Abstract
Background
Right ventricular (RV) function is an important prognostic factor in heart failure. Patients with impaired right ventricular function have a poorer prognosis. The ratio between a tricuspid annular plane systolic excursion (TAPSE) and pulmonary artery systolic pressure (PASP) is a simple non-invasive parameter that has shown a good correlation with invasively estimated right ventricle (RV)-pulmonary artery (PA) coupling. The current study aimed to determine the value of the non-invasive evaluation of RV-PA coupling using the TAPSE/PASP ratio in predicting in-hospital mortality in patients with acute heart failure.
Methods
We included 200 patients with (heart failure and reduced ejection fraction) HFrEF presented by acute heart failure. Echocardiographic evaluation for left ventricle systolic and diastolic function was performed at the time of admission. RV functions were evaluated by calculating the following (TAPSE, PSAP, TAPSE/PASP ratio). Data were analyzed to find the predictors of in-hospital mortality.
Results
The study cohort included two hundred consecutive patients who were hospitalized for a diagnosis of acute decompensation of chronic heart failure. The in-hospital mortality rate was 12%. TAPSE/PASP was an independent predictor for in-hospital mortality (odd ratio = 3.470; 95% confidence interval, 1.240–9.705, p-value = 0.018) and (odd ratio = 18.813; 95% confidence interval, 1.974–179.275, p-value = 0.011) in univariate and multivariable logistic regression analyses respectively. In ROC curve analysis, TAPSE/PASP with a cut-off value < 0.4 mm/mmHg had a sensitivity of 79.17, a specificity of 47.73, and an area under ROC curve = 0.666 for predicting in-hospital mortality.
Conclusions
The non-invasive TAPSE/PASP ratio could be an independent predictor of mortality in HErEF patients presenting with acute heart failure.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Acute heart failure (AHF) has a poor prognosis, with a dramatic increase in mortality and rehospitalization [1, 2]. The in-hospital mortality of patients with AHF was reported to range from 4 to 11% in most published AHF registries [3,4,5] and quite high post-discharge rehospitalization rates at 60- to 90-day from 25 to 30% [6].
In heart failure patients, right ventricular (RV) function is an important prognostic factor. Patients with impaired (RV) function have a poorer prognosis than those with normal RV systolic function with a two- to threefold increase in the risk of cardiac death, regardless of the degree of left ventricular (LV) dysfunction [7, 8].
Pulmonary hypertension (PH) is the most important cause of right ventricular failure (RVF) in heart failure patients [9], with both PH [9] and RVF being related to worsening clinical outcomes in heart failure (HF) patients [7, 10].
The evaluation of tricuspid annular plane systolic excursion (TAPSE) is a simple, and non-invasive method for assessing the RV systolic function in patients with heart failure [11, 12].
Maintaining right ventricular -pulmonary artery coupling refers to the ability of right ventricular contractility to compensate for increased afterload [13].
The gold standard for assessment of right ventricular-pulmonary artery (RV-PA) coupling requires invasive recordings of pressure–volume loops for measurement of the end-systolic/arterial elastance (Ees/Ea) ratio [14, 15].
Echocardiographic measurement of the ratio between (TAPSE) and pulmonary artery systolic pressure (PASP) is a simple noninvasive parameter that has shown a good correlation with invasively estimated RV-PA coupling [15].
A lower TAPSE/PASP ratio (indicating poor RV-PA coupling) has been associated with adverse prognoses in patients with cardiovascular disease [15,16,17,18,19,20,21,22].
The present study aims to determine the value of the TAPSE/ PASP ratio in predicting in-hospital mortality in patients with acute heart failure.
Methods
Study population
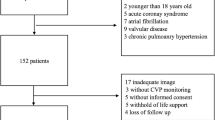
The present study prospectively included 200 consecutive patients aged ≥ 18 with acute decompensation of a previously known HF and reduced ejection fraction (HFrEF), admitted to the Cardiology department, Tanta University between January 2021 till December 2021.
HFrEF was defined as HF with a (left ventricular ejection fraction) LVEF < 40% [22]. Acute decompensated heart failure was defined as worsening HF symptoms resulting in the need for unplanned emergency department (ED) visits, or hospitalization. The diagnosis of prior HF was based on history, echocardiography, radionuclide studies findings, and patients’ medical records.
Patients' New York Heart Association Functional (NYHA) functional class were evaluated depending on the history taken from patients about their condition in the last 2 weeks before admission.
