Abstract
Background
RpoN, also known as σ54, first reported in Escherichia coli, is a subunit of RNA polymerase that strictly controls the expression of different genes by identifying specific promoter elements. RpoN has an important regulatory function in carbon and nitrogen metabolism and participates in the regulation of flagellar synthesis, bacterial motility and virulence. However, little is known about the effect of RpoN in Plesiomonas shigelloides.
Results
To identify pathways controlled by RpoN, RNA sequencing (RNA-Seq) of the WT and the rpoN deletion strain was carried out for comparison. The RNA-seq results showed that RpoN regulates ~ 13.2% of the P. shigelloides transcriptome, involves amino acid transport and metabolism, glycerophospholipid metabolism, pantothenate and CoA biosynthesis, ribosome biosynthesis, flagellar assembly and bacterial secretion system. Furthermore, we verified the results of RNA-seq using quantitative real-time reverse transcription PCR, which indicated that the absence of rpoN caused downregulation of more than half of the polar and lateral flagella genes in P. shigelloides, and the ΔrpoN mutant was also non-motile and lacked flagella. In the present study, the ability of the ΔrpoN mutant to kill E. coli MG1655 was reduced by 54.6% compared with that of the WT, which was consistent with results in RNA-seq, which showed that the type II secretion system (T2SS-2) genes and the type VI secretion system (T6SS) genes were repressed. By contrast, the expression of type III secretion system genes was largely unchanged in the ΔrpoN mutant transcriptome and the ability of the ΔrpoN mutant to infect Caco-2 cells was also not significantly different compared with the WT.
Conclusions
We showed that RpoN is required for the motility and contributes to the killing ability of P. shigelloides and positively regulates the T6SS and T2SS-2 genes.
Similar content being viewed by others
Background
After many years in the family Vibrionaceae, the genus Plesiomonas, represented by a single species, Plesiomonas shigelloides, currently resides in the family Enterobacteriaceae [1]. P. shigelloides is a gram-negative opportunistic pathogen that causes acute secretory gastroenteritis, an invasive shigellosis-like disease, and a cholera-like illness [2,3,4]. Extra intestinal infections are also associated with P. shigelloides, such as bacteremia, pseudoappendicitis and meningitis [5,6,7]. RpoN (σ54) is widely found in pathogenic bacteria. It binds to the core RNA polymerase and regulates the transcription of many functional genes in an enhancer-binding protein (EBP)-dependent manner, in general, bacteria contain one or two RpoNs but multiple EBPs [8]. Regulation of RpoN has been extensively studied in many bacterias. In Escherichia coli, RpoN affects the nitrogen and carbon metabolism, fermentation, cell envelope biogenesis, stress fitness, and pathogenesis [9, 10]. The RpoN regulon is very diverse, controlling genes involved in the response to nitrogen limitation, nitric oxide stress, availability of alternative carbon sources, toxic levels of zinc and nucleic acid damage in Salmonella typhimurium [11]. In Pseudomonas putida, RpoN affects the utilization of nitrate, urea, and uncharged amino acids as nitrogen sources, as well as lysine, C4-dicarboxylates, and alpha-ketoglutarate as carbon sources [12, 13]. RpoN also regulates the susceptibility to tobramycin [14], quinolones, and carbapenems [15, 16] in P. aeruginosa. In addition, RpoN controls bacterial exopolysaccharide production and biofilm formation [17, 18], quorum sensing [19, 20], environmental adaptation [21, 22], and antibiotic resistance [23, 24] in other bacteria.
P. shigelloides is a unique member of the Enterobacteriaceae family that contains two different gene clusters, one exclusively for lateral flagella biosynthesis and the other one containing the biosynthetic polar flagella genes [25]. However, the transcriptional regulatory network of polar and lateral flagella is currently unreported. RpoN is known to be required for bacterial motility and flagellar synthesis, such as in V. cholerae [26,27,28], Vibrio parahaemolyticus [29], Vibrio campbellii [30], Aeromonas hydrophila [31, 32], Pseudomonas aeruginosa [33, 34], Caulobacter crescentus [35], Helicobacter pylori [36] and Rhodobacter sphaeroides [37]. In E. coli, σ70 is mainly involved in the synthesis and regulation of flagella; however, recent studies have also reported that σ54 also has an important regulatory role on the motility [38, 39].
The Type VI secretion system is a protein translocation nanomachine that is widespread among gram-negative bacteria and is also a contact-dependent bacterial weapon that allows for the direct killing of competitors through the translocation of proteinaceous toxins [40, 41]. These proteinaceous toxins have a wide variety of functions within target cells that ultimately help the secreting cell gain a competitive advantage [42]. The primary role of the T6SS appears to be to act against competitor bacteria. T6SS genes are distributed over the P. shigelloides chromosome and consist of two clusters: a large cluster and an auxiliary clusters. The large cluster encodes the majority of the structural T6SS components, including the outer sheath proteins, key proteins for the tip of the T6SS and proteins that assemble at the inner and outer membranes [43]. Additionally, the large cluster encodes a gene necessary for disassembly of the T6SS, clpV, and an essential transcriptional regulator, vasH [44, 45]. VasH is a σ54-dependent transcription factor encoded in the large T6SS cluster that positively regulates the two auxiliary clusters essential for T6SS activity [46].
The T2SS is a multi-protein complex used by many gram-negative bacteria to move substrates across their cell membrane [47]. It is a key virulence factor in many human pathogens including Acinetobacter baumannii [48], Klebsiella pneumoniae [49], Pseudomonas aeruginosa [50], Vibrio cholerae [51] and enterotoxigenic Escherichia coli [52]. Many diverse effectors and toxins depend on the T2SS for secretion [53,54,55], such substrates are involved in adhesion, biofilm formation, nutrient acquisition, colonization, and invasion [56]. The canonical T2SS operon contains approximately 13 genes, often arranged in a single operon, named gspC to gspO [57, 58]. In the present study, we also found a certain association between RpoN and the type II secretion system.
The effects of RpoN in P. shigelloides are unknown; therefore, we used P. shigelloides as the research object and revealed the function of RpoN in P. shigelloides.
Results
Phylogenetic analysis of RpoN
To analyze the function of the RpoN, a phylogenetic tree based on the RpoN amino acid sequences was constructed and the RopD protein of P. shigelloides was selected as the outgroup control (Fig. 1). The neighbor-joining tree consisting of 21 species of bacteria, RpoN of P. shigelloides was closer to Escherichia coli and Salmonella typhi than Vibrio. Moreover, RpoN of P. aeruginosa formed a separate evolutionary branch.
