Abstract
Background
Rhododendron pudingense, firstly discovered in Puding county of Guizhou province in 2020, have adapted to living in rocky fissure habitat, which has important ornamental and economic values. However, the genetic diversity and population structure of this species have been rarely described, which seriously affects the collection and protection of wild germplasm resources.
Results
In the present study, 13 pairs of primers for polymorphic microsatellite were used to investigate the genetic diversity of 65 R. pudingense accessions from six different geographic populations. A total of 254 alleles (Na) were obtained with an average of 19.5 alleles per locus. The average values of polymorphic information content (PIC), observed heterozygosity (Ho), and expected heterozygosity (He) were 0.8826, 0.4501, and 0.8993, respectively, These results indicate that the microsatellite primers adopted demonstrate good polymorphism, and the R. pudingense exhibits a high level of genetic diversity at the species level. The average genetic differentiation coefficient (Fst) was 0.1325, suggested that moderate divergence occurred in R. pudingense populations. The average values of genetic differentiation coefficient and gene flow among populations were 0.1165 and 3.1281, respectively. The analysis of molecular variance (AMOVA) indicated that most of the population differences (88%) were attributed to within-population variation. The PCoA results are consistent with the findings of the UPGMA clustering analysis, supporting the conclusion that the six populations of R. pudingense can be clearly grouped into two separate clusters. Based on Mantel analysis, we speculate that the PD population may have migrated from WM-1 and WM-2. Therefore, it is advised to protect the natural habitat of R. pudingense in situ as much as possible, in order to maximize the preservation of its genetic diversity.
Conclusions
This is the first comprehensive analysis of genetic diversity and population structure of R. pudingense in Guizhou province. The research results revealed the high genetic diversity and moderate population diferentiation in this horticulture plant. This study provide a theoretical basis for the conservation of wild resources of the R. pudingense and lay the foundation for the breeding or cultivation of this new species.
Similar content being viewed by others
Background
Rhododendron pudingense, commonly called Puding Azalea, is an evergreen shrub belonging to the Rhododendron genus in the Ericaceae family. R. pudingense was first found in Puding county of Guizhou province in 2020, which possess a low number of individuals dispersedly distributed in small habitat areas [1].
Although previous studies have revealed that the Rhododendron species prefer to inhabit acidic soil, R. pudingense as a unique species in Guizhou can thrives in alkaline karst soils. R. pudingense plants were mainly distributed in four counties, such as Puding, Qinglong, Wangmo, and Zhenning [2]. Although R. pudingense have been discovered in multiple locations, they live in alkaline karst crevices, and seed reproduction rate of R. pudingense communities is low, with difficulties in the survival of seedlings. Based on the IUCN Red List criteria and standards (version 3.1), this species is defined as endangered (EN) and requires urgent research and conservation efforts [1]. Therefore, it is of utmost importance to protect this species, as both genetic resource conservation and plant breeding require assessment of the genetic diversity and outcomes of endangered species [3, 4]. To date, potential habitats, population structure, spatial distribution patterns, soil microbial community structure, functional diversity, and enzyme activities of R. pudingense plants have been reported in previous researchs [2, 5,6,7], the genetic diversity and population structure of R. pudingense population were still unclear, which may cause it difficult to plan conservation strategies for this R. pudingense.
Genetic diversity is an aspect of biodiversity that helps us understand conservation strategies for rare and endangered species [8]. For example, scholars have demonstrated through the summarization of molecular marker methods in studies on numerous species of angiosperms that endangered species possess lower genetic diversity compared to non-endangered species, and the loss of genetic diversity can cause loss of adaptive responses and evolutionary potential to complex environmental changes [9,10,11]. Studying the genetic diversity of endangered or rare and endemic species at the level of individuals or ecosystems can not only help understand the species’ evolutionary history and mechanisms for species endangerment, but also lay the foundation for developing scientifically effective protection measures. Furthermore, such studies can provide important guidance for the conservation of large-scale biodiversity and adaptation strategies for evolution. Therefore, quantifying genetic variation and diversity patterns within and between different populations is of paramount importance for the conservation and management planning of small population species.
Plant population genetics plays a crucial role in promoting plant breeding and conservation strategies. Several molecular marker methods, including microsatellite markers, also known as simple sequence repeats (SSR), Random Amplified Polymorphic DNA (RAPD), Amplified Fragment Length Polymorphism (AFLP), Inter Simple Sequence Repeat (ISSR), and Restriction Fragment Length Polymorphism (RFLP), were used to analyze genetic diversity in plants [12,13,14,15]. For genetic diversity evaluation, microsatellite markers have been widely applied in many plant species to evaluate genetic diversity, to construct genetic maps, and to determine species lineages due to four advantages: (1) high abundance, genome-wide coverage, and high polymorphism level; (2) high information content provided by multiple alleles at each locus; (3) Mendelian inheritance and co-dominance; (4) primers designed for each locus, facilitating collaboration and exchange between different laboratories. Microsatellite markers have been extensively used in genetic mapping, marker-assisted selection, variety identification, pedigree analysis, estimation of genetic distances among populations, and studies on evolution and genetic diversity [16]. In the present study, microsatellite markers were employed to analyze the genetic diversity indices, genetic differentiation, gene flow, and population structure of R. pudingense population collected from four different countries in Guizhou province. Our results aimed: (1) to systematically reveal genetic diversity and population structure of R. pudingense plants located in four countries in Guizhou province; (2) to explore the reasons of the current genetic patterns of this species; (3) to provide references for the conservation and breeding of germplasm resources of R. pudingense.
