Abstract
Background
Deprescribing, defined as discontinuing or reducing the dose of medications that are no longer needed or for which the risks outweigh the benefits is a way to reduce polypharmacy. In 2022, the US Deprescribing Research Network (USDeN) published recommendations concerning the measurement of outcomes for deprescribing intervention studies. The objectives of this systematic review were to identify the outcome categories used in deprescribing intervention trials and to relate them to the previously published recommendations.
Methods
We searched MEDLINE, Embase, PsychInfo, and the Cochrane library from January 2012 through January 2022. Studies were included if they were randomized controlled trials evaluating a deprescribing intervention. After data extraction, outcomes were categorized by type: medication outcomes, clinical outcomes, system outcomes, implementation outcomes, and other outcomes based on the previously published recommendations.
Results
Thirty-six studies were included. The majority of studies focused on older adults in nursing homes and targeted inappropriate medications or polypharmacy. In 20 studies, the intervention was a medication review; in seven studies, the intervention was educational or informative; and three studies based their intervention on motivational interviewing or patient empowerment. Thirty-one studies presented a medication outcome (primary outcome in 26 studies), 25 a clinical outcome, 18 a system outcome, and seven an implementation outcome. Only three studies presented all four types of outcomes, and 10 studies presented three types of outcomes.
Conclusions
This review provides an update on the implementation of gold standard deprescribing studies in clinical practice. Implementation outcomes need to be developed and specified to facilitate the implementation of these practices on a larger scale and clinical outcome need to be prioritized. Finally, this review provides new elements for future real-life deprescribing studies.
Similar content being viewed by others
Introduction
Deprescribing: a topical issue
Deprescribing has become a priority today because polypharmacy can increase the risk of drug-related problems [1,2,3,4,5,6,7,8]. Deprescribing can be defined as a patient-centered process conducted under the supervision of a healthcare professional for discontinuing or reducing the dose of medications that are no longer needed, for which the risks outweigh the benefits, or that are incompatible with the goals of care [9,10,11]. In deprescribing implementation trials, an intervention (educational intervention, medication review, motivational interviewing, etc.) is applied to one or more participants to increase deprescribing. The main objective of these studies is generally to assess the success or not of the intervention. Clinical outcomes are usually secondary, depending on the success of the intervention as well as unintended benefits that were not a direct result of deprescribing [11]. These trials are to be distinguished from medication cessation trials in which all participants in the intervention group stop taking the deprescribed drugs. These trials provide direct information on the clinical benefits and the harm of deprescribing in the target population.
Heterogeneous methodologies, new recommendations
Many systematic reviews have focused on assessing the impact of deprescribing in a targeted population, such as older adults [12,13,14], on a specific type of medication, such as drugs that increase the risk of falls (FRIDs) or benzodiazepines (BZD) and benzodiazepine-related drugs [15,16,17], or on a specific outcome, such as compliance [18]. The conclusions of these systematic reviews are consistently limited because of a large heterogeneity when it comes to comparing deprescribing interventions and their methodologies.
In 2022, following the recommendations of Aubert et al. published two years earlier [19], the US Deprescribing Research Network (USDeN) published guidelines concerning the measurement of outcomes for deprescribing intervention studies [20]. These recommendations take the form of a conceptual framework that includes different categories of outcomes to be followed in deprescribing implementation studies especially when it comes to performing randomized clinical trials, considered the gold standard in research: medication outcomes that directly reflect the deprescribing intervention by quantifying changes in the total number or dose of drugs, clinical outcomes that reflect the downstream effects of drug reduction on patients, system outcomes that reflect population-level effects (hospitalization, quality of care …) and finally implementation outcomes such as effectiveness and setting that are essential for large-scale implementation of interventions. The objectives of this review were (i) to identify the outcome categories used in deprescribing implementation trials over the past 10 years and (ii) to relate them to the previously published recommendations.
Methods
Study design
A review of the literature was conducted to select randomized controlled trials evaluating a deprescribing implementation intervention. The outcomes used in each study were identified and categorized according to the classification proposed in the recommendations of the US Deprescribing Research Network (USDeN) concerning the measurement of outcomes for deprescribing intervention studies [20]. These recommendations are proposed in the form of a conceptual framework that includes different categories of outcomes: medication outcomes that directly reflect the deprescribing intervention by quantifying changes in the total number or dose of drugs, clinical outcomes that reflect the downstream effects of drug reduction on patients (function, quality of life, adverse drug withdrawal events, etc.), system outcomes that reflect population-level effects (hospitalization, quality of care, cost of care, etc.), and finally implementation outcomes such as the effectiveness and setting that are essential for large-scale implementation of interventions (reach, effectiveness, adoption, etc.).
Systematic review
-
Protocol and registration
The protocol of this review was registered on PROSPERO (CRD42022360796) and was conducted in compliance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Extension for Systematic Reviews (PRISMA) Additional file 2: Appendix 2 [21].
