Abstract
Background
Discriminating acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) from cardiogenic pulmonary edema (CPE) is often challenging. This systematic review examines studies using biomarkers or images to distinguish ALI/ARDS from CPE.
Methods
Three investigators independently identified studies designed to distinguish ALI/ARDS from CPE in adults. Studies were identified from PubMed, and the Cochrane Central Register of Controlled Trials database until July 3, 2017.
Results
Of 475 titles and abstracts screened, 38 full texts were selected for review, and we finally included 24 studies in this systematic review: 21 prospective observational studies, two retrospective observational studies, and one retrospective combined with prospective study. These studies compared various biomarkers to differentiate subjects with ALI/ARDS and in those with CPE, and 13 calculated the area under the receiver operator characteristic curve (AUC). The most commonly studied biomarker (four studies) was brain natriuretic peptide (BNP) and the discriminatory ability ranged from AUC 0.67–0.87 but the timing of measurement varied. Other potential biomarkers or tools have been reported, but only as single studies.
Conclusions
There were no identified biomarkers or tools with high-quality evidence for differentiating ALI/ARDS from CPE. Combining clinical criteria with validated biomarkers may improve the predictive accuracy.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Differentiating between cardiogenic pulmonary edema (CPE) and acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) is challenging in the early stages of illness [1]. The most widely accepted definition of ALI/ARDS had been based on the American-European Consensus Conference (AECC) definition, of acute onset respiratory failure with bilateral infiltrates on chest radiograph, and pulmonary capillary wedge pressure (PCWP) <18 mmHg, or absence of elevated left atrial pressure [2]. However, pulmonary artery catheterization is rarely used in clinical practice because clinical estimation of PCWP is invasive, costly, and does not aid in the diagnosis of ALI/ARDS [3,4,5,6,7]. There were potential inconsistencies in this definition, including a lack of explicit criteria for defining acute respiratory failure, the sensitivity of the PaO2/FiO2 (P/F) ratio to ventilator settings, poor reliability of the chest radiograph criteria, and difficulties distinguishing ARDS from CPE including that these diagnoses can coexist [8, 9]. Based on these limitations, the Berlin definition for ARDS was published in 2012 and is reported to have better predictive validity for mortality, than this earlier definition [8]. Pulmonary capillary wedge pressure measurement was removed from this definition. Patients were presumed to have ARDS if they had respiratory failure not fully explained by cardiac failure or fluid overload as judged by the treating physician using all available data.
In current practice and most clinical studies, ALI/ARDS is usually differentiated from CPE by the clinical circumstances and by physical findings, but this distinction is often made only by post hoc review after patient’ discharge or death, and is often based on the response to therapy [10, 11]. The ARDS Clinical Trial Network reported that fluid management to decrease cardiogenic fluid retention and the effects of lung permeability and edema, will shorten the duration of mechanical ventilation and intensive care without increasing nonpulmonary organ failure [12]. The differentiation between ALI/ARDS and CPE is important in order to avoid delaying treatment of fluid retention and avoiding unnecessary testing [13]. Several biomarkers to distinguish ALI/ARDS from CPE have been reported. The aim of this systematic review was to review published studies of potential biomarkers to distinguish ALI/ARDS from CPE.
Methods
This systematic review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [14].
Search criteria
We included prospective or retrospective cohort studies written in English, which evaluated biomarkers or images for differentiating ALI/ARDS from CPE in adults. Studies that did not refer ALI/ARDS based on the AECC or the Berlin definition were excluded from this systematic review [2, 8]. We identified studies from the PubMed database using the search terms: “acute lung injury [All Fields] OR acute respiratory distress syndrome [All Fields] OR pneumonia [All Fields] AND cardiogenic pulmonary edema [All Fields] OR hydrostatic pulmonary edema [All Fields] OR ARDS diagnostics [All Fields] OR decompensated heart failure [All Fields]”, and from the Cochrane Central Register of Controlled Trials database using the search terms: “acute lung injury AND cardiogenic pulmonary edema”, “acute respiratory distress syndrome AND cardiogenic pulmonary edema”, and “pneumonia AND cardiogenic pulmonary edema” (accessed on July 3, 2017). All included studies focused on distinguishing “pure” ALI/ARDS from “pure” CPE. Mixed cases were excluded from analysis. Studies published only in abstract form were excluded. Full texts of articles were further evaluated by three investigators (KK, TA, and YK).
