Abstract
Background
LIM (Lineage-11 (LIN-11), Insulin-1 (ISL-1), and Mechanotransduction-3 (MEC-3)) genes belong to a family that hold ubiquitous properties contributing to organ, seed, and pollen development as well as developmental and cellular responses to biotic and abiotic stresses. Lettuce (Lactuca sativa) is a highly consumed vegetable crop susceptible heat stress. High temperatures limit lettuce’s overall yield, quality and marketability. Lettuce LIM genes have not been identified and their role in response to high temperatures is not known. Aiming to identify potential new targets for thermoresilience, we searched for LIM genes in lettuce and compared them with orthologous of several dicotyledons and monocotyledons plant species.
Results
We identified fourteen lettuce LIM genes distributed into eight different subgroups using a genome-wide analysis strategy. Three belonging to DAR (DA means “large” in Chinese) class I, two DAR class II, one in the WLIM1, two in the WLIM2, one in the PLIM1, two in the PLIM2 class, one ßLIM and two δLIMs. No DAR-like were identified in any of the species analyzed including lettuce. Interestingly, unlike other gene families in lettuce which underwent large genome tandem duplications, LIM genes did not increase in number compared to other plant species. The response to heat stress induced a dynamic transcriptional response on LsLIM genes. All heat stress regimes, including night stress, day stress and day and night stress were largely responsible for changes in LIM transcriptional expression.
Conclusions
Our global analysis at the genome level provides a detailed identification of LIM genes in lettuce and other dicotyledonous and monocotyledonous plant species. Gene structure, physical and chemical properties as well as chromosomal location and Cis-regulatory element analysis together with our gene expression analysis under different temperature regimes identified LsWLIM1, LsWLIM2b, LsDAR3 and LsDAR5 as candidate genes that could be used by breeding programs aiming to produce lettuce varieties able to withstand high temperatures.
Similar content being viewed by others
Background
Recent climate data has shown an increase in global temperature at an average rate of 0.08 degrees Celsius per decade since 1880 [1]. However, a more drastic increase has been observed since 1981, when the average rate of increase was calculated at 0.18 °C; more than twice from previous decades [1]. As global temperatures are on the rise, crop production is threatened by the current climate change scenario represented by variable increase of high temperatures [2,3,4,5,6]. These temperature regimes vary in duration and intensity. Major yield losses due to high temperatures have been reported for many crop species [2,3,4,5,6]. Among the plant species that are severely affected by high temperatures is lettuce (Lactuca sativa). Lettuce production is largely based on field and control environment conditions. High temperature during lettuce field production results in less yield and the appearance of disorders including tip-burn, and bolting and other not desirable traits that limit its marketability [7]. Most studies investigating lettuce in response to high temperatures have been focused on the effects on crop physiology, yield, and quality [8,9,10,11,12]. Lettuce that tolerates higher temperatures are prone to less transpiration and produce more sugars in response to warmer environment [8, 10]. At the genomic level, many gene families have shown to be stress responsive. In plants, many gene families are heat-stress responsive [13]. However, the role of LIM genes in response to environmental stresses has not been characterized specially in response to increased temperatures.
LIM proteins (Lineage-11 (LIN-11), insulin-1 (ISL-1), and mechanotransduction-3 (MEC-3)) belong to a small gene family class found in plant and animals [14,15,16]. In plants, LIM proteins have been shown to be involved in a variety of processes during vegetative and reproductive development (See review [14]). LIM proteins have been proposed to protect against biotic stresses by regulating salicylic acid (SA), jasmonic acid (JA) and abscisic acid (ABA) under stress conditions [17]. LIM proteins contain one or several (up to five) double zinc finger motifs, known as LIM domains, which function by mediating protein–protein interactions. LIM domains have been found in a wide variety of eukaryotic proteins of diverse functions [16]. LIM domain-containing transcription factors without the homeodomain have also been described. Specifically, the LIM-only protein LMO2 was found to act as a bridging molecule in assembly of the erythroid DNA-binding complex, while other LIM-domain-containing proteins such as LIM kinases are known to participate in regulation of actin dynamics through phosphorylation of cofilin [18]. In contrast, plants possess two distinct sets of LIM proteins, one that is plant–specific and has been partially functional characterized. Another, cysteine rich protein (CRP)-like that comprises CRPs exhibiting the same overall structure found in animals (i.e., two very similar LIM domains separated by an ≈ 50 amino acid–long inter LIM domain and a relatively short and variable C–terminal domain).
Here, we describe a global identification and analysis of LIM genes under a diverse set of temperature regimes. A comprehensive description of each LIM subclass including gene structure, physical and chemical properties as well as chromosomal location and cis-regulatory element analysis is provided. This study provides a new set of candidate genes that can be used for plant breeders to generate thermotolerant lettuce germplasm.
Results
Genome-wide identification and phylogenetic analysis of LIM genes in lettuce and other plant species
With the aim of identifying potential new targets for thermoresilience, we searched for LIM genes in several dicotyledons and monocotyledons plant species. In the model plant Arabidopsis thaliana, thirteen LIM domain-containing genes have been previously identified: Seven DA1/DAR genes (containing one LIM domain) and six 2LIM or CRP-like genes (containing two LIM domains) [19, 20]. We used these Arabidopsis LIM proteins as query sequences and performed an automated local BLASTP search to identify LIM family members of lettuce (Lactuca sativa), sunflower (Helianthus annuus), tobacco (Nicotiana tabacum), Brachypodium distachyon, barley (Hordeum vulgare), rice (Oryza sativa), maize (Zea mays), sorghum (Sorghum bicolor), and foxtail millet (Setaria italica) (Additional File 1: Table S1). Only LIM proteins that we confirmed to possess the expected numbers of LIM domains or DA1-like domains were retained for further analysis. In lettuce, we identified five DARs gene and nine 2LIM genes.