-
Search strategy
The search strategy was developed with a senior librarian. We searched MEDLINE from January 2012 to January 2022 using Medical Subject Headings (MeSH) and keywords for deprescribing. Keywords were selected when they were synonymous with deprescribing, stopping or reducing medication. We used terms ceas*, cessation, decreas*, deprescrib*, de-prescrib*, de-prescrip*, discontinu*, eliminate*, reduc*, stop*, taper*, substitut*, withdraw*, optimiz*, remov*, interrupt*, step-down*, restriction, deintensification, diminish* and drop* in the research equation. Embase, PsychInfo, and the Cochrane Central Register of Controlled Trials electronic databases were searched using a strategy based on the MEDLINE strategy. This strategy was adapted for searching in other databases. The MEDLINE search strategy is available in Additional file 1: Appendix S1.
-
Study eligibility criteria
Three investigators independently evaluated each title first, then each abstract among the selected articles, which moved to full-text review if two investigators considered the citation eligible. Disputes were resolved by discussion with input from a fourth investigator if needed.
Studies were included if they were randomized controlled trials evaluating a deprescribing implementation intervention. The comparator was defined as usual care.
Excluded studies were protocols, qualitative studies, medico-economic studies, conference abstracts, academic theses, commentaries, and opinion articles, or that were not published in English and for which the full text was not available. In addition, medication cessation trials were excluded, i.e., when one or more drug was discontinued in all patients in the intervention arm.
Outcomes categorization according to the new recommendations
Two investigators independently extracted data regarding the study characteristics. Disagreements were resolved through consensus and a thorough review of the article. The extracted data were as follows: author, publication year, country, target population (study location and minimum age), target medication, intervention type, duration of the follow-up, and outcomes.
After data extraction, the outcomes were categorized by type based on the previously published recommendations [20]: medication outcomes, clinical outcomes, system outcomes, implementation outcomes, and other outcomes. The primary outcome was identified in each study and the studies were classified alphabetically by the author’s name.
Results
Study selection
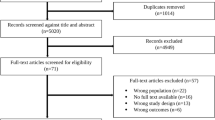
A total of 26 516 records were retrieved from the databases. After the removal of duplicates, 18 476 titles, and abstracts were screened for eligibility. Full-text articles were sought and screened, yielding 147 eligible articles. Ultimately, 36 studies were included [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57] (Fig. 1).
Characteristics of the included studies
Participants and settings
The 36 included studies took place in 16 different countries: 19 (52.8%) in Europe [22, 25, 28,29,30,31,32,33, 35, 36, 38, 41, 46, 48, 52,53,54, 56, 57], 11 (30.6%) in North America [23, 24, 26, 27, 34, 39, 43, 44, 49,50,51], four (11.1%) in Asia [40, 42, 47, 55], and two (5.6%) in Australia [37, 45]. The general characteristics of the selected studies are presented in Table 1.
The majority of studies were on older adults in institutions, and the duration of the follow-up period was heterogenous
The majority of studies (26; 72.2%) specifically targeted older adults, either by age criteria [23,24,25, 27, 28, 30, 32, 33, 36,37,38, 42,43,44,45,46, 50, 52, 55, 57] or by the use of tools for identifying inappropriate drugs in older adults [22, 24, 25, 28,29,30,31,32,33,34, 42, 45, 46, 55,56,57]. In 18 studies (50%), the patients were over 65 years of age [23,24,25, 27, 28, 30, 32, 33, 36,37,38, 42, 44,45,46, 50, 52, 55]. Regarding the setting, 13 studies (36.1%) took place in primary care [23, 27, 30, 35, 43, 44, 46, 49, 50, 52,53,54, 57], 12 (33.3%) in nursing homes [24, 28, 29, 31, 32, 36, 38, 41, 42, 45, 48, 56], seven (19.4%) in hospitals (emergency department, surgery unit, intensive care unit, trauma unit) [25, 26, 33, 34, 37, 39, 51], two (5.6%) in a rehabilitation hospital [40, 55], one (2.8%) in an outpatient department of psychiatry [47], and one (2.8%) in subacute medical outpatient [22]. The follow-up period ranged from one to 36 months, with 13 studies (36.1%) with a duration of follow-up of 12 months [22, 23, 25, 27, 29, 30, 33, 35, 39, 42, 45, 53, 57] and four studies (11.1%) with a follow-up period longer than 12 months [31, 46, 48, 54].
Targeting inappropriate medication and polypharmacy in older adults
In most cases, the studies were conducted to address the prevalence of inappropriate medications or to reduce polypharmacy in older adults. Regarding the drugs targeted by the deprescribing intervention, the results were heterogeneous. Indeed, 15 studies (41.7%) targeted potentially inappropriate medications (PIMs) [22, 24, 28,29,30,31,32,33,34, 42, 45, 46, 55,56,57] in older adults using different lists or criteria (STOPP criteria [58, 59], Beer’s list [60], Laroche list [61], or a country-specific list). Other studies targeted specific drugs such as benzodiazepines and BZD-related drugs (n = 7; 19.4%) [23, 37, 43, 44, 50, 53, 54], anticholinergic drugs (n = 3; 8.3%) [27, 41, 47], psychotropic drugs (n = 2; 5.6%) [36, 48], or opioids (n = 3; 8.3%) [39, 49, 51].