Results
Genetic charaterisitc of 13 microsatellite markers
In the present study, 13 primer pairs were selected for detection of genetic diversity of R. pudingense populations (Table 1). And a total of 254 alleles at 13 polymorphic microsatellite loci were amplified across 65 individual plants from 4 natural populations (Table 2). The number of alleles per locus ranged from 10 (RDW38) to 27 (RDW46 and RDE11) with an average of 19.5. The total number of effective alleles (Ne) across all loci in this study was 139.6401, and the number of effective alleles per locus ranged from 4.1711 (RDW38) to 15.6260 (RDW46) with an average of 10.7415. The Shannon diversity index (I) ranged from 1.6605 (RDW38) to 2.9735 (RDW46) with an average of 2.5597. The polymorphic information content (PIC) ranged from 0.7241 (RDW38) to 0.9324 (RDW46), with an average of 0.8826, suggested that each locus exhibited high polymorphism (PIC ≥ 0.5). The observed heterozygosity (Ho) ranged from 0.2787 (RDW31) to 0.6667 (R557) with an average of 0.4501. The expected heterozygosity (He) ranged from 0.7662 (RDW38) to 0.9435 (RDW46) with an average of 0.8993.
In addition, the within-population inbreeding coefficient (Fis) ranged from 0.1784 (R557) to 0.6036 (RDW31), with an average of 0.4169. The total inbreeding coefficient (Fit) ranged from 0.2424 (R557) to 0.6661 (RDW31), with an average of 0.4942. The genetic differentiation coefficient (Fst) ranged from 0.0779 (R557) to 0.2150 (N8), with an average of 0.1325, indicating that only 13.25% of the genetic variation occurred between populations, while the remaining 86.75% occurred within populations, suggesting the presence of inbreeding among the selected genetic loci. The gene flow (Nm) ranged from 0.9127 (N8) to 2.9589 (R557), with an average of 1.6366. Among them, N8 (Nm=0.9127) was less than 1, indicating that the population at this locus is more prone to genetic drift and differentiation (Table 2).
Genetic diversity of populations in R. pudingense
To further investigate the genetic diversity among different population, we performed comparative analysis the genetic diversity of six populations based on microsatellite markers (Table 3). Our results showed that the number of allele loci in the six populations ranged from 5.9231 (PD) to 8.2308 (WM-3) with an average of 7.5. The range of effective alleles (Ne) ranged from 3.8988 (PD) to 6.0061 (WM-3) with an average of 5.1477. This result indicated differences in genetic diversity among different populations. The values of the Shannon diversity index (I) range from 1.4842 (PD) to 1.8751 (WM-3), with an average of 1.7414, and the differences between the populations are small, indicating relatively similar levels of genetic diversity among the populations. The observed heterozygosity (Ho) ranges from 0.3706 (WM-2) to 0.5058 (ZN), with an average of 0.4515. The expected heterozygosity (He) ranges from 0.7188 (PD) to 0.8073 (WM-3), with an average of 0.7808, this suggests that the genetic diversity of different populations R. pudingense in this study is relatively rich. Furthermore, Ho is lower than He in all populations, indicating a certain degree of heterozygote deficiency within the populations. Combining the expected heterozygosity and Shannon diversity index, WM-3 population has the highest genetic diversity, while PD has the lowest genetic diversity. The values of fixation index (F) range from 0.3622 (QL) to 0.5466 (WM-2), all greater than 0.25, and with an average of 0.4258, indicating the presence of inbreeding within different populations of R. pudingense, and the and the inbreeding phenomenon in the WM-2 population is more severe, and more prone to genetic differentiation compared to other populations. In addition, we also tested whether 13 pairs of microsatellite loci followed the Hardy-Weinberg equilibrium in the central distribution of the R. pudingenseg. The results showed that most loci deviated from genetic equilibrium, indicating that R. pudingense populations was not in Hardy-Weinberg equilibrium. In the linkage disequilibrium test, no significant linkage disequilibrium was found between any pair of loci. Therefore, the reason why the natural population of R. pudingense does not follow the random mating pattern may be due to the existence of null alleles at loci, leading to insufficient heterozygote individuals (Table 4).
Genetic differentiation and gene flow among populations in R. pudingense
Analysis of molecular variance showed that that 88% of the genetic variation mainly come from between-individual variations within the population variation, while only 12% is due to between-population variation (Table 5).