Patients with acute coronary syndrome, myocarditis, congenital heart disease, and cardiogenic shock were excluded from the study.
Informed consent was taken from all patients and the study was approved by the local ethical committee.
Echocardiographic evaluation
Two dimensional transthoracic echocardiographic and Doppler studies were performed at the time of admission of all patients using the commercially available (M5S probe, GE Vivid E9 echocardiographic system) with a 2.5 MHz transducer.
LV function
The biplane method of discs was used to measure LVEF. From the trans-mitral flow profile, trans-mitral pulsed-wave Doppler was obtained, and the peaks of both early diastolic filling (E) and late diastolic filling (A) were measured. In the apical four-chamber view tissue Doppler imaging (TDI) of the mitral annulus was performed by placing the1- to 2-mm sample volume over the septal mitral valve annulus. The value of è was measured and E/è was calculated. The Maximal LA Volume was measured in the apical 4-chamber view at the ventricular end-systolic frame just before the mitral valve opening from the apical views. LA volumes were indexed to the body surface area (LAVI). [22].
RV function
TAPSE was calculated in 2-dimensional M-mode echocardiograms from the 4-chamber view by positioning the M-mode cursor on the lateral tricuspid annulus and calculating the amount of longitudinal displacement of the annulus at peak systole [23].
Using the peak velocity (Vmax) of the tricuspid regurgitation Continuous-wave Doppler tracing, the pulmonary artery systolic pressure (PSAP) was determined as the difference in pressures between the right ventricle and the right atrium. The simplified Bernoulli equation (PSAP = 4(Vmax)2 + right atrial pressure) was used [24].
Right atrial pressure (RAP) was derived based on the size and distensibility of the inferior vena cava (IVC) during respiration. IVC diameter of 2.1 cm collapsed > 50% with a sniff indicated normal RA pressure of 3 mmHg, but IVC diameter > 2.1 cm collapsed 50% with a sniff indicated high RAP of 15 mmHg. If the IVC diameter and collapse did not suit this paradigm, an 8-mmHg intermediate value was employed [25].
RV-PA coupling was calculated using the ratio between TAPSE and PASP (TAPSE/PASP) [26].
Right Ventricle Fraction area change (RVFAC) was obtained by tracing the RV end-diastolic area (RVEDA) and end-systolic area (RVESA) in the apical 4-chamber view using the formula (RVEDA − RVESA)/RVEDA × 100[23].
The RA Volume was measured using the 4-chamber at the ventricular end-systolic frame just before the tricuspid valve opening from the apical views. Right atrial volume index (RAVI) was indexed to the body surface area. [27].
The severity of tricuspid regurgitation was also evaluated in from the apical four-chamber view and assessed semiquantitatively from the Color Doppler Flow (mild degree: up to 1/3 of the right atrium (RA), moderate degree: 1/3–2/3 of RA, severe degree: 2/3–the full length of RA) [28].
An experienced echocardiographer carried out all the measurements.
Intraobserver and interobserver variability were assessed n 15 randomly selected patients by repeated analysis on the same cine loop by the same investigator or independently by two separate investigators using intraclass correlation coefficient.
Laboratory evaluation
All patients had a measurement of B-type natriuretic peptide (BNP), hemoglobin level, serum sodium, serum potassium, glucose, C-Reactive Protein (CRP), creatinine, bilirubin, AST (aspartate aminotransferase), and ALT (Alanine aminotransferase).
All clinical, demographic, echocardiographic data and laboratory investigations were evaluated at the time of admission.
Statistical analysis
Statistical studies were carried out using analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp). The quantitative variables are expressed as mean + standard deviation (SD). The qualitative data are expressed as counts and percentages. Verification of normality of distribution was performed using The Kolmogorov–Smirnov test.
To compare qualitative values chi-square (Fisher or Monte Carlo) was used. Student t-test was used to compare two groups for normally distributed quantitative variables. Mann Whitney test was used to compare two groups for not normally distributed quantitative variables. Univariate and multivariable logistic regression analyses were performed to detect independent predictors of in-hospital mortality. Receiving operator characteristics (ROC) curve is used to detect optimal cut-off values of TAPSE/PASP for predicting in-hospital mortality. A p-value < 0.05 is considered statistically significant.
In addition, the power of the sample size was calculated by the G Power tool (Franz Faul, University of Kiel, Germany, version 3.1.9.4) with 0.05 alpha and 0.6 effect size. The calculated power value was 0.86 according to to post hoc-type power analysis.