Phylogenetic analysis of RpoN. An unrooted phylogenetic tree constructed using the neighbor joining method based on RpoN amino acid sequences, Bootstrap values were based on 1000 replications and only values greater than 70% are shown. All amino acid sequences were downloaded from the National Center for Biotechnology Information
Transcriptome sequencing revealed gene expression related to RpoN of the P. shigelloides
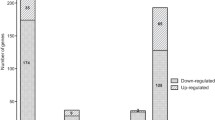
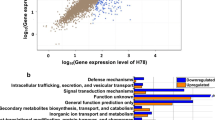
To investigate the regulatory role of RpoN in P. shigelloides, transcriptome profiles of the WT and ΔrpoN strains were analyzed using RNA-seq. The RNA-seq results showed that RpoN regulates approximately 13.2% of the P. shigelloides transcriptome: a total of 398 DEGs in the ΔrpoN strain were identified in comparison with the WT strain, including 210 downregulated genes and 188 upregulated genes (Fig. 2A, Table S1A). GO enrichment analysis of the DEGs showed that they were mainly classified as Cellular component, followed by Molecular function, and finally by Biological process (Fig. 2B and C). KEGG signaling pathway analysis showed that the upregulated genes were involved in flagellar assembly, microbial metabolism in diverse environments, biosynthesis of cofactors, two-component system, nucleotide metabolism, pyrimidine metabolism, quorum sensing and oxidative phosphorylation (Fig. 2D). The downregulated genes were involved in biosynthesis of secondary metabolites, biosynthesis and metabolism of amino acids, the bacterial secretion system, 2 − Oxocarboxylic acid metabolism, flagellar assembly, bacterial chemotaxis, pantothenate and CoA biosynthesis, butanoate and glycerophospholipid metabolism (Fig. 2E). Among the upregulated DEGs were genes responsible for the biosynthesis of cofactors (iscS, pyrF, pdxJ, yjjG, thiH), nucleotide metabolism (guaA, guaB, pyrH, tdk), and oxidative phosphorylation (ppk2, cydB). Among the downregulated DEGs were genes responsible for biosynthesis and metabolism of amino acids (argA/B/C/D/H, leuA/B/C/D, thrA/B/C, metL, cysE, lysC, trpD, hisD), glycerophospho-lipid metabolism (glpA/C/D/Q/T), pantothenate and CoA biosynthesis (ilvD/E/G/M), and butanoate metabolism (phbB, putA).
Transcriptomic analysis of the P. shigelloides between WT and ΔrpoN strains. A The volcano plot of differential expressed genes (DEGs), red circle was up- regulated genes, green circle was down-regulated genes, blue circle was no DEGs; B GO enrichment of up-regulated DEGs; C GO enrichment of down-regulated DEGs, The enrichment analysis of GO function is divided into three major categories: Biological process and Cellular component and Molecular function. D KEGG enrichment of up-regulated DEGs; E KEGG enrichment of down-regulated DEGs, the GeneRatio refers to the ratio of the number of DEGs in the pathway and the number of all annotated genes in the pathway
RpoN influenced the motility, flagellar synthesis, motor assembly and growth of P. shigelloides
In this study, the effect of RpoN on the motility of P. shigelloides was validated by constructing the ΔrpoN mutant and ΔrpoN/pBAD33-rpoN+ to observe their migration in swimming agar plates. The swimming agar plate results showed that the motility of ΔrpoN mutant was basically lost; however, the motility of the ΔrpoN/pBAD33-rpoN+ complementation strain was restored to the WT level. Thus, RpoN positively controls the motility of P. shigelloides (Fig. 3A). Meanwhile, the flagella produced by the WT, ΔrpoN, and ΔrpoN/pBAD33-rpoN+ strains were observed using TEM, which indicated that a lack of RpoN influences the flagellar synthesis and assembly in P. shigelloides, consistent with the motility assay (Fig. 3B and C). In our RNA-seq analysis, we found that over half of all polar and lateral flagella genes had significantly lower expression in the ΔrpoN mutant relative to the WT (Fig. S1A and S1B). Subsequently three greatest downregulated genes, flaC and fliAL for flagellar synthesis and motA for motor assembly, were selected for validation using qRT-PCR in the WT, ΔrpoN, and ΔrpoN/pBAD33-rpoN+ strains (Fig. 3D). The results of qRT-PCR were consistent with RNA-seq analysis, suggesting the effects of RpoN on motility gene expression could be caused by RpoN-mediated regulation. Given the significant downregulation of flaC, motA, and fliAL in the RNA-seq data, we further validate the effect of RpoN on the three genes by constructing flaC, motA and fliAL promoter-lux fusion in the ΔrpoN mutant and WT strains. The expression levels of flaC, motA, and fliAL promoter-lux fusions in the ΔrpoN mutant were lower than those in the WT (Fig. 3E). In addition, the differences in growth between the WT and ΔrpoN mutant in LB liquid medium and DMEM were compared. When grown in LB liquid, the growth of the ΔrpoN mutant slightly lagged behind that of the WT in the lag phase (Fig. 3F). When grown in DMEM, the growth of ΔrpoN mutant lagged behind that of the WT in the log phase (Fig. 3G). Growth of the rpoN complementation strain completely restored to the WT level indicated that growth metabolism genes of P. shigelloides in the lag and log phase were regulated by RpoN, which is in line with the RNA-seq results that RpoN plays an important regulatory function in carbon and nitrogen metabolism in P. shigelloides.
RpoN is essential for the motility of P. shigelloides and affects the growth of P. shigelloides. A The motility of WT, ΔrpoN and ΔrpoN/pBAD33-rpoN+ strains grown in swimming agar plate. B TEM visualization of the flagella produced by the WT, ΔrpoN and ΔrpoN/pBAD33-rpoN+ strains. The hollow bacterial flagella were pointed by the colored arrows. C The picture is drawn based on the observed difference in the number of all flagella produced by WT, ΔrpoN and ΔrpoN/pBAD33-rpoN+. D Transcription verification of flaC, motA and fliAL by qRT-PCR. E Expression of flaC-lux, motA-lux, and fliAL-lux in the WT and ΔrpoN mutant. F The differences in growth between the WT, ΔrpoN mutant and ΔrpoN/pBAD33-rpoN+ in LB liquid medium, and the optical density at 600 nm (OD600) was monitored. G The differences in growth between the WT, ΔrpoN mutant and ΔrpoN/pBAD33-rpoN.+ in DMEM, and the optical density at 600 nm (OD600) was monitored. Significant differences were indicated by asterisks (*** p ≤ .001; ** p ≤ .01; * p ≤ .05)
RpoN contributes to the killing ability in P. shigelloides by positively regulating the expression of T6SS clusters
The T6SS protects P. shigelloides by directly translocating toxins to its neighboring bacteria and secreting virulence effectors into the host cells. The T6SS machinery contains two parts, a bacteriophage-like structure and a membrane anchor, to penetrate target cell membranes and secrete effector proteins. Here, transcriptomic profiling of the ΔrpoN mutant revealed a positive regulatory role of RpoN on both the large cluster and auxiliary cluster of the T6SS (Fig. 4A). At the same time, transcription of the two clusters of T6SS was verified using qRT-PCR, the results of which were consistent with those of the RNA-seq analysis (Fig. 4B). Subsequently, the killing assay was used to compare the ability of the WT, ΔrpoN, and ΔrpoN/pBAD33-rpoN+ strains to kill E. coli MG1655, with the aim of verifying that RpoN is required for the killing ability in P. shigelloides by positively regulating the expression of T6SS clusters. Compared with the WT, the ΔrpoN mutant showed a 54.6% reduction in its ability to kill MG1655, whereas the ΔrpoN/pBAD33-rpoN+ complementation strain could restore the killing ability partially, reaching only about 70% levels compared with that of the WT (Fig. 4C). Hemolysin Co-Regulated Protein (Hcp) is a key effector protein, and the normal expression and secretion of Hcp is a sign of T6SS function. Interestingly, we found RpoN in the DNA pull-down assay previously carried out for the hcp gene (Table S1B), suggesting that RpoN could directly regulate hcp expression.