And the values of the genetic differentiation between different populations ranges from 0.0125 (between WM-1 and WM-2) to 0.1830 (between QL and ZN), with an average of 0.1165 (Table 6), which is consistent with the result of AMOVA, indicating that the genetic variation in R. pudingense populations is mainly caused by between-individual variation within the populations. Moreover, the gene flow associated with genetic differentiation ranges from 1.1162 (between ZN and QL) to 19.7687 (between WM-1 and WM-2) among different populations, with an average of 3.1281 (Table 6), indicating that there is significant gene flow between R. pudingense populations. This results further revealed that genetic differentiation among populations is inhibited. This is also a reason for the relatively low genetic differentiation in the populations of R. pudingense.
To further evaluate the genetic divergence between populations, Popgen32 software was used to calculate the genetic distance between R. pudingense populations (Table 7). The genetic distance between six R. pudingense populations ranges from 0.4315 (between WM-1 and WM-2) to 1.6190 (between QL and ZN), with an average of 1.0410, and the coefficient of genetic similarity ranges from 0.1981 (between QL and ZN) to 0.6495 (between WM-1 and WM-2), with an average of 0.3714, indicating that the farthest genetic relationships with QL and ZN, while between WM-1 and WM-2 are the closest.
Population structure and genetic relationships
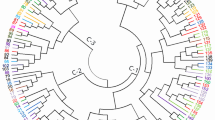
The result revealed that maximum value of delta K was at K = 5, thus, the all six populations in this study can be divided into five genetic clusters (Fig. 1; Table S1). The genetic composition of Zhenning (ZN) contained 10 individuals, mainly originates from genetic cluster 5 (red squares). The genetic composition of Puding (PD) contained 11 individuals, mainly originates from genetic cluster 4 (yellow squares).The genetic composition of Qinglong (QL) contained 13 individuals, mainly originates from genetic cluster 3 (green squares), and the genetic composition of Wangmo (WM) contained 31 individuals, among which the genetic composition of WM-3 includes 10 individuals, mainly from originates cluster 2 (blue square), and the genetic compositions of WM-1 and WM-2 include 10 and 11 individuals respectively, mainly from originates cluster 1 (purple square)(Fig. 1b). Although most individuals are assigned to different genetic clusters, it should be noted that the populations are not completely independent from each other. To further assess the genetic relationships among six R. pudingense populations, principal coordinate analysis (PCoA) was performed based on Nei’s genetic distance for the 6 populations (Figs. 2) and 65 plant samples (Fig. S1). The cumulative variance percentage of the first three axes was 17.86% (Axis 1–7.88%, Axis 2–5.12%, Axis 3–4.86%) (Fig. 2). The closer the distance between two populations in the graph, the smaller genetic background differences between them. And the results of the PCoA were consistent with those of the structure analysis and supported the UPGMA clustered tree, as described below.
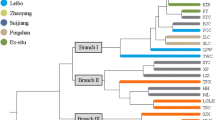
The UPGMA dendrogram was constructed from Nei’s genetic distance values and is an accurate reflection of the genetic relationships among and within populations (Fig. 3). The UPGMA dendrogram indicated that the six R. pudingense populations could be divided into two major clusters (Fig. 3). This clustering result is consistent with the results of genetic similarity and genetic distance between populations.
A Mantel test conducted for R. pudingense indicated correlation between genetic distance and geographic distance among populations was not significant (r = 0.1685, P = 0.210) (Fig. 4), this suggests that genetic differentiation among R. pudingense was not caused by geographic distance .
Discussion
The magnitude of genetic diversity is a product of long-term biological evolution and serves as a prerequisite for the survival, development, and evolution of organisms. Maintaining genetic diversity within natural populations is crucial for ensuring the continued survival, fitness, and evolutionary potential of a species [10]. Traditionally, diversity has been evaluated through analysis of morphological and physiological traits in plants. However, due to the instability of these traits under different environmental conditions, and as the size of plant populations decreases, loss of genetic diversity reduces their ability to adapt to changes in the environment, with inbreeding and reduced fitness inevitable consequences for most species.In recent years, the development of molecular DNA molecular marker methods has advanced our understanding of genetic resources [17,18,19,20,21]. And among a rang of DNA molecular marker techniques, microsatellite molecular marker technology is widely regarded as the most practical method in population genetic studies due to its ability to measure codominant alleles and exhibit a high level of polymorphism [22]. Several studies have been conducted on the genetic diversity of the Rhododendron genus using microsatellite technology [23,24,25,26]. This study is the first to investigate the genetic diversity and population structure of R. pudingense through molecular marker technology, it is important for the protection, management and understanding of their genetic relationships.
Genetic diversity of populations in R. pudingense
Differences in genetic diversity are influenced by several factors, such as unique evolutionary history, distribution patterns, and human-induced disturbances. Higher genetic diversity or greater genetic variation indicates a stronger adaptive capacity of organisms to environmental changes. Understanding the magnitude, spatial and temporal distribution, and relationship with environmental conditions of within-species variation helps us further conserve rare or endangered species [27].
