Abstract
Background
The effect of a combination of a goal-directed fluid protocol and preoperative carbohydrate loading on postoperative complications in elderly patients still remains unknown. Therefore, we designed this trial to evaluate the relative impact of preoperative carbohydrate loading and intraoperative goal-directed fluid therapy versus conventional fluid therapy (CFT) on clinical outcomes in elderly patients following gastrointestinal surgery.
Methods
This prospective randomized controlled trial with 120 patients over 65 years undergoing gastrointestinal surgery were randomized into a CFT group (n = 60) with traditional methods of fasting and water-deprivation, and a GDFT group (n = 60) with carbohydrate (200 ml) loading 2 h before surgery. The CFT group underwent routine monitoring during surgery, however, the GDFT group was conducted by a Vigileo/FloTrac monitor with cardiac index (CI), stroke volume variation (SVV), and mean arterial pressure (MAP). For all patients, demographic data, intraoperative parameters and postoperative outcomes were recorded.
Results
Patients in the GDFT group received significantly less crystalloids fluid (1111 ± 442.9 ml vs 1411 ± 412.6 ml; p < 0.001) and produced significantly less urine output (200 ml [150–300] vs 400 ml [290–500]; p < 0.001) as compared to the CFT group. Moreover, GDFT was associated with a shorter average time to first flatus (56 ± 14.1 h vs 64 ± 22.3 h; p = 0.002) and oral intake (72 ± 16.9 h vs 85 ± 26.8 h; p = 0.011), as well as a reduction in the rate of postoperative complications (15 (25.0%) vs 29 (48.3%) patients; p = 0.013). However, postoperative hospitalization or hospitalization expenses were similar between groups (p > 0.05).
Conclusions
Focused on elderly patients undergoing open gastrointestinal surgery, we found perioperative fluid optimisation may be associated with improvement of bowel function and a lower incidence of postoperative complications.
Trial registration
ChiCTR, ChiCTR1800018227. Registered 6 September 2018 - Retrospectively registered.
Similar content being viewed by others
Background
Patients ≥ 65 years old are termed elderly [1]. Elderly patients typically present with a range of physical, pharmacological, and psychological comorbidities that must be carefully considered by clinicians and anesthesiologists prior to any major surgical operation [2]. Indeed, more advanced age remains a key risk factor associated with higher rates of postoperative morbidity and mortality [3].
Elderly patients are more susceptible to deleterious effects such as dehydration, hypovolemia and hemodynamic instability induced by prolonged fasting [4]. Intraoperative hypovolemia can, in turn, result in a range of serious postoperative complications including hypotension and severe arrhythmia, whereas hypervolemia can also cause serious problems such as anastomotic leaks, infection, pulmonary edema, or even death [5,6,7,8,9,10]. As such, it is clear that both insufficient and excess fluid infusion can cause harm in patients [11]. Owing to age-related declines in organ function and greater difficulty adjusting fluid preloading, elderly patients are thus at a substantially higher risk of postoperative mortality.
Preoperative fluid therapy mainly aims to prevent the patients from a dehydrated or hypovolemic state before anesthesia induction. Therefore, multiple ERAS (Enhanced Recovery After Surgery) guidelines include the oral intake of carbohydrate loading (200 ml) 2 h before surgery, which may help to decrease postoperative complications, such as postoperative nausea and vomiting and wound infection. Besides, the use of routine hemodynamic measurements (such as arterial blood pressure or heart rate) is often a relatively imprecise means of monitoring for changes in blood volume [12, 13]. In contrast, goal-directed fluid therapy approaches rely upon monitoring more advanced hemodynamic variables such as SVV and pulse pressure variability, which are more sensitive to hypovolemia and therefore allow for optimal preloading by enabling clinicians to appropriate titrate fluids and inotropic substances [14, 15].
