Abstract
Background
This study aimed to explore whether the tricuspid annular systolic excursion (TAPSE)/mitral annular systolic excursion (MAPSE) ratio was associated with the occurrence of cardiogenic pulmonary edema (CPE) in critically ill patients.
Materials and methods
This was a prospective observational study conducted in a tertiary hospital. Adult patients admitted to the intensive care unit who were on mechanical ventilation or in need of oxygen therapy were prospectively screened for enrolment. The diagnosis of CPE was determined based on lung ultrasound and echocardiography findings. TAPSE ≥ 17 mm and MAPSE ≥ 11 mm were used as normal references.
Results
Among the 290 patients enrolled in this study, 86 had CPE. In the logistic regression analysis, the TASPE/MAPSE ratio was independently associated with the occurrence of CPE (odds ratio 4.855, 95% CI: 2.215–10.641, p < 0.001). The patients’ heart function could be categorized into four types: normal TAPSE in combination with normal MAPSE (TAPSE↑/MAPSE↑) (n = 157), abnormal TAPSE in combination with abnormal MAPSE (TAPSE↓/MAPSE↓) (n = 40), abnormal TAPSE in combination with normal MAPSE (TAPSE↓/MAPSE↑) (n = 50) and normal TAPSE in combination with abnormal MAPSE (TAPSE↑/MAPSE↓) (n = 43). The prevalence of CPE in patients with TAPSE↑/MAPSE↓ (86.0%) was significantly higher than that in patients with TAPSE↑/MAPSE↑ (15.3%), TAPSE↓/MAPSE↓ (37.5%), or TAPSE↓/MAPSE↑ (20.0%) (p < 0.001). The ROC analysis showed that the area under the curve for the TAPSE/MAPSE ratio was 0.761 (95% CI: 0.698–0.824, p < 0.001). A TAPSE/MAPSE ratio of 1.7 allowed the identification of patients at risk of CPE with a sensitivity of 62.8%, a specificity of 77.9%, a positive predictive value of 54.7% and a negative predictive value of 83.3%.
Conclusions
The TAPSE/MAPSE ratio can be used to identify critically ill patients at higher risk of CPE.
Similar content being viewed by others
Background
Acute cardiogenic pulmonary edema (CPE) is a common medical emergency and its prevalence continues to rise over time; an estimated 6.2 million American adults ≥ 20 years of age had heart failure between 2013 and 2016 [1]. CPE is among the major causes of acute respiratory failure and can result in higher mortality, longer hospital stay and increased cost [2,3,4]. Lung ultrasound and echocardiography can be used to detect CPE with the aid of clinical data [5]. The widespread use of echocardiography in the intensive care unit (ICU) also enables physicians to identify various types of myocardial dysfunction at bedside [6].
Left ventricular (LV) systolic dysfunction and/or LV diastolic dysfunction are usually deemed the main risk factors for acute respiratory failure or weaning failure from mechanical ventilation [7,8,9,10]. However, the mismatch between right ventricular (RV) and LV stroke volumes is a prerequisite for acute pulmonary edema to occur as fluid is lost from the circulation into the airspaces [11, 12]. It appears to be a basic concept that a strong RV might contribute to the occurrence of CPE in patients with LV dysfunction. However, which index might help assess RV and LV function mismatch and whether their mismatch was associated with CPE has seldomly been reported. Tricuspid annular plane systolic excursion (TAPSE) is a commonly used parameter of RV systolic function [13]. Similarly, mitral annular plane systolic excursion (MAPSE), a parameter that can be easily measured at the bedside, can reflect LV longitudinal systolic function as well as LV diastolic function [14, 15]. We hypothesize that the TAPSE/MAPSE ratio can be used as an index of RV-LV function mismatch, which constitutes a risk factor for CPE in critically ill patients. Thus, the aim of this study was to assess whether the TAPSE/MAPSE ratio is associated with the occurrence of CPE in critically ill patients.
Methods
Study population
This prospective observational study screened adult patients who were admitted to the ICU of a tertiary hospital from 1 May 2018 to 1 March 2021.