In this study, microsatellite marker was used to amplify 65 samples from the four populations of R. pudingense using 13 primer pairs. A total of 254 allele loci were amplified, and the Fis and Fit values for the selected primers were both greater than 0, indicating a low level of hybridization among the 13 microsatellite loci [28, 29], and that indicating high genetic diversity within the population. Additionally, the average Shannon’s information index (I) for the 13 primers was 2.5597, and the average expected heterozygosity (He) was 0.8993, the average eobserved heterozygosity (Ho) was 0.4501. In general, a higher genetic diversity index indicates a greater diversification of the genome within a population and a greater amount of genetic variation among individuals [10]. The results of this study are higher than the findings of [22] in terms of genetic diversity using microsatellite markers (Ho=0.450, He=0.899, I = 2.560). These results indicate the high polymorphism of the microsatellite marker technology and support the feasibility of using microsatellite markers to investigate the genetic diversity of R. pudingense.
Indices such as polymorphic information content (I), observed heterozygosity (Ho) [30], expected heterozygosity (He) [31], and fixation index (F) [32] are commonly used to describe population genetic diversity. The expected heterozygosity (He) values for the populations of R. pudingense ranged from 0.7188 to 0.8073, and the observed heterozygosity (Ho) values ranged from 0.3706 to 0.5058. In all six populations, He was greater than Ho, and F were greater than 0, this indicates the presence of some degree of inbreeding in the population of R. pudingense. And these results are consistent with previous studies on the genetic structure of endemic plants by using microsatellite technology [24, 25, 30]. Based on these findings, we speculate that there is a high level of inbreeding within the populations of R. pudingense, which increases population homozygosity and exacerbates genetic differentiation among populations. According to the principles of conservation genetics, inbreeding can reduce the survival and reproductive capacity of a species, and leading to low genetic diversity, that may cause a population to decline [10, 33]. Therefore, when conserving R. pudingense, the phenomenon of population inbreeding should also be taken into consideration.
Generally, a small population size, narrow distribution range, large spatial distance and high altitude between populations can restrict pollination between groups, leading to self-fertilization or inbreeding and even reducing genetic diversity [34, 35]. In this study, the PD population is situated at an altitude of approximately 1500 m, where they face the challenges of a harsh mountain environment. This high-altitude region imposes significant selection pressure on the PD population. Moreover, the lack of birds and animals that aid in seed dispersal makes it difficult for them to engage in gene exchange with the outside world [34]. Additionally, the high humidity in the mountain air hinders pollen from being carried long distances by the wind, further limiting internal genetic exchange within the population. Consequently, these factors contribute to a reduced level of genetic diversity within the PD population. The result also suggest a diminished capacity of the population to adapt to evolving environments, consequently elevating the risk of species extinction [10, 19].
Genetic differentiation and gene flow among populations in R. pudingense
The genetic differentiation coefficient (Fst) and gene flow (Nm) are commonly used indices to describe the degree of differentiation between natural populations [36, 37]. If gene flow is low, there is limited genetic exchange between the two populations, resulting in a high genetic differentiation coefficient and a distant genetic relationship between the populations [38, 39] and [33] suggested that when the Fst coefficient ranges from 0.05 to 0.15, natural populations are at a moderate level of differentiation. In this study, the average genetic differentiation coefficient (Fst) of R. pudingense was 0.1138, indicating a low degree of genetic differentiation between the populations. Gene flow (Nm) refers to the transfer and exchange of genes between different populations, and it can weaken the genetic differences between populations [40]. Generally, when Nm > 1, gene flow is high, and the degree of genetic differentiation between populations is low, allowing populations to resist the effects of genetic drift. When Nm < 1, populations with smaller numbers are more likely to undergo genetic drift [41]. In this study, the average Nm of R. pudingense populations was 3.1281, and the gene flow between the WM population and the other three populations was relatively high, with values exceeding 2, theoretically preventing genetic differentiation caused by genetic drift [27]. This result suggests frequent gene flow between the WM-1,WM-2,WM-3 populations and the other three populations. However, genetic drift is not the primary factor influencing changes in plant genetic outcomes, and populations are also affected by habitat fragmentation and destruction. Genetic drift may gradually occur as a result [42]. Meanwhile, the results of molecular variance analysis in this study indicated that 88% of the genetic variation originated within populations, while 12% of the genetic variation originated among populations (Table 5), which is consistent with the results of gene flow and genetic differentiation. The AMOVA results also support population differentiation. AMOVA reveals molecular differences between populations and within populations, primarily highlighting molecular differences within populations rather than between populations. This situation is the same as in the studies of [24] and [25] using microsatellite markers. The magnitude of genetic variation is influenced by multiple factors, and complex ecological environments are one of the reasons for genetic variation in species. Among them, the Ericaceae is an outcrossing plant, and its floral scent can attract insects for pollination, facilitating gene flow between different populations [34].
Population structure and genetic relationships
In genetic diversity analysis, multiple methods are usually used in combination to obtain a more comprehensive result and interpretation [21, 43,44,45]. The PCoA and UPGMA analyses have the ability to cluster populations based on genetic distance or dissimilarity, constructing a hierarchical clustering dendrogram that visually represents the differences between samples, and the STRUCTURE software can infer population genetic structure using genetic markers. In this study, the UPGMA clustering analysis divided six populations from four different regions into two distinct clusters, indicating the presence of two separate genetic populations in these areas. and the results of the PCoA analysis are in agreement with the STRUCTURE plot and support the UPGMA clustering tree, further validating these findings.