Data extraction
We extracted the following information from included studies: study design, sample size, diagnostic methods of ALI/ARDS or CPE, assessed markers, mean value of the markers in ALI/ARDS or CPE, the area under the receiver operator characteristic curve (AUC), and specificity and sensitivity for ALI/ARDS or CPE at a cutoff.
Assessing risk of bias
The risk of bias in the included studies was assessed according to the recommendations outlined in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. and MOOSE guidelines for the following items: selection, performance, detection, attrition, and publication bias [14]. Each study included in this review was assessed for quality as good, moderate, or poor based on biases using the modified Hayden’s criteria [15], which included source population, sample size, inclusion criteria, how to determine the final diagnosis of ALI/ARDS or CPE, and analysis providing sufficient presentation of data. Three investigators independently determined the quality based on these points. Disagreements among the investigators were resolved by review of the assessments to reach consensus.
Results
Database search and characteristics of included studies
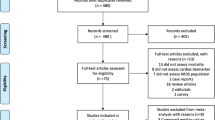
We identified 475 studies through PubMed and CENTRAL databases, and then excluded 437 studies as the abstract did not meet the inclusion criteria. We excluded 14 of the remaining 38 records after retrieving and inspecting the full text (Fig. 1).
We finally included 24 studies in this systematic review: ten studies using systemic biomarkers which were measured in plasma [the quality of these studies by the modified Hayden’s criteria was, good (n = 6) [10, 16,17,18,19,20], moderate (n = 1) [21], and poor (n = 3) [22,23,24]], and 11 studies using “lung-specific” biomarkers measured in bronchoalveolar lavage (BAL) or pulmonary edema fluid [quality was good (n = 2) [25, 26] and poor (n = 9) [27,28,29,30,31,32,33,34,35]], three studies of chest ultrasonography or computed tomography (CT) [good (n = 1) [36] and moderate (n = 2) [37, 38]]. Most were reported as prospective cohort studies but two studies were retrospective and one study combined a retrospective cohort with a prospective cohort. These studies were published from the USA (n = 10), Japan (n = 5), Germany (n = 2), Australia (n = 2), China (n = 2), Italy (n = 1), Belgium (n = 1), The Netherlands (n = 1), Taiwan (n = 1), and Switzerland (n = 1).
Sixteen studies diagnosed either ALI/ARDS or CPE using clinical information [10, 16,17,18, 21, 22, 24,25,26,27, 29, 30, 34, 36,37,38], and in eight studies the final diagnosis was confirmed by at least two independent reviewers [10, 16,17,18, 21, 26, 36, 37]. Six studies [19, 20, 23, 32, 33, 35] used the results of PCWP by pulmonary artery catheterization and two studies used the edema fluid protein/plasma protein ratio to differentiate ALI/ARDS from CPE [28, 31].
Systemic biomarkers
Nine of ten studies that evaluated systemic markers to distinguish ALI/ARDS from CPE assessed predictive power using AUC. Brain natriuretic peptide (BNP) was the most commonly assessed biomarker (Table 1). The discriminatory ability to differentiate CPE from ARDS varied among four studies with AUC, 0.67–0.83. The levels of plasma CRP in patients with ALI/ARDS were significantly higher than those with CPE [18, 33]. In these studies, subjects thought to have both ALI/ARDS and CPE were explicitly excluded from the analysis. Komiya and colleagues showed that when C-reactive protein was used to differentiate CPE from ALI/ARDS, the AUC was as good as BNP, and the AUC when the combination of BNP and CRP was used to differentiate CPE from ALI/ARDS was significantly higher than either BNP or CRP alone.
The plasma soluble suppression of tumorigenicity-2 [20], heparin-binding protein [39], and copeptin [16] were evaluated in single studies that showed high predictive value for differentiating ALI/ARDS from CPE. Arif and colleagues reported that pulmonary leak index was significantly higher in ARDS than in CPE patients and the AUC for ARDS was 0.98 for transferrin, 0.95 for total protein, and 0.80 for albumin levels in plasma [22]. Other studies compared mean value of mucin-associated antigen in serum, or arteriovenous differences in lactate between ALI/ARDS and CPE but the sample size for each of these studies was small, and the methods used as the standard for diagnosis were unclear.