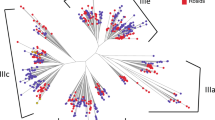
After our genome-wide identification of LIM genes in lettuce, sunflower, tobacco, Arabidopsis, brachypodium, barley, rice, maize, sorghum, and foxtail millet, we performed a phylogenetic analysis aiming to elucidate the evolutionary relationship of LIM genes within the dicotyledons and monocotyledons species used in this study (Fig. 1A; Additional Fig. S1). Our results separate the identified LIM genes into nine subgroups. The WLIM1 subgroup contains members of all the ten plant species. Interestingly, most species only have one WLIM1 gene. However, maize has two and tobacco four (Fig. 1B). Evolutionarily, dicotyledons and monocotyledons WLIM1 genes were grouped in two clear separated clades. Within the dicotyledons, WLIM1a genes from lettuce, sunflower, and tobacco clustered together. A different cluster was formed by the other three WLIM1 genes from tobacco and the Arabidopsis ortholog (Fig. 1A). Similarly, WLIM2 genes clustered based on their monocotyledonous or dicotyledonous origin. However, Arabidopsis WLIM2 diverged from the other dicotyledonous plant species analyzed (Fig. 1A).
Phylogenetic analysis of LIM genes in different plant species. A The amino acid sequences of LIM proteins from A. thaliana (At), B. distachyon (Bd), H. annuus (Ha), H. vulgare (Hv), L. sativa (Ls), N. tabacum (Nt), O. sativa (Os), S. italica (Si), S. bicolor (Sb), and Z. mays (Zm) were aligned and used to construct a phylogenetic tree was using the Maximum Likelihood method and Whelan and Goldman (WAG) model [21] with 1000 Bootstrap replications. Genes belonging to different classes were differently colored. Maroon, WLIM1; olive, PLIM1; green, βLIM1; teal, WLIM2; navy, δLIM2; purple, PLIM2; red, DAR-class I; fuchsia, DAR-class II; blue, DAR-like. B Number of LIM genes identified in diverse plant species. Phylogenetic relationship among different plant species was constructed based on Zhao et al. [22]
δLIM2 forms an interesting subgroup with only genes from lettuce, sunflower, and tobacco. Remarkably, our analysis shows that tobacco δLIM2 genes diverged earlier form lettuce and sunflower δLIMs (Fig. 1A). Within the ßLIM genes, two independent clades were found. One formed by dicotyledons, and another formed by monocotyledons. Although, no ßLIM genes were identified in barley (Fig. 1B).
Developmentally, PLIM1 and PLIM2 are relevant since the name derived from the preferential expression in pollen. Similarly, as δLIM, PLIM1 genes were only found in lettuce, sunflower, and tobacco, which might suggest a unique and shared function between both subgroups. Unlike PLIM1, we identified PLIM2 genes in dicotyledons and monocotyledons (Fig. 1B). Lettuce PLIM2 have the same evolutionary origin than sunflower PLIM2c and PLIM2d. In monocotyledons, a clear irradiation was seen in all the species analyzed (Fig. 1A).
DAR genes are divided in three groups, DAR-Class I, DAR-Class II, and DAR-like genes. DAR-Class I and II genes were found in dicotyledons and monocotyledons. Interestingly, DAR-like was only found in Arabidopsis (Fig. 1B). We identified three lettuce DAR-Class I. LsDAR1 and LsDAR3 cladded with AtDAR1, HaDAR5 and HaDAR7. LsDAR4 formed a separated clade with HaDAR2 (Fig. 1A). We also identified two DAR-Class II which cluster as expected with sunflower genes from the same subgroup. Monocotyledons DAR-Class I genes were more abundant than DAR-Class II and showed a strong conservation as only formed three clusters. Monocotyledons DAR-Class II have a common origin as all the members clustered together (Fig. 1A; Additional Fig. S1).
Subcellular localization and physiochemical properties of LIM proteins
To further characterize the lettuce LIM family, we first predicted their subcellular localization (Fig. 2A) and collected their physiochemical characteristics including molecular weight (MW) (Fig. 2B), exon number (Fig. 2C), theoretical isoelectric point (pI), instability index, aliphatic index, chromosomal coordinates, and hydropathicity (Additional File 2: Table S2). Members of the lettuce LIM family showed similar MWs by subgroup (Fig. 2B). For instance, PLIM2, WLIM1 and WLIM2 average 20 kDa in weight. However, the DAR-related class average 50 kDa in weight in MWs ranging from 30 to 70 kDa (Fig. 2B). Interestingly, while PLIM2, WLIM1 and WLIM2 showed relatively similar number of exons, DAR-related genes showed a larger variability (Fig. 2C). It is noteworthy that even though LIM genes are described as transcription factors, a DAR-Like gene was predicted to be localized in the peroxisome. Similarly, a WLIM2 and a δLIM which were predicted to be localized in the mitochondria and chloroplast, respectively (Fig. 2A).
General characteristics of LIM genes of a set of dicotyledonous and monocotyledonous plant species. A Subcellular localization prediction of LIM proteins. B Molecular weight analysis of LIM proteins of a set of dicotyledonous and monocotyledonous plant species. C Exon number distribution of the LIM genes. D dN/dS ratios of LIM genes. E Mapping of lettuce LIM genes onto the linkage groups using the LinkageMapView package of R software. Genes were colored differently based on their classification
We then quantified the ratio of substitution rates at non-synonymous and synonymous sites in each LIM class of the dicotyledonous and monocotyledonous plant species analyzed to explore the evolutionary pressures on proteins. All the LIM classes in each of the dicotyledonous and monocotyledonous plants showed dN/dS ratio of lower that 0.10 (Fig. 2D). In general, no evidence of positive selection was found on any other member of the LIM genes based on the dN/dS ratio analysis (Fig. 2D; Additional File 3: Table S3), due to the fact that the hallmark signature of positive selection is accepted to be dN/dS > 1 [23].