Results
The study cohort included two hundred consecutive patients who were hospitalized for a diagnosis of acute decompensation of chronic heart failure. Patients were divided into two groups: the in-hospital mortality group (n = 24 [12%], and the Survival group (n = 176 [88%]).
Baseline clinical, hemodynamic, and laboratory characteristics
The baseline clinical characteristics hemodynamic and laboratory characteristics are shown in Tables 1 and 2. There were no significant differences between both groups regarding age, sex, body mass index, NYHA class prior to decompensation, length of stay in the intensive care unit, length of hospital stay, heart failure etiology, hypertension, dyslipidemia, diabetes mellitus, smoking status, atrial fibrillation, stroke, peripheral vascular disease, chronic obstructive pulmonary disease or asthma, major medication prior to decompensation, the incidence of implantation of Implantable Cardioverter Defibrillator (ICD)/permanent pacemaker, need for the vasodilator, non-invasive ventilation, diastolic blood pressure, heart rate, oxygen saturation, hemoglobin concentration and levels of sodium, potassium, Glucose, CRP, bilirubin, AST, ALT. Patients in the in-hospital mortality group were older, with more previous admission due to heart failure excerption, had a longer hospital stay, and had a higher need for vasopressors, inotropes, invasive ventilation, lower systolic blood pressure (mmHg), lower oxygen saturation, higher CRP level, higher Serum creatinine and BNP levels (p-value = 0.005, < 0.001, < 0.001, 0.001, 0.001, 0.001, < 0.001, 0.036, < 0.001, and < 0.001 respectively).
Echocardiographic characteristics
-
a.
Left ventricle function echocardiography study (Table 3): Both groups did not differ with respect to peak mitral E wave velocity, peak mitral A wave velocity LV IVRT, mitral E/e′ ratio, and LAVI. LVEF% was lower in the in-hospital mortality group.
-
b.
RV function (Table 4): There were no significant differences between both groups regarding peak tricuspid E wave velocity and peak tricuspid A wave velocity, tricuspid E/è, RVFAC, and severity of tricuspid regurgitation. The pulmonary artery systolic pressure (PASP) and RAVI were higher and TAPSE, TAPSE/PASP (mm/mmHg) were lower in the in-hospital mortality group (p-value 0.010, < 0.001, 0.009, and 0.005 respectively).
Univariate and multivariable logistic regression analyses were built to identify predictors of in-hospital mortality. The results showed that TAPSE/PASP was an independent predictor for in-hospital mortality (odd ratio = 5.0; 95% confidence interval, (1.890–13.230), p-value = 0.001) and (odd ratio = 119.868; 95% confidence interval, (1.246–11,530.0), p-value = 0.040) in univariate and multivariable logistic regression analyses respectively (Table 5).
Also, the need for vasopressor, elevated BNP, CRP, serum creatinine, lower systolic pressure, and EF% were independent predictors of mortality.
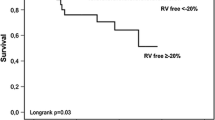
In ROC curve analysis, TAPSE/PASP with a cut-off value < 0.4 mm/mmHg had a sensitivity of 79.17, a specificity of 47.73, and an area under ROC curve = 0.666 for predicting in-hospital mortality (Fig. 1).
Furthermore, patients in each group were classified into three tertiles, tertile1 with TAPSE/PASP < 0.4, tertile2 with TAPSE/PASP between (0.4 & 0.7) and tertile3 with TAPSE/PASP > 0.7. Most of the patients in the mortality group fall within the first tertile 79.2% and there is a statistically significant difference between both groups p-value = 0.043. (Table 6).
Finally, the cause of mortality was ventricular fibrillation (16.7%), ventricular tachycardia (8.3%), asystole (12.5%), renal failure (12.5%), and cardiogenic shock (50.0%) ( Table 6).
Reproducibility
Intra-observer and inter-observer variability for conventional two-dimensional/Doppler measurements and TDI-derived parameters ranged from 0.94 and 0.97 and 0.92 and 0.94 respectively.
Discussion
Acute heart failure (AHF) is a leading cause of hospital admissions and is linked with a marked increase in morbid and fatal events [29]. The prognosis of AHF is poor despite advances in therapeutic options with in-hospital mortality rates between 4 and 7% [6].