Lung biomarkers
Only one of the 11 studies that evaluated “lung-specific” biomarkers used AUC to evaluate their ability to distinguish ALI/ARDS from CPE (Table 2). Ware and colleagues showed that the fluid-to-plasma protein ratio had a high AUC and good sensitivity and specificity for differentiating ALI from CPE, and that a fluid to plasma ratio >0.65 was associated with higher mortality and more days requiring mechanical ventilation [25]. Schutte and colleagues reported that the protein concentration in BALF from ALI/ARDS subjects was higher than in CPE [33]. In two studies, surfactant apoprotein (SP)-A was significantly greater in BALF from subjects with CPE compared to those with ALI/ARDS [32, 35]. Laminin gamma-2 fragments are parts of laminin-5, which is a cellular adhesion molecule expressed solely by epithelium, and promotes epithelial cell migration and repair of injured epithelium [40]. The concentration of these fragments in epithelial lining fluid from subjects with ALI/ARDS was significantly higher than those with CPE, and the concentration of laminin gamma-2 fragments at 5 days after onset also was associated with mortality [27].
Imaging studies
Copetti and colleagues evaluated the ability of chest ultrasound to detect characteristic signs of ALI/ARDS vs CPE [38] (Table 3). During normal breathing, sonography can detect the lung moving or “sliding” along the pleura, but this sliding is impaired when there are inflammatory adhesions. While subjectively, normal lung sliding is seen in subjects with CPE, it is absent or decreased in subjects with ALI/ARDS. “B lines” on chest sonography (distinct from Kerly B lines on plain radiography, and previously called comet-tail artifacts), are generated from the thickened interlobular septa (e.g., seen in interstitial edema) at the lung wall interface [41]. Sekiguchi and colleagues reported that a higher “B-line ratio” (proportion of chest zones with positive B lines relative to all zones examined) was specific for the diagnosis of CPE, and that findings of a left-sided pleural effusion >20 mm, moderate or severe left ventricle dysfunction, and minimal diameter of inferior vena cava >23 mm were helpful to distinguish CPE from ALI/ARDS using a derived, simplified prediction score as shown in Table 3 [36]. Some features on chest CT were reported to better differentiate ARDS from CPE. Small ill-defined opacities, defined as patchy areas of ground-glass attenuation or airspace consolidation, and left-dominant pleural effusion had high specificity for ALI/ARDS in a single-center retrospective study [37].
Discussion
We systematically reviewed serum and pulmonary biomarkers, and imaging used to differentiate ALI/ARDS from CPE. BNP, released from ventricular cardiomyocytes in response to both ventricle volume expansion and pressure overload, was the most commonly evaluated biomarker; but the predictive ability was variable. While two studies measured BNP levels early in the clinical presentation, and these showed a good discriminatory ability [18, 19], other studies allowed BNP to be tested up to 3 hours (IQR 0.5–14) [10] or 48 hours after presentation and these were less able to distinguish ARDS/ALI from CPE [21]. BNP is known to decrease after treatment for heart failure [42] and this could explain the higher discriminatory ability before starting treatment. Replicate and prospective studies with consistent timing of measurements are required in order to improve the quality of evidence.
As well, the subjects in the study by Levitt [21] were younger than in the other studies [10, 18, 19] and in the younger subjects BNP was a less sensitive biomarker. Because elderly patients may respond less well to diuretics, ACE inhibitors, and inotropic agents compared to younger patients [43], the younger patients may also respond to treatment more rapidly. Renal failure often accompanies severe sepsis and ARDS and this can increase BNP despite normal cardiac function [44]. Due to these different patient characteristics in each study, we did not collect these raw data to combine for a meta-analysis.
CRP is widely used as a marker of systemic inflammation, and in one study by the authors of this review; AUC when CRP was used to differentiate ALI/ARDS from CPE was as good as when BNP was used [18]. While BNP levels can increase in some conditions such as renal failure or sepsis despite normal cardiac function [44], CRP is not directly influenced by cardiac function. CRP combined with BNP may have greater discriminatory ability than either BNP or CRP alone [18].