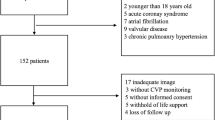
The inclusion criteria were as follows: patients on mechanical ventilation or those in need of oxygen therapy to maintain arterial SpO2 above 90%. Patients were excluded if they met any of the following criteria: age below 18 years; admitted after thoracotomy; history of chronic heart failure; moderate-to-severe chronic pulmonary hypertension; diffuse parenchymal lung disease; pneumothorax or subcutaneous emphysema; moderate-to-severe mitral or aortic valve disease; ACS complicated by ventricular septal rupture; insufficient image quality for echocardiography measurement; absence of an echocardiography examiner; or refusal to provide informed consent.
Lung ultrasound and echocardiography
Lung ultrasound and echocardiograms were recorded within 24 h of ICU admission. One experienced physician (H Z) who was blinded to the patients’ clinical data performed the lung ultrasound and echocardiographic examination. Images were saved for offline analysis.
We scanned four chest areas per side to evaluate the presence of interstitial syndrome including the upper anterior, lower anterior, upper lateral and basal lateral areas with patients in the supine position [16]. The echocardiographic results were reported based on the PRICES statement [17]. At least three cardiac cycles were analysed and averaged. M-mode and Doppler echocardiographic measurements were taken according to standard protocols. The measurements of tricuspid annular plane systolic excursion (TAPSE), RV fractional area change (FAC), peak velocity of tricuspid regurgitation (TR), left ventricular ejection fraction (LVEF), mitral annular plane systolic excursion (MAPSE), mitral peak E velocity, averaged tissue Doppler velocity of lateral and medial mitral annuli at early diastole (e’), left ventricular outflow tract velocity-time integral (LVOT-VTI) and diameter of inferior vena cava (DIVC) were performed as previously described [18, 19]. Left atrial (LA) volume was measured based on tracings of the blood-tissue interface on apical four- and two-chamber views, which was then indexed to body surface area [20]. TAPSE ≥ 17 mm and MAPSE ≥ 11 mm were used as normal references [13, 21].
CPE was diagnosed based on the integration of lung ultrasound and echocardiographic signs (Fig. 1): two or more regions with at least three B-lines bilaterally in the absence of pleural line abnormalities, reduction of lung sliding, anterior subpleural consolidations, and spared areas of normal parenchyma and E/e’ ≥ 13 or E/e’ 9–12 with at least 2 of the 4 specific conditions, namely, LAVI > 34ml/m2, PASP ≥ 40 mmHg, LVEF ≤ 45%, and LV hypertrophy [9, 22].
Lung ultrasound and echocardiographic examination. A. Lung ultrasound showing diffuse B-lines; B. TAPSE measurement; C. MAPSE measurement; D. LVEF measurement; E. Mitral E peak velocity; F. Septal e’ measurement; G. LA volume measurement; 1 H. TR measurement
TAPSE: tricuspid annular plane systolic excursion; MAPSE: mitral annular plane systolic excursion; LVEF: left ventricular ejection fraction; LA: left atrium; TR: tricuspid regurgitation
Other parameters evaluated
Data on the following parameters for each patient were collected: heart rate (HR), mean arterial pressure (MAP), central venous pressure (CVP), mechanical ventilation (MV) support, norepinephrine (NE) use, and partial pressure of arterial oxygen to fraction of inspired oxygen ratio (PaO2/FiO2). Demographic information and data on the diagnosis, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Sequential Organ Failure Assessment (SOFA) score, comorbidities, length of ICU stay and ICU mortality were also collected for all patients.
Statistical analyses
Data analyses were performed using the statistical software package SPSS 22.0 (SPSS, Inc., Chicago, Illinois, USA). All p values were two tailed, and statistical significance was defined as p < 0.05. A previous study found a correlation between MAPSE and E/e’ in critically ill patients (r = 0.38) [14]. We anticipated at least a modest correlation between the TAPSE/MAPSE ratio and E/e’(r = 0.20–0.40). To detect an effect size of 0.20 at an alpha error of 0.05 and statistical power of 0.90, at least 259 participants were required for this study. All lung ultrasound and echocardiographic data were prospectively collected, and patients were excluded without TAPSE, MAPSE, LVEF, and E/e’ measurements due to inadequate echocardiographic image quality. Continuous data were expressed as the mean ± standard deviation or median (25th-75th percentiles). The distributions of the continuous values were assessed for normality by the Kolmogorov‒Smirnov test. Group differences were analysed using Student’s unpaired t test, the Mann‒Whitney U test, the chi-squared test, or Fisher’s exact test, as appropriate. We performed a binary logistic analysis to assess the independent factors of CPE. The variables that had p < 0.1 in the univariable model were included in the multivariable model and the odds ratio was calculated, together with their 95% confidence intervals (CI). Spearman’s correlation coefficients and their corresponding p values were calculated to assess the variable relationships. ROC curves were generated to determine the sensitivity and specificity of parameters to predict CPE. Sensitivity analyses were performed to test the TAPSE/MAPSE ratio for the detection of CPE in patients with normal LVEF and abnormal LVEF. Intra-observer variability in TAPSE, MAPSE and LVEF was assessed in 20 randomly selected patients and was tested using intraclass correlation coefficients (ICCs). An ICC > 0.8 was considered excellent agreement.