GDFT has been shown to reduce the duration of hospitalization and to decrease the incidence of postoperative complications by 20–50% in previous systematic reviews [16,17,18]. However, there have been few studies regarding whether preoperative carbohydrate loading combined with intraoperative GDFT is similarly beneficial in elderly patient populations. As such, in the present study we conducted fluid optimisation in an elderly patient population via the use of hemodynamic indicators (CI, SVV, MAP) and vasoactive drugs, as necessary to. We hypothesized that preoperative carbohydrate (200 ml) loading and intraoperative GDFT based upon SVV, CI and MAP may lower postoperative hospitalization and postoperative complications in elderly patients undergoing gastrointestinal surgery.
Methods
Patients
This prospective randomized trial was performed at the Sichuan Provincial People’s Hospital (Chengdu, China). The trial was registered in the center of Chinese Clinical Trial Registry (ChiCTR1800018227). After the Ethics Committees of the Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital approved this study (Approval Number 2018157), we enrolled patients between May 2018 and November 2019 to receive two different protocols for perioperative fluid therapy. Eligible patients were individuals > 65 years old that were scheduled to undergo elective open gastrointestinal surgery (anticipated operating time > 2 h) and that matched the ASA (American Society of Anesthesiologists) class 2–4 criteria. Prior studies have shown that the accuracy of Vigileo/FloTrac is reduced in patients with abnormal sinus rhythm or intraabdominal pressure values [19], and as such patients were excluded from this study if they had no sinus rhythm, or if they had a history of gastrointestinal surgery, peripheral artery disease, or with high risk of reflux and aspiration (pyloric obstruction or achalasia of the cardia). All patients provided written informed consent before participation in the trial.
Randomization and blinding
A series of random numbers generated by SPSS software were used to randomize the grouping of patients. The patients numbered 1–120 according to the time of elective surgery were randomized either to the GDFT group or the CFT group. The blinding of group assignment was ensured by the opaque envelopes. The random serial numbers and the envelopes were kept by an investigator who was not involved in the conduct of the trial. Subjects, surgeons, patients themselves, ICU physicians and professionals for data recording, collection and analysis were blind to group assignment. However, the attending anesthesiologist and investigators could not be blinded because of the existence of cardiac index trending monitor in the GDFT group.
Intraoperative management
All patients were monitored for pulse oximetry, five-lead-electrocardiogram, blood pressure, heart rate, temperature and end-tidal carbon dioxide concentrations. Central venous pressure (CVP) monitoring was conducted in some patients as decided by the attending anesthesiologist based on the patients’ physical situation and surgical needs after the surgery. Patients in both groups received a minimum of one peripheral intravenous access (18G), with a central venous catheter being inserted via the internal jugular vein and with radial arterial catheters being introduced via the non-dominant forearm under low-dose dexmedetomidine for sedation in the operating room at 7:30 AM on the day of surgery. For each patient, the following was recorded: baseline characteristics, surgery type, and basic hemodynamics variables (blood pressure, MAP, HR). An initial arterial blood gas analysis was also conducted at this time. For patients in the GDFT group, the radial arterial lines were attached to the fourth generation Vigileo/FloTrac monitor (Edwards Life Sciences, CA, USA). The technique of point-of-care gastric ultrasound was performed to make sure that the patient was not in high risk of reflux aspiration on account of carbohydrate loading 2 h before the gastrointestinal operation. Anesthesia was then induced by using propofol (2–2.5 mg.kg− 1) or etomidate (0.2 mg.kg− 1) in combination with sufentanil (15–25 μg) and cisatracurium (0.15–0.2 mg.kg− 1). Anesthesia was maintained with remifentanil (0.2–0.25 μg.kg− 1 min− 1), dexmedetomidine (0.4–0.7 μg.kg− 1 h− 1) and sevoflurane at a MAC (minimum alveolar concentration) of 1–1.3 in order to achieve a BIS (bispectral index) value of 40–60. Each patient was ventilated with a tidal volume of 8 ml/kg without PEEP (positive end-expiratory pressure) at a respiratory rate sufficient to achieve an end-tidal carbon dioxide value of 35–45 mmHg. Flow rates were set to 2–3 L/min, and 60% oxygen was administered for the operative duration.