Plasma soluble suppression of tumorigenicity-2, an IL-1 receptor family member which is a mediator of inflammation and immunity, showed excellent discrimination [20]. Heparin-binding protein is an antimicrobial protein stored in neutrophil granules, and it induces cytoskeletal rearrangement of endothelial cells, which leads to breakdown of cell barriers and an increase in macromolecular efflux [39]. Copeptin, the C-terminal portion of the arginine vasopressin precursor, is secreted together with arginine vasopressin precursor from the neurohypophysis. This secretion is thought to reflect the inflammatory cytokine response and the presence of hemodynamic and osmoregulatory disturbances [45, 46]. These biomarkers appeared to be robust in discriminating ALI/ARDS from CPE in single studies; so validation in replicate studies will be necessary.
Sample size in each study for lung-specific biomarkers was small compared with studies of serum biomarkers, and airway sampling by BAL may be difficult in the emergency department setting. When pulmonary edema is present, pulmonary edema fluid can be obtained by inserting a suction catheter into an endotracheal tube until frothy fluid is obtained by suctioning [25]. The pulmonary edema fluid-to-plasma protein ratio has been studied for decades as a tool to differentiate pulmonary permeability edema from hydrostatic edema [47].
Combining cardiac and thoracic ultrasonography could help to determine the cause of acute pulmonary edema [36]. However, these techniques are operator-skill dependent. Chest CT may be better at discriminating ALI/ARDS from CPE than chest radiography; although CT is rarely performed for acute respiratory failure in the emergency department setting. Milne and colleagues performed an independent two-observer study of chest radiographs from 61 subjects with cardiac disease, and 28 with capillary permeability edema, not described as ALI or ARDS [48]. The overall accuracy for distinguishing capillary permeability edema from cardiac edema was 91%. Another study reported that 87% of subjects with hydrostatic edema but only 60% of those with increased permeability edema were correctly identified in critical ill patients [49]. It is controversial if chest radiography can be recommended to differentiate the type of pulmonary edema.
A limitation of studies focusing on biomarkers or images for discriminating ALI/ARDS from CPE is that these can coexist [8]. Some degree of hydrostatic edema is present in many cases of ALI/ARDS, in fact the pulmonary capillary wedge pressure is reported to be elevated in 30% of ARDS patients [12]. Schmickl and colleagues developed a decision support algorithm to distinguish CPE from ARDS based on clinical data [11]. However, while all studies included in this systematic review specifically excluded mixed cases of ALI/ARDS with CPE, these authors included these cases in the ALI/ARDS group. Protein biomarkers such as BNP showed no statistically significant difference when comparing “pure” CPE with serum from subjects with ALI/ARDS both with and without CPE. BNP levels were elevated for both ALI/ARDS with and without CPE and pure CPE (708 pg/mL vs 749 pg/mL; p = 0.18). Strict fluid management that addresses cardiogenic pulmonary edema and pulmonary permeability edema increases ventilator-free days [12]. This suggests that biomarkers like BNP could be useful for differentiating CPE from ALI/ARDS and for initiating fluid restriction and diuretics early to decrease the risk of CPE.
We identified that some biomarkers, e.g., soluble suppression of tumorigencity-2, BNP plus CRP, heparin-binding protein, and plasma transferrin had high AUCs for differentiating ALI/ARDS from CPE, but these were each only assessed in a single study. All studies compared the biomarker measurement to the clinical diagnosis, but no study compared accuracy of the clinical diagnosis alone to that of clinical diagnosis plus biomarkers. Since ALI/ARDS and CPE can certainly co-exist, biomarkers need to be considered to evaluate the relative role of CPE in contributing to the morbidity of ALI/ARDS.
Because there are no accepted criteria for differentiating CPE from ALI/ARDS at the time of clinical presentation, for these studies, the decision to classify as ALI/ARDS versus CPE was made by clinical expert(s) reviewing clinical information and response to therapy including diuretics. The fundamental questions are: are biomarkers measured at clinical presentation more reliable (and accessible) than post hoc experts’ opinion in differentiating these conditions and can biomarkers identify patients who should have therapy for both conditions (coexisting). Neither question was answered by this systematic review, largely because there is no true gold standard for distinguishing ARDS/ALI from CPE. Given this limitation we understood that the purpose of this review was to identify potential biomarkers that would most closely correlate with expert clinical judgement, which was often post hoc – after the diagnosis became clear. If these biomarkers could be then used for earlier detection and intervention, as suggested by the studies showing that BNP appeared to be most useful when measured at the time of presentation in the emergency department and before initiating therapy, this may allow guidance of appropriate intervention before such time that the clinical differentiation is clear.