Results
Baseline characteristics of the study population
During the study period, 990 critically ill patients on mechanical ventilation or in need of oxygen therapy were screened for enrolment, and 700 patients were excluded (Fig. 2). Among the 290 patients enrolled in this study, 86 were categorized into the CPE group, and the remaining 204 were categorized into the nonCPE group. Table 1 summarizes the general characteristics of the two groups. No significant differences were found between the two groups regarding sex, illness severity, comorbidities, or length of ICU stay. The two groups had similar proportions of sepsis and MV support. In comparison with the nonCPE group, the CPE group had older age (66 vs. 63, p = 0.007), lower PaO2/FiO2 (236 vs. 323 mmHg, p < 0.001) and higher ICU mortality (22.1% vs. 11.3%, p = 0.017).
Comparison of hemodynamic and echocardiographic parameters between the two groups
The lung ultrasound, TAPSE, FAC, MAPSE, E/e’, LAVI, and LVEF data were complete. TR was undetectable in 27 patients, IVCD was unavailable in 15 patients and LVOT-VTI was unavailable in 6 patients. The two groups had similar HRs and MAPs. The CPE group had a higher CVP level than the nonCPE group (p = 0.008). No significant difference was found regarding TAPSE (p = 0.657) and mitral peak A wave velocity (p = 0.509) between the two groups. The CPE group had lower FAC, LVEF, e’ velocity and cardiac index (CI) than the nonCPE group (p < 0.05). The CPE group had higher mitral peak E wave velocity, E/e’ ratio, LAVI, TR and DIVC than the nonCPE group (p < 0.001) (Table 2).
Factors associated with the presence of CPE
In the logistic regression analysis, age (OR 1.028, 95% CI:1.005–1.052, p = 0.016), CVP (OR 1.148, 95% CI: 1.021–1.291, p = 0.022), DIVC (OR 3.995, 95% CI:1.554–10.274, p = 0.004), LVEF (OR 0.953, 95% CI: 0.928–0.978, p < 0.001), and the TAPSE/MAPSE ratio (OR 4.855, 95% CI: 2.215–10.641, p < 0.001) were independently associated with the occurrence of CPE (Table 3).
Prevalence of CPE in patients with different ventricular functions
Among all the patients, 83 (28.6%) displayed abnormal MAPSE and 90(31.0%) displayed abnormal TAPSE. We categorized the patients’ ventricular function into four types: normal TAPSE in combination with normal MAPSE (TAPSE↑/MAPSE↑) (n = 157), abnormal TAPSE in combination with abnormal MAPSE (TAPSE↓/MAPSE↓) (n = 40), abnormal TAPSE in combination with normal MAPSE (TAPSE↓/MAPSE↑) (n = 50) and normal TAPSE in combination with abnormal MAPSE (TAPSE↑/MAPSE↓) (n = 43). The prevalence of CPE in patients with TAPSE↑/MAPSE↓ (86.0%) was significantly higher than that in patients with TAPSE↑/MAPSE↑ (15.3%), TAPSE↓/MAPSE↓ (37.5%) or TAPSE↓/MAPSE↑ (20.0%) (p < 0.001) (Fig. 3).
Prevalence of CPE in patients with different ventricular functions. The prevalence of CPE in patients with patients with TAPSE↑/MAPSE↓ (86.0%) was significantly higher than patients with TAPSE↑/MAPSE↑ (15.3%), TAPSE↓/MAPSE↓ (37.5%) and TAPSE↓/MAPSE↑ (20.0%) (p < 0.001)
CPE: cardiogenic pulmonary edema; TAPSE: tricuspid annular plane systolic excursion; MAPSE: mitral annular plane systolic excursion; TAPSE↑/MAPSE↑: normal TAPSE in combination with normal MAPSE; TAPSE↓/MAPSE↓: abnormal TAPSE in combination of abnormal MAPSE; TAPSE↓/MAPSE↑: abnormal TAPSE in combination with normal MAPSE; TAPSE↑/MAPSE↓: normal TAPSE in combination with abnormal MAPSE.