Using medication reviews in a multidisciplinary context
In 20 studies, the intervention was a medication drug review, carried out most of the time by a pharmacist (n = 9; 25%) [22, 24, 28, 29, 34, 40, 42, 45, 52] or a multidisciplinary team (n = 8; 22.2%) [27, 30, 36, 38, 41, 48, 55, 56]. In seven studies (19.4%), the intervention was educational or informative, provided to either patients (n = 5; 13.9%) [23, 37, 44, 49, 51] or general practitioners (n = 2; 5.6%) [53, 54], or both (n = 1; 2.8%) [43]. In two other studies (5.6%), the deprescribing intervention was carried out using computerized decision support for physicians [26, 46]. Three studies based their intervention on motivational interviewing or patient empowerment [39, 49, 50].
Outcomes of the included studies
Among the 36 included studies, 31 (86.1%) presented a medication outcome [22,23,24, 27,28,29,30,31,32,33,34,35,36, 38,39,40, 42,43,44,45,46, 48,49,50,51,52,53,54,55,56,57], 25 (69.4%) a clinical outcome [24,25,26, 28, 29, 32, 33, 35, 36, 38,39,40,41,42, 45,46,47,48,49, 52,53,54,55,56,57], 18 (50%) a system outcome [23, 25, 26, 28,29,30, 32, 34, 38, 40, 42, 43, 45, 46, 51, 55,56,57], and seven (19.4%) an implementation outcome [22, 34, 37, 40, 49, 55, 57]. The outcome type of the included studies is presented in Table 2, and their characteristics are presented in Tables 3, 4, and 5.
A total of three studies (8.3%) presented all four types of outcomes (medication, clinical, system, and implementation) [40, 55, 57], and 10 studies (27.8%) presented three types of outcomes: medication, clinical, and system outcomes for eight studies (22.2%) [28, 29, 32, 38, 42, 45, 46, 56], medication, clinical and implementation for one [49], and medication, system, and implementation for another [34]. These elements are described in the Table 2. Finally, 16 outcomes (n = 12; 33.3% of studies) were classified as "other outcomes" [22, 26, 28, 29, 34, 37, 41, 42, 44, 47, 49, 57].
The primary outcome was a medication outcome in almost three out of four studies (n = 26; 72.2%) [22,23,24, 27,28,29,30,31,32,33,34,35,36, 39, 43,44,45, 49,50,51,52,53,54,55,56,57]. In most studies, this outcome type focused on the number of drugs deprescribed or the number or percentage of patients for whom the deprescribing intervention was successful. The choice of medication outcomes was rarely justified or explained, and the methods for collecting these data were poorly developed. Indeed, the data were mostly collected from the patients’ charts, pharmacy dispensing databases, or GP reports but we had no further details. In some cases, this data was self-reported by the patients.
The primary outcome was clinical in five studies (13.9%), whether measuring cognitive function (n = 2; 5.6%) [41, 47], falls (n = 2; 5.6%) [25, 42], or delirium severity (n = 1; 2.8%) [26]. The majority of studies (n = 24; 66.7%) that included one or more clinical outcomes selected at least one outcome that was directly and specifically related to the drug deprescribed [24,25,26, 28, 29, 32, 35, 36, 38,39,40,41,42, 45,46,47,48,49, 52,53,54,55,56,57]. However, as with medication outcomes, the choice of the clinical outcomes was rarely justified. The other primary outcomes could be system outcomes (n = 1; 2.8%, cost saving of deprescribing) [40], both clinical and system outcomes (n = 1; 2.8%, unplanned hospital admission and death) [46], or other outcomes (n = 1; 2.8%, proportion of patients who initiated a discussion with a healthcare professional about deprescribing) [37]. The implementation outcomes were never primary outcomes. Two studies (5.6%) did not specify the primary outcome [38, 48].
Discussion
The majority of studies included in this systematic review targeted potentially inappropriate medications in older adults. The follow-up periods of the studies were very heterogeneous and the interventions most often included a medication review carried out by a pharmacist or a multidisciplinary team. The majority of studies presented at least one medication outcome, which was very often the study's primary endpoint, making it possible to conclude whether or not the deprescribing intervention was successful. Many of the studies presented at least one clinical outcome, but the choice of outcome was very rarely justified or applied, as was the method of measurement. All studies included in this review were published before the USDeN recommendations were published. The comparison of deprescribing implementation trial outcomes with USDeN recommendations [20] shows that work will be needed in the coming years to harmonize practices and increase the level of evidence of deprescribing studies. Indeed, only 3 studies presented the 4 types of outcomes recommended [40, 55, 57]. Two of these 3 studies targeted the deprescribing of PIMS in older adults [55, 57], and all 3 interventions were medication reviews [40, 55, 57]. Therefore, it was not shown that drug-specific or multidisciplinary interventions adhered more to the guidelines than other interventions. The studies to date are not very comparable because of their characteristics and the heterogeneity of the selected outcomes. It would be interesting to repeat this work in 10 years' time to measure the impact of these recommendations.