Study protocol
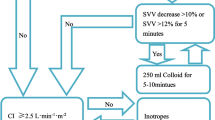
The bowel preparation was performed by the surgeons’ instruction 1 day before surgery. The GDFT group was fasted for 8 h and drunk 200 ml carbohydrate (Shuneng; Yi Chang Ren Fu Pharmaceutical Co Ltd., China) 2 h before the operation. The CFT group was fasted for 8 h and prohibited drinking for 4 h before surgery. The CFT group was initially infused with a balanced crystalloid solution at 2 ml/kg/h for baseline fluid therapy and 6 ml/kg/h during surgery (from incision to suturing). In addition, additional bolus (crystalloid or colloids) could be infused at the discretion of the attending anesthesiologist, or vasoactive drugs could be administered to ensure that routine monitoring variables remained within the normal range (heart rate < 100 bpm, MAP > 65 mmHg, urine output > 0.5 ml/kg/h). Patients in the GDFT treatment group were infused based on the Vigileo/FloTrac instrument. If CI was ≥ 2.5 l/min/m2, MAP was > 65 mmHg, and SVV was < 12%, the administration rate was maintained at 6 ml/kg/h for the entire surgical operation. When CI reached ≤ 2.5 l/min/m2 with SVV > 12%, additional colloid 250 ml boluses were administered within 10 min. When CI was ≤ 2.5 l/min/m2 and SVV was < 12%, dobutamine was injected to achieve a CI of ≥ 2.5 l/min/m2. Ephedrine boluses of 3–18 mg or norepinephrine infusions were administered when CI was ≥ 2.5 l/min/m2 and MAP was < 65 mmHg (Fig. 1). All hemodynamic variables were reassessed after 10 min.
Data collection
We recorded lactate values at 5 min prior to the induction of anesthesia (T1), 60 min after the operation was initiated (T2), and at the end of surgery (T3). All intraoperative data were collected upon the completion of the operation, including operative duration, duration of anesthesia, types and amounts of fluid infusion, estimated blood loss, urine output, and vasoactive agent use. Following intensive care unit (ICU) or ward admission, the management of all patients was conducted by medical staff blinded to patient group assignments. Time to first flatus and time to first oral intake was recorded. Following patient discharge, we recorded the postoperative hospital stay, amount of hospital expenses, admission to ICU, postoperative complications and mortality. The predefined criteria for complications in the study protocol were described in Additional file 1.
Statistical analysis
The sample size calculation was based on a study by Wackeling et al. [20], which showed the postoperative complication rate was 59.3% in patients receiving standard of care fluid therapy as compared to 37.5% in the GDFT group. For a reduction in the rate of postoperative complication from 60 to 32% in the GDFT group with type I error of 0.05 and a power of 80%, a sample size of 50 patients was calculated by our researchers for each group. Owing to loss of about 20% of patients allocated to groups as a result of changes in scheduled surgical procedure, our study finally included 60 patients per group. SPSS 26.0 (IBM, IL, USA) was used for all statistical testing. Data were given as mean ± SD, absolute values (percentages) or medians (25th–75th percentiles). Data were compared via Student’s t-tests (for normally distributed variables), Wilcoxon rank-sum tests (for non-normally distributed variables), and chi-squared or Fisher’s exact tests (for categorical variables). Normality was assessed via the Kolmogorov-Smirnov test. Arterial blood lactate concentrations were normally compared via analysis of variance (ANOVA) on repeated measurements with Bonferroni correction against baseline, whereas they were compared via the Friedman test when non-normally distributed. p < 0.05 was the significance threshold.