Relationship between the TAPSE/MAPSE ratio and CPE
The TAPSE/MAPSE ratio was correlated with E/e’ (r = 0.343, p < 0.001). To evaluate the sensitivity and specificity of the TAPSE/MAPSE ratio in the prediction of CPE, ROC curves were generated. The ROC analysis showed that the area under the curve for the TAPSE/MAPSE ratio was 0.761 (95% CI: 0.698–0.824, p < 0.001). The optimum cut-off value of the TAPSE/MAPSE ratio for the prediction of CPE was 1.7, which resulted in a sensitivity of 62.8%, a specificity of 77.9%, a positive predictive value of 54.7% and a negative predictive value of 83.3% (Fig. 4).
ROC analysis of the TAPSE/MAPSE ratio for the prediction of CPE. The ROC analysis showed that the areas under the curve for the TAPSE/MAPSE ratio for prediction of CPE was 0.761 (95%CI 0.698–0.824, p < 0.001)
CPE: cardiogenic pulmonary edema; TAPSE: tricuspid annular plane systolic excursion; MAPSE: mitral annular plane systolic excursion
Sensitivity analysis
We performed sensitivity analysis in patients with LVEF ≥ 50% (n = 185) and in patients with LVEF < 50% (n = 105) separately and found that the AUCs of the TAPSE/MAPSE ratio for the detection of CPE were 0.679 (95% CI: 0.569–0.789, p = 0.003) and 0.743 (95% CI: 0.649–0.837, p < 0.001), respectively. We also performed subgroup analysis of hemodynamic and echocardiographic parameters among CPE patients with different types of ventricular function and found that patients with TAPSE↑/MAPSE↓ had similar E/e’ values and had significantly lower CVP than the other three types (p < 0.05) (Supplemental Fig. 1).
Reproducibility
The intra-observer variability analysis revealed that the ICCs for TAPSE, MAPSE and LVEF were: 0.975 (95% CI: 0.937–0.990), 0.955 (95% CI: 0.890–0.982) and 0.930 (95% CI: 0.832–0.972), respectively.
Discussion
In this study, we prospectively assessed the lung ultrasound and echocardiography of critically ill patients on mechanical ventilation or in need of oxygen therapy. We found that the TAPSE/MAPSE ratio was independently associated with the occurrence of CPE. We also found that patients with TAPSE↑/MAPSE↓ were common and were more prone to CPE than patients with TAPSE↑/MAPSE↑, TAPSE↓/MAPSE↓ or TAPSE↓/MAPSE↑.
We integrated lung ultrasound and echocardiography to detect CPE, which could be missed by clinical examination [23]. In comparison with a prior study on weaning failure patients, the PaO2/FiO2 ratio was higher in this study (median 306 vs. 167 mmHg) possibly due to the identification of CPE patients without evident respiratory stress [24]. Therefore, ultrasound examination could enable physicians to identify and manage CPE patients at an earlier stage, thus avoiding dire consequences.
This study revealed that normal RV function in combination with abnormal LV function was not rare in critically ill patients. In patients with acute coronary syndrome (ACS) or Takotsubo cardiomyopathy, isolated LV systolic dysfunction is common [25,26,27]. Hania et al. reported that within 5 days of myocardial infarction, LV systolic dysfunction was detected in 46% of AMI patients [28]. In a medical ICU, left ventricular apical ballooning was found in 28% of critically ill patients [27]. LV systolic function, LV diastolic function and RV function can be compromised independently or collectively during sepsis [29, 30]. Therefore, RV and LV function mismatch can also exist in septic patients.
We found that patients with normal TAPSE and lower MAPSE were prone to CPE. There is a close interrelationship between systolic and diastolic function. Energy is stored during systole, which will be released during early diastole improving forwards flow into the ventricle. If LV contractile function is reduced, diastolic forwards flow will be compromised [12]. Thus, diastolic function is usually worse in heart failure patients with reduced LVEF than in those with preserved LVEF [31]. Additionally, some researchers found that MAPSE had a close relationship with LV diastolic function in obese patients [15]. This study found that patients with TAPSE↑/MAPSE↓ had a higher prevalence of CPE than those with TAPSE↓/MAPSE↓ (86% vs. 37.5%), which suggests that RV systolic function contributes to the occurrence of CPE. A previous study on patients with acute heart failure also noted that preserved RV function was associated with the development of pulmonary edema [32]. Interestingly, Kobayashi et al. contended that impaired RV function was associated with severe pulmonary congestion [33]. However, they incorporated only patients with pulmonary congestion and focused on the severity rather than the risk factors for pulmonary edema. Furthermore, they enrolled only patients with decompensated heart failure. The pulmonary arterial pressure was much higher than that in this study, which suggested the existence of a longstanding higher RV afterload induced by LV failure.