The need to consider outcomes selection in deprescribing trials
The USDeN recommendations state that clinical outcomes should be the primary outcome assessed in deprescribing trials. The conclusion made by Gnjidic and Reeve in 2020, pointing out that these studies are typically focused on the success of the intervention, and, therefore, the primary outcomes are often the proportion of participants who stopped a medication [11], supports our findings. Thus, it seems crucial to harmonize practices and terminology regarding the primary outcome of deprescribing trials to increase the levels of evidence in these studies.
As pointed out by the USDeN, it is essential that the outcomes that quantify drug switching benefit from more standardized definitions. For example, is it more relevant to measure the number of potentially inappropriate medicines prescribed to older adults or to measure the number of patients with at least one potentially inappropriate medicine? These results may vary depending on how they are presented. It is also critical to consider the substitution of a drug for another substance, whether or not it is a medication, when discussing the success of an intervention. This pragmatic aspect of the impact of deprescribing is all too often not measured in the included studies of this review. For example, when successfully deprescribing a BZD, can the intervention be considered a success if the patient has switched to other substances such as alcohol, cannabis, or doxylamine? The same applies to deprescribing an opioid and new or increased cannabis use. It should be noted that it is often difficult to measure these outcomes with any rigor, as they are often self-reported by the patient. In this regard, the data found in the patient’s medical records or the health insurance databases should always be considered with caution. Indeed, it is a common occurrence for drugs dispensed without a prescription to not be recorded, or a drug dispensed to not be a drug taken by the patient. Thus, cross-referencing several sources, such as official databases with patient-reported data, provides more robust indicators of the effectiveness of the intervention.
Deprescribing trials need to include other categories of outcomes (clinical, system, implementation), as specified by the USDeN. These recommendations do not address the number and types of outcomes to be selected for trials, nor do they address the need to measure medication, clinical, system, and implementation outcomes. In our opinion, this choice depends on the objectives of each study and the drugs targeted by the deprescribing intervention. The acceptability of the measurement of many patient-reported outcomes must also be considered to avoid patient fatigue during the assessment and thus biased results. However, it appears to us to be a priority to associate a clinical outcome to assess the clinical impact of the drug deprescribed and to ultimately justify the intervention. For example, Kersten et al. [41] chose to measure mouth dryness after deprescribing anticholinergic drugs in older adults. The choice of this outcome is questionable in terms of the risk/benefit of the drug and the patient's quality of life. The patient's quality of life or the measure of a functional dimension appear to be more relevant clinical outcomes. In this case, the timing of the measurement and the appropriateness of the instrument used should also be considered and justified.
In our review, only seven studies out of 36 proposed an evaluation of implementation outcomes [22, 34, 37, 40, 49, 55, 57], mostly assessing the time required for the intervention or the perception of healthcare professionals regarding deprescribing. Cost assessment could be classified as an implementation outcome or a system outcome. Implementation science was born out of the need to effectively translate research findings into practice in order to bridge the gap between research and practice [62]. This involves measuring different criteria, such as acceptability, adoption, feasibility, or even sustainability in real life [63]. Our results show that this area of research on deprescribing is still in its early stages and that there is an urgent need to integrate implementation criteria into deprescribing trials. This is what many researchers in this field [62, 64] have called for, leading to the publication of recommendations [65, 66], tools [67], or frameworks [68, 69] for which the objective is to translate deprescribing, or as some call it "de-implementation" [70], into practice. In addition to the other outcomes measured during clinical trials, these implementation outcomes guarantee that the interventions can indeed be implemented and not just remain in the literature.
Going beyond the confines and extending the follow-up time
The majority of the included studies were conducted in nursing homes, hospitals, or rehabilitation hospitals. These settings can be an obstacle to deprescribing, particularly for the deprescribing of BZDs or antipsychotic drugs in an anxiety-inducing environment. Performing deprescribing upstream, i.e., in primary care, could prevent patients from being institutionalized or hospitalized. However, it should be kept in mind that having the patient under clinical supervision is a key element in the feasibility of a deprescribing trial. The hospital or tertiary care setting allows withdrawal events to be followed and monitored more readily. In addition, multidisciplinary teams in these settings are also a lever for deprescribing. Giving greater consideration to more frequent implementation of deprescribing trials in real life should be undertaken because hospitalization or institutionalization only affect a minority of patients, which leads to lower generalizability. In this review, the reported follow-up period was very heterogeneous, and the timing of measurement was rarely justified. This heterogeneity may be related to the time required to deprescribe certain classes of drugs, particularly for medications used on a long-term basis. For example, deprescribing a drug that does not cause withdrawal syndrome (e.g., aspirin in primary prevention) is certainly faster than deprescribing a BZD or an opioid. It would appear that in most studies these follow-up timelines were chosen based on the study feasibility, which appears to be a limitation to the success of the intervention. A long follow-up period (more than 12 months) allows for conclusion that the intervention is safe and sustainable. However, it makes it likely that patients will experience events leading to treatment resumption or to be lost to follow-up. Hence, it is essential to justify the choice of the follow-up period and the measure of the intervention according to the objective of the study. This is in line with USDeN recommendations stating that clinical outcomes are often measured too early or too late concerning the clinical effect. Finally, when it comes to deprescribing PIMs, it is necessary to homogenize practices and establish a consensus on the classification of inappropriate drugs in older adults, as different studies use different lists to select included patients.