Distribution of LIM genes in the lettuce genome
Because the lettuce genome underwent whole-genome triplication [24], we explored the impact of this event on the distribution of the LIM gene family. LIM and DAR genes were randomly distributed in eight out of the nine lettuce chromosomes (Fig. 2E). No LIM genes were found in chromosome nine. Chromosome two has the largest number of LIM genes within a single chromosome with three members. Interestingly, no members of the same gene family are localized in the same chromosome but LsDAR1 and LsDAR2 in chromosome three. We did not find any duplication or triplication evidence in the lettuce LIM gene family (Fig. 2E).
LIM genes share syntenic regions with dicotyledons and monocotyledons species
To further characterize the evolutionary relationship of LIM genes, we first constructed a comparative syntenic map using lettuce, sunflower and Arabidopsis (Fig. 3A). These three plant species share large syntenic regions in each of their chromosomes. Interestingly, we found more LIM genes in the syntenic regions shared with Arabidopsis than with sunflower (Fig. 3A). Our syntenic analysis showed that LsßLIM1, sited on chromosome 5, is syntenic to HaßLIM1a localized on chromosome 5 of sunflower. Interestingly, both LsßLIM1 and HaßLIM1a are localized at the end of the chromosome likely closer to telomeric regions (Fig. 3B). The other syntenic LIM gene between sunflowers in lettuce is PLIM2b. While LsPLIM2b sits on chromosome seven, HaPLIM2b is localized on chromosome four (Fig. 3B). In Arabidopsis, all members of the PLIM subgroup, AtPLIM1a, AtPLIM1b, and AtPLIM1c share synteny with LsPLIM2b (Fig. 3C). Similarly, LsWLIM1 and LsWLIM2b are syntenic to AtWLIM1 and AtWLIM2b, respectively (Fig. 3C). We then expanded our syntenic analysis and found that lettuce shares LIM contained-syntenic regions with all monocotyledons and dicotyledons included in this study. However, the collinear regions of lettuce were larger with Arabidopsis, sunflower and tobacco compared to monocotyledon species (Additional Fig. S2).
Syntenic analysis between lettuce LIM genes and other plant species. A Macrosynteny analysis of LIM genes in A. thaliana, L. sativa, and H. annuus. MCScan (python version) was utilized to identify syntenic pairs among A. thaliana, L. sativa, and H. annuus genomes. Gray lines represent all collinear blocks between the genomes of lettuce and other plant species, and green lines highlight syntelogs containing LIM genes. B Microsynteny analysis of LIM genes in L. sativa and H. annuus. Gray lines connect homologs in the collinear regions between lettuce and sunflower genomes, and red lines highlight LIM gene pairs. C Microsynteny analysis of LIM genes in A. thaliana and L. sativa. Gray lines connect homologs in the collinear regions between Arabidopsis and lettuce genomes, and red lines highlight LIM gene pairs
Gene structure of lettuce LIM genes
LIM genes in lettuce have a variable number of exons and gene sizes (Fig. 4A). While LIM genes have a highly conserved gene structure, DAR genes have a more diverse motif composition and have larger proteins (Fig. 4A; Additional Fig. S3). Another interesting feature of the DAR genes compared to the LIM gene family is their variability in the intron phase. Unlike 2LIM genes in lettuce which only contain phase 0 and phase 2 intron, LsDAR genes contain phase 1 introns in addition of phase 0 and 2 intron (Fig. 4A). Motif sequence information is available in Additional Fig. S3 and Additional File 4: Table S4.
Structural analysis and developmental expression of LsLIM genes. A Exon–intron structures of lettuce LIM genes (the left panel) were drawn using Gene Structure Display Server (GSDS) v2.0. Blue boxes, green boxes, and black lines represent untranslated region (UTR), coding sequence (CDS), and intron, respectively. The numbers on each intron represent the intron phase (0, 1, or 2). Conserved motifs in the LIM protein sequences (the right panel) were discovered using the Multiple Em for Motif Elicitation (MEME) Suite tool. Lettuce LIM proteins with 10 identified motifs were shown. B Tissue expression (root, stem, leaf, flower, and seed) of lettuce LIM genes obtained from the LettuceGDB. C Cis-regulatory element analysis of promoter regions of LIM genes in lettuce. 2-kb upstream regions of LsLIM genes were analyzed to discover putative cis-regulatory elements using the PlantCARE tool. Four groups of cis-regulatory elements (stress response, hormone response, metabolism, and circadian rhythm) are displayed. The enrichment of each cis-regulatory element was represented using different colors. White, no occurrence; yellow to red, one to seven
Developmental gene expression of lettuce LIM genes
To better understand the function of lettuce LIM genes, we examined their transcriptional expression in different tissues including roots, stems, leaves, flowers, and seeds using the transcriptome data of different tissues derived from LettuceGDB database [25] (Fig. 4B; Additional File 5: Table S5). Similarly, as reported in other plant species, most PLIM genes were expressed only in floral tissues. However, LsPLIM2b was ubiquitously expressed. Lettuce WLIM were expressed in all tissues analyzed as well as most of the DAR genes. Interestingly, lettuce δLIM genes did not show any expression of any tissue analyzed, while only LsδLIM2a shows a basal expression in flowers. The lack of expression of δLIM genes suggest a tissue specific expression and function in a different tissue than the ones explored in this study.
Cis-regulatory element analysis of lettuce LIM gene promoters
In order to understand how gene expression of LIM genes is regulated, we performed a cis-regulatory element analysis of LIM gene promoters using a 2 kb upstream region of each gene. We particularly focused on cis-regulatory elements related to stress and hormone responses as well as metabolism and circadian rhythm (Fig. 4C; Additional File 6: Table S6). We identified 43 cis-regulatory elements among LIM gene promoters. Interestingly, within the stress responsive cis-elements, box 4, G-Box, GATA-motif, GT1 motif, ARE and w box were highly abundant in all LIM gene families. Interestingly, ARE motifs were found across the promoter of most LIM gene families except WLIM2. A similar enrichment of ARE motif was found on heat shock factor and heat shock proteins in lettuce [26], which suggest a role of this motif in the stress response. When we particularly looked at the WLIMs gene families, TCT-motif and box 4 were the most abundant in the stress responsive category (Fig. 4C). In the hormone stress category, CGTCA-motif, ERE, TGACG motif and ABRE were ubiquitously present in the LIM gene promoters. Interestingly, ABRE2, ABRE3a and ABRE4 motifs were underrepresented in promoter regions of most LIM genes. Within the cis-elements related to metabolism only O2-site was present across most LIM gene promoters. In contrast, MBSI was only present in three genes. Similarly, cis-elements related to circadian rhythm were overrepresented in all LIM gene promoters.