This study reminds us to pay more attention to RV systolic function when dealing with CPE. For Patients with TAPSE↑/MAPSE↓, CPE could occur when CVP or DIVC was still lie in the “normal” range. A prior study on heart failure patients with reduced LVEF reported that β blockers were associated with lower mortality in patients with preserved RVEF but not in those with reduced RVEF [34]. This study also suggests that downregulating hyperdynamic RV could be an alternative treatment in critically ill patients with CPE. Adequate sedation or analgesia or administration of β blockers with close monitoring of cardiac output has the potential to reduce the adrenal level and thus improve the mismatch of RV and LV function. Therefore, this study provided us with a new perspective to address critically ill patients with CPE.
Limitations
First, this is a single-centre observational study, and sepsis patients accounted for a large part of the enrolled population, which might affect the external validity. Furthermore, we could not identify the exact aetiologies for RV-LV function mismatch, which can result from hypertension, ACS, Takotsubo cardiomyopathy, septic cardiomyopathy or other causes. Second, lung ultrasound and echocardiography were not performed serially. Thus, we did not know how myocardial function and CPE developed. Future serial studies are warranted. Third, the differentiation between ARDS and CPE can be challenging [35]. However, the lung ultrasound and echocardiography examination enabled us to identify ARDS patients according to the specific pleural abnormalities and the lack of evidence of LV filling pressure elevation [36].
Conclusion
The TAPSE/MAPSE ratio can be used to identify critically ill patients at higher risk of CPE.
Data Availability
Data cannot be made available for the public because the patient consent did not include such an agreement. However, data may be made available for selected research questions on request to the corresponding author.
Abbreviations
- APACHE:
-
Acute physiology and chronic health evaluation.
- ARDS:
-
Acute respiratory distress syndrome
- CPE:
-
Cardiogenic pulmonary edema
- CVP:
-
Central venous pressure
- DIVC:
-
Diameter of inferior vena cava
- HR:
-
Heart rate
- ICU:
-
Intensive care unit
- LV:
-
Left ventricle
- LVEF:
-
Left ventricular ejection fraction
- MAPSE:
-
Mitral annular plane systolic excursion FAC:fractional area change
- NE:
-
Norepinephrine
- PEEP:
-
Positive end-expiratory pressure
- Pplat:
-
Plateau pressure
- RV:
-
Right ventricle
- SOFA:
-
Sequential organ failure assessment
- TAPSE:
-
Tricuspid annular plane systolic excursion
References
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind M, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson U, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW. Heart Disease and Stroke Statistics-2020 update: a Report from the American Heart Association. Circulation. 2020;141:e139–e596.
Vincent JL, Akça S, De Mendonça A, Haji-Michael P, Sprung C, Moreno R, Antonelli M, Suter PM, SOFA Working Group. Sequntial organ failure assessment: the epidemiology of acute respiratory failure in critically ill patients(*). Chest. 2002;121:1602–9.
Franca SA, Toufen C Jr, Hovnanian AL, Albuquerque AL, Borges ER, Pizzo VR, Carvalho CR. The epidemiology of acute respiratory failure in hospitalized patients: a brazilian prospective cohort study. J Crit Care. 2011;26:330e1–8.
Schmickl CN, Biehl M, Wilson GA, Gajic O. Comparison of hospital mortality and long-term survival in patients with acute lung injury/ARDS vs cardiogenic pulmonary edema. Chest. 2015;147:618–25.
Lichtenstein DA. Lung ultrasound for the cardiologist-a basic application: the B-profile of the Bedside Lung Ultrasound in Emergencies protocol for diagnosing haemodynamic pulmonary oedema. Arch Cardiovasc Dis. 2020;113:489–91.
Salem R, Vallee F, Rusca M, Mebazaa A. Hemodynamic monitoring by echocardiography in the ICU: the role of the new echo techniques. Curr Opin Crit Care. 2008;14:561–8.