Results
In total, 225 patients were eligible for inclusion in the present study between May 2018 and November 2019. After excluding patients based on our exclusion criteria and failure to give consent, the remaining 120 patients were randomized into the GDFT (n = 60) and CFT (n = 60) groups. Among these patients, 5 (3 and 2 in the GDFT and CFT groups, respectively) dropped out of the study, 3 (2 and 1 in the GDFT and CFT groups, respectively) were found to be inoperable (Fig. 2). In total, our final analyses thus included 55 patients in the GDFT group and 57 patients in the CFT group.
There were no significant differences between groups with respect to demographic characteristics, surgical category, or ASA classification (p > 0.05). Operative duration and duration of anesthesia were also similar between groups. Intraoperative blood loss was comparable between groups. Patients in the CFT group were administered a significantly larger volume of fluids relative to patients in the GDFT group (2033 ± 468.7 ml vs 1697 ± 536.9 ml; p < 0.01), with the difference primarily being in the form of crystalloid infusion (1411 ± 412.6 ml vs 1111 ± 442.9 ml; p < 0.001). Urine output was also significantly greater in the CFT group compared with the GDFT group (400 ml [290–500] vs 200 ml [150–300]; p < 0.001). There were no significant differences between groups with respect to intraoperative colloid infusion volume. With respect to vasoactive drugs, dobutamine was used more frequently in the GDFT group. There was also a significant increase in arterial lactate concentration in the CFT group relative to baseline at the end of surgery (1.0 mmol/l (0.8–1.2) vs 0.7 mmol/l (0.6–0.95); p < 0.001). No other differences with respect to lactate values were detected between these two groups. For full details regarding these findings, see Tables 1 and 2.
An intention-to-treat analysis and per protocol analysis for postoperative outcomes were presented in Table 3. With respect to postoperative bowel function, we found that GDFT was associated with a shorter time to first flatus (56 ± 14.1 h vs 64 ± 22.3 h; p = 0.002) and to first oral intake (72 ± 16.9 h vs 85 ± 26.8 h; p = 0.011) relative to the CFT group. In addition, there were significantly fewer instances of postoperative complications among patients in the GDFT group relative to the CFT group (15 (25.0%) vs 29 (48.3%) patients; p = 0.013). Further analysis of predefined types of complications found that there was a lower rate of infection complications in in the GDFT group relative to the CFT group (5 (9.1%) vs 13 (22.8%) patients; p = 0.048). However, GDFT was not associated with any significant differences in postoperative hospitalization duration (9.1 ± 2.8 days vs 9.7 ± 3.2 days; p = 0.290), ICU admission (6 (10.0%) vs 7 (11.7%) patients; p = 0.769), or hospitalization expenses (57,599 ± 16,363 RMB vs 62,802 ± 19,891 RMB; p = 0.120). In the per protocol analysis, the GDFT group was also associated with a lower rate of complications (14 (25.5%) vs 27(47.4%) patients; p = 0.016) relative to the CFT group. Again, there was no difference in hospital stay (9.2 ± 2.9 days vs 9.7 ± 3.1 days; p = 0.397) and hospitalization expenses (56,845 ± 16,446 RMB vs 60,571 ± 16,579 RMB; p = 0.235). Although there were no significant differences between groups with respect to postoperative mortality (0 (0%) vs 2 (3.3%) patients; p = 0.496), two patients in the CFT group did die on postoperative day 3 as a result of acute massive gastrointestinal hemorrhage and acute myocardial infraction respectively.
Discussion
In this prospective randomized study, we evaluated the effect of using a standard of care intraoperative fluid therapy as compared to an intraoperative goal-directed fluid therapy combined with a preoperative carbohydrate loading on the incidence of postoperative complications in elderly patients undergoing open gastrointestinal surgery. We observed a significantly faster restoration in bowel function and lower rate of postoperative complications in the GDFT group as compared to the standard of care group.