RpoN is involved in killing E.coli MG1655 by positively regulating the expression of T6SS clusters. A RNA-seq analysis of the transcription levels of T6SS clusters. B Transcription verification of T6SS clusters in the WT, ΔrpoN and ΔrpoN/pBAD33-rpoN+ by qRT-PCR. C Killing assay of the WT, ΔrpoN and ΔrpoN/pBAD33-rpoN+. Killing ability of ΔrpoN and ΔrpoN/pBAD33-rpoN.+ were reported as percentage relative to the WT. Significant differences were indicated by asterisks (*** p ≤ .001; ** p ≤ .01; * p ≤ .05)
RpoN positively regulates the T2SS-2 cluster associated with the killing ability of P. shigelloides
In addition to the differential expression of the two gene clusters of T6SS in the transcriptomic profile of the ΔrpoN mutant, we also identified another secretion system, T2SS-2, which is positively regulated by RpoN. Many diverse effectors and toxins involved in adhesion, biofilm formation, nutrient acquisition, colonization, and invasion, depend on the T2SS for secretion. We found that two types of T2SS were distributed in P. shigelloides genomes: The T2SS-1 and T2SS-2 clusters consisted of 12 and 11 genes, respectively. RNA-seq analysis of the transcription levels of the T2SS-2 cluster is shown in Fig. 5A and the qRT-PCR verification of the transcription of the T2SS-2 cluster in the WT, ΔrpoN, and ΔrpoN/pBAD33-rpoN+ is shown in Fig. 5B. The results indicated that RpoN positively regulates the T2SS-2 cluster in P. shigelloides. However, the expression of the T2SS-1 cluster in the transcriptomic profiling of the ΔrpoN mutant was not significantly different (Fig. S1C). Next, we deleted the T2SS-2 cluster to verify whether it was associated to the killing ability of P. shigelloides. The killing assay indicated that deletion of the T2SS-2 cluster reduced the ability of P. shigelloides to kill E. coli MG1655 (Fig. 5C). Combined with the differential expression of the T2SS-2 cluster in the RNA-seq analysis, verified by qRT-PCR, we hypothesized that RpoN positively regulates the T2SS-2 cluster, which is associated with the killing ability of P. shigelloides.
RpoN positively regulates the T2SS-2 cluster associated with the killing ability of P. shigelloides. A RNA-seq analysis of the transcription levels of T2SS-2 cluster. (B) Transcription verification of T2SS-2 cluster in the WT, ΔrpoN and ΔrpoN/pBAD33-rpoN.+ by qRT-PCR. C Killing assay of the WT and ΔT2SS-2. Killing ability of ΔT2SS-2 was reported as percentage relative to the WT. Significant differences were indicated by asterisks (*** p ≤ .001; ** p ≤ .01; * p ≤ .05)
The overall regulation of T3SS cluster expression by RpoN showed no significant difference, and RpoN had little effect in the ability of P. shigelloides to infect Caco-2 cells
Gram-negative bacteria are known to subvert eukaryotic cell physiological mechanisms using a wide array of virulence factors, among which the T3SS system is often one of the most important. T3SS can remodel cytoskeletal integrity to promote intracellular invasion, as well as silencing specific eukaryotic cell signals, notably to hinder or elude the immune response and cause apoptosis. A previous study reported that RpoN has an important regulatory effect on the T3SS cluster; however, in the transcriptomic profiling of the ΔrpoN mutant, we found that the overall change in the expression of the T3SS cluster was not obvious (Fig. 6A), which was verified using qRT-PCR in the WT, ΔrpoN, and ΔrpoN/pBAD33-rpoN+ strains (Fig. 6B). The results of the T3SS cluster analysis showed that only a few genes showed differences in expression. However, we still wanted to phenotype the strains by invasion assay to verify whether RpoN affects P. shigelloides' ability to infect Caco-2 cells. Invasion assays of the WT, ΔrpoN, and ΔrpoN/pBAD33-rpoN+ strains indicated that RpoN had little effect in the ability of P. shigelloides to infect Caco-2 cells (Fig. 6C).
RpoN showed no significant difference in the ability of P. shigelloides to infect Caco-2 cells and regulate the T3SS cluster expression. A RNA-seq analysis of the transcription levels of T3SS cluster. B Transcription verification of T3SS cluster in the WT, ΔrpoN and ΔrpoN/pBAD33-rpoN+ by qRT-PCR. C Invasion assay of the WT, ΔrpoN and ΔrpoN/pBAD33-rpoN+ strains. Invasion ability of ΔrpoN and ΔrpoN/pBAD33-rpoN.+ were reported as percentage relative to the WT. Significant differences were indicated by asterisks (*** p ≤ .001; ** p ≤ .01; * p ≤ .05)
Discussion
Plesiomonas shigelloides, which causes intestinal infections, is often isolated from seafood, uncooked food, and contaminated water [59]. To date, few molecular studies on the pathogenic mechanisms of P. shigelloides have been published. Transcription factors play a crucial role in microbial growth and the response to environmental changes by regulating the expression of target genes [8]. Sigma factors are the most widely occurring transcription factors, especially, σ54 factors acts as multifunctional regulators of many important biological processes. In this study, using RNA sequencing, we found that RpoN regulates approximately 13.2% of the P. shigelloides transcriptome, comprising 398 DEGs, which include 210 downregulated genes and 188 upregulated genes. Based on number of the DEGs, the main DEG groups were related to biosynthesis of cofactors, nucleotide metabolism, oxidative phosphorylation, pantothenate and CoA biosynthesis, biosynthesis and metabolism of amino acids, butanoate and glycerophospholipid metabolism, flagellar assembly, bacterial chemotaxis and bacterial secretion system, except for groups with function unknown and general function prediction only. The RNA-seq-dependent transcriptomics analysis indicated that RpoN significantly regulated amino acid biosynthesis and metabolism of nitrogen and carbon in P. shigelloides, including valine, leucine, isoleucine, arginine, and lysine biosynthesis, and glycine, serine, threonine, cysteine, and methionine metabolism.