Goudelin M, Champy P, Amiel JB, Evrard B, Fedou AL, Daix T, François B, Vignon P. Left ventricular overloading identified by critical care echocardiography is key in weaning-induced pulmonary edema. Intensive Care Med. 2020;46:1371–81.
Vignon P. Ventricular diastolic abnormalities in the critically ill. Curr Opin Crit Care. 2013;19:242–9.
Blanco PA, Cianciulli TF. Pulmonary edema assessed by Ultrasound: impact in Cardiology and Intensive Care Practice. Echocardiography. 2016;33:778–87.
Caille V, Amiel JB, Charron C, Belliard G, Vieillard-Baron A, Vignon P. Echocardiography: a help in the weaning process. Crit Care. 2010;14:R120.
MacIver DH, Clark AL. The vital role of the right ventricle in the pathogenesis of acute pulmonary edema. Am J Cardiol. 2015;115:992–1000.
MacIver DH, Dayer MJ, Harrison AJ. A general theory of acute and chronic heart failure. Int J Cardiol. 2013;165:25–34.
Zaidi A, Knight DS, Augustine DX, Harkness A, Oxborough D, Pearce K, Ring L, Robinson S, Stout M, Willis J, Sharma V. Echocardiographic assessment of the right heart in adults: a practical guideline from the british Society of Echocardiography. Echo Res Pract. 2020;7:G19–G41.
Bergenzaun L, Ohlin H, Gudmundsson P, Willenheimer R, Chew MS. Mitral annular plane systolic excursion (MAPSE) in shock: a valuable echocardiographic parameter in intensive care patients. Cardiovasc Ultrasound. 2013;11:16.
Taşolar H, Mete T, Çetin M, Altun B, Ballı M, Bayramoğlu A, Otlu Y. Mitral annular plane systolic excursion in the assessment of left ventricular diastolic dysfunction in obese adults. Anatol J Cardiol. 2015;15:558–64.
Volpicelli G, Mussa A, Garofalo G, Cardinale L, Casoli G, Perotto F, Fava C, Frascisco M. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689–96.
Sanfilippo F, Huang S, Herpain A, Balik M, Chew MS, Clau-Terré F, Corredor C, De Backer D, Fletcher N, Geri G, Mekontso-Dessap A, McLean A, Morelli A, Orde S, Petrinic T, Slama M, van der Horst I, Vignon P, Mayo P, Vieillard-Baron A. The PRICES statement: an ESICM expert consensus on methodology for conducting and reporting critical care echocardiography research studies. Intensive Care Med. 2021;47:1–13.
Zhang H, Lian H, Zhang Q, Chen X, Wang X, Liu D. Prognostic implications of tricuspid annular plane systolic excursion/pulmonary arterial systolic pressure ratio in septic shock patients. Cardiovasc Ultrasound. 2020;18:20.
Zhang H, Huang W, Zhang Q, Chen X, Wang X, Liu D. Prevalence and prognostic value of various types of right ventricular dysfunction in mechanically ventilated septic patients. Ann Intensive Care. 2021;11:108.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70.
Matos JD, Balachandran I, Heidinger BH, Mohebali D, Feldman SA, McCormick I, Litmanovich D, Manning WJ, Carroll BJ. Mitral annular plane systolic excursion and tricuspid annular plane systolic excursion for risk stratification of acute pulmonary embolism. Echocardiography. 2020;37:1008–13.
Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–91.
Alsaddique A, Royse AG, Royse CF, Mobeirek A, El Shaer F, AlBackr H, Fouda M, Canty DJ. Repeated monitoring with Transthoracic Echocardiography and Lung Ultrasound after Cardiac surgery: feasibility and impact on diagnosis. J Cardiothorac Vasc Anesth. 2016;30:406–12.
Moschietto S, Doyen D, Grech L, Dellamonica J, Hyvernat H, Bernardin G. Transthoracic Echocardiography with Doppler tissue imaging predicts weaning failure from mechanical ventilation: evolution of the left ventricle relaxation rate during a spontaneous breathing trial is the key factor in weaning outcome. Crit Care. 2012;16:R81.
Chockalingam A, Mehra A, Dorairajan S, Dellsperger KC. Acute left ventricular dysfunction in the critically ill. Chest. 2010;138:198–207.