Transcriptional expression analysis of LIM genes under different heat stress regimes
To investigate the role of LIM genes under increased temperatures, we grew three different lettuce types, leaf (‘Bambino’), romaine (‘Manatee’) and butterhead (60,179) under different heat stress regimes. After germination, lettuce plants were placed into one of the following four temperature regimes: Control (25 °C/15 °C), night stress (25 °C/25 °C), day stress (35 °C/20 °C) and day/night stress (35 °C/25 °C). Light conditions were set at 450 ± 5 mmol·m−2·s−1 for an 11-h photoperiod, and CO2 levels were maintained at an optimum 400 ppm. The duration of each controlled heat treatment was dictated by the time from sow until the lettuce began to show preliminary signs of stem elongation (bolting) (Fig. 5A).
Transcriptional expression analysis of lettuce LIM genes under different heat stress regimes. A Phenotype of L. sativa cultivars Bambino (leaf), Manatee (romaine) and 60,179 (butterhead) at maturity after Control (25 °C/15 °C); night stress (25 °C/25 °C); day stress (35 °C/20 °C); day/night stress (35 °C/25 °C) treatments. B The relative expression of lettuce LIM genes. mRNA levels were determined by RT-qPCR analysis and normalized to LsTUB expression levels. Relative expression of treatment (night stress, day stress, and day/night stress) to the control are presented. Asterisks indicate significant differences between treatment and control determined by Student’s t-test (* p < 0.05, ** p < 0.01, *** p < 0.001)
All tissue samples were collected before bolting began, at peak maturity, and used for RT-qPCR analysis to explore the transcriptional responses of lettuce LIM genes under different heat stress regimes. LsWLIM1 did not show significant difference among the temperature regimes (Fig. 5B). LsβLIM1 transcriptional expression changed in all three types of lettuce and in all temperature regimes analyzed. While LsWLIM2a expression was only significantly different in all temperature regimes in ‘Bambino’ and during day stress in 60,179, no differences were found in ‘Manatee’. LsWLIM2b expression changed in almost all temperature treatments. Interestingly, LsWLIM2b expression was downregulated during night stress in ‘Bambino’ and 60,179 but upregulated in ‘Manatee’ (Fig. 5B). LsPLIM2b was not expressed under control conditions, but all heat stress treatments upregulated its expression in all genotypes.
DAR genes also showed variability in gene expression after heat stresses. For instance, LsDAR1 was significantly different during night stress in ‘Bambino’, and during day stress in 60,179 and ‘Manatee’ (Fig. 5B). LsDAR2 showed downregulation in ‘Bambino’ during night and day/night stress. Interestingly, in 60,179 and ‘Manatee’ the low expression was only detected during combined day/night stress. LsDAR2 also showed also mostly downregulation under all temperature regimes but upregulation during day stress in 60,179. LsDAR4 was upregulated in ‘Bambino’ and 60,179 but not in ‘Manatee’ during night and day stress, respectively. LsDAR5 was upregulated in ‘Bambino’, 60,179 and ‘Manatee’ during day stress. LsPLIM1, LsPLIM2a, LSδLIM2a and LSδLIM2a were not included in the analysis as their expression in leaves was not detected (Fig. 4B).
Discussion
Long-term increased temperatures represent one of the most pressing threads for global food production as heat stress results in an overall decrease in plant growth and development and thus yield [2,3,4,5,6]. Current changes in human diets have positioned lettuce as one of the most highly consumed leafy vegetables. Therefore, breeding stress-tolerant lettuce cultivars able to withstand high temperature is one of the strategies needed to reduce the loss caused by abiotic and biotic stresses. Members of the LIM gene family have been identified in response to biotic and abiotic stressors [6, 17, 27, 28]. Lettuce proteins contain LIM domains with a unique double-zinc finger motif able to recognize DNA from downstream genes and thus good candidates for breeding purposes.
Whole-genome duplication did not contribute to the diversification of LIM genes in dicotyledons and monocotyledons
Gene family expansion through gene duplication is a major driving force that generates gene diversification. In lettuce, a whole-genome triplication event occurred in lettuce since its divergence from the grape lineage [24]. While the increase in gene number in some gene families have been attributed to this event, others, for instance, the heat shock protein family 70 did increase due to whole-genome triplication but rather to independent tandem duplication events [26]. The identification of LIM genes has been mostly conducted in dicotyledonous. For instance, 15 members were found in 15 LIM in tomato (Solanum lycopersicum L.) [17], twelve in poplar (Populus trichocarpa) [19] and 21 in alfalfa (Medicago sativa L.) [27]. Our syntenic analysis comparing lettuce to a set of dicotyledonous and monocotyledonous species reveals that, despite the presence of LIM genes in syntenic regions, their numbers did not undergo massive diversification as compared with other gene families [26], as shown by their number and chromosomal location (Fig. 1 and 2; Additional Fig. S2). These results indicate that LIM genes were not only conserved during the domestication of lettuce but are also highly conserved in angiosperms plants.