Previous studies have been reported that RpoN controls bacterial growth [60,61,62], swimming and twitching motility [63]. In this study, the differences in growth between the WT and ΔrpoN mutant in LB liquid medium and DMEM were compared, which showed that the growth of the ΔrpoN mutant slightly lagged behind that of the WT in the lag phase when grown in LB liquid and lagged behind that of the WT in the log phase when grown in DMEM. The migration in the swimming agar plate of the WT, ΔrpoN, and ΔrpoN/pBAD33-rpoN+ strains suggested that RpoN positively regulates the motility of P. shigelloides. The TEM results also showed that the absence of RpoN caused failure of flagellar formation in P. shigelloides. Furthermore, transcriptome analyses revealed that the expression of more than half of the flagellar genes were reduced in the ΔrpoN mutant relative to that in the WT. The results indicated that RpoN is essential for the motility and flagellar synthesis of P. shigelloides. However, we also observed that certain flagella genes were upregulated, and some flagella genes were not significantly changed (Fig. S1A and S1B). This cannot change the phenomenon that the ΔrpoN mutant was non-motile and lacked flagella. For this, we speculate that the expression of some flagella genes, such as flaC and fliAL for flagellar synthesis and motA for motor assembly, were significantly affected by RpoN and dominantly contributed to the motility of P. shigelloides. In subsequent studies we will explore which flagellar genes are directly regulated by RpoN and identify the core regulators of the flagellar hierarchy for P. shigelloides.
Bacteria have evolved multiple strategies to survive and develop optimal fitness in their ecological niche. They deploy protein secretion systems for robust and efficient delivery of antibacterial toxins into their target cells, thereby inhibiting their growth or killing them [64]. The T6SS system is a contact-dependent bacterial weapon that allows for direct killing of competitors through the translocation of proteinaceous toxins. Many studies have reported that RpoN also controls the bacterial T6SS [65,66,67,68] and virulence [69, 70]. Previous studies have also reported that RpoN directly binds to the promoter regions of hcpA and hcpB, which encode Hcp1-family T6SS effectors [71]. In the present study, transcriptomic profiling of the ΔrpoN mutant revealed a positive regulatory role of RpoN on both the large cluster and auxiliary cluster of the T6SS. In addition, we found RpoN in the DNA pull-down assay previously carried out for the hcp gene. Meanwhile, killing assay verified that RpoN contributes to the killing ability of P. shigelloides by positively regulating the expression of T6SS clusters. In a subsequent study, we will purify the RpoN protein to further verify which genes are directly regulated by RpoN in the T6SS clusters.
In addition to the two gene clusters of T6SS, we also found another secretion system, T2SS-2, which was positively regulated by RpoN. Two types of T2SS were distributed in P. shigelloides genomes, T2SS-1 and T2SS-2. The results of RNA-seq indicated that RpoN positively regulates the T2SS-2 cluster in P. shigelloides. However, the expression of T2SS-1 cluster in the transcriptomic profile of ΔrpoN mutant was not significantly different. Moreover, the killing assay indicated that deletion of the T2SS-2 cluster reduced the ability of P. shigelloides to kill E. coli MG1655, suggesting that the T2SS-2 cluster was associated with the killing ability of P. shigelloides. Moreover, this is the first report that RpoN positively regulates the expression of T2SS-2. Previous studies reported the RpoN-dependent cascade regulation of T3SS, which proved that RpoN activates hrpL through the interaction between HrpR and HrpS, thereby regulating hrp gene transcription [72, 73]. In Erwinia amylovora and Dickeya dadantii, HrpS, but not HrpR, interacts with RpoN and activates hrpL, thus regulating the transcription of T3SS-associated genes [74,75,76]. However, we found that the overall change in the expression of the T3SS cluster in the transcriptomics profiling of the ΔrpoN mutant was not obvious and the invasion assay verified that RpoN had little effect in the ability of P. shigelloides to infect Caco-2 cells.
In the present study, we revealed the RpoN-controlled pathways, and our proposal regarding the major metabolic pathways regulated by RpoN in P. shigelloides is outlined in Fig. 7. These findings and knowledge support the key regulatory role of RpoN in bacterial growth and pathogenesis, as well as laying the groundwork for further determination of the complex regulatory network of RpoN in bacteria.
Conclusions
In this work, the RNA-seq results showed that RpoN regulates ~ 13.2% of the P. shigelloides transcriptome, and is involved in amino acid transport and metabolism, glycerophospholipid metabolism, pantothenate and CoA biosynthesis, ribosome biosynthesis, flagellar assembly, and bacterial secretion system. In addition, we showed that T2SS-2 is related to the killing ability of P. shigelloides: RpoN is required for the motility and contributes to the killing ability of P. shigelloides, and affects the killing ability by positively regulating T6SS and T2SS-2 genes.
Materials and methods
Bacterial strains, growth conditions, and plasmids
The bacterial strains, as well as the plasmids used, are listed in Table 1. Bacteria were grown in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 20% fetal bovine serum (FBS) and in Luria–Bertani (LB) liquid, solid, and semi-solid medium at 37 ℃ (statically or in a shaking incubator) or at 30 ℃ statically. If necessary, the media were supplemented with ampicillin (25 μg/ml), chloramphenicol (25 μg/ml), or kanamycin (50 μg/ml).
Gene deletion and complementation
In this study, the suicide vector pRE112 was used to make deletion mutations of the rpoN gene and the T2SS-2 cluster of P. shigelloides [77]. The complementation strain, ΔrpoN/pBAD33-rpoN+, was constructed by introducing the recombinant vector pBAD33-rpoN+ into the ΔrpoN strain via electroporation. Agarose gel electrophoresis and DNA sequencing of PCR products was used to confirm the presence of the correct deletion mutations and complementation strains. Confirmation of the deletion of rpoN and T2SS-2 cluster and the complementation of rpoN in P. shigelloides was shown in Fig. S1D to K. All primers used in this study are shown in Table 2.
Transcriptome sequencing
Cultures of P. shigelloides WT and the ΔrpoN mutant were grown in LB at 37 ℃ for 12 h to the stationary phase (OD600 ≈ 1.5), and then were harvested using centrifugation. Total RNA of the WT and ΔrpoN mutant strains was extracted using the TRIzol® Reagent (Invitrogen) according to the manufacturer's protocol, followed by treatment with an RNase-Free DNase. RNA degradation and contamination was monitored using 1% agarose gels. The total amount and integrity of the RNA were assessed using an RNA Nano 6000 Assay Kit on the Bioanalyzer 2100 system. cDNA was prepared and modified according to the manufacturer’s protocol. After the cDNA library was tested and qualified, it was sequenced on an Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA) to generate 150 bp paired-ends reads. Three independent libraries were prepared for each of the RNA-seq samples. After filtering the raw reads, the clean reads were mapped to the genome of P. shigelloides (GenBank assembly accession GCA_009183595.1). HTSeq (v0.9.1) was used to quantify of gene expression levels, and then the Fragments Per Kilobase of transcript sequence per Millions base pairs sequenced (FPKM) value of each gene was calculated based on the length of the gene and read count mapped to this gene. The criteria for a significant difference in expression were |log2 fold change|≥ 1 and adjusted P-value (padj) ≤ 0.05. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway databases [78,79,80] were used to analyze the functions and enriched pathways of the DEGs.