Strengths and limits
The main strengths of this study lie in the quality of the articles included. Randomized controlled trials represent the gold standard of trials, for which there is a great deal of methodological reflection. In addition, three investigators independently evaluated each title and abstract. We acknowledge several limitations of this review. First, we only included studies published in the past 10 years. This choice was made because the majority of deprescribing trials have been conducted over the past decade [71, 72]. Secondly, our search equation only queried the titles in the databases. We made this choice by constructing a search equation with numerous terms directly related to deprescribing. Indeed, researchers often devote a great deal of effort to choosing appropriate titles that reflect the main objective and design of their studies. Thirdly, we only included studies with usual care as a comparator, which appeared to be the most relevant to us for adaptation in primary care. Fourthly, we did not analyze the references of the selected articles, which could have resulted in the omission of some eligible articles. Finally, as with any review, a publication bias is likely due to the frequent non-publication of non-significant results [73].
Conclusion
In conclusion, this analysis confirmed our hypotheses regarding the importance of harmonizing deprescribing study methods to generate usable clinical evidence. There is a need to propose recommendations for real-life deprescribing trials, starting with the integration of deprescribing as soon as prescriptions go beyond the appropriate use of the drug, with sufficiently long follow-up periods, relevant outcome measurement times, and implementation measures allowing reproducibility of interventions. Switching from a discontinued drug to another drug or non-drug substance needs to be more extensively measured to conclude the success of deprescribing interventions. Implementation outcomes need to be developed and specified to facilitate the application and the reproducibility of these practices on a larger scale. Finally, clinical outcomes need to be justified and prioritized.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Bao Y, Shao H, Bishop TF, Schackman BR, Bruce ML. Inappropriate Medication in Home Health Care. J Gen Intern Med. 2012;27(5):491.
Cannon KT, Choi MM, Zuniga MA. Potentially inappropriate medication use in elderly patients receiving home health care: a retrospective data analysis. Am J Geriatr Pharmacother juin. 2006;4(2):134–43.
Tsao CH, Tsai CF, Lee YT, Weng MC, Lee HC, Lin DB, et al. Drug Prescribing in the Elderly Receiving Home Care. Am J Med Sci août. 2016;352(2):134–40.
Ibrahim IA, Kang E, Dansky KH. Polypharmacy and possible drug-drug interactions among diabetic patients receiving home health care services. Home Health Care Serv Q. 2005;24(1–2):87–99.
Steinman MA, Miao Y, Boscardin WJ, Komaiko KDR, Schwartz JB. Prescribing quality in older veterans: a multifocal approach. J Gen Intern Med. 2014;29(10):1379–86.
Saraf AA, Petersen AW, Simmons SF, Schnelle JF, Bell SP, Kripalani S, et al. Medications associated with geriatric syndromes and their prevalence in older hospitalized adults discharged to skilled nursing facilities. J Hosp Med. 2016;11(10):694–700.
Lohman MC, Scherer EA, Whiteman KL, Greenberg RL, Bruce ML. Factors Associated With Accelerated Hospitalization and Re-hospitalization Among Medicare Home Health Patients. J Gerontol A Biol Sci Med Sci. 2018;73(9):1280–6.
Jyrkkä J, Enlund H, Korhonen MJ, Sulkava R, Hartikainen S. Polypharmacy status as an indicator of mortality in an elderly population. Drugs Aging. 2009;26(12):1039–48.
Hilmer SN, Gnjidic D. Deprescribing: the emerging evidence for and the practice of the « geriatrician’s salute ». Age Ageing. 2018;47(5):638–40.
Scott IA, Hilmer SN, Reeve E, Potter K, Le Couteur D, Rigby D, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med mai. 2015;175(5):827–34.
Gnjidic D, Reeve E. Deprescribing: What do we know, and where to next? Br J Clin Pharmacol. 2021;87(3):722–4.
Ibrahim K, Cox NJ, Stevenson JM, Lim S, Fraser SDS, Roberts HC. A systematic review of the evidence for deprescribing interventions among older people living with frailty. BMC Geriatr. 2021;21(1):258.