Furthermore, the UPGMA clustering analysis revealed that PD is genetically closer to WM-1 and WM-2, indicating a close genetic affinity despite the geographical distance between these populations. The Mantel analysis results highlighted a non-significant correlation between geographical distance and genetic distance (r = 0.1685, P = 0.210). Based on the findings of gene flow, genetic differentiation, and the Mantel test analysis, it can be hypothesized that the PD population migrated from WM-1 and WM-2.
Conservation of populations
The genetic diversity of organisms forms the basis for their adaptation to dynamic environments. The higher the genetic diversity or the greater the genetic variation within a species, the stronger its capacity to adapt to environmental changes, expand its distribution range, and explore new habitats. It is evident that the evolutionary potential, resistance to adverse environments, ecosystem resilience, and stability of a species are all contingent upon the magnitude of genetic diversity [26, 46, 47]. Although R. pudingense exhibits high genetic diversity, its distribution range is actually narrow, and the population is small. There is high genetic variation within populations of R. pudingense, but low genetic differentiation among populations, and the correlation between genetic distance and geographic distance is not significant. Therefore, the following suggestions are proposed: First, it is recommended to expand the habitat of R. pudingense and conduct large-scale regional protection efforts. Second, suitable locations can be selected based on the habitat requirements of R. pudingense for seedling cultivation. Third, under favorable conditions, efforts can be made to domesticate and cultivate R. pudingense, expanding its ecological niche and enabling it to survive and reproduce in a more diverse environment.
Conclusions
The genetic information from this study offers primary data for understanding the genetic diversity and population structure of R. pudingense, which can contribute to the formulation of conservation and management measures for endangered plants. Natural populations showed moderate to high levels of genetic diversity, high gene flow, and low genetic differentiation among populations. These populations serve as valuable genetic resources for future breeding programs and conservation strategies. This is the first study to utilize microsatellite markers to investigate the genetic diversity of R. pudingense, providing valuable references for improving germplasm resources and parental selection in breeding strategies.
The markers used in this study can be used to study population structure, genetic diversity, germplasm resource collection, and conservation strategies. They provide important information on genetic structure and contribute significantly to future improvements, provides a deeper understanding of the reasons for the endangerment of R. pudingense and offers scientific support for the conservation of its genetic resources.
Methods
Plant materials
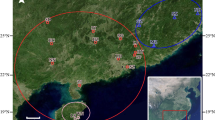
The 65 R. pudingense materials used in this study were collected from six different populations in Guizhou province. Specifically, 11 samples were collected from PD county, 13 samples from QL county, 31 samples from WM county, and 10 samples from ZN county. Details of the sampling are listed in Table 8; Fig. 5. Fresh leaves were collected and then labeled with sample numbers, then placed in sealed preservation bags and stored in a cooler box for transportation back to the laboratory. The samples were subsequently frozen rapidly using liquid nitrogen and stored at -80 degrees Celsius in a freezer for DNA extraction. The formal identification of the samples used in this study was performed by Xiao-Yong Dai. Voucher specimens were deposited in the Herbarium of Guizhou Provincial Academy of Forestry (GF) and Kunming Institute of Botany, Chinese Academy of Sciences (PE), the deposition number was 180,507,112 (fl., Holotype GF!, isotypes KUN!, PE!). Our field investigation and experimental studies comply with the regulations of local legislative bodies, national and international guidelines.
DNA extraction and PCR amplification
The plant genomic DNA was extracted from leaf samples using the plant Genomic DNA extraction Kit (200) (Qingke Biotech Co., Ltd.; TSP102-200). a total of 13 pairs of highly polymorphic and amplified microsatellite primers [48,49,50,51] were screened by combining with the R. pudingense materials, which served as amplification primers for subsequent analysis. Based on the 13 pairs of polymorphic primers, forward primers were synthesized with FAM, HEX, ROX, and TAMRA fluorescent labels at the 5’ end, and all 65 samples were amplified and analyzed by capillary electrophoresis. The amplification system was as follows: The PCR reaction system consists of 17 µl of Golden Mix (Green), 1 µl of 10 µM Primer F, 1 µl of 10 µM Primer R, and 1 µl of Template (gDNA), making a total volume of 20 µl. The PCR reaction program is as follows: 98 °C for 2 min, one cycle; 98 °C for 10 s, annealing temperature (Tm) for 10 s, 72 °C for 10 s, 35 cycles; 72 °C for 5 min, one cycle. The amplified PCR products are subjected to agarose gel electrophoresis (2 µl of the sample + 6 µl of bromophenol blue) at 300 V for 12 min to obtain the gel image. The gel image is used to determine the template concentration, and then it is diluted with water to the required concentration for capillary electrophoresis. Finally, specific and polymorphic loci with high specificity and good polymorphism are selected for statistical analysis.