Quantitative real-time reverse transcription PCR (qRT-PCR)
To confirm the RNA-Seq results, differentially expressed genes (DEGs) from the RNA-Seq analysis related to the observed phenotypic changes were selected, and qRT-PCR was performed to verify their expression changes. Briefly, Total RNA of the WT, ΔrpoN and ΔrpoN/pBAD33-rpoN+ strains was extracted using the TRIzol® Reagent (Invitrogen), followed by dissolution in RNase-Free water. cDNA synthesis was performed by using a PrimeScript™ RT reagent Kit (Takara) with 1.2 μg total RNAs in each reaction mix. Gene specific primers for the qRT-PCR are listed in Table 2. Using the cDNA as the template, qPCR analysis was conducted on an Applied Biosystems ABI 7500 sequence detection system (Applied Biosystems, Foster City, CA, USA) with the SYBR green fluorescence dye. The P. shigelloides gyrB gene was used as the internal control for qRT-PCR, and the relative expression levels were calculated as fold change values using the 2−△△CT method [81]. Each experiment was carried out in triplicate.
Motility assay and transmission electron microscopy of flagella
To measure motility, motility assays of P. shigelloides WT, ΔrpoN, and ΔrpoN/pBAD33-rpoN+ strains were performed as described previously [82]. Briefly, freshly grown bacterial colonies were transferred using a sterile toothpick onto swimming agar plates. The swimming agar plates were incubated for 12 h at 30 °C and motility was examined by the migration of bacteria through the agar from the center toward the plate periphery. We conducted the experiments at three time points with six repetitions for each time. Transmission electron microscopy (TEM) and negative staining were used to visualize the flagella of the WT, ΔrpoN, and ΔrpoN/pBAD33-rpoN+ strains, as previously described [83].
Luminescence screening assay
The procedures of the lux reporter assay were described in a previous study [84]. The amplification products of the respective promoter regions were digested and cloned into the XhoI-BamHI site, upstream of the lux genes, in the plasmid pMS402. Briefly, flaC, motA, and fliAL promoter-lux fusions were constructed in the ΔrpoN mutant and WT, and bacterial cultures were grown in LB medium at 37 °C to the mid-logarithmic phase. The cultures were transferred into a black 96-well plate with a transparent bottom. Promoter activities was measured at OD600 using a Synergy 2 plate reader (Agilent BioTek, Santa Clara, CA, USA). Primers for the lux reporter assay are listed in Table 2. Each experiment was carried out in triplicate.
Growth assay
Growth assays were performed as described previously [85]. The WT, ΔrpoN, and ΔrpoN/pBAD33-rpoN+ strains were cultured overnight at 37 °C with shaking in sterile LB medium. Then, the bacterial solution was added to five wells of a 96-well cell plate containing 200 μl of LB or DMEM at a ratio of 1:200 per well. Fresh LB or DMEM was added to the surrounding wells as controls. Finally, the prepared 96-well cell plate was placed in a Molecular Devices Spectramax 190 full-wavelength microplate reader (Molecular Devices LLC, San Jose, CA, USA) to carry out the dynamic growth experiment. We conducted the experiments at three time points with five repetitions for each time.
Killing assay
The E. coli MG1655 killing assay was carried out as described previously [86], with some modifications. Overnight cultures were diluted (1:100) in LB medium and grown aerobically at 37 °C until the optical density at 600 nm reached 1.5 for P. shigelloides and E. coli, respectively. The cells were harvested and concentrated. The predator and prey bacteria were mixed at a ratio of 1:1, and 20 µl of this mixture was placed onto LB agar plates without antibiotics. After 3 h of static incubation at 37 °C, the bacteria were removed from the LB agar plates by vortexing in 3 ml of phosphate buffered saline (PBS), and serial dilutions were spotted onto antibiotic-containing LB agar plates to enumerate colony forming units. The killing ability of ΔrpoN, ΔrpoN/pBAD33-rpoN+, and ΔT2SS-2 strains were reported as a percentage relative to that of the WT. The experiments were performed at least three times.
Invasion assay
The invasion assay was carried out as described previously [87]. Briefly, the WT, ΔrpoN, and ΔrpoN/pBAD33-rpoN+ strains were grown overnight in LB. The next day, the overnight bacterial solution was transferred to fresh LB and the bacteria were grown to OD600 = 0.6. Approximately 5 × 107 the WT, ΔrpoN, and ΔrpoN/pBAD33-rpoN+ bacterial cells were layered onto confluent monolayers of approximately 1 × 105 Caco-2 cells (a human epithelial cell line originally derived from colon carcinoma) per well in 24-well plates. The plates were centrifuged at 1000 × g for 30 s to promote sinking of the bacteria, followed by incubation at 37 °C in 5% CO2 for 1 h. The monolayer was washed extensively with PBS, and fresh, pre-warmed DMEM containing gentamycin was added to kill extracellular bacteria. After 1 h of incubation, the monolayer was washed with PBS twice, and the cells were lysed using 0.1% Triton X-100 for 10 min. The released intracellular bacteria were enumerated using the plate counting method. The invasive ability was expressed as the percentage of the inoculum that survived the gentamycin treatment. We conducted the assay at four time points with six repetitions for each time.
Statistical analysis
Statistical analysis of the data was performed using GraphPad Prism v7.0 software (GraphPad Inc., La Jolla, CAS, USA) [88]. All data are expressed as means ± standard deviation (SD). Differences between two groups were calculated using independent-samples t-test or Mann–Whitney U test. A probability value (P) ≤ 0.05 was considered statistically significant (in the figures, *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; ns indicates not significant). Construction of the RpoN evolutionary tree used the Molecular Evolutionary Genetics Analysis (MEGA 6.0) software package [89].
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the Sequence Read Archive, with accession numbers PRJNA902854.
Abbreviations
- RNA-Seq:
-
RNA transcriptome sequencing
- DEGs:
-
Differential expressed genes
- GO:
-
GeneOntology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- WT:
-
Wild-type; ΔrpoN, rpoN isogenic deletion mutant strain
- ΔrpoN/pBAD33-rpoN + :
-
Complementation strain of rpoN
- LB:
-
Luria–Bertani
- DMEM:
-
Dulbecco’s Modified Eagle’s medium
- PBS:
-
Phosphate-buffered saline
- T2SS:
-
Type II secretion system
- T6SS:
-
Type VI secretion system
- qRT-PCR:
-
Quantitative real time Polymerase Chain Reaction
References
Janda JM, Abbott SL, McIver CJ. Plesiomonas shigelloides Revisited. Clin Microbiol Rev. 2016;29(2):349–74.