Cis-elements composition and developmental specific expression
Cis-regulatory sequences located in promoters regulate gene expression and thus playing important roles in development and physiology. We identified that LsLIM genes contain many cis-elements related to stress responses (Fig. 4C). For instance, we identified anaerobic responsive elements (ARE), are bipartite elements consisting of GC and GT motifs, in the promoter regions of LsPLIM genes. AREs are essential for anaerobic induction [29]. Thus, it might be tentative to speculate that this cis-element may help in the resilience and resistance to decay under anaerobic conditions [30]. We also found the ABA responsive element (ABRE) in most of the LsLIM genes. Interestingly, we also identified ABRE2, ABRE3a and ABRE4. However, their expression was not widely distributed in LsLIM genes as the ABRE cis-element (Fig. 4C). The identification of cis-regulatory elements in vegetable crops open opportunities to study and engineer gene regulatory regions able to respond faster to changes in temperature and capable to maintain productivity under stressful conditions.
LIM genes are also strongly associated with their phylogenetic classifications. Three lettuce LIM genes among the fourteen identified in lettuce are only expressed in flowers (Fig. 4B). In some plant species, the PLIM1 and PLIM2 family genes are pollen specific and likely function in pollen growth and development. For instance, Lilium longiflorum, LiLIM play a role in pollen tube growth [31] and in Nicotiana tabacum, NtPLIM1 function in pollen germination and pollen tube growth [32]. Similarly, in sunflower, HaPLIM1a is highly expressed in maturing pollen, suggesting its role in pollen tube growth and pollen germination [19]. The other ten genes were expressed across all the different tissues analyses which suggest the function in different developmental processes.
Transcriptional response of LIM genes to increased temperatures
Transcription factors are fundamental for plant growth, development, and stress responses [13]. The ability of plants to respond to heat stress is strongly associated with the integration of gene regulators of more elaborated responses. Modulation of the transcriptional levels of transcription factor has been used as a strategy to regulate multiple downstream genes to improve stress tolerance [2, 33]. LIM genes have been identified in response to biotic and abiotic stresses in particular to heat stress [6], salinity, drought, and to the Fusarium graminearum infection [14, 17, 28]. We identified LsLIM genes that showed dynamic transcriptional expression under higher temperatures. Out of the fourteen identified LsLIM genes, ten belonging to the WLIM, βLIM, PLIM, DAR subgroups were responsive to at least one of the stress regimes imposed to lettuce plants during development (Fig. 5). Our transcriptional data show that LsWLIM1, LsWLIM2b, LsDAR3 and LsDAR5 participate in the heat stress response process to different heat stress regimes. In other plant species including tomato (Solanum lycopersicum) [17] and alfalfa (Medicago sativa) [27], exposure to high temperatures resulted in the activation of orthologous LIM genes indicating that these genes may have similar physiological functions. Noteworthy, it has been shown that LIM genes interact with b-ZIP transcription factors AREB1, AREB2, and ABF3 to jointly regulate plant response to abiotic stress [34]. Therefore, indicating that LIM genes might be part of conserved regulatory network involved in the stress response.
Conclusions
Our detailed genome-wide analysis on the LIM gene family identified 14 genes in lettuce. Phylogenic analysis of LIM genes in monocotyledons and dicotyledons plant species highlight a close relationship with their orthologous LIM genes within these two clades. We identified reproductive specific members in several plant species as well as lettuce LIM genes responsive to increased temperatures. Our data show that LsWLIM1, LsWLIM2b, LsDAR3 and LsDAR5 as potential candidates for breeding programs aiming to produce lettuce varieties able to withstand higher temperatures under the current climate change scenario.
Methods
Plant material and growth conditions
Seeds from three different lettuce (L. sativa) types, leaf (‘Bambino’), romaine (‘Manatee’) and butterhead (60,179) were obtained from the lettuce breeding program at the University of Florida and pre-germinated for 48 h in a dark growth chamber until radicle emergence was observed [26]. Germinated seeds were transplanted into Pro-Mix® high-porosity (HP) Mycorrhizal soil and moved into light at room temperature. After 15 days following sowing, seedlings were transplanted into 3.78 L trade nursery pots prepared with 15 g 18–6-12 (N-P-K) osmocote and Pro-Mix® high-porosity (HP) Mycorrhizal soil inside a walk-in growth chamber using an average photosynthetic photon flux of 450 ± 5 mmol·m−2·s−1 with eleven-hour photoperiod (07:00 to 18:00).
Heat stress regimes
Lettuce seedlings of similar size were used for each temperature regime. Plants from each cultivar and breeding line were grown for 15 days in the conditions previously described and then transferred inside growth chambers equipped with two multi-level shelves to one of the following heat stress regimes. Temperature regimes (day/night) were divided in 4 groups as follows: HT0: Control 25 °C/15 °C; HT1: 25 °C/25 °C; HT2: 35 °C/20 °C; HT3: 35 °C/25 °C, with day spanning from 07:00 to 18:00 and night from 18:01 to 06:59. Temperatures were gradually adjusted until they reached the set-up temperature. CO2 concentration and the relative humidity were set at 400 ppm and 60%, respectively. Each shelf contained plants distributed using a complete randomize block design.
LIM sequences retrieval and Identification
The amino acid sequences of LIM genes in A. thaliana were collected from public database [19, 20, 35]. To identify LIM genes in other plant species (B. distachyon, H. annuus, H. vulgare, L. sativa, N. tabacum, O. sativa, S. italica, S. bicolor, and Z. mays), retrieved protein sequences were used as queries in BLASTP search against protein sequences of all nine plant species [24, 36,37,38,39,40,41,42,43] using 1·E−10 as an E-value threshold. The presence of the LIM domains (IPR001781) and DA1-like domain (IPR022087) was confirmed using InterProScan [44] and used as a criteria to retaining for further analyses.
Phylogenetic Analysis and classification of LIM genes in plants
Once LIM genes were identified, multiple sequence alignment for 132 LIM genes of ten plant species were performed using the Multiple Sequence Comparison by Log-Expectation (MUSCLE) method [45]. The phylogenetic relationship among these genes was inferred by the Maximum Likelihood method based on Whelan and Goldman (WAG) model [21] with 1000 Bootstrap replications using MEGA11 software [46]. A discrete Gamma distribution was used to model evolutionary rate differences among sites [47]. Gene names and subgroups of LIM genes were assigned based on their phylogenetic relationship with previously reported genes (Additional File 1: Table S1).