Thillainadesan J, Gnjidic D, Green S, Hilmer SN. Impact of Deprescribing Interventions in Older Hospitalised Patients on Prescribing and Clinical Outcomes: A Systematic Review of Randomised Trials. Drugs Aging avr. 2018;35(4):303–19.
Bloomfield HE, Greer N, Linsky AM, Bolduc J, Naidl T, Vardeny O, et al. Deprescribing for Community-Dwelling Older Adults: a Systematic Review and Meta-analysis. J Gen Intern Med. 2020;35(11):3323–32.
Lee J, Negm A, Peters R, Wong EKC, Holbrook A. Deprescribing fall-risk increasing drugs (FRIDs) for the prevention of falls and fall-related complications: a systematic review and meta-analysis. BMJ Open. 2021;11(2):e035978.
Soni A, Thiyagarajan A, Reeve J. Feasibility and effectiveness of deprescribing benzodiazepines and Z-drugs: systematic review and meta-analysis. Addict Abingdon Engl. 2022;118(1):7–16.
Reeve E, Ong M, Wu A, Jansen J, Petrovic M, Gnjidic D. A systematic review of interventions to deprescribe benzodiazepines and other hypnotics among older people. Eur J Clin Pharmacol août. 2017;73(8):927–35.
Ulley J, Harrop D, Ali A, Alton S, Fowler Davis S. Deprescribing interventions and their impact on medication adherence in community-dwelling older adults with polypharmacy: a systematic review. BMC Geriatr. 2019;19(1):15.
Aubert CE, Kerr EA, Maratt JK, Klamerus ML, Hofer TP. Outcome Measures for Interventions to Reduce Inappropriate Chronic Drugs: A Narrative Review. J Am Geriatr Soc. 2020;68(10):2390–8.
Bayliss EA, Albers K, Gleason K, Pieper LE, Boyd CM, Campbell NL, et al. Recommendations for outcome measurement for deprescribing intervention studies. J Am Geriatr Soc. 2022;70(9):2487–97.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Aharaz A, Rasmussen JH, McNulty HBØ, Cyron A, Fabricius PK, Bengaard AK, et al. A Collaborative Deprescribing Intervention in a Subacute Medical Outpatient Clinic: A Pilot Randomized Controlled Trial. Metabolites. 2021;11(4):204.
Ashworth N, Kain N, Wiebe D, Hernandez-Ceron N, Jess E, Mazurek K. Reducing prescribing of benzodiazepines in older adults: a comparison of four physician-focused interventions by a medical regulatory authority. BMC Fam Pract. 2021;22(1):68.
Balsom C, Pittman N, King R, Kelly D. Impact of a pharmacist-administered deprescribing intervention on nursing home residents: a randomized controlled trial. Int J Clin Pharm août. 2020;42(4):1153–67.
Boyé NDA, van der Velde N, de Vries OJ, van Lieshout EMM, Hartholt KA, Mattace-Raso FUS, et al. Effectiveness of medication withdrawal in older fallers: results from the Improving Medication Prescribing to reduce Risk Of FALLs (IMPROveFALL) trial. Age Ageing. 2017;46(1):142–6.
Campbell NL, Perkins AJ, Khan BA, Gao S, Farber MO, Khan S, et al. Deprescribing in the Pharmacologic Management of Delirium: A Randomized Trial in the Intensive Care Unit. J Am Geriatr Soc avr. 2019;67(4):695–702.
Campbell NL, Holden RJ, Tang Q, Boustani MA, Teal E, Hillstrom J, et al. Multicomponent behavioral intervention to reduce exposure to anticholinergics in primary care older adults. J Am Geriatr Soc juin. 2021;69(6):1490–9.
Cateau D, Ballabeni P, Niquille A. Effects of an interprofessional deprescribing intervention in Swiss nursing homes: the Individual Deprescribing Intervention (IDeI) randomised controlled trial. BMC Geriatr. 2021;21(1):655.
Cateau D, Ballabeni P, Niquille A. Effects of an interprofessional Quality Circle-Deprescribing Module (QC-DeMo) in Swiss nursing homes: a randomised controlled trial. BMC Geriatr. 2021;21(1):289.
Clyne B, Smith SM, Hughes CM, Boland F, Cooper JA, Fahey T, et al. Sustained effectiveness of a multifaceted intervention to reduce potentially inappropriate prescribing in older patients in primary care (OPTI-SCRIPT study). Implement Sci IS. 2016;11(1):79.
Cool C, Cestac P, McCambridge C, Rouch L, de Souto BP, Rolland Y, et al. Reducing potentially inappropriate drug prescribing in nursing home residents: effectiveness of a geriatric intervention. Br J Clin Pharmacol juill. 2018;84(7):1598–610.
Curtin D, Jennings E, Daunt R, Curtin S, Randles M, Gallagher P, et al. Deprescribing in Older People Approaching End of Life: A Randomized Controlled Trial Using STOPPFrail Criteria. J Am Geriatr Soc avr. 2020;68(4):762–9.