This prospective cohort study aimed to explore the value of the TAPSE/PASP ratio in predicting in-hospital mortality in patients with acute heart failure in HFrEF patients admitted with acute heart failure.
The main findings of the present study were: (1) The in-hospital mortality of AHF patients was 12%. (2) TAPSE/PASP was an independent predictor for in-hospital mortality in patients with acute decompensation of HFrEF. (3) TAPSE/PASP with a cut-off value < 0.4 mm/mmHg had a sensitivity of 79.17, a specificity of 47.73, and an area under ROC curve = 0.666 for predicting in-hospital mortality (4) The majority of patients in the mortality group (79.2%) had TAPSE/PASP of < 0.4.
The in-hospital mortality in the current study is similar to the finding of Wang HK et al. [30] and close to the mortality rate reported in some large registries as the study submitted by Wajner A et al. [31], in brazil tertiary hospitals.
While in other studies where the number of patients is larger, the in-hospital mortality was slightly lower, like in the study of C. Lombardi et al.[32], who studied 728 patients with AHF, the in-hospital deaths were 8.9%. These differences could be attributed to the differences in the number of patients included in these studies and to the facilities and hearth care quality offered to the patients.
In normal hearts, the synchronization between the RV and pulmonary circulation is important and makes both work as a single cardiopulmonary unit resulting in matching between contractility and afterload, and this is called (RV-PA coupling)[33].
In patients who develop pulmonary hypertension, the pulmonary vascular resistance is increased and the compliance in vascular bed is reduced with increased afterload and alteration in RV-PA coupling resulting in poor outcomes in patients with pulmonary hypertension [34].
Tello et al. proved that TAPSE/PASP is a reliable method for evaluation of RV-PA coupling in patients with severe idiopathic and thromboembolic pulmonary hypertension and, they showed that a value of this ratio < 0.31 mm/mm could be used as a predictor of RV-PA uncoupling [14].
TAPSE/PASP ratio reflects the RV response to changes in the afterload [35] with the higher the ratio, the better the RV function [13] with maintained RV-PA coupling in the initial stages of chronic PH the RV enhances its contractility to counteract the increased afterload, which is frequently accompanied by RV hypertrophy and dilatation. As chronic PH progresses to RV failure, RV-PA uncoupling causes a decline in RV systolic function [36].
The current study adds to the prior reports on the importance of non-invasive echocardiographic measurement of RV-PA coupling in risk stratification in critically ill patients and patients with heart failure.
Jentzer. et al., in their cohort study, found that the loss of RV-PA coupling was linked to increased mortality risk during hospitalization and after discharge among patients admitted to the cardiac intensive care unit [37]. Also, Guazzi. et al. found that TAPSE/PASP was inversely related to NYHA functional class, and they concluded that this ratio which combined measurement of both longitudinal RV fiber shortening (TAPSE) to the developed pressure in the pulmonary artery is a good clinical index that relates the length of RV contraction to the force generated and the use of this ratio is better than the use of both separately and this was vailed for both HFrEF and HFpEF patients [13].
Bragança et al. conducted a retrospective analysis on 70 HF patients who had CRT implantation. TAPSE/PASP ratio demonstrated the best predictive capacity to detect non-response to cardiac resynchronization therapy (CRT [16].
Santas et al. reported that TAPSE/PASP was a predictor of readmission in a prospective study of 1,127 patients with HFpEF fraction discharged with a diagnosis of AHF. Patients in the lowest quintile (TAPSE/PASP < 0.28) had the highest incidence of repeat admissions [19].
The above-mentioned data come with our results that TAPSE/PASP could be used as an independent predictor of in-hospital mortality in patients admitted with acute heart failure.
The association between reduced TAPSE/PASP and mortality in patients with acute decompensation of HFrEF reported in the present study suggests that measures that improve RV-PA coupling may lead to a better outcome. This emphasizes the importance of RV function in patients with LV failure and suggests that optimizing biventricular function in this group of patients is necessary [38].
Therapeutic interventions that improve coupling may have a favorable effect on outcomes. These interventions may include treatments that reduce the RA and pulmonary artery pressure [39]. Such therapeutic interventions might include treatments using either diuretic, drugs that affect the pulmonary vasculature, fluid removal, and short-term inotropic. These options may result in short-term enhancement of RV-PA coupling during the hospital stay and enable recovery from the acute stage [40], 41.