The primary outcome was initially the length of hospitalization. The reduction in the hospital stay of 1 day was used for simple size calculation. However, during the pilot experiments, we noted that the improvement in the length of hospitalization was lower than that we had been predicted in the original study design and the study may not be finished with the power originally planned. We found that hospitalization duration can be influenced by a range of factors such as patients’ wishes, preoperative physical condition, health care system requirements, and institution-specific differences in treatment regimens. All of these factors thus have the potential to influence the relationship between GDFT and postoperative hospitalization duration. However, we found the interventions may have more effect on the rate of postoperative complications than the length of hospitalization. Therefore, we finally determined the rate of postoperative complications as the primary outcome and the length of hospitalization as one of the secondary outcomes.
For this study, we opted to utilize CI rather than the oxygen delivery index as the key target variable for our GDFT protocol as it can be readily and continuously monitored via radial artery pulse waveform analysis. When arterial oxygen saturation and hemoglobin levels are adequate, CI can serve as an effective measurement to evaluate oxygen supply within tissues and organs [21]. As the use of CI and SVV with the Vigileo/FloTrac monitor has been found to be potentially unreliable in patients with irregular heart rhythms [19, 22] and in patients with poorly controlled intraoperative ventilation [23], we did not include patients without in sinus rhythm in the present study, and all patients were ventilated using a tidal volume of 8 ml/kg.
Excess fluid administration can result in a range of problems including increased rates of postoperative cardiac morbidity, pneumonia, respiratory failure, delayed wound healing, and anastomotic leak as a consequence of intestinal edema in patients undergoing colorectal surgery [8]. As shown in some prior trials [21, 24, 25], our study also found that the GDFT protocol is associated with reductions in the rate of postoperative complications. However, the rates of postoperative complications in some previous studies were higher than in our present analysis. There are two potential reasons for this discrepancy. For one, Mayer et al. [21] selected high-risk patients with at least two risk factors according to risk index of Lee [26] as their experimental subjects. Furthermore, Benes et al. [25] recruited patients that had to meet both operation-related and patient-related high-risk criteria. In contrast, all patients we included were over the age of 65, some of whom may be in the low-to-moderate risk category. In addition, we only monitored the incidence of postoperative complications that occurred during hospitalization, whereas Benes et al. monitored patients for 30 days [25]. Lastly, the lower incidence of postoperative complications in our study may also be related to preoperative oral carbohydrate loading, which may reduce the metabolic and inflammatory response after surgery and improved surgical clinical outcomes [27]. Together, our results suggest that perioperative fluid optimisation reduces the incidence of postoperative complications in elderly patients, rather than only in high-risk patients.
The traditional view considered that the preoperative 8-h food fasting and 4-h liquid fasting accompanied by a decrease in the gastric volume and acidity can reduce the risk of reflux aspiration and misabsorption [28]. However, a long time for preoperative fasting can result in harms to the body, such as loss of water and nutrients, instability of hemodynamics, delayed wound healing, the extension of hospital stays [29]. Therefore, ERAS (Enhanced Recovery After Surgery) guidelines suggested the oral intake of carbohydrate loading 2 h before surgery [30]. In our study, we performed goal-directed fluid administration in combination with preoperative carbohydrate loading as compared to conventional clinical care. The preoperative oral carbohydrate loading combined with intraoperative ERAS may be worth popularizing in the clinical practice owing to their beneficial effect on postoperative outcomes.
Increased arterial lactate levels are closely linked to tissue hypoxia and blood volume insufficiency [31]. In the context of suboptimal fluid management, postoperative restoration of gastrointestinal motility and oral food intake may be delayed due to higher lactate levels. In our study, we did not observe any significant differences in arterial lactate values at analyzed time points between groups. However, we did find that lactate values in the CFT group increased at the end of surgery relative to baseline values. As a systematic review and meta-analysis demonstrated that perioperative GDFT facilitated gastrointestinal functional recovery such as shortening the first time to exhaust and the first time to take oral diet compared with CFT [32]. The slower recovery of bowel function observed in the CFT group may thus be associated with gastrointestinal mucosal hypoperfusion and increase in lactate production [33].
