Abstract
Background
The early 2 factor (E2F) family is characterized as a kind of transcription factor that plays an important role in cell division, DNA damage repair, and cell size regulation. However, its stress response has not been well revealed.
Results
In this study, ZmE2F members were comprehensively identified in the maize genome, and 21 ZmE2F genes were identified, including eight E2F subclade members, seven DEL subfamily genes, and six DP genes. All ZmE2F proteins possessed the DNA-binding domain (DBD) characterized by conserved motif 1 with the RRIYD sequence. The ZmE2F genes were unevenly distributed on eight maize chromosomes, showed diversity in gene structure, expanded by gene duplication, and contained abundant stress-responsive elements in their promoter regions. Subsequently, the ZmE2F6 gene was cloned and functionally verified in drought response. The results showed that the ZmE2F6 protein interacted with ZmPP2C26, localized in the nucleus, and responded to drought treatment. The overexpression of ZmE2F6 enhanced drought tolerance in transgenic Arabidopsis with longer root length, higher survival rate, and biomass by upregulating stress-related gene transcription.
Conclusions
This study provides novel insights into a greater understanding and functional study of the E2F family in the stress response.
Similar content being viewed by others
Background
Environmental stimuli, including drought, salinity, and high temperature, have frequently occurred in recent decades and led to yield loss of crops in agricultural production. Drought stress is a main misfortune in these abiotic stressors and will be a more severe challenge and threat to agriculture and humanity by 2050 [1]. In adaption to drought, plants activate a series of physiological, morphological, and molecular changes [1,2,3]. Among them, many transcription factors (TFs) can be dominated by stress to regulate gene expression and coordinate plant antagonism to adverse factors [4,5,6].
The early E2 factor (E2F) family proteins are initially discovered as TFs of the E2 gene and play key roles in cell proliferation control in adenoviruses [7]. E2Fs can be classified into typical and atypical E2Fs according to protein structure. Typically, E2Fs have only one DNA-binding domain (DBD) and form heterodimeric complexes with dimerization proteins (DPs) to bind the promoters of downstream genes, but atypical E2Fs possess duplicated two DBD [8]. In higher plants, E2Fs are also categorized into three subclades, including E2F, DP, and DEL (DP-E2F-like), due to the difference in the composition of conserved domains [9, 10]. Over the past decade, E2Fs have well been revealed to play significant roles in the cell cycle and DNA damage repair [11,12,13,14,15,16]. For instance, ectopic expression of DcE2F1 of Daucus carota promotes cell proliferation in Arabidopsis seedlings [14]. In Arabidopsis, eight E2F members exhibit antagonistic roles in cell proliferation, such as E2Fa/b, which acts as a positive regulator, but E2Fc is a negative regulator [17,18,19]. E2Fa/b can also activate the DNA damage response and cell cycle progression by differentially regulating the expression of genes [11]. Additionally, AtE2Fa/DPa also inhibits growth, and AtE2Fa/DPa-overexpressing plants show an abnormal phenotype owing to ectopic cell division or enhanced DNA endoreduplication [12, 20].
In addition to the crucial role in the cell cycle, few reports show that atypical E2F inhibits the accumulation of salicylic acid to balance growth and defense [21, 22]. Furthermore, AtE2Fa acts downstream of ERECTA kinase, which is involved in cell size and stomatal density [23]. Via expression analysis, it is suggested that TaE2F-DP of wheat, PheE2F/DPs of Moso bamboo, E2F/DP genes of Medicago truncatula, and PvE2F/DPs of Phaseolus vulgaris respond to drought or salt stress, suggesting their potential roles in regulating stress tolerance [9, 24,25,26]. To date, however, the role of E2F in plant stress tolerance remains obscure.