Conclusions
The non-invasive TAPSE/PASP ratio could be an independent predictor of mortality in HErEF patients presenting with acute heart failure. We recommend further multicentre studies on a larger number of patients to validate our results.
Study limitations
A relatively small number of patients is a limitation of the current study, also further studies over a longer period of follow-up are recommended as the study analysis was limited to outcomes during hospitalization. Studies with follow-up estimation of TAPSE/PASP and evaluation of the effect of different AHF therapeutic options on RV-PA coupling are required.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AHF:
-
Acute heart failure
- RV:
-
Right ventricular
- PH:
-
Pulmonary hypertension
- RVF:
-
Right ventricular failure
- HF:
-
Heart failure
- TAPSE:
-
Tricuspid annular plane systolic excursion
- (Ees/Ea):
-
End-systolic/arterial elastance ratio
- RV-PA:
-
Right ventricular-pulmonary artery
- HFrEF:
-
HF and reduced ejection fraction
- NYHA:
-
New York heart association
- LVEF:
-
Left ventricular ejection fraction
- ED:
-
Emergency department
- E/ è:
-
The ratio of early flow velocity to the early annular velocity
- E:
-
Early diastolic filling
- A:
-
Late diastolic filling
- TDI:
-
Tissue Doppler imaging
- Vmax:
-
Peak velocity
- RAP:
-
Right atrial pressure
- LAVI:
-
Left atrial volume index
- IVC:
-
Inferior vena cava
- RVFAC:
-
Right ventricular fractional area change
- RAVI:
-
Right atrial volume index
- RA:
-
Right atrium
- HFpEF:
-
Heart failure with preserved
- HFmrEF:
-
Heart failure with a mid-range ejection fraction
- CRP:
-
C-reactive protein
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine transaminase
- CRT:
-
Cardiac resynchronization therapy
References
Metra M, Teerlink JR. Heart failure. Lancet. 2017;390:1981–95. https://doi.org/10.1016/S0140-6736(17)31071-1.
Crespo-Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, et al. European society of cardiology heart failure long-term registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail. 2016;18:613–25. https://doi.org/10.1002/ejhf.566.
Galvao M, Kalman J, Demarco T, Fonarow GC, Galvin C, Ghali JK, et al. Gender differences in in-hospital management and outcomes in patients with decompensated heart failure: analysis from the acute decompensated heart failure national registry (ADHERE). J Cardiac Fail. 2006;12:100–7. https://doi.org/10.1016/j.cardfail.2005.09.005.
Follath F, Yilmaz MB, Delgado JF, Parissis JT, Porcher R, Gayat E, et al. Clinical presentation, management and outcomes in the acute heart failure global survey of standard treatment (ALARM-HF). Intensive Care Med. 2011;37:619–26. https://doi.org/10.1007/s00134-010-2113-0.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;2021:42. https://doi.org/10.1093/eurheartj/ehab368.
Farmakis D, Parissis J, Lekakis J, Filippatos G. Acute heart failure: epidemiology, risk factors, and prevention. Revista Española de Cardiología (English Edition). 2015;68:245–8. https://doi.org/10.1016/j.rec.2014.11.004.
de Groote P, Fertin M, Goéminne C, Petyt G, Peyrot S, Foucher-Hossein C, et al. Right ventricular systolic function for risk stratification in patients with stable left ventricular systolic dysfunction: comparison of radionuclide angiography to echoDoppler parameters. Eur Heart J. 2012;33:2672–9. https://doi.org/10.1093/eurheartj/ehs080.
Damy T, Kallvikbacka-Bennett A, Goode K, Khaleva O, Lewinter C, Hobkirk J, et al. Prevalence of, associations with, and prognostic value of tricuspid annular plane systolic excursion (TAPSE) among out-patients referred for the evaluation of heart failure. J Cardiac Fail. 2012;18:216–25. https://doi.org/10.1016/j.cardfail.2011.12.003.
Guazzi M, Galie N. Pulmonary hypertension in left heart disease. Eur Respir Rev. 2012;21:338–46. https://doi.org/10.1183/09059180.00004612.
Damy T, Viallet C, Lairez O, Deswarte G, Paulino A, Maison P, et al. Comparison of four right ventricular systolic echocardiographic parameters to predict adverse outcomes in chronic heart failure. Eur J Heart Fail. 2009;11:818–24. https://doi.org/10.1093/eurjhf/hfp111.