Conclusions
We found that there were no identified biomarkers or tools with high-quality evidence for differentiating ALI/ARDS from CPE. Because there is no objective “gold standard” for diagnosing ALI/ARDS or CPE, a clear distinction between ALI/ARDS and CPE may not have been possible in any of these reported studies. The eventual diagnosis was determined by post hoc expert review, blinded to target marker. These limitations pose an obstacle to developing a reliable method to differentiate these disorders. However, differentiating the cause of pulmonary edema is important because the therapy of ALI/ARDS and CPE are fundamentally different. Although fluid restriction might be used to treat both CPE and ARDS/ALI, early recognition of ALI/ARDS allows an emphasis on lung-protective ventilation and in the treatment of the underlying cause of the ARDS whilst recognition of CPE may lead to the appropriate use of diuretics, inotropic therapy, and afterload reduction. Combining clinical criteria with validated biomarkers may improve the predictive accuracy and improve the outcomes of ALI/ARDS even it co-exists with CPE.
Abbreviations
- ALI:
-
Acute lung injury
- ARDS:
-
Acute respiratory distress syndrome
- AUC:
-
Area under the curve
- BAL:
-
Bronchoalveolar lavage
- BNP:
-
Brain natriuretic peptide
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- CPE:
-
Cardiogenic pulmonary edema
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- ELF:
-
Epithelial lining fluid
- IQR:
-
Interquartile range
- MOOSE:
-
Meta-analysis of Observational Studies in Epidemiology
- PCWP:
-
Pulmonary capillary wedge pressure
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- PVPI:
-
Pulmonary vascular permeability index
- SP-A:
-
Surfactant protein-A
- SP-B:
-
Surfactant protein-B
References
Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353(26):2788–96.
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–24.
Shah MR, Hasselblad V, Stevenson LW, Binanay C, O'Connor CM, Sopko G, Califf RM. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA. 2005;294(13):1664–70.
Mermel LA, Maki DG. Infectious complications of Swan-Ganz pulmonary artery catheters. Pathogenesis, epidemiology, prevention, and management. Am J Respir Crit Care Med. 1994;149(4 Pt 1):1020–36.
Connors Jr AF, Speroff T, Dawson NV, Thomas C, Harrell Jr FE, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA. 1996;276(11):889–97.
Ferguson ND, Meade MO, Hallett DC, Stewart TE. High values of the pulmonary artery wedge pressure in patients with acute lung injury and acute respiratory distress syndrome. Intensive Care Med. 2002;28(8):1073–7.
Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, de Boisblanc B, Connors Jr AF, Hite RD, Harabin AL. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354(21):2213–24.
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. Jama. 2012;307(23):2526–33.
Ferguson ND, Davis AM, Slutsky AS, Stewart TE. Development of a clinical definition for acute respiratory distress syndrome using the Delphi technique. J Crit Care. 2005;20(2):147–54.
Rana R, Vlahakis NE, Daniels CE, Jaffe AS, Klee GG, Hubmayr RD, Gajic O. B-type natriuretic peptide in the assessment of acute lung injury and cardiogenic pulmonary edema. Crit Care Med. 2006;34(7):1941–6.
Schmickl CN, Shahjehan K, Li G, Dhokarh R, Kashyap R, Janish C, Alsara A, Jaffe AS, Hubmayr RD, Gajic O. Decision support tool for early differential diagnosis of acute lung injury and cardiogenic pulmonary edema in medical critically ill patients. Chest. 2012;141(1):43–50.
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, de Boisblanc B, Connors Jr AF, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–75.
Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–49.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12.
Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144(6):427–37.
Lin Q, Fu F, Chen H, Zhu B. Copeptin in the assessment of acute lung injury and cardiogenic pulmonary edema. Respir Med. 2012;106(9):1268–77.
Lin Q, Shen J, Shen L, Zhang Z, Fu F. Increased plasma levels of heparin-binding protein in patients with acute respiratory distress syndrome. Crit Care. 2013;17(4):R155.
Komiya K, Ishii H, Teramoto S, Takahashi O, Eshima N, Yamaguchi O, Ebi N, Murakami J, Yamamoto H, Kadota J. Diagnostic utility of C-reactive protein combined with brain natriuretic peptide in acute pulmonary edema: a cross sectional study. Respir Res. 2011;12:83.