Physiochemical properties of LIM proteins
The subcellular localization of LIM proteins from ten plant species were estimated using WoLF PSORT online tool [48]. The physical and chemical parameters including molecular weight, theoretical isoelectric point, instability index, aliphatic index, and hydropathicity of all LIM proteins were computationally predicted using ProtParam tool in the ExPASy server [49].
dN/dS ratio analysis
The protein sequence alignment between LIM genes from ten species and their corresponding Marchantia polymorpha homologs were performed using EMBOSS Water pairwise alignment in the EMBL-EBI data resources [50]. The non-synonymous to synonymous substitution ratio (dN/dS) was calculated based on the sequence alignment using the PAL2NAL server [51].
Chromosomal mapping of LIM genes in lettuce
The chromosomal locations of LIM genes in the lettuce genome were based on annotation gff3 file, which was retrieved from Phytozome, Lactuca sativa V8 genome database [24, 52]. The R package LinkageMapView was used to depict the chromosomal distribution of lettuce LIM genes.
Syntenic analysis of LIM genes
MCScanX tool was utilized to identify collinear blocks among plant genomes from A. thaliana, B. distachyon, H. annuus, H. vulgare, L. sativa, N. tabacum, O. sativa, S. italica, S. bicolor, and Z. mays [53]. Genome-wide syntenic relationship was displayed using the Circos tool [54]. Macro- and microsynteny visualization among Arabidopsis, lettuce, and sunflower genomes was conducted using MCScan (python version) from jcvi [55].
Gene structure, motif, and cis-regulatory element analysis
Exon–intron organization of lettuce LIM genes were illustrated using Gene Structure Display Server (GSDS) v2.0 [56]. Multiple Em for Motif Elicitation (MEME) Suite online tool was used to identify conserved motifs in LIM proteins across ten plant species [57]. The following parameters were used for MEME motif discovery: discovery mode = classic, number of repetitions = any, number of motifs = 10, optimum motif length = 6 to 50 residues. Cis-regulatory elements in the 2-kb upstream regions of LsLIM genes were discovered using the PlantCARE database [58].
Expression analysis of lettuce LIM genes
To analyze the expression patterns of lettuce LIM genes, we obtained RNA-seq data from the lettuce genome across different tissue types from LettuceGDB [25]. The Reads Per Kilobase Million (RPKM) values were transformed by log2(1 + RPKM). The heatmap of each LsLIM gene was visualized using R software.
RNA isolation and RT-qPCR analysis
Total RNA was extracted using Trizol® reagent (Invitrogen) according to the manufacturer’s instruction. DNase treatment was performed using DNase I (Thermo Scientific). First-strand cDNA synthesis was conducted using iScript™ cDNA Synthesis Kit (Bio-Rad). Quantitative real-time PCR was performed using AzuraView™ GreenFast qPCR Blue Mix LR reagent (Azura Genomics) in the QuantStudio™ 3 Real-Time PCR System (Applied Biosystems) on a 96-well reaction plate. Reaction conditions consisted of pre-denaturation step at 95 °C for 5 min and 45 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. The melting curve analysis was followed using 95 °C for 15 s, 60 °C for 60 s, and 95 °C for 15 s [59]. Relative transcript levels were normalized using LsTUB gene [26] and calculated based on 2−ΔΔCt method [60]. Student’s t-test was used to assess the significant differences between control condition and stress regime within the same genotypes. Primers used in RT-qPCR analysis are listed in Additional Table 7: Table S7.
Statistical analysis
R software/environment was used for the statistical analyses of the RT-qPCR data. We used data from three independent experiments. Three biological replicates with three technical repetitions per biological replicate were used. Student’s t-test was used to compare gene expression among the different heat stress regimes and the control conditions. Differences in means were considered significant at p-value < 0.05.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
NOAA National Centers for Environmental Information, Monthly Global Climate Report for Annual 2023. Published online January 2024. Retrieved on January 25, 2024 from https://www.ncei.noaa.gov/access/monitoring/monthly-report/global/202313.
Chen C, Begcy K, Liu K, Folsom JJ, Wang Z, Zhang C, et al. Heat stress yields a unique MADS box transcription factor in determining seed size and thermal sensitivity. Plant Physiol. 2016;171:606–22.
Folsom JJ, Begcy K, Hao X, Wang D, Walia H. Rice fertilization-Independent Endosperm1 regulates seed size under heat stress by controlling early endosperm development. Plant Physiol. 2014;165:238–48.
Begcy K, Nosenko T, Zhou L-Z, Fragner L, Weckwerth W, Dresselhaus T. Male Sterility in Maize after Transient Heat Stress during the Tetrad Stage of Pollen Development. Plant Physiol. 2019;181:683–700.
Nadakuduti SS, Laforest LC, Tachev M, Decker AN, Egesa AO, Shirazi AS, et al. Heat stress during seed development leads to impaired physiological function and plasticity in seed oil accumulation in Camelina sativa. Front Plant Sci. 2023;14:1284573.
Li X, Bruckmann A, Dresselhaus T, Begcy K. Heat stress at the bicellular stage inhibits sperm cell development and transport into pollen tubes. Plant Physiol. 2024;195:2111–28.
Hao J, Yang J, Liu X, Pan G, Li Y, Zhang X, et al. Molecular basis of high temperature-induced bolting in lettuce revealed by multi-omics analysis. BMC Genomics. 2022;23:580.
Sublett W, Barickman T, Sams C. Effects of Elevated Temperature and Potassium on Biomass and Quality of Dark Red ‘Lollo Rosso’ Lettuce. Horticulturae. 2018;4:11.
Carotti L, Graamans L, Puksic F, Butturini M, Meinen E, Heuvelink E, et al. Plant Factories Are Heating Up: Hunting for the Best Combination of Light Intensity, Air Temperature and Root-Zone Temperature in Lettuce Production. Front Plant Sci. 2021;11:592171.