Bleeker GB, Steendijk P, Holman ER, Yu CM, Breithardt OA, Kaandorp TAM, et al. Assessing right ventricular function: the role of echocardiography and complementary technologies. Heart. 2006. https://doi.org/10.1136/hrt.2005.082503.
Meluzín J, Špinarová L, Bakala J, Toman J, Krejči J, Hude P, et al. Pulsed Doppler tissue imaging of the velocity of tricuspid annular systolic motion. A new, rapid, and non-invasive method of evaluating right ventricular systolic function. Eur Heart J. 2001. https://doi.org/10.1053/euhj.2000.2296.
Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol-Heart Circ Physiol. 2013;305:H1373–81. https://doi.org/10.1152/ajpheart.00157.2013.
Tello K, Wan J, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, et al. Validation of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular-arterial coupling in severe pulmonary hypertension. Circ Cardiovasc Imaging. 2019. https://doi.org/10.1161/CIRCIMAGING.119.009047.
Spruijt OA, de Man FS, Groepenhoff H, Oosterveer F, Westerhof N, Vonk-Noordegraaf A, et al. The effects of exercise on right ventricular contractility and right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med. 2015. https://doi.org/10.1164/rccm.201412-2271OC.
Bragança B, Trêpa M, Santos R, Silveira I, Fontes-Oliveira M, Sousa MJ, et al. Echocardiographic assessment of right ventriculo-arterial coupling: clinical correlates and prognostic impact in heart failure patients undergoing cardiac resynchronization therapy. J Cardiovasc Imaging. 2020;28:109. https://doi.org/10.4250/jcvi.2019.0094.
Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, et al. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail. 2017;19:873–9. https://doi.org/10.1002/ejhf.664.
Guazzi M, Dixon D, Labate V, Beussink-Nelson L, Bandera F, Cuttica MJ, et al. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2017;10:1211–21. https://doi.org/10.1016/j.jcmg.2016.12.024.
Santas E, Palau P, Guazzi M, de la Espriella R, Miñana G, Sanchis J, et al. Usefulness of right ventricular to pulmonary circulation coupling as an indicator of risk for recurrent admissions in heart failure with preserved ejection fraction. Am J Cardiol. 2019;124:567–72. https://doi.org/10.1016/j.amjcard.2019.05.024.
Nie L, Li J, Zhang S, Dong Y, Xu M, Yan M, et al. Correlation between right ventricular–pulmonary artery coupling and the prognosis of patients with pulmonary arterial hypertension. Medicine. 2019;98: e17369. https://doi.org/10.1097/MD.0000000000017369.
Vriz O, Pirisi M, Bossone E, Fadl ElMula FEM, Palatini P, Naeije R. Right ventricular–pulmonary arterial uncoupling in mild-to-moderate systemic hypertension. J Hypertens. 2020;38:274–81. https://doi.org/10.1097/HJH.0000000000002238.
Ristow B, Ali S, Whooley MA, Schiller NB. Usefulness of left atrial volume index to predict heart failure hospitalization and mortality in ambulatory patients with coronary heart disease and comparison to left ventricular ejection fraction (from the heart and soul study). Am J Cardiol. 2008;102:70–6. https://doi.org/10.1016/j.amjcard.2008.02.099.
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American society of echocardiography endorsed by the European association of echocardiography, a registered branch of the European society of cardiology, and the Canadian society of echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. https://doi.org/10.1016/J.ECHO.2010.05.010.
Parasuraman S, Walker S, Loudon BL, Gollop ND, Wilson AM, Lowery C, et al. Assessment of pulmonary artery pressure by echocardiography-A comprehensive review. Int J Cardiol Heart Vasc. 2016;12:45–51. https://doi.org/10.1016/J.IJCHA.2016.05.011.
Darahim K. Usefulness of right atrial volume index in predicting outcome in chronic systolic heart failure. J Saudi Heart Assoc. 2014;26:73–9. https://doi.org/10.1016/J.JSHA.2013.09.002.
Fortuni F, Butcher SC, Dietz MF, van der Bijl P, Prihadi EA, de Ferrari GM, et al. Right ventricular-pulmonary arterial coupling in secondary tricuspid regurgitation. Am J Cardiol. 2021;148:138–45. https://doi.org/10.1016/j.amjcard.2021.02.037.
Ebtia M, Murphy D, Gin K, Lee PK, Jue J, Nair P, et al. Best method for right atrial volume assessment by two-dimensional echocardiography: validation with magnetic resonance imaging. Echocardiography. 2015;32:734–9. https://doi.org/10.1111/echo.12735.