Data analyses
The specific bands of each individual were counted based on their band sizes (bp). The peaks were analyzed using GeneMapper 4.1 software [52], with signal values above 400 and no other interfering peaks. Additionally, the peaks obtained from the same locus exhibited similar shapes. If the peak shapes were dissimilar, even if the peak value exceeded 400 and there was no other interfering peak, the data were not considered acceptable. This criterion was used to filter out usable data and establish the raw data matrix.
The population genetic structure was analyzed using the Bayesian model-based clustering method in STRUCTURE version 2.3.3 software [53]. The Markov Chain Monte Carlo (MCMC) method was employed, allowing for pre-defined population grouping (K) and calculating, sampling, and assigning individuals based on allele frequencies. The parameter settings were as follows: K values ranged from 1 to 10, with 10 independent runs for each K value, and a total of 100,000 iterations per run for repeated sampling. Finally, the most suitable K value was determined based on the method described in [54] using STRUCTURE HARVESTER (http://taylor0.biology.ucla.edu/struct_harvest/) website. The Hardy-Weinberg equilibrium (HWE) of each population at each locus was evaluated using the online software Genepop v.4.7 (https://genepop.curtin.edu.au/) [55].
Based on this criterion, genetic distances were calculated using the unweighted pair-group method with arithmetic means (UPGMA) to construct a clustering tree of individuals [56]. Specifically, the UPGMA tree was built using the populations-1.2.30 software, with the value set to 1000. The visualization and editing of the clustering tree were carried out using FigTree version 1.4.2 software.
According to the results of the analysis of population genetic structure, variation and differentiation between populations were calculated using GenAlEx version 6.5 software. Significance tests were conducted [56]. Gene flow (Nm) was calculated using [41] formula: Nm = 0.25(1 - Fst)/Fst.
To further investigate the genetic relationship among individuals of R. pudingense in four different populations, principal coordinate analysis (PCoA) was conducted using GenAlEx v6.5 [57].
Data availability
The sequencing data of the 13 polymorphic microsatellite primers were listed in the manuscript, and no other DNA sequences were applied to this study.
Abbreviations
- AFLP:
-
Amplified fragment length polymorphism
- AMOVA:
-
Analysis of molecular variance
- ISSR:
-
Inter simple sequence repeat
- PCoA:
-
Principal coordinate analysis
- RAPD:
-
Random amplified polymorphic DNA
- RFLP:
-
Restriction fragment length polymorphism
- ISSR:
-
Inter simple sequence repeat
- SSR:
-
Simple sequence repeat
References
Dai XY, Yang CH, Yang B, Chen P, Ma YP. A new species of Rhododendron (Ericaceae) from Guizhou, China[J]. PhytoKeys. 2020;146:53–9.
Wang HD, Yuan CJ, Yang R, Zhao HJ, Ma JB, Guo JR, Dai XY, Ding FJ. Prediction of potential suitable growth areas for the endemic plant Rhododendron pudingense in Guizhou based on MaxEnt model[J]. J South Agric. 2023;54(03):938–47.
Cohen JI, Williams JT, Plucknett DL, Shands H. Ex situ conservation of plant genetic resources: global development and environmental concerns[J]. Science. 1991;253(5022):866–72.
Ouborg NJ. Integrating population genetics and conservation biology in the era of genomics[J]. Biol Lett. 2010;6(1):3–6.
Zhao HJ, Yang R, Yuan CJ, Dai XY, Wang HD, Ma JB, Guo JR. Population structure and spatial distribution pattern of Rhododendron pudingense[J]. Journal of Tropical and Subtropical Botany; 2023.
Yuan CJ, Wang HD, Yang XW, Yang R, Dai XY, Ding FJ, Wu HL. Characteristics of soil enzyme activity variation in Rhododendron pudingense under different karst habitats[J]. J South Agric. 2023;54(02):525–34.
Yuan CJ, Wang HD, Dai XY, Chen M, Luo J, Yang R, Ding FJ. Effect of karst microhabitats on the structure and function of the rhizosphere soil microbial community of Rhododendron pudingense[J]. Sustainability. 2023;15(9):7104.
Gordon SP, Sloop CM, Davis HG, Cushman JH. Population genetic diversity and structure of two rare vernal pool grasses in central California[J]. Conserv Genet. 2012;13(1):117–30.
Kaljund K, Jaaska V. No loss of genetic diversity in small and isolated populations of Medicago sativa subsp. falcata[J]. Biochem Syst Ecol. 2010;38(4):510–20.
Frankham R, Briscoe A, Ballou JD. Introduction to conservation genetics[M]. Cambridge university press; 2002.
Frankham R. Stress and adaptation in conservation genetics[J]. J Evol Biol. 2005;18(4):750–5.
Zhang XJ, Liu XF, Liu DT, Cao YR, Li ZH, Ma YP, Ma H. Genetic diversity and structure of Rhododendron Meddianum, a plant species with extremely small populations[J]. Plant Divers. 2021;43(6):472–9.