Zhao X, Sui X, Zhao L, Gao X, Wang J, Wen X, et al. Morphological and physiological response mechanism of lettuce (Lactuca Sativa L.) to consecutive heat stress. Sci Hortic. 2022;301:111112.
Joukhadar I, Tonnessen B, Coon D, Walker S. Performance of Heat-tolerant Lettuce Cultivars in Southern New Mexico in 2020–21. HortTechnology. 2023;33:313–6.
Chase K, Belisle C, Ahlawat Y, Yu F, Sargent S, Sandoya G, et al. Examining preharvest genetic and morphological factors contributing to lettuce (Lactuca sativa L.) shelf-life. Sci Rep. 2024;14:6618.
Huang L-Z, Zhou M, Ding Y-F, Zhu C. Gene Networks Involved in Plant Heat Stress Response and Tolerance. Int J Mol Sci. 2022;23:11970.
Srivastava V, Verma PK. The plant LIM proteins: unlocking the hidden attractions. Planta. 2017;246:365–75.
Smith MA, Hoffman LM, Beckerle MC. LIM proteins in actin cytoskeleton mechanoresponse. Trends Cell Biol. 2014;24:575–83.
Winkelman JD, Anderson CA, Suarez C, Kovar DR, Gardel ML. Evolutionarily diverse LIM domain-containing proteins bind stressed actin filaments through a conserved mechanism. Proc Natl Acad Sci. 2020;117:25532–42.
Khatun K, Robin AHK, Park J-I, Ahmed NU, Kim CK, Lim K-B, et al. Genome-wide identification, characterization and expression profiling of LIM family genes in Solanum lycopersicum L. Plant Physiol Biochem. 2016;108:177–90.
Wadman IA. The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins. EMBO J. 1997;16:3145–57.
Arnaud D, Déjardin A, Leplé J-C, Lesage-Descauses M-C, Pilate G. Genome-Wide Analysis of LIM Gene Family in Populus trichocarpa, Arabidopsis thaliana, and Oryza sativa. DNA Res. 2007;14:103–16.
Li Y, Zheng L, Corke F, Smith C, Bevan MW. Control of final seed and organ size by the DA1 gene family in Arabidopsis thaliana. Genes Dev. 2008;22:1331–6.
Whelan S, Goldman N. A General Empirical Model of Protein Evolution Derived from Multiple Protein Families Using a Maximum-Likelihood Approach. Mol Biol Evol. 2001;18:691–9.
Zhao T, Zwaenepoel A, Xue J-Y, Kao S-M, Li Z, Schranz ME, et al. Whole-genome microsynteny-based phylogeny of angiosperms. Nat Commun. 2021;12:3498.
Kryazhimskiy S, Plotkin JB. The Population Genetics of dN/dS. PLoS Genet. 2008;4:e1000304.
Reyes-Chin-Wo S, Wang Z, Yang X, Kozik A, Arikit S, Song C, et al. Genome assembly with in vitro proximity ligation data and whole-genome triplication in lettuce. Nat Commun. 2017;8:14953.
Guo Z, Li B, Du J, Shen F, Zhao Y, Deng Y, et al. LettuceGDB: The community database for lettuce genetics and omics. Plant Commun. 2023;4:100425.
Kim T, Samraj S, Jiménez J, Gómez C, Liu T, Begcy K. Genome-wide identification of heat shock factors and heat shock proteins in response to UV and high intensity light stress in lettuce. BMC Plant Biol. 2021;21:185.
Nian L, Liu X, Yang Y, Zhu X, Yi X, Haider FU. Genome-wide identification, phylogenetic, and expression analysis under abiotic stress conditions of LIM gene family in Medicago sativa L. PLoS ONE. 2021;16:e0252213.
Li Y, Liu X, Xiao Y, Wen Y, Li K, Ma Z, et al. Genome-wide characterization and function analysis uncovered roles of wheat LIMs in responding to adverse stresses and TaLIM8-4D function as a susceptible gene. Plant Genome. 2022;15:e20246.
Kaur A, Pati PK, Pati AM, Nagpal AK. In-silico analysis of cis-acting regulatory elements of pathogenesis-related proteins of Arabidopsis thaliana and Oryza sativa. PLoS ONE. 2017;12:e0184523.
Ellison JC. Long-term retrospection on mangrove development using sediment cores and pollen analysis: A review. Aquat Bot. 2008;89:93–104.
Wang B-J, Hsu Y-F, Chen Y-C, Wang C-S. Characterization of a lily anther-specific gene encoding cytoskeleton-binding glycoproteins and overexpression of the gene causes severe inhibition of pollen tube growth. Planta. 2014;240:525–37.
Sweetman J, Spurr C, Eliasson Å, Gass N, Steinmetz A, Twell D. Isolation and characterisation of two pollen-specific LIM domain protein cDNAs from Nicotiana tabacum. Sex Plant Reprod. 2000;12:339–45.
Begcy K, Mariano ED, Lembke CG, Zingaretti SM, Souza GM, Araújo P, et al. Overexpression of an evolutionarily conserved drought-responsive sugarcane gene enhances salinity and drought resilience. Ann Bot. 2019;124:691–700.
Kim J-S, Mizoi J, Yoshida T, Fujita Y, Nakajima J, Ohori T, et al. An ABRE promoter sequence is involved in osmotic stress-responsive expression of the DREB2A gene, which encodes a transcription factor regulating drought-inducible genes in Arabidopsis. Plant Cell Physiol. 2011;52:2136–46.
Cheng C, Krishnakumar V, Chan AP, Thibaud-Nissen F, Schobel S, Town CD. Araport11: a complete reannotation of the Arabidopsis thaliana reference genome. Plant J. 2017;89:789–804.
The International Brachypodium Initiative. Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature. 2010;463:763–8.