Mandal BK, Whale K, Morson BC. Acute colitis due to Plesiomonas shigelloides. Br Med J (Clin Res Ed). 1982;285(6354):1539–40.
McNeeley D, Ivy P, Craft JC, Cohen I. Plesiomonas: biology of the organism and diseases in children. Pediatr Infect Dis. 1984;3(2):176–81.
Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond). 1978;80(2):275–80.
Billiet J, Kuypers S, Van Lierde S, Verhaegen J. Plesiomonas shigelloides meningitis and septicaemia in a neonate: report of a case and review of the literature. J Infect. 1989;19(3):267–71.
Fischer K, Chakraborty T, Hof H, Kirchner T, Wamsler O. Pseudoappendicitis caused by Plesiomonas shigelloides. J Clin Microbiol. 1988;26(12):2675–7.
Pennycook KM, Pennycook KB, McCready TA, Kazanowski D. Severe cellulitis and bacteremia caused by Plesiomonas shigelloides following a traumatic freshwater injury. IDCases. 2019;19: e00637.
Yu C, Yang F, Xue D, Wang X, Chen H. The Regulatory Functions of σ54 Factor in Phytopathogenic Bacteria. Int J Mol Sci. 2022;22(23):12692.
Riordan JT, Mitra A. Regulation of Escherichia coli Pathogenesis by Alternative Sigma Factor N. EcoSal Plus. 2017;7(2).
Reitzer L, Schneider BL. Metabolic context and possible physiological themes of sigma(54)-dependent genes in Escherichia coli. Microbiol Mol Biol Rev. 2001;65(3):422–44 table of contents.
Hartman CE, Samuels DJ, Karls AC. Modulating Salmonella Typhimurium’s response to a changing environment through bacterial enhancer-binding proteins and the RpoN regulon. Front Mol Biosci. 2016;3:41.
Köhler T, Harayama S, Ramos JL, Timmis KN. Involvement of Pseudomonas putida RpoN sigma factor in regulation of various metabolic functions. J Bacteriol. 1989;171(8):4326–33.
Herrera MC, Duque E, Rodríguez-Herva JJ, Fernández-Escamilla AM, Ramos JL. Identification and characterization of the PhhR regulon in Pseudomonas putida. Environ Microbiol. 2010;12(6):1427–38.
Viducic D, Murakami K, Amoh T, Ono T, Miyake Y. RpoN promotes Pseudomonas aeruginosa survival in the presence of tobramycin. Front Microbiol. 2017;8:839.
Viducic D, Ono T, Murakami K, Katakami M, Susilowati H, Miyake Y. rpoN gene of Pseudomonas aeruginosa alters its susceptibility to quinolones and carbapenems. Antimicrob Agents Chemother. 2007;51(4):1455–62.
Viducic D, Murakami K, Amoh T, Ono T, Miyake Y. RpoN modulates Carbapenem tolerance in pseudomonas aeruginosa through pseudomonas quinolone signal and PqsE. Antimicrob Agents Chemother. 2016;60(10):5752–64.
Hao B, Mo ZL, Xiao P, Pan HJ, Lan X, Li GY. Role of alternative sigma factor 54 (RpoN) from Vibrio anguillarum M3 in protease secretion, exopolysaccharide production, biofilm formation, and virulence. Appl Microbiol Biotechnol. 2013;97(6):2575–85.
Shang L, Yan Y, Zhan Y, Ke X, Shao Y, Liu Y, Yang H, Wang S, Dai S, Lu J, Yan N, Yang Z, Lu W, Liu Z, Chen S, Elmerich C, Lin M. A regulatory network involving Rpo, Gac and Rsm for nitrogen-fixing biofilm formation by Pseudomonas stutzeri. NPJ Biofilms Microbiomes. 2021;7(1):54.
Cai Z, Liu Y, Chen Y, Yam JK, Chew SC, Chua SL, Wang K, Givskov M, Yang L. RpoN regulates virulence factors of pseudomonas aeruginosa via modulating the PqsR quorum sensing regulator. Int J Mol Sci. 2015;16(12):28311–9.
Tague JG, Hong J, Kalburge SS, Boyd EF. Regulatory small RNA Qrr2 Is expressed independently of sigma factor-54 and can function as the Sole Qrr small RNA To control quorum sensing in vibrio parahaemolyticus. J Bacteriol. 2022;204(1): e0035021.
Feng L, Bi W, Chen S, Zhu J, Liu X. Regulatory function of sigma factors RpoS/RpoN in adaptation and spoilage potential of Shewanella baltica. Food Microbiol. 2021;97: 103755.
Zhang JJ, Hu WL, Yang Y, Li H, Picardeau M, Yan J, Yang XF. The sigma factor σ54 is required for the long-term survival of Leptospira biflexa in water. Mol Microbiol. 2018. https://doi.org/10.1111/mmi.13967.
Xu T, Yu M, Liu J, Lin H, Liang J, Zhang XH. Role of RpoN from Labrenzia aggregata LZB033 (Rhodobacteraceae) in formation of flagella and biofilms, motility, and environmental adaptation. Appl Environ Microbiol. 2019;85(7):e02844-e2918.
Sapi E, Theophilus PA, Pham TV, Burugu D, Luecke DF. Effect of RpoN, RpoS and LuxS pathways on the biofilm formation and antibiotic sensitivity of borrelia burgdorferi. Eur J Microbiol Immunol (Bp). 2016;6(4):272–86.
Merino S, Aquilini E, Fulton KM, Twine SM, Tomás JM. The polar and lateral flagella from Plesiomonas shigelloides are glycosylated with legionaminic acid. Front Microbiol. 2015;6:649.
Klose KE, Mekalanos JJ. Distinct roles of an alternative sigma factor during both free-swimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol Microbiol. 1998;28(3):501–20.
Correa NE, Peng F, Klose KE. Roles of the regulatory proteins FlhF and FlhG in the Vibrio cholerae flagellar transcription hierarchy. J Bacteriol. 2005;187(18):6324–32.
Prouty MG, Correa NE, Klose KE. The novel sigma54- and sigma28-dependent flagellar gene transcription hierarchy of Vibrio cholerae. Mol Microbiol. 2001;39(6):1595–609.
Stewart BJ, McCarter LL. Lateral flagellar gene system of Vibrio parahaemolyticus. J Bacteriol. 2003;185(15):4508–18.
Petersen BD, Liu MS, Podicheti R, Yang AY, Simpson CA, Hemmerich C, Rusch DB, van Kessel JC. The polar flagellar transcriptional regulatory network in Vibrio campbellii deviates from canonical vibrio species. J Bacteriol. 2021;203(20): e0027621.