Dalleur O, Boland B, Losseau C, Henrard S, Wouters D, Speybroeck N, et al. Reduction of potentially inappropriate medications using the STOPP criteria in frail older inpatients: a randomised controlled study. Drugs Aging avr. 2014;31(4):291–8.
Edey R, Edwards N, Von Sychowski J, Bains A, Spence J, Martinusen D. Impact of deprescribing rounds on discharge prescriptions: an interventional trial. Int J Clin Pharm févr. 2019;41(1):159–66.
Eveleigh R, Muskens E, Lucassen P, Verhaak P, Spijker J, van Weel C, et al. Withdrawal of unnecessary antidepressant medication: a randomised controlled trial in primary care. BJGP Open. 2018;1(4):bjgpopen17X101265.
Gedde MH, Husebo BS, Mannseth J, Kjome RLS, Naik M, Berge LI. Less Is More: The Impact of Deprescribing Psychotropic Drugs on Behavioral and Psychological Symptoms and Daily Functioning in Nursing Home Patients. Results From the Cluster-Randomized Controlled COSMOS Trial. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry. 2021;29(3):304–15.
Gnjidic D, Ong HMM, Leung C, Jansen J, Reeve E. The impact of in hospital patient-education intervention on older people’s attitudes and intention to have their benzodiazepines deprescribed: a feasibility study. Ther Adv Drug Saf. 2019;10:2042098618816562.
Gulla C, Flo E, Kjome RL, Husebo BS. Deprescribing antihypertensive treatment in nursing home patients and the effect on blood pressure. J Geriatr Cardiol JGC avr. 2018;15(4):275–83.
Hah JM, Trafton JA, Narasimhan B, Krishnamurthy P, Hilmoe H, Sharifzadeh Y, et al. Efficacy of motivational-interviewing and guided opioid tapering support for patients undergoing orthopedic surgery (MI-Opioid Taper): A prospective, assessor-blind, randomized controlled pilot trial. EClinicalMedicine. 2020;28: 100596.
Ee C, Lee K, Tan H, Low L. Effectiveness and feasibility of deprescribing of symptomatic medications in a Singapore rehabilitation hospital. Proc Singap Healthc. 2018;28:201010581878200.
Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2013;68(3):271–8.
Kua CH, Yeo CYY, Tan PC, Char CWT, Tan CWY, Mak V, et al. Association of Deprescribing With Reduction in Mortality and Hospitalization: A Pragmatic Stepped-Wedge Cluster-Randomized Controlled Trial. J Am Med Dir Assoc janv. 2021;22(1):82-89.e3.
Kuntz JL, Kouch L, Christian D, Hu W, Peterson PL. Patient Education and Pharmacist Consultation Influence on Nonbenzodiazepine Sedative Medication Deprescribing Success for Older Adults. Perm J. 2019;23:18–161.
Navy HJ, Weffald L, Delate T, Patel RJ, Dugan JP. Clinical Pharmacist Intervention to Engage Older Adults in Reducing Use of Alprazolam. Consult Pharm J Am Soc Consult Pharm. 2018;33(12):711–22.
Potter K, Flicker L, Page A, Etherton-Beer C. Deprescribing in Frail Older People: A Randomised Controlled Trial. PLoS ONE. 2016;11(3): e0149984.
Rieckert A, Reeves D, Altiner A, Drewelow E, Esmail A, Flamm M, et al. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial. BMJ. 2020;369:m1822.
Sathienluckana T, Unaharassamee W, Suthisisang C, Suanchang O, Suansanae T. Anticholinergic discontinuation and cognitive functions in patients with schizophrenia: a pharmacist-physician collaboration in the outpatient department. Integr Pharm Res Pract. 2018;7:161–71.
Smeets CHW, Smalbrugge M, Koopmans RTCM, Nelissen-Vrancken G, van der Spek K, Teerenstra S, et al. Can the PROPER intervention reduce psychotropic drug prescription in nursing home residents with dementia? Results of a cluster-randomized controlled trial. Int Psychogeriatr. 2021;33(6):577–86.
Sullivan MD, Turner JA, DiLodovico C, D’Appollonio A, Stephens K, Chan YF. Prescription Opioid Taper Support for Outpatients With Chronic Pain: A Randomized Controlled Trial. J Pain mars. 2017;18(3):308–18.
Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med juin. 2014;174(6):890–8.
Tseng ES, Zolin SJ, Young BT, Claridge JA, Conrad-Schnetz KJ, Curfman ET, et al. Can educational videos reduce opioid consumption in trauma inpatients? A cluster-randomized pilot study. J Trauma Acute Care Surg. 2021;91(1):212–8.
van der Meer HG, Wouters H, Pont LG, Taxis K. Reducing the anticholinergic and sedative load in older patients on polypharmacy by pharmacist-led medication review: a randomised controlled trial. BMJ Open. 2018;8(7):e019042.
