid beneficiaries. AIDS Res Ther. 2020;17(1):12.
Wang X, Schmerold L, Naito T. Real-world medication persistence among HIV-1 patients initiating integrase inhibitor-based antiretroviral therapy in Japan. J Infect Chemother. 2022;28(11):1464–70.
Apostolova N, et al. Efavirenz and the CNS: what we already know and questions that need to be answered. J Antimicrob Chemother. 2015;70(10):2693–708.
Qin P, et al. Efavirenz use and neurocognitive performance among older people living with HIV who were on antiretroviral therapy. AIDS Care. 2020;32(1):12–20.
Xia H, et al. Switching from efavirenz to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide reduces central nervous system symptoms in people living with HIV. Chin Med J (Engl). 2021;134(23):2850–6.
Law JKC, Butler LT, Hamill MM. Predictors of discontinuation of Efavirenz as Treatment for HIV, due to Neuropsychiatric Side Effects, in a multi-ethnic sample in the United Kingdom. AIDS Res Hum Retroviruses. 2020;36(6):459–66.
Leutscher PD, et al. Discontinuation of efavirenz therapy in HIV patients due to neuropsychiatric adverse effects. Scand J Infect Dis. 2013;45(8):645–51.
Fernández-Bargiela N, et al. Discontinuation due to neuropsychiatric adverse events with efavirenz- and dolutegravir-based antiretroviral therapy: a comparative real-life study. Eur J Hosp Pharm. 2022;29(4):207–11.
Niu L, et al. The Mental Health of People living with HIV in China, 1998–2014: a systematic review. PLoS ONE. 2016;11(4):e0153489.
“If you get AIDS… you have to endure it alone”: understanding the social constructions of HIV/AIDS in China Soc Sci Med, 2007. 65(2): p. 284 – 95.
Hua W, et al. Neuropsychiatric adverse events during 12 months of treatment with Efavirenz in Treatment-Naïve HIV-Infected patients in China: a prospective cohort study. Front Psychiatry. 2021;12:579448.
Surial B, et al. Changes in renal function after switching from TDF to TAF in HIV-Infected individuals: a prospective cohort study. J Infect Dis. 2020;222(4):637–45.
Abe K, et al. Renal function in japanese HIV-1-positive patients who switch to tenofovir alafenamide fumarate after long-term tenofovir disoproxil fumarate: a single-center observational study. AIDS Res Ther. 2021;18(1):94.
Hikasa S, et al. Effect of switching from tenofovir disoproxil fumarate to tenofovir alafenamide on estimated glomerular filtration rate slope in patients with HIV: a retrospective observational study. J Infect Chemother. 2022;28(3):396–400.
Seo JW, et al. Recovery of Tenofovir-induced nephrotoxicity following switch from Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide in Human Immunodeficiency Virus-Positive patients. Infect Chemother. 2020;52(3):381–8.
Tao X, et al. Virologically suppressed HIV-infected patients on TDF-containing regimens significantly benefit from switching to TAF-containing regimens: a meta-analysis of randomized controlled trials. Int J Infect Dis. 2019;87:43–53.
Zhou C, et al. Characterization of HIV-1 molecular epidemiology and transmitted drug-resistance in newly diagnosed HIV-infected patients in Sichuan, China. BMC Infect Dis. 2022;22(1):602.
Lin B, et al. HIV drug resistance in HIV positive individuals under antiretroviral treatment in Shandong Province, China. PLoS ONE. 2017;12(7):e0181997.
Yuan H, et al. Prevalence of transmitted HIV-1 drug resistance among treatment-naive individuals in China, 2000–2016. Arch Virol. 2021;166(9):2451–60.
Zhang F, et al. An analysis of drug resistance among people living with HIV/AIDS in Shanghai, China. PLoS ONE. 2017;12(2):e0165110.
Wei Q, et al. High rate of HIV-1 Drug Resistance in antiretroviral therapy-failure patients in Liaoning Province, China. AIDS Res Hum Retroviruses. 2022;38(6):502–9.
Yuan D, et al. Prevalence and determinants of virological failure, genetic diversity and drug resistance among people living with HIV in a minority area in China: a population-based study. BMC Infect Dis. 2020;20(1):443.
Lan Y, et al. Transmitted drug resistance and transmission clusters among HIV-1 treatment-naïve patients in Guangdong, China: a cross-sectional study. Virol J. 2021;18(1):181.
Cao X, et al. Prevalence of primary drug resistance among newly diagnosed HIV-1 infected individuals in Hunan province, China. AIDS Res Hum Retroviruses; 2023.
Zhuang X, et al. Analysis of hospitalization expenses of 610 HIV/AIDS patients in Nantong, China. BMC Health Serv Res. 2020;20(1):813.
Zhou F, et al. Expenditures for the care of HIV-infected patients in rural areas in China’s antiretroviral therapy programs. BMC Med. 2011;9:6.
Acknowledgements
We would like to express our appreciation for all the patients included in this study as well as their families.
Funding
The study was supported by grants of the National Major Infectious Prevention and Treatment Program in the 13th Five-Year Plan (2018zx10302104-001) and Special Foundation for Innovation Development in Hunan Province (2020SK21362). The funding bodies had no role in the design of the study, data collection, data analysis and interpretation, and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
CJ, WXZ, ZGQ: study concept and design; collection, analysis and interpretation of data, drafting of manuscript; critical revision of manuscript. TW, WN, ZF, WM: data collection; critical revision of manuscript. ZGQ, WXZ, XG: study concept and design; critical revision of manuscript; final approval for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki. The ethics committee of the First Hospital of Changsha approved the study protocol. The data used in this study was anonymized before its use. The need for informed consent was waived by the ethics committee/Institutional Review Board of the First Hospital of Changsha because of the retrospective nature of the study.
Consent for publication
Not applicable. No individual person’s data was shown separately. All data are presented as a whole.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jing, C., Wei, T., Ning, W. et al. Treatment persistence of bictegravir/emtricitabine/tenofovir alafenamide and efavirenz + lamivudine + tenofovir disoproxil among HIV-1 patients newly starting treatment in Hunan Province in China. BMC Infect Dis 23, 396 (2023). https://doi.org/10.1186/s12879-023-08359-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08359-w