Karmpaliotis D, Kirtane AJ, Ruisi CP, Polonsky T, Malhotra A, Talmor D, Kosmidou I, Jarolim P, de Lemos JA, Sabatine MS, et al. Diagnostic and prognostic utility of brain natriuretic Peptide in subjects admitted to the ICU with hypoxic respiratory failure due to noncardiogenic and cardiogenic pulmonary edema. Chest. 2007;131(4):964–71.
Bajwa EK, Volk JA, Christiani DC, Harris RS, Matthay MA, Thompson BT, Januzzi JL. Prognostic and diagnostic value of plasma soluble suppression of tumorigenicity-2 concentrations in acute respiratory distress syndrome. Crit Care Med. 2013;41(11):2521–31.
Levitt JE, Vinayak AG, Gehlbach BK, Pohlman A, Van Cleve W, Hall JB, Kress JP. Diagnostic utility of B-type natriuretic peptide in critically ill patients with pulmonary edema: a prospective cohort study. Crit Care. 2008;12(1):R3.
Arif SK, Verheij J, Groeneveld AB, Raijmakers PG. Hypoproteinemia as a marker of acute respiratory distress syndrome in critically ill patients with pulmonary edema. Intensive Care Med. 2002;28(3):310–7.
De Backer D, Creteur J, Zhang H, Norrenberg M, Vincent JL. Lactate production by the lungs in acute lung injury. Am J Respir Crit Care Med. 1997;156(4 Pt 1):1099–104.
Shih JY, Yang SC, Yu CJ, Wu HD, Liaw YS, Wu R, Yang PC. Elevated serum levels of mucin-associated antigen in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;156(5):1453–7.
Ware LB, Fremont RD, Bastarache JA, Calfee CS, Matthay MA. Determining the aetiology of pulmonary oedema by the oedema fluid-to-plasma protein ratio. Eur Respir J. 2010;35(2):331–7.
Kushimoto S, Taira Y, Kitazawa Y, Okuchi K, Sakamoto T, Ishikura H, Endo T, Yamanouchi S, Tagami T, Yamaguchi J, et al. The clinical usefulness of extravascular lung water and pulmonary vascular permeability index to diagnose and characterize pulmonary edema: a prospective multicenter study on the quantitative differential diagnostic definition for acute lung injury/acute respiratory distress syndrome. Crit Care. 2012;16(6):R232.
Katayama M, Ishizaka A, Sakamoto M, Fujishima S, Sekiguchi K, Asano K, Betsuyaku T, Kotani T, Ware LB, Matthay MA, et al. Laminin gamma2 fragments are increased in the circulation of patients with early phase acute lung injury. Intensive Care Med. 2010;36(3):479–86.
Kropski JA, Fremont RD, Calfee CS, Ware LB. Clara cell protein (CC16), a marker of lung epithelial injury, is decreased in plasma and pulmonary edema fluid from patients with acute lung injury. Chest. 2009;135(6):1440–7.
Colucci G, Domenighetti G, Della Bruna R, Bonilla J, Limoni C, Matthay MA, Martin TR. Comparison of two non-bronchoscopic methods for evaluating inflammation in patients with acute hypoxaemic respiratory failure. Crit Care. 2009;13(4):R134.
Chandel NS, Budinger GR, Mutlu GM, Varga J, Synenki L, Donnelly HK, Zirk A, Eisenbart J, Jovanovic B, Jain M. Keratinocyte growth factor expression is suppressed in early acute lung injury/acute respiratory distress syndrome by smad and c-Abl pathways. Crit Care Med. 2009;37(5):1678–84.
Newman V, Gonzalez RF, Matthay MA, Dobbs LG. A novel alveolar type I cell-specific biochemical marker of human acute lung injury. Am J Respir Crit Care Med. 2000;161(3 Pt 1):990–5.
Shimura S, Masuda T, Takishima T, Shirato K. Surfactant apoprotein-A concentration in airway secretions for the detection of pulmonary oedema. Eur Respir J. 1996;9(12):2525–30.
Schutte H, Lohmeyer J, Rosseau S, Ziegler S, Siebert C, Kielisch H, Pralle H, Grimminger F, Morr H, Seeger W. Bronchoalveolar and systemic cytokine profiles in patients with ARDS, severe pneumonia and cardiogenic pulmonary oedema. Eur Respir J. 1996;9(9):1858–67.