Intraoperative reduced urine output is an independent predictor of AKI (acute kidney injury) after major abdominal surgery [34]. In this study, we found that patients in the GDFT treatment group required reduced crystalloid administration and exhibited reduced urine output relative to patients in the CFT group. However, postoperative AKI occurred in one patient in the GDFT group and two patients in the CFT group. This outcome may be explained by work conducted by Kheterpal et al. [35], as these authors suggested that urine is unreliable when used as a marker of blood volume and renal function, given that it can be influenced by neurohormonal signaling in response to operative stress. The administration of diuretics and vasoactive agents has also been linked to the incidence of acute renal failure. Furthermore, Myles et al. [36] suggested that patients undergoing major abdominal surgery in the restrictive fluid group was associated with a significantly higher rate of postoperative AKI and was not associated with a significantly higher risk of renal-replacement therapy at 90 days than those in the liberal fluid group. However, in our study, we found no statistically differences in the postoperative AKI between the CFT group and the GDFT group. Therefore, the intraoperative goal-directed fluid therapy appeared to be at lower risk of postoperative AKI than the restrictive regimen, which deserves more larger, higher-quality RCTs to further study [37].
There are multiple limitations to the present analysis. For one, in an effort to avoid potential compounds we did not utilize the CI and SVV trending monitor for patients in the CFT group, so we were not able to compare these parameters between groups. Moreover, we were not able to blind investigators intraoperatively to patient treatment strategies. Additionally, the professional level of the attending anesthesiologists and physicians for postoperative treatment in the ICU and hospital wards should be equivalent between groups. Otherwise, it is possible that higher rates of postoperative complications may result from poorer postoperative care in the CFT group. Moreover, although the two interventions including preoperative carbohydrate loading and intraoperative GDFT are the most efficient measures of the ERAS protocols to perform perioperative fluid optimization, it seems that we may not differentiate the beneficial effects between them on postoperative outcomes due to the trial design, which was worthy of further study. Lastly, we did not pre-define standardized discharge criteria in the present study, as discharge may be influenced by a range of factors including patient demands, health care system capacity, and specific treatment regimens used. Thus, the duration of postoperative hospitalization could be also biased.
Conclusion
The results of this study indicate that preoperative carbohydrate loading and intraoperative fluid optimization guided by CI, SVV, and MAP may be associated with more rapid improvements in bowel function and a decreased incidence of postoperative complications in elderly patients undergoing open gastrointestinal surgery. Therefore, the combination of a goal-directed fluid protocol and preoperative carbohydrate loading can achieve some clinical benefits and is worth to be applied for elderly patients undergoing open gastrointestinal surgery.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and supporting data can be obtained from the corresponding author.
Abbreviations
- GDFT:
-
Goal-directed fluid therapy
- CTF:
-
Conventional fluid therapy
- BMI:
-
Body mass index
- ASA:
-
American Society of Anesthesiologists
- MAP:
-
Mean arterial pressure
- HR:
-
Heart rate
- RMB:
-
Renminbi
- ICU:
-
Intensive care unit
- AKI:
-
Acute kidney injury
References
Lohsiriwat V. Outcome of enhanced recovery after surgery (ERAS) for colorectal surgery in early elderly and late elderly patients. Ann Acad Med Singap. 2019;48:347–53.
Evers BM, Townsend CM, Thompson JC. Organ physiology of aging. Surg Clin North Am. 1994;74(1):23–39. https://doi.org/10.1016/S0039-6109(16)46226-2.
Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203(6):865–77. https://doi.org/10.1016/j.jamcollsurg.2006.08.026.
Yeniay O, Tekgul ZT, Okur O, Koroglu N. Unexpectedly prolonged fasting and its consequences on elderly patients undergoing spinal anesthetics. A prospective observational study 1. Acta Cir Bras. 2019;34(3):e201900309.
Nisanevich V, Felsenstein I, Almogy G, Weissman C, Einav S, Matot I. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology. 2005;103(1):25–32. https://doi.org/10.1097/00000542-200507000-00008.