Maize is a crucial crop worldwide and is widely used as food and livestock feed. During its growth, maize plants show sensitivity to water deficit due to its high water demand, leading to maize yield being greatly affected by drought stress [27,28,29,30]. Therefore, it becomes imperative to identify and explore drought tolerance-related genes that can be used to enhance maize resilience through molecular breeding [31,32,33,34,35]. In our previous study, we found that ZmPP2C26 regulated drought tolerance [36] and targeted maize ZmE2F (Zm00001d048412, data not shown), indicating that ZmE2F might be involved in drought response. Hence, in this study, we comprehensively investigated ZmE2F genes in the maize genome. Thereafter, phylogenetic relationships, conserved motifs and domains, gene structures and duplication, and protein-protein interaction networks were analyzed. Additionally, the ZmE2F6 (Zm00001d048412) was functionally verified by performing subcellular localization, expression patterns in drought treatment, and ectopic expression in Arabidopsis under drought stress. This study will significantly contribute to a better understanding of E2Fs in stress response.
Methods
Identification of ZmE2Fs in the maize genome
The genome and amino acid data of maize B73 were downloaded from the MaizeGDB database (https://download.maizegdb.org/Zm-B73-REFERENCE-GRAMENE-4.0/). Meanwhile, the coding sequences and amino acid sequences of 8 AtE2Fs and 9 OsE2Fs of Arabidopsis and rice were downloaded from the Arabidopsis Information Resource (TAIR) (https://www.arabidopsis.org/) and the Rice Genome Annotation Project (RGAP) database (http://rice.uga.edu/) and were used as queries to perform local BLASTp with an E-value of 1e− 10 in the maize protein database for maize E2F searching, respectively. After removing the redundant sequences manually, the candidate sequences were further analyzed for the presence of the E2F_DP domain (PF02319) by using PFAM (http://pfam.xfam.org/). The candidates possessing the E2F domain were identified as maize ZmE2F members. The secondary structure and physicochemical properties, including molecular weights, isoelectric point (PI), stability coefficient, and grand average of hydropathicity (GRAVY), of ZmE2Fs were analyzed using SOPMA (https://npsa.lyon.inserm.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html) and EXPASY (https://www.expasy.org/). The subcellular localization of ZmE2Fs was predicted using cNLS Mapper (https://nls-mapper.iab.keio.ac.jp/cgi-bin/NLS_Mapper_form.cgi).
Conserved motifs, domains, and phylogenetic analysis
To further identify the conserved motifs and domains, the amino acid sequences of ZmE2Fs were analyzed using MEME (http://meme-suite.org/tools/meme) and NCBI-CDD (https://www.ncbi.nlm.nih.gov/cdd), respectively. The motif and domain composition of each ZmE2F was visualized by TBtools [37]. The protein sequences of all ZmE2F, AtE2F, and OsE2F were multiple-aligned using ClustalW with default parameters. The maximum likelihood tree was built with 1000 bootstrap replications by MEGA11 (https://www.megasoftware.net/). Meanwhile, protein-protein interaction (PPI) analysis among ZmE2F members was performed using the STRING tool [38].
Gene structure, promoter, duplication, and synteny analyses
The chromosomal location of each ZmE2F gene was obtained from the maizeGDB database. The coding sequences and genomic DNA sequences of every ZmE2F were downloaded and used to analyze exon-intron composition using Gene Structure Display Server 2.0 (GSDS) (http://gsds.gao-lab.org/). The 2000 bp upstream sequence of the transcription start site of each ZmE2F gene was retrieved from maizeGDB and used for cis-acting element analysis using PlantCARE (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/). Meanwhile, the gene duplication events and the synteny relationship between ZmE2F, AtE2F, and OsE2F gene members were analyzed using MCScanX with default parameters. The chromosomal location, duplications, and synteny relationships of the ZmE2F, AtE2F, and OsE2F genes were visualized using TBtools [37]. The non-synonymous (Ka) and synonymous (Ks) substitution rates per site of the duplicated gene pairs were calculated using TBtools [37]. Then, the divergence time in millions of years (Mya) was also calculated using the following formula: T = Ks/2λ × 10− 6 Mya (λ = 6.5 × 10− 9 for grasses) [39].