Vicens C, Bejarano F, Sempere E, Mateu C, Fiol F, Socias I, et al. Comparative efficacy of two interventions to discontinue long-term benzodiazepine use: cluster randomised controlled trial in primary care. Br J Psychiatry J Ment Sci. 2014;204(6):471–9.
Vicens C, Sempere E, Bejarano F, Socias I, Mateu C, Fiol F, et al. Efficacy of two interventions on the discontinuation of benzodiazepines in long-term users: 36-month follow-up of a cluster randomised trial in primary care. Br J Gen Pract J R Coll Gen Pract. 2016;66(643):e85-91.
Wong APY, Ting TW, Charissa EJM, Boon TW, Heng KY, Leng LL. Feasibility & Efficacy of Deprescribing rounds in a Singapore rehabilitative hospital- a randomised controlled trial. BMC Geriatr. 2021;21(1):584.
Wouters H, Scheper J, Koning H, Brouwer C, Twisk JW, van der Meer H, et al. Discontinuing Inappropriate Medication Use in Nursing Home Residents: A Cluster Randomized Controlled Trial. Ann Intern Med. 2017;167(9):609–17.
Zechmann S, Senn O, Valeri F, Essig S, Merlo C, Rosemann T, et al. Effect of a patient-centred deprescribing procedure in older multimorbid patients in Swiss primary care - A cluster-randomised clinical trial. BMC Geriatr. 2020;20(1):471.
Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83.
Gallagher P, Baeyens JP, Topinkova E, Madlova P, Cherubini A, Gasperini B, et al. Inter-rater reliability of STOPP (Screening Tool of Older Persons’ Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment) criteria amongst physicians in six European countries. Age Ageing sept. 2009;38(5):603–6.
By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227–46.
Laroche ML, Charmes JP, Merle L. Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol août. 2007;63(8):725–31.
Ailabouni NJ, Reeve E, Helfrich CD, Hilmer SN, Wagenaar BH. Leveraging implementation science to increase the translation of deprescribing evidence into practice. Res Soc Adm Pharm RSAP mars. 2022;18(3):2550–5.
Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for Implementation Research: Conceptual Distinctions, Measurement Challenges, and Research Agenda. Adm Policy Ment Health Ment Health Serv Res. 2011;38(2):65–76.
Scott S. Deprescribing: a call for research that supports implementation in practice. Int J Pharm Pract. 2021;29(6):525–6.
Farrell B, Conklin J, Dolovich L, Irving H, Maclure M, McCarthy L, et al. Deprescribing guidelines: An international symposium on development, implementation, research and health professional education. Res Soc Adm Pharm RSAP juin. 2019;15(6):780–9.
Hamilton M, Gnjidic D, Christine Lin CW, Jansen J, Weir KR, Shaheed CA, et al. Opioid deprescribing: Qualitative perspectives from those with chronic non-cancer pain. Res Soc Adm Pharm. 2022;18(12):4083–91.
Thompson W, Lundby C, Graabæk T, Nielsen DS, Ryg J, Søndergaard J, et al. Tools for Deprescribing in Frail Older Persons and Those with Limited Life Expectancy: A Systematic Review. J Am Geriatr Soc. 2019;67(1):172–80.
Linsky A, Gellad WF, Linder JA, Friedberg MW. Advancing the Science of Deprescribing: A Novel Comprehensive Conceptual Framework. J Am Geriatr Soc. 2019;67(10):2018–22.
Scott S, Twigg MJ, Clark A, Farrow C, May H, Patel M, et al. Development of a hospital deprescribing implementation framework: A focus group study with geriatricians and pharmacists. Age Ageing. 2019;49(1):102–10.
Steinman MA, Boyd CM, Spar MJ, Norton JD, Tannenbaum C. Deprescribing and deimplementation: Time for transformative change. J Am Geriatr Soc déc. 2021;69(12):3693–5.
Desai M, Park T. Deprescribing practices in Canada: A scoping review. Can Pharm J. 2022;155(5):249–57.
Brunner L, Rodondi N, Aubert CE. Barriers and facilitators to deprescribing of cardiovascular medications: a systematic review. BMJ Open. 2022;12(12):e061686.
DeVito NJ, Goldacre B. Catalogue of bias: publication bias. BMJ Evid-Based Med avr. 2019;24(2):53–4.
Acknowledgements
The authors sincerely acknowledge Sophie Domingues for her help in editing and translating this paper.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
JFH and CVV were in charge of the project and were responsible for the review with substantial contributions to the conception, design, drafting, and completion of the manuscript. PN made a substantial contribution to the design and the acquisition of data and analysis, was involved in drafting the manuscript, and contributed critically to important intellectual content. EB and AE made substantial contributions to the design and the acquisition of data and analysis and contributed to the drafting of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nizet, P., Evin, A., Brociero, E. et al. Outcomes in deprescribing implementation trials and compliance with expert recommendations: a systematic review. BMC Geriatr 23, 428 (2023). https://doi.org/10.1186/s12877-023-04155-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-04155-y