Badouin H, Gouzy J, Grassa CJ, Murat F, Staton SE, Cottret L, et al. The sunflower genome provides insights into oil metabolism, flowering and Asterid evolution. Nature. 2017;546:148–52.
Beier S, Himmelbach A, Colmsee C, Zhang X-Q, Barrero RA, Zhang Q, et al. Construction of a map-based reference genome sequence for barley. Hordeum vulgare L Sci Data. 2017;4:170044.
Edwards KD, Fernandez-Pozo N, Drake-Stowe K, Humphry M, Evans AD, Bombarely A, et al. A reference genome for Nicotiana tabacum enables map-based cloning of homeologous loci implicated in nitrogen utilization efficiency. BMC Genomics. 2017;18:448.
Ouyang S, Zhu W, Hamilton J, Lin H, Campbell M, Childs K, et al. The TIGR rice genome annotation resource: improvements and new features. Nucleic Acids Res. 2007;35 Database:D883-887.
Bennetzen JL, Schmutz J, Wang H, Percifield R, Hawkins J, Pontaroli AC, et al. Reference genome sequence of the model plant Setaria. Nat Biotechnol. 2012;30:555–61.
McCormick RF, Truong SK, Sreedasyam A, Jenkins J, Shu S, Sims D, et al. The Sorghum bicolor reference genome: improved assembly, gene annotations, a transcriptome atlas, and signatures of genome organization. Plant J. 2018;93:338–54.
Jiao Y, Peluso P, Shi J, Liang T, Stitzer MC, Wang B, et al. Improved maize reference genome with single-molecule technologies. Nature. 2017;546:524–7.
Jones P, Binns D, Chang H-Y, Fraser M, Li W, McAnulla C, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30:1236–40.
Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7.
Tamura K, Stecher G, Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol. 2021;38:3022–7.
Kim T, Dias FO, Gentile A, Menossi M, Begcy K. ScRpb4, Encoding an RNA Polymerase Subunit from Sugarcane, Is Ubiquitously Expressed and Resilient to Changes in Response to Stress Conditions. Agriculture. 2022;12:81.
Horton P, Park K-J, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, et al. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35 Web Server:W585–7.
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, et al. Protein Identification and Analysis Tools on the ExPASy Server. In: Walker JM, editor., et al., The Proteomics Protocols Handbook. Totowa, NJ: Humana Press; 2005. p. 571–607.
Madeira F, Pearce M, Tivey ARN, Basutkar P, Lee J, Edbali O, et al. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022;50:W276-279.
Suyama M, Torrents D, Bork P. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006;34 Web Server:W609–12.
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012;40:D1178-1186.
Wang Y, Tang H, DeBarry JD, Tan X, Li J, Wang X, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49–e49.
Krzywinski M, Schein J, Birol İ, Connors J, Gascoyne R, Horsman D, et al. Circos: An information aesthetic for comparative genomics. Genome Res. 2009;19:1639–45.
Tang H, Bowers JE, Wang X, Ming R, Alam M, Paterson AH. Synteny and Collinearity in Plant Genomes. Science. 2008;320:486–8.
Hu B, Jin J, Guo A-Y, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31:1296–7.
Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37 Web Server:W202-208.
Lescot M. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–7.
Preciado J, Begcy K, Liu T. The Arabidopsis HDZIP class II transcription factor ABA INSENSITIVE TO GROWTH 1 functions in leaf development. J Exp Bot. 2022;73:1978–91.
Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–8.
Acknowledgements
Not applicable.
IUCN policy statement
Lettuce seeds were obtained from the lettuce breeding program at the University of Florida. Our experimental research on lettuce plants, including the collection of plant material, complied with relevant institutional, national, and international guidelines and legislation. Plant species at risk of extinction were not used in this study.
Funding
This work was supported by the Competitive Seed Grant Research Initiative (Grant No. 00129910) from the College of Agricultural and Life Sciences at UF, the Hatch project FLA-ENH-005853 from the United States Department of Agriculture and the Global Food Systems Institute to K.B.
Author information
Authors and Affiliations
Contributions
K.B. conceived the projects and experiments. T.K. and C.Q performed the bioinformatics analysis. H.M performed the controlled environment experiments. T.K., A.O.E., and C.Q performed the molecular analysis. G.S. provided plant material. T.K, A.O.E, C.Q and K.B. analyzed the data. T.K. prepared the figures. K.B. wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12870_2024_5466_MOESM8_ESM.pdf
Additional file 8: Supplemental Figure S1. Phylogenetic analysis of LIM genes from ten plant species. The protein sequences from A. thaliana (At), B. distachyon (Bd), H. annuus (Ha), H. vulgare (Hv), L. sativa (Ls), N. tabacum (Nt), O. sativa (Os), S. italica (Si), S. bicolor (Sb), and Z. mays (Zm) were utilized for multiple sequence alignment and phylogenetic tree construction. Phylogenetic relationship was inferred using the Maximum Likelihood method, employing Whelan and Goldman (WAG) model and a discrete Gamma distribution. The numbers at the nodes represent the percentage of bootstrap values, based on 1000 replications. Additional Figure S2. Syntenic analysis of LIM genes of ten plant species. Syntelog anchors among different plant genomes of A. thaliana (Ath), B. distachyon (Bdi), H. annuus (Han), H. vulgare (Hvu), L. sativa (Lsa), N. tabacum (Nta), O. sativa (Osa), S. italica (Sit), S. bicolor (Sbi), and Z. mays (Zma) were identified using MCScanX [53]. The syntenic relationships among genomes were depicted using Circos [54]. Gray, orange, and red lines indicate all syntelogs, syntelogs among LIM genes, and LsLIM-containing syntelogs, respectively. Additional Figure S3. Conserved motifs of LIM proteins discovered using MEME analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, T., Egesa, A., Qin, C. et al. Global identification of LIM genes in response to different heat stress regimes in Lactuca sativa. BMC Plant Biol 24, 751 (2024). https://doi.org/10.1186/s12870-024-05466-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05466-x