Šmíd M, Čech J, Rokyta R, Roučka P, Hájek T. Mild to moderate functional tricuspid regurgitation: retrospective comparison of surgical and conservative treatment. Cardiol Res Pract. 2010;2010:1–5. https://doi.org/10.4061/2010/143878.
Crespo-Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, et al. European society of cardiology heart failure long-term registry (<scp>ESC-HF-LT</scp>): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail. 2016;18:613–25. https://doi.org/10.1002/ejhf.566.
Wang HK, Hu SC, Wang TD, Chen WJ, Huang CH. Prediction of outcome of in-hospital mortality for acute heart failure. J Acute Med. 2017. https://doi.org/10.6705/j.jacme.2017.0703.003.
Wajner A, Zuchinali P, Olsen V, Polanczyk CA, Rohde LE. Causes and predictors of in-hospital mortality in patients admitted with or for heart failure at a tertiary hospital in Brazil. Arq Bras Cardiol. 2017. https://doi.org/10.5935/abc.20170136.
Lombardi C, Peveri G, Cani D, Latta F, Bonelli A, Tomasoni D, et al. In-hospital and long-term mortality for acute heart failure: analysis at the time of admission to the emergency department. ESC Heart Fail. 2020. https://doi.org/10.1002/ehf2.12847.
Todaro MC, Carerj S, Zito C, Trifirò MP, Consolo G, Khandheria B. Echocardiographic evaluation of right ventricular-arterial coupling in pulmonary hypertension. Am J Cardiovasc Dis. 2020;10(4):272–283.
Vanderpool RR, Pinsky MR, Naeije R. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart. 2015;101:37–43. https://doi.org/10.1136/heartjnl-2014-306142.
Amsallem M, Mercier O, Kobayashi Y, Moneghetti K, Haddad F. Forgotten no more. JACC Heart Fail. 2018;6:891–903. https://doi.org/10.1016/j.jchf.2018.05.022.
Vonk Noordegraaf A, Westerhof BE, Westerhof N. The Relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol. 2017;69:236–43. https://doi.org/10.1016/j.jacc.2016.10.047.
Jentzer JC, Anavekar NS, Reddy YNV, Murphree DH, Wiley BM, Oh JK, et al. Right ventricular pulmonary artery coupling and mortality in cardiac intensive care unit patients. J Am Heart Assoc. 2021. https://doi.org/10.1161/JAHA.120.019015.
Kukulski T, She L, Racine N, Gradinac S, Panza JA, Velazquez EJ, et al. Implication of right ventricular dysfunction on long-term outcome in patients with ischemic cardiomyopathy undergoing coronary artery bypass grafting with or without surgical ventricular reconstruction. J Thorac Cardiovasc Surg. 2015;149:1312–21. https://doi.org/10.1016/j.jtcvs.2014.09.117.
Jentzer JC, Mathier MA. Pulmonary hypertension in the intensive care unit. J Intensive Care Med. 2016;31:369–85. https://doi.org/10.1177/0885066615583652.
Harjola V-P, Mebazaa A, Čelutkienė J, Bettex D, Bueno H, Chioncel O, et al. Contemporary management of acute right ventricular failure: a statement from the heart failure association and the working group on pulmonary circulation and right ventricular function of the European society of cardiology. Eur J Heart Fail. 2016;18:226–41. https://doi.org/10.1002/ejhf.478.
Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, et al. Evaluation and management of right-sided heart failure: a scientific statement from the American heart association. Circulation. 2018. https://doi.org/10.1161/CIR.0000000000000560.
Acknowledgements
Not applicable
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M.N: Conception of the work, Performing echocardiography, Preparing the manuscript. A.ALK: Data collection, Data analysis and interpretation, Drafting the article. A.ALA: Data analysis and interpretation, Performing echocardiography, Draft revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed written consent was taken from all patients and the study was approved by the local ethical committee of Tanta University, Faculty of Medicine, all methods were performed in accordance with the relevant guidelines and regulations (committee reference number: 35287–01-21).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Naseem, M., Alkassas, A. & Alaarag, A. Tricuspid annular plane systolic excursion/pulmonary arterial systolic pressure ratio as a predictor of in-hospital mortality for acute heart failure. BMC Cardiovasc Disord 22, 414 (2022). https://doi.org/10.1186/s12872-022-02857-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02857-6