Wilhelms M, Gonzalez V, Tomás JM, Merino S. Aeromonas hydrophila lateral flagellar gene transcriptional hierarchy. J Bacteriol. 2013;195(7):1436–45.
Wilhelms M, Molero R, Shaw JG, Tomás JM, Merino S. Transcriptional hierarchy of Aeromonas hydrophila polar-flagellum genes. J Bacteriol. 2011;193(19):5179–90.
Jyot J, Dasgupta N, Ramphal R. FleQ, the major flagellar gene regulator in Pseudomonas aeruginosa, binds to enhancer sites located either upstream or atypically downstream of the RpoN binding site. J Bacteriol. 2002;184(19):5251–60.
Dasgupta N, Wolfgang MC, Goodman AL, Arora SK, Jyot J, Lory S, Ramphal R. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in pseudomonas aeruginosa. Mol Microbiol. 2003;50(3):809–24.
Wu J, Newton A. Regulation of the Caulobacter flagellar gene hierarchy; not just for motility. Mol Microbiol. 1997;24(2):233–9.
Tsang J, Hoover TR. Basal body structures differentially affect transcription of RpoN- and FliA-dependent flagellar genes in helicobacter pylori. J Bacteriol. 2015;197(11):1921–30.
Poggio S, Osorio A, Dreyfus G, Camarena L. The flagellar hierarchy of Rhodobacter sphaeroides is controlled by the concerted action of two enhancer-binding proteins. Mol Microbiol. 2005;58(4):969–83.
Zhao K, Liu M, Burgess RR. Promoter and regulon analysis of nitrogen assimilation factor, sigma54, reveal alternative strategy for E. coli MG1655 flagellar biosynthesis. Nucleic Acids Res. 2010;38(4):1273–83.
Dong T, Yu R, Schellhorn H. Antagonistic regulation of motility and transcriptome expression by RpoN and RpoS in Escherichia coli. Mol Microbiol. 2011;79(2):375–86.
Coulthurst SJ. The Type VI secretion system - a widespread and versatile cell targeting system. Res Microbiol. 2013;164(6):640–54.
Joshi A, Kostiuk B, Rogers A, Teschler J, Pukatzki S, Yildiz FH. Rules of engagement: the type vi secretion system in vibrio cholerae. Trends Microbiol. 2017;25(4):267–79.
Hernandez RE, Gallegos-Monterrosa R, Coulthurst SJ. Type VI secretion system effector proteins: effective weapons for bacterial competitiveness. Cell Microbiol. 2020;22(9): e13241.
Cianfanelli FR, Monlezun L, Coulthurst SJ. Aim, load, fire: the type VI secretion system, a bacterial nanoweapon. Trends Microbiol. 2016;24(1):51–62.
Bönemann G, Pietrosiuk A, Diemand A, Zentgraf H, Mogk A. Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. EMBO J. 2009;28(4):315–25.
Kitaoka M, Miyata ST, Brooks TM, Unterweger D, Pukatzki S. VasH is a transcriptional regulator of the type VI secretion system functional in endemic and pandemic Vibrio cholerae. J Bacteriol. 2011;193(23):6471–82.
Metzger LC, Stutzmann S, Scrignari T, Van der Henst C, Matthey N, Blokesch M. Independent regulation of type VI secretion in Vibrio cholerae by TfoX and TfoY. Cell Rep. 2016;15(5):951–8.
Naskar S, Hohl M, Tassinari M, Low HH. The structure and mechanism of the bacterial type II secretion system. Mol Microbiol. 2021;115(3):412–24.
Lin Y, Jiao Y, Yuan Y, Zhou Z, Zheng Y, Xiao J, Li C, Chen Z, Cao P. Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell apoptosis via the TLR2/JNK/mitochondrial-mediated pathway. Emerg Microbes Infect. 2018;7(1):1.
Tomás A, Lery L, Regueiro V, Pérez-Gutiérrez C, Martínez V, Moranta D, Llobet E, González-Nicolau M, Insua JL, Tomas JM, Sansonetti PJ, Tournebize R, Bengoechea JA. Functional genomic screen identifies Klebsiella pneumoniae factors implicated in blocking nuclear factor κB (NF-κB) signaling. J Biol Chem. 2015;290(27):16678–97.
Jyot J, Balloy V, Jouvion G, Verma A, Touqui L, Huerre M, Chignard M, Ramphal R. Type II secretion system of Pseudomonas aeruginosa: in vivo evidence of a significant role in death due to lung infection. J Infect Dis. 2011;203(10):1369–77.
Overbye LJ, Sandkvist M, Bagdasarian M. Genes required for extracellular secretion of enterotoxin are clustered in Vibrio cholerae. Gene. 1993;132(1):101–6.
Connell TD, Holmes RK. Mutational analysis of the ganglioside-binding activity of the type II Escherichia coli heat-labile enterotoxin LT-IIb. Mol Microbiol. 1995;16(1):21–31.
Cianciotto NP. Many substrates and functions of type II secretion: lessons learned from legionella pneumophila. Future Microbiol. 2009;4(7):797–805.
Shutinoski B, Schmidt MA, Heusipp G. Transcriptional regulation of the Yts1 type II secretion system of Yersinia enterocolitica and identification of secretion substrates. Mol Microbiol. 2010;75(3):676–91.
Sikora AE, Zielke RA, Lawrence DA, Andrews PC, Sandkvist M. Proteomic analysis of the Vibrio cholerae type II secretome reveals new proteins, including three related serine proteases. J Biol Chem. 2011;286(19):16555–66.
Cianciotto NP, White RC. Expanding role of type II secretion in bacterial pathogenesis and beyond. Infect Immun. 2017;85(5):e00014-17.
Nivaskumar M, Francetic O. Type II secretion system: a magic beanstalk or a protein escalator. Biochim Biophys Acta. 2014;1843(8):1568–77.
Pugsley AP. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57(1):50–108.
Maciejewska A, Bednarczyk B, Lugowski C, Lukasiewicz J. Structural studies of the Lipopolysaccharide Isolated from Plesiomonas shigelloides O22:H3 (CNCTC 90/89). Int J Mol Sci. 2020;21(18):6788.
Bittner M, Saldías S, Altamirano F, Valvano MA, Contreras I. RpoS and RpoN are involved in the growth-dependent regulation of rfaH transcription and O antigen expression in Salmonella enterica serovar Typhi. Microb Pathog. 2004;36(1):19–24.
Wang K, Liu E, Song S, Wang X, Zhu Y, Ye J, Zhang H. Characterization of Edwardsiella tarda rpoN: roles in σ(70) family regulation, growth, stress adaption and virulence toward fish. Arch Microbiol. 2012;194(6):493–504.