Yilmaz A. Analysis of genetic variation among some Turkish oaks using random amplified polymorphic DNA (RAPD) method[J]. J Biotechnol, 2016 (231): 17–8.
Stone BW, Ward A, Farenwald M, Lutz AW, Wolfe AD. Genetic diversity and population structure in Cary’s Beardtongue Penstemon caryi (Plantaginaceae), a rare plant endemic to the eastern Rocky Mountains of Wyoming and Montana[J]. Conserv Genet. 2019;20:1149–61.
Everaert H, De-Wever J, Tang TKH, Vu TLA, Maebe K, Rottiers H, Lefever S, Smagghe G, Dewettinck K, Messens K. Genetic classification of Vietnamese cacao cultivars assessed by SNP and SSR markers[J]. Tree Genet Genomes. 2020;16(3):43.
Wang JG, Li WJ, Zhang HJ, Wu D, Zhang JL, Jia ZZ. Study on the diversity and stability of plant communities in Baili Rhododendron scenic area in Guizhou province[J]. For Resour Manage, 2020, (2): 120–5.
Marian CO, Krebs SL, Arora R. Dehydrin variability among rhododendron species: a 25-kDa dehydrin is conserved and associated with cold acclimation across diverse species[J]. New Phytol. 2004;161(3):773–80.
Zhao X, Zhou ZQ, Lin QB, Pan KY, Hong DY. Molecular evidence for the interspecific relationships in Paeonia sect. Moutan: PCR-RFLP and sequence analysis of glycerol-3-phosphate acyltransferase (GPAT) gene[J]. J Syst Evol. 2004;42(3):236.
Su X, Zhang H, Dong LN, Zhang JQ, Zhu XT, Sun K. RAPD classification and identification of Paeonia rockii varieties planted in Gansu province[J]. Acta Bot Boreali Occidentalia Sinica. 2006;26(4):696–701.
Suo ZL, Zhou SL, Zhang HJ, Zhang ZM. DNA molecular evidences of the inter-specific hybrids between Paeonia ostii and P. Suffruticosa based on ISSR markers[J]. Forestry Sci Res. 2004;17(6):700–5.
Liu P, Wang ZC, Shang FD. AFLP analysis of genetic diversity of Paeonia suffruticosa cultivars in Henan province[J]. Hortic Plant J. 2006;33(6):1369–72.
Wang SQ. Genetic diversity and population structure of the endangered species Paeonia decomposita endemic to China and implications for its conservation[J]. BMC Plant Biol. 2020;20(1):1–14.
Li TQ. A study on the conservation biology of the extremely small population wild plant Rhododendron elongatum[D]. Beijing: China Academy of Forestry Sciences; 2018.
Man L. Study on the genetic structure of Xing’an Rhododendron population based on SSR molecular markers[D]. Northeast: Northeast Normal University; 2016.
Liu YY. A study on the conservation genetics of the endangered plant Rhododendron Grandis endemic to southwest China[D]. Kunming: Yunnan University; 2019.
Wang SL. Genetic diversity of kapok population in the dry hot valley area of Yunnan province[D]. Chinese Academy of Sciences: Xishuangbanna tropical botanical garden,; 2007.
Hamrick JL, Godt MJW, Sherman-Broyles SL. Factors influencing levels of genetic diversity in woody plant species[C]. Population Genetics of Forest Trees: Proceedings of the International Symposium on Population Genetics of Forest Trees Corvallis, Oregon, USA, July 31–August 2, 1990. Springer Netherlands, 1992: 95–124.
Klocke JA, Mei-Ying H, Shin-Foon C, Kubo I. Grayanoid diterpene insect antifeedants and insecticides from Rhododendron molle[J]. Phytochemistry. 1991;30(6):1797–800.
Wang SZ, Zhang L, Yang W, Luo Y, Zheng Z, Fang YP, XiangJ, Jin WB. SSR analysis of genetic diversity in different age groups of Rhododendron simsii population in Dabie mountains [J]. Forestry Sci Res. 2018;31(5):125–30.
Sun S. Genetic variation of natural populations of Acer griseum based on SSR markers [D]. Beijing: China Academy of Forestry Sciences; 2014.
Nei M. Molecular population genetics and evolution[M]. North-Holland Publishing Company; 1975.
Barrett SCH, Kohn JR. Genetic and evolutionary consequences of small population size in plants: implications for conservation[J]. Genetics and Conservation of Rare Plants; 1991. pp. 3–30.
Balloux F, Lugon-Moulin N. The estimation of population differentiation with microsatellite markers[J]. Mol Ecol. 2002;11(2):155–65.
Nybom H. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol. 2004;13(5):1143–55.
Man-Kyu HUH, Hong-Wook HUH. Patterns of genetic diversity and population structure of the clonal herb, Potentilla fragarioides var. Sprengeliana (Rosaceae) in Korea[J]. J Integr Plant Biol. 2000;42(1):64–70.
Niswander JD, Brown KS, Iba BY, Leyshon WC, Workman PL. Population studies on southwestern Indian tribes. I. history, culture, and genetics of the Papago[J]. Am J Hum Genet. 1970;22(1):7–23.