Cloning and subcellular localization of the ZmE2F6 gene
The specific primers (Table S1) were designed by Primer 5.0, synthesized at Tsingke Biotech (Beijing, China), and used to amplify the sequence of the ZmE2F6 gene from maize B73 cDNA using Phanta Max Super-Fidelity DNA Polymerase (Vazyme, Nanjing). After amplification, the PCR product was purified by a gel recovery kit subcloned, and inserted into the pMD19-T vector to generate pMD19-T-ZmE2F6 and verified by sequencing. The sequencing result was aligned with the candidate sequence of the ZmE2F6 gene using DNAMAN. The open reading frame (ORF) sequence of ZmE2F6 without stop codon was amplified from the pMD19-T-ZmE2F6 plasmid using the specific primers designed by CE Design V1.04 (Table S1) with the Xba I and Spe I recognition sites. The PCR products and pCAMBIA2300-35 S-eGFP plasmids were digested using Xba I and Spe I. Subsequently, they were inserted into the Xba I and Spe I sites of pCAMBIA2300-35 S-eGFP to produce the fusion expression vector 35 S-ZmE2F6-eGFP using the ClonExpress II One Step Cloning Kit (Vazyme, Nanjing). Each construct was introduced into Agrobacterium tumefaciens strain GV3101 and then used for transient expression in the leaves of Nicotiana benthamiana. As described by Sun et al. [40], the constructs were infiltrated into the leaves of five-week-old N. benthamiana. The GFP fluorescence was observed and imaged using a confocal laser scanning microscope (Zeiss 800). The empty vector 35 S-eGFP was served as the positive control.
Plant treatment, RNA extraction, and quantitative real-time PCR (qRT‒PCR) analysis
The seeds of maize B73 lines were soaked in 10% H2O2 for 15 min, rinsed twice using sterile water, soaked in distilled water for 8 h, then wrapped in filter paper and cultured at 28 °C until germination. Subsequently, the seedlings of the same size were transferred to the hydroponic cassette containing hoagland nutrient solution and cultured at 16 h light at 28 °C /8 h dark at 24 °C. Three-leaf-stage seedlings were subjected to 16% PEG-6000 treatment mimicking drought stress, and then shoots containing leaf, stem and leaf sheath, and roots were sampled at 0, 3, 6, 12, and 24 h of treatment, respectively. The total RNA of each sample was extracted using an RNAison plus kit (Takara, Dalian), examined for quality using NanoDrop OneC (ThermoFisher Scientific), treated with DNase to remove DNA contamination, reverse-transcribed into cDNA using a PrimeScript™ RT Regent Kit (Takara, Dalian), and used to perform qRT-PCR. The specific primers of ZmE2F6 were designed using Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome), synthesized at TsingkeBiotech (Beijing, China), and listed in Table S1. The ZmGAPDH gene was amplified using specific primers (Table S1) and used as an internal reference. The qRT-PCR was performed in the Bio-Rad CFX96™ Real-Time PCR system using 2 × Universal SYBR Green Fast qPCR Mix (ABclonal, Wuhan). The 20 µL reaction mixture contained 10 µL of 2 × Universal SYBR Green Fast qPCR Mix, 0.4 µL of each forward and reverse primer, 1.0 µL of each cDNA as template, and 8.2 µL of ddH2O. The reaction protocol was set as a two-step temperature cycle including 95 °C for 3 min, followed by 40 cycles at 95 °C for 5 s and 60 °C for 30 s. The relative expression level of ZmE2Fs was calculated and normalized using the 2−ΔΔCt method [41].
Y2H and GST pull-down analysis
The ORF of ZmE2F6 was amplified using specific primers designed by CE Design V1.04 with the Nde I and EcoR I recognition sites (Table S1) and inserted into the pGADT7 plasmid to generate AD-ZmE2F6 as described above. The BD-ZmPP2C26 plasmid was constructed in our previous study [36]. The AD-ZmE2F6 and BD-ZmPP2C26 plasmids were cotransformed into the yeast strain Y2H Gold using a yeast transformation kit (Coolaber, Beijing). Subsequently, yeast cells were cultured on synthetic dropout (SD) medium without Trp and Leu (SD/-Trp/-Leu) at 30℃ for 2 days, and then positive clones were transferred onto SD/-Trp/-Leu/-His/-Ade plates with X-α-gal and cultured at 30℃ for 2 days. Meanwhile, the ORF of ZmE2F6 was amplified and inserted into the EcoR I and Xho I sites of pGEX-6P-1 to generate GST-ZmE2F6 and used for the GST pull-down assay. The His-ZmPP2C26 plasmids were produced in our previous study. The GST pull-down was performed as described by Lu et al. [36].