Zhu L, Gong T, Wood TL, Yamasaki R, Wood TK. σ54 -Dependent regulator DVU2956 switches Desulfovibrio vulgaris from biofilm formation to planktonic growth and regulates hydrogen sulfide production. Environ Microbiol. 2019;21(10):3564–76.
Schulz T, Rydzewski K, Schunder E, Holland G, Bannert N, Heuner K. FliA expression analysis and influence of the regulatory proteins RpoN, FleQ and FliA on virulence and in vivo fitness in Legionella pneumophila. Arch Microbiol. 2012;194(12):977–89.
Lin HH, Filloux A, Lai EM. Role of recipient susceptibility factors during contact-dependent interbacterial competition. Front Microbiol. 2020;11: 603652.
Dong TG, Mekalanos JJ. Characterization of the RpoN regulon reveals differential regulation of T6SS and new flagellar operons in Vibrio cholerae O37 strain V52. Nucleic Acids Res. 2012;40(16):7766–75.
Wang Y, Li Y, Wang J, Wang X. FleQ regulates both the type VI secretion system and flagella in pseudomonas putida. Biotechnol Appl Biochem. 2018;65(3):419–27.
Sana TG, Soscia C, Tonglet CM, Garvis S, Bleves S. Divergent control of two type VI secretion systems by RpoN in pseudomonas aeruginosa. PLoS ONE. 2013;8(10): e76030.
Sheng L, Gu D, Wang Q, Liu Q, Zhang Y. Quorum sensing and alternative sigma factor RpoN regulate type VI secretion system I (T6SSVA1) in fish pathogen Vibrio alginolyticus. Arch Microbiol. 2012;194(5):379–90.
Mahmud AKMF, Nilsson K, Fahlgren A, Navais R, Choudhury R, Avican K, Fällman M. Genome-Scale Mapping Reveals Complex Regulatory Activities of RpoN in Yersinia pseudotuberculosis. mSystems. 2020;5(6):e01006-20.
Arnold WK, Savage CR, Lethbridge KG, Smith TC 2nd, Brissette CA, Seshu J, Stevenson B. Transcriptomic insights on the virulence-controlling CsrA, BadR, RpoN, and RpoS regulatory networks in the Lyme disease spirochete. PLoS ONE. 2018;13(8): e0203286.
Shao X, Zhang X, Zhang Y, Zhu M, Yang P, Yuan J, Xie Y, Zhou T, Wang W, Chen S, Liang H, Deng X. RpoN-dependent direct regulation of quorum sensing and the type VI secretion system in pseudomonas aeruginosa PAO1. J Bacteriol. 2018;200(16):e00205-e218.
Hutcheson SW, Bretz J, Sussan T, Jin S, Pak K. Enhancer-binding proteins HrpR and HrpS interact to regulate hrp-encoded type III protein secretion in pseudomonas syringae strains. J Bacteriol. 2001;183(19):5589–98.
Jovanovic M, James EH, Burrows PC, Rego FG, Buck M, Schumacher J. Regulation of the co-evolved HrpR and HrpS AAA+ proteins required for pseudomonas syringae pathogenicity. Nat Commun. 2011;2:177.
Lee JH, Sundin GW, Zhao Y. Identification of the HrpS binding site in the hrpL promoter and effect of the RpoN binding site of HrpS on the regulation of the type III secretion system in Erwinia amylovora. Mol Plant Pathol. 2016;17(5):691–702.
Ramos LS, Lehman BL, Sinn JP, Pfeufer EE, Halbrendt NO, McNellis TW. The fire blight pathogen Erwinia amylovora requires the rpoN gene for pathogenicity in apple. Mol Plant Pathol. 2013;14(8):838–43.
Yi X, Yamazaki A, Biddle E, Zeng Q, Yang CH. Genetic analysis of two phosphodiesterases reveals cyclic diguanylate regulation of virulence factors in Dickeya dadantii. Mol Microbiol. 2010;77(3):787–800.
Xi D, Jing F, Liu Q, Cao B. Plesiomonas shigelloides sipD mutant, generated by an efficient gene transfer system, is less invasive. J Microbiol Methods. 2019;159:75–80.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28(11):1947–51.
Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49(D1):D545–51.
Jiang L, Feng L, Yang B, Zhang W, Wang P, Jiang X, Wang L. Signal transduction pathway mediated by the novel regulator LoiA for low oxygen tension induced salmonella typhimurium invasion. PLoS Pathog. 2017;13(6): e1006429.
Wilhelms M, Fulton KM, Twine SM, Tomás JM, Merino S. Differential glycosylation of polar and lateral flagellins in Aeromonas hydrophila AH-3. J Biol Chem. 2012;287(33):27851–62.
Evans MR, Fink RC, Vazquez-Torres A, Porwollik S, Jones-Carson J, McClelland M, Hassan HM. Analysis of the ArcA regulon in anaerobically grown Salmonella enterica sv. Typhimurium. BMC Microbiol. 2011;11:58.
Li Y, Yan J, Guo X, Wang X, Liu F, Cao B. The global regulators ArcA and CytR collaboratively modulate vibrio cholerae motility. BMC Microbiol. 2022;22(1):22.
Yan J, Li Y, Guo X, Wang X, Liu F, Li A, Cao B. The effect of ArcA on the growth, motility, biofilm formation, and virulence of Plesiomonas shigelloides. BMC Microbiol. 2021;21(1):266.
Borgeaud S, Metzger LC, Scrignari T, Blokesch M. The type VI secretion system of Vibrio cholerae fosters horizontal gene transfer. Science. 2015;347(6217):63–7.
Schubert RH, Holz-Bremer A. Cell adhesion of Plesiomonas shigelloides. Zentralbl Hyg Umweltmed. 1999;202(5):383–8.
Yang S, Xi D, Wang X, Li Y, Li Y, Yan J, Cao B. Vibrio cholerae VC1741 (PsrA) enhances the colonization of the pathogen in infant mice intestines in the presence of the long-chain fatty acid, oleic acid. Microb Pathog. 2020;147: 104443.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–9.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Key Programs for Infectious Diseases of China (grant numbers 2017ZX10303405-001, 2017ZX10104002-001–006, 2018ZX10712001-017). The funding bodies had no role in the design of the study, collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Junxiang Yan: Investigation, Conceptualization, Project administration, Methodology, Writing-original draft. Xueqian Guo: Project administration, Methodology. Jinghao Li: Data curation, Formal analysis. Yuehua Li: Methodology, Formal analysis. Hongmin Sun: Software, Visualization. Ang Li: TEM, negative staining. Boyang Cao: Investigation, Conceptualization, Writing-original draft, Funding acquisition, Supervision, Writing—review & editing. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yan, J., Guo, X., Li, J. et al. RpoN is required for the motility and contributes to the killing ability of Plesiomonas shigelloides. BMC Microbiol 22, 299 (2022). https://doi.org/10.1186/s12866-022-02722-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-022-02722-8