Barton NH, Slatkin M. A quasi-equilibrium theory of the distribution of rare alleles in a subdivided population[J]. Heredity. 1986;56(3):409–15.
Zhao QS, Zhao YY, Wang P, Deng XJ, Ma QB, Nian M, Yang CY. Genetic structure and spatial distribution relationship of wild soybean population in Daguanling, Xintian, Hunan[J]. J South China Agricultural Univ. 2014;35(4):42–9.
Wright S. Evolution and the genetics of populations, volume 4: variability within and among natural populations[M]. University of Chicago press; 1984.
Li ZH, Liu ZL, Wang ML, Qian ZQ, Zhao P, Zhu J, Yang YX, Yan XH, Li YY, Zhao GF. A review of species formation under the condition of gene flow: evolution of reproductive isolation mechanisms [J]. Biodivers Sci. 2014;22(1):88–96.
Wright S. Evolution in mendelian populations[J]. Genetics. 1931;16(2):97–159.
Setoguchi H, Mitsui Y, Ikeda H, Nomura N, Tamura A. Genetic structure of the critically endangered plant Tricyrtis ishiiana (Convallariaceae) in relict populations of Japan[J]. Conserv Genet. 2011;12:491–501.
Zong XX, Guan JP, Gu J, Wang H, Ma Y. Differentiation on population structure and genetic diversity of pea core collections separately constituted from Chinese land races and international genetic resources[J]. J Plant Genetic Resour. 2009;10:347–53.
Tan X, Wu Z, Cheng W, Wang T, Li Y. Association analysis and its application in plant genetic research[J]. Chin Bull Bot. 2011;46(1):108–18.
Lai GT, Lai ZX, Liu WH, Ye W, Lin YL, Liu SC, Chen YK, Zhang ZH, Wu GJ. ISSR analysis of 3 natural populations of the wild banana distributed in the middle of Fujian Province based on NTSYS and STRUCTURE software[J]. Chin J Trop Crops. 2014;35(2):223–31.
Foster SA. The evolutionary process: A critical study of evolutionary theory by verne rant[J]. The Quarterly Review of Biology, 1993, 68(Volume 68, Number 1):107.
Solbrig OT. From genes to ecosystems: a research agenda for biodiversity: report of a IUBS-SCOPE-UNESCO workshop[M]. International Union of Biological Sciences; 1991.
Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue[J]. Phytochemical Bull. 1987;19:11–5.
Wang XQ, Huang Y, Long CL. Cross-amplification and characterization of microsatellite loci for the genus Rhododendron[J]. HortScience. 2010;45(9):1394–7.
Li LF, Yin DX, Song N, Tang EN, Xiao HX. Genomic and EST microsatellites for Rhododendron Aureum (Ericaceae) and cross-amplification in other congeneric species[J]. Am J Bot. 2011;98(9):e250–2.
Wang N, Qin ZC, Yang JB, Zhang JL. Development and characterization of 15 microsatellite loci for Rhododendron Delavayi Franch.(Ericaceae)[J]. Hort Sci. 2010;45(3):457–9.
Currie-Fraser E, Shah P, True S. Data analysis using gene mapper v4. 1: comparing the newest generation of GeneMapper software to legacy Genescan and Genotyper software[J]. J Biomol Techniques. 2010;21(3):31.
Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data[J]. Genetics. 2000;155(2):945–59.
Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study[J]. Mol Ecol. 2005;14(8):2611–20.
Mahesh BA, Kannan E, Davis G, Venkatesan P, Ragunath PK. GenPop-An online tool to analyze human population genetic data. Bioinformation. 2020;16(2):149–52.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms[J]. Mol Biol Evol. 2018;35(6):1547–9.
Peakall ROD, Smouse PE. GenALEx 6.5: genetic analysis in Excel, population genetic software for teaching and research[J]. Mol Ecol Notes. 2006;6(1):288–95.
Acknowledgements
I am grateful to related local institutions and staff in Guizhou for providing research permission inside the forests and help to collect experimental materials.
Funding
This study was supported by the Guizhou Provincial Science and Technology Foundation Project (Guizhou Science and Technology Foundation - ZK [2021] General 097, Guizhou Science and Technology Foundation - ZK [2021] General 089); Guizhou Provincial Science and Technology Conditions and Service Function Construction Project (Guizhou Science and Technology Service Enterprise [2020] 4010).
Author information
Authors and Affiliations
Contributions
CJ designed the research and revised the manuscript. Pl, HD, Dl, XY performed the experiments. S H analyzed the data and wrote the main manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was carried out by the Rhododendron Research Team of Guizhou Academy of Forestry in the field work of the “Guizhou Tianbao Project Benefit Monitoring” and “Rhododendron Research” projects, I confirmed that I complied with all relevant institutional, national and international guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, S., Yuan, C., Zhang, P. et al. Study on the characteristics of genetic diversity of different populations of Guizhou endemic plant Rhododendron pudingense based on microsatellite markers. BMC Plant Biol 24, 77 (2024). https://doi.org/10.1186/s12870-024-04759-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-04759-5