Bajaj A, Sethi A, Rathor P, Suppogu N, Sethi A. Acute Complications of myocardial infarction in the current era: diagnosis and management. J Investig Med. 2015;63:844–55.
Park JH, Kang SJ, Song JK, Kim HK, Lim CM, Kang DH, Koh Y. Left ventricular apical ballooning due to severe physical stress in patients admitted to the medical ICU. Chest. 2005;128:296–302.
Hanania G, Cambou JP, Guéret P, Vaur L, Blanchard D, Lablanche JM, Boutalbi Y, Humbert R, Clerson P, Genès N, Danchin N. USIC 2000 investigators: management and in-hospital outcome of patients with acute myocardial infarction admitted to intensive care units at the turn of the century: results from the french nationwide USIC 2000 registry. Heart. 2004;90:1404–10.
Vieillard-Baron A, Cecconi M. Understanding cardiac failure in sepsis. Intensive Care Med. 2014;40:1560–3.
Pulido JN, Afessa B, Masaki M, Yuasa T, Gillespie S, Herasevich V, Brown DR, Oh JK. Clinical spectrum, frequency, and significance of myocardial dysfunction in severe sepsis and septic shock. Mayo Clin Proc. 2012;87:620–8.
Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–16.
Yamamoto M, Ishizu T, Seo Y, Nakagawa D, Sato K, Kawamatsu N, Machino-Ohtsuka T, Hamada-Harimura Y, Sai S, Sugano A, Nishi I, Ieda M. Pathophysiological role of right ventricular function and interventricular functional mismatch in the development of pulmonary edema in acute heart failure. J Cardiol. 2022;79:711–8.
Kobayashi M, Gargani L, Palazzuoli A, Ambrosio G, Bayés-Genis A, Lupon J, Pellicori P, Pugliese NR, Reddy Y, Ruocco G, Duarte K, Huttin O, Rossignol P, Coiro S, Girerd N. Association between right-sided cardiac function and ultrasound-based pulmonary congestion on acutely decompensated heart failure: findings from a pooled analysis of four cohort studies. Clin Res Cardiol. 2021;110:1181–92.
Lam PH, Keramida K, Filippatos GS, Gupta N, Faselis C, Deedwania P, George B, Iskandrian A, Cleland JG, Choudhary G, Wu WC, Morgan CJ, Fonarow GC, Ahmed A. Right ventricular ejection Fraction and Beta-Blocker Effect in Heart failure with reduced ejection fraction. J Card Fail. 2022;28:65–70.
Komiya K, Akaba T, Kozaki Y, Kadota JI, Rubin BK. A systematic review of diagnostic methods to differentiate acute lung injury/acute respiratory distress syndrome from cardiogenic pulmonary edema. Crit Care. 2017;21:228.
Sekiguchi H, Schenck LA, Horie R, Suzuki J, Lee EH, McMenomy BP, Chen TE, Lekah A, Mankad SV, Gajic O. Critical care ultrasonography differentiates ARDS, pulmonary edema, and other causes in the early course of acute hypoxemic respiratory failure. Chest. 2015;148:912–8.
Acknowledgements
We would like to thank Dr. Hua Zhao, from Department of Critical Care Medicine, Peking Union Medical College Hospital and Dr. Ye Liu, from Department of Medicine, University of Alabama at Birmingham Heersink School of Medicine, Alabama, USA, for their kind suggestions on the statistical issue.
Funding
Nil.
Author information
Authors and Affiliations
Contributions
H Z designed the study, analyzed and interpreted data, performed the statistical analysis, and drafted the manuscript. H L analyzed data and revised the manuscript. X W analyzed data and revised the manuscript. Q Z analyzed data and revised the manuscript. D L designed the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was conducted in compliance with the Declaration of Helsinki and was approved by the ethics committee of Peking Union Medical College Hospital, Beijing, China (ZS-1422). Written informed consent was obtained from the representatives or the next of kin of each patient.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12871_2023_2142_MOESM1_ESM.docx
Supplementary Material 1: Echocardiographic and hemodynamic parameters of CPE patients with different ventricular function.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, H., Lian, H., Wang, X. et al. Tricuspid annular plane systolic excursion/mitral annular plane systolic excursion ratio in critically ill patients: an index of right- and left-ventricular function mismatch and a risk factor for cardiogenic pulmonary edema. BMC Anesthesiol 23, 175 (2023). https://doi.org/10.1186/s12871-023-02142-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-023-02142-9