Miller EJ, Cohen AB, Matthay MA. Increased interleukin-8 concentrations in the pulmonary edema fluid of patients with acute respiratory distress syndrome from sepsis. Crit Care Med. 1996;24(9):1448–54.
Gunther A, Siebert C, Schmidt R, Ziegler S, Grimminger F, Yabut M, Temmesfeld B, Walmrath D, Morr H, Seeger W. Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med. 1996;153(1):176–84.
Sekiguchi H, Schenck LA, Horie R, Suzuki J, Lee EH, McMenomy BP, Chen TE, Lekah A, Mankad SV, Gajic O. Critical care ultrasonography differentiates ARDS, pulmonary edema, and other causes in the early course of acute hypoxemic respiratory failure. Chest. 2015;148(4):912–8.
Komiya K, Ishii H, Murakami J, Yamamoto H, Okada F, Satoh K, Takahashi O, Tobino K, Ichikado K, Johkoh T, et al. Comparison of chest computed tomography features in the acute phase of cardiogenic pulmonary edema and acute respiratory distress syndrome on arrival at the emergency department. J Thorac Imaging. 2013;28(5):322–8.
Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16.
Gautam N, Olofsson AM, Herwald H, Iversen LF, Lundgren-Akerlund E, Hedqvist P, Arfors KE, Flodgaard H, Lindbom L. Heparin-binding protein (HBP/CAP37): a missing link in neutrophil-evoked alteration of vascular permeability. Nat Med. 2001;7(10):1123–7.
Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24(5):326–32.
Hasan AA, Makhlouf HA. B-lines: Transthoracic chest ultrasound signs useful in assessment of interstitial lung diseases. Ann Thorac Med. 2014;9(2):99–103.
Gackowski A, Isnard R, Golmard JL, Pousset F, Carayon A, Montalescot G, Hulot JS, Thomas D, Piwowarska W, Komajda M. Comparison of echocardiography and plasma B-type natriuretic peptide for monitoring the response to treatment in acute heart failure. Eur Heart J. 2004;25(20):1788–96.
Man JP, Jugdutt BI. Systolic heart failure in the elderly: optimizing medical management. Heart Fail Rev. 2012;17(4-5):563–71.
Rudiger A, Gasser S, Fischler M, Hornemann T, von Eckardstein A, Maggiorini M. Comparable increase of B-type natriuretic peptide and amino-terminal pro-B-type natriuretic peptide levels in patients with severe sepsis, septic shock, and acute heart failure. Crit Care Med. 2006;34(8):2140–4.
Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52(1):112–9.
Groeneveld AB. Radionuclide assessment of pulmonary microvascular permeability. Eur J Nucl Med. 1997;24(4):449–61.
Fein A, Grossman RF, Jones JG, Overland E, Pitts L, Murray JF, Staub NC. The value of edema fluid protein measurement in patients with pulmonary edema. Am J Med. 1979;67(1):32–8.
Milne EN, Pistolesi M, Miniati M, Giuntini C. The radiologic distinction of cardiogenic and noncardiogenic edema. AJR Am J Roentgenol. 1985;144(5):879–94.
Aberle DR, Wiener-Kronish JP, Webb WR, Matthay MA. Hydrostatic versus increased permeability pulmonary edema: diagnosis based on radiographic criteria in critically ill patients. Radiology. 1988;168(1):73–9.
Funding
None.
Availability of data and materials
All data are available in the cited journal publications or in the mentioned internet databases.
Author information
Authors and Affiliations
Contributions
KK reviewed and prepared the manuscript, contributed to the protocol design, performed manuscript screening, data extraction and analyses for this systematic review. BKR reviewed and prepared the manuscript and contributed to the protocol design. JK contributed to the protocol design, and performed manuscript screening, data extraction and analyses for this systematic review. TA performed manuscript screening, data extraction and analyses for this systematic review. YK performed manuscript screening, data extraction and analyses for this systematic review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable due to type of article with the exclusive use of previously published data for review article.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Komiya, K., Akaba, T., Kozaki, Y. et al. A systematic review of diagnostic methods to differentiate acute lung injury/acute respiratory distress syndrome from cardiogenic pulmonary edema. Crit Care 21, 228 (2017). https://doi.org/10.1186/s13054-017-1809-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-017-1809-8