Lobo DN, Bostock KA, Neal KR, Perkins AC, Rowlands BJ, Allison SP. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet. 2002;359(9320):1812–8. https://doi.org/10.1016/S0140-6736(02)08711-1.
Brandstrup B, Tønnesen H, Beier-Holgersen R, Hjortsø E, Ørding H, Lindorff-Larsen K, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens. Ann Surg. 2003;238(5):641–8. https://doi.org/10.1097/01.sla.0000094387.50865.23.
Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002;89(4):622–32. https://doi.org/10.1093/bja/aef220.
Strunden MS, Heckel K, Goetz AE, Reuter DA. Perioperative fluid and volume management: physiological basis, tools and strategies. Ann Intensive Care. 2011;1(1):1–8.
Shin CH, Long DR, McLean D, Grabitz SD, Ladha K, Timm FP, et al. Effects of intraoperative fluid management on postoperative outcomes: a hospital registry study. Ann Surg. 2018;267(6):1084–92. https://doi.org/10.1097/SLA.0000000000002220.
Miller TE, Roche AM, Mythen M. Fluid management and goal-directed therapy as an adjunct to enhanced recovery after surgery (ERAS). Can J Anaesth. 2014;62(2):158–68. https://doi.org/10.1007/s12630-014-0266-y.
Pierrakos C, Velissaris D, Scolletta S, Heenen S, De Backer D, Vincent J-L. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med. 2012;38(3):422–8. https://doi.org/10.1007/s00134-011-2457-0.
Meregalli A, Oliveira RP, Friedman G. Occult hypoperfusion is associated with increased mortality in hemodynamically stable, high-risk, surgical patients. Crit Care. 2004;8(2):R60–5. https://doi.org/10.1186/cc2423.
Tote SP, Grounds RM. Performing perioperative optimization of the high-risk surgical patient. Br J Anaesth. 2006;97(1):4–11. https://doi.org/10.1093/bja/ael102.
Davies SJ, Wilson RJT. Preoperative optimization of the high-risk surgical patient. Br J Anaesth. 2004;93(1):121–8. https://doi.org/10.1093/bja/aeh164.
Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, et al. Effect of a perioperative, cardiac output–guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery. JAMA. 2014;311(21):2181–90. https://doi.org/10.1001/jama.2014.5305.
Gurgel ST, do Nascimento P. Maintaining tissue perfusion in high-risk surgical patients. Anesth Analg. 2011;112(6):1384–91. https://doi.org/10.1213/ANE.0b013e3182055384.
Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112(6):1392–402. https://doi.org/10.1213/ANE.0b013e3181eeaae5.
Lansdorp B, Lemson J, van Putten MJAM, de Keijzer A, van der Hoeven JG, Pickkers P. Dynamic indices do not predict volume responsiveness in routine clinical practice. Br J Anaesth. 2012;108(3):395–401. https://doi.org/10.1093/bja/aer411.
Wakeling HG, McFall MR, Jenkins CS, Woods WGA, Miles WFA, Barclay GR, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth. 2005;95(5):634–42. https://doi.org/10.1093/bja/aei223.
Mayer J, Boldt J, Mengistu AM, Röhm KD, Suttner S. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: a randomized, controlled trial. Crit Care. 2010;14(1):R18. https://doi.org/10.1186/cc8875.
Umgelter A, Reindl W, Schmid RM, Huber W. Is supra-ventricular arrhythmia a reason for the bad performance of the FlowTrac device? Crit Care. 2007;11(1):406. https://doi.org/10.1186/cc5154.
Kubitz JC, Annecke T, Forkl S, Kemming GI, Kronas N, Goetz AE, et al. Validation of pulse contour derived stroke volume variation during modifications of cardiac afterload. Br J Anaesth. 2007;98(5):591–7. https://doi.org/10.1093/bja/aem062.