Plant transformation, phenotyping, and RNA-seq
The ORF of ZmE2F6 was amplified without a stop codon and inserted into the Xba I and Nde I sites of pRI201-35 S-GUS to produce 35 S-ZmE2F6-GUS as described above. The 35 S-ZmE2F6-GUS construct was transformed into Agrobacterium tumefaciens strain GV3101 and then used to transform Arabidopsis thaliana (Col-0) by the floral-dip method [42]. According to the method of Sun et al. [40], the positive transformants were screened on 1/2 MS plates with 50 mg/L kanamycin, used for harvesting seeds individually. The homozygous lines without segregation on 1/2 MS plates with 50 mg/L kanamycin were screened and used for PCR detection. Meanwhile, the leaves of homozygous lines were sampled and used to perform GUS staining using the GUS Staining Kit (Coolaber, Beijing).
According to the methods described by Sun et al. [40] with minor modifications, for drought stress, the seeds of homozygous lines and wild type (WT) were surface-sterilized, planted on 1/2 MS plates supplemented with 0 (control), 150, and 250 mM mannitol, vernalized for 2 days in the dark at 4 °C, and vertically cultured in a chamber under 10 h light/14 h dark at 22 °C with 60–70% humidity. At 14 days of treatment, the seedlings were photographed and measured for root length. Moreover, another batch of overexpressed lines and WT were sown in soil and incubated in a greenhouse under the same conditions. After 2 weeks, the seedlings were subjected to withholding water for two weeks, then rewatered for at least 2 days, and monitored for phenotyping. Subsequently, the survival number of each line was counted and used to calculate the survival rate. The leaf of each line was sampled, dried at 80 °C for 3 days, and used for biomass measurement.
Meanwhile, three-week-old seedlings of homozygous lines and WT were sampled and used for RNA sequencing at Sanshubio Company (Jiangsu, China). As described by Sun et al. [40], the total RNA of each sample was extracted, qualified for quality and integrity, and used to construct a sequencing library. Then, library sequencing was conducted using the NovaSeq 6000 system. The sequencing adapters and low-quality reads of raw data were removed to generate clean data, which were mapped to the Arabidopsis genome by hisat2 [43] and used for assembling transcripts of every gene using StringTie [44]. The differentially expressed genes (DEGs) were confirmed with a p-value < 0.05 and |FoldChange| > 2 using DESeq2 [45]. The GO analysis of DEGs was performed using KOBAS [46].
Data analysis
All assays were performed with three replicates. The data are shown as the mean values ± standard error (SE). The significance was analyzed by Student’s t-test at the p < 0.05 or p < 0.01 level.
Results
ZmE2F members in maize
To identify the maize ZmE2F family, a local BLASTp search against the maize protein database was performed using the amino acid sequences of 8 AtE2F and 9 OsE2F members as query references [10, 47]. As shown in Table 1, a total of 21 ZmE2F members were identified and designated as ZmE2F1 to ZmE2F21. The CDS length of the ZmE2F genes ranged from 648 (ZmE2F18) to 1608 bp (ZmE2F14), encoding 215 to 535 amino acids with molecular weight varying from 23.39 to 59.21 kDa. The theoretical isoelectric point (pI) of ZmE2F proteins ranged from 4.71 (ZmE2F11) to 9.41 (ZmE2F13), and the grand average hydropathicity (GRAVY) of all ZmE2F proteins was less than 0 and ranged from − 0.062 (ZmE2F1) to -0.603 (ZmE2F5). The instability indices of ZmE2F proteins varied from 31.45 (ZmE2F20) to 62.39 (ZmE2F5), and 19 ZmE2F members contained the highest random coil in their secondary structure, implying that they were unstable hydrophilic proteins. All ZmE2F proteins were predicted to be localized in the nucleus.
Phylogenetic analysis
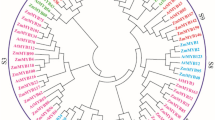
To investigate the evolutionary relationships of ZmE2Fs and model plants, a total