Salzwedel C, Puig J, Carstens A, Bein B, Molnar Z, Kiss K, et al. Perioperative goal-directed hemodynamic therapy based on radial arterial pulse pressure variation and continuous cardiac index trending reduces postoperative complications after major abdominal surgery: a multi-center, prospective, randomized study. Crit Care. 2013;17(5):R191. https://doi.org/10.1186/cc12885.
Benes J, Chytra I, Altmann P, Hluchy M, Kasal E, Svitak R, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care. 2010;14(3):R118. https://doi.org/10.1186/cc9070.
Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–9. https://doi.org/10.1161/01.CIR.100.10.1043.
Rizvanović N, Nesek Adam V, Čaušević S, Dervišević S, Delibegović S. A randomised controlled study of preoperative oral carbohydrate loading versus fasting in patients undergoing colorectal surgery. Int J Color Dis. 2019;34(9):1551–61. https://doi.org/10.1007/s00384-019-03349-4.
Nygren J. The metabolic effects of fasting and surgery. Best Pract Res Clin Anaesthesiol. 2006;20(3):429–38. https://doi.org/10.1016/j.bpa.2006.02.004.
Nygren J, Thacker J, Carli F, Fearon KC, Norderval S, Lobo DN, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: enhanced recovery after surgery (ERAS) society recommendations. Clin Nutr. 2012;31(6):801–16. https://doi.org/10.1016/j.clnu.2012.08.012.
American Society of Anesthesiologists committee. Practice guidelines for preoperative fasting and the use of pharmacogic agent to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126(3):376–93.
Forget P, Lois F, de Kock M. Goal-directed fluid management based on the pulse Oximeter–derived Pleth variability index reduces lactate levels and improves fluid management. Anesth Analg. 2010;111(4):910–4. https://doi.org/10.1213/ANE.0b013e3181eb624f.
Sun Y, Chai F, Pan C, Romeiser JL, Gan TJ. Effect of perioperative goal-directed hemodynamic therapy on postoperative recovery following major abdominal surgery-a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2017;21(1):141–58. https://doi.org/10.1186/s13054-017-1728-8.
Giglio MT, Marucci M, Testini M, Brienza N. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2009;103(5):637–46. https://doi.org/10.1093/bja/aep279.
Mizota T, Yamamoto Y, Hamada M, Matsukawa S, Shimizu S, Kai S. Intraoperative oliguria predicts acute kidney injury after major abdominal surgery. Br J Anaesth. 2017;119(6):1127–34. https://doi.org/10.1093/bja/aex255.
Kheterpal S, Tremper Kevin K, Englesbe Michael J, O’Reilly M, Shanks Amy M, Fetterman Douglas M, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology. 2007;107(6):892–902. https://doi.org/10.1097/01.anes.0000290588.29668.38.
Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378(24):2263–74. https://doi.org/10.1056/NEJMoa1801601.
Wrzosek A, Jakowicka-Wordliczek J, Zajaczkowska R, et al. Perioperative restrictive versus goal-directed fluid therapy for adults undergoing major non-cardiac surgery. Cochrane Database Syst Rev. 2019;12(12):CD012767.
Acknowledgements
Not applicable.
Funding
This work was Funded by the Sichuan science and technology department research projects, China (No. 2017FZ0042).
Author information
Authors and Affiliations
Contributions
PZ, XCW and DF contributed to the concept and design of the study. XL, MXL and JLM collected and analyzed the data. XL wrote the original draft. PZ, XCW and DF critically reviewed and revised the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committees of the Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital and written informed consent was obtained from all patients.
Consent for publication
Not Applicable.
Competing interests
The authors declare they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Types and definitions of postoperative complications.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, X., Zhang, P., Liu, M.X. et al. Preoperative carbohydrate loading and intraoperative goal-directed fluid therapy for elderly patients undergoing open gastrointestinal surgery: a prospective randomized controlled trial. BMC Anesthesiol 21, 157 (2021). https://doi.org/10.1186/s12871-021-01377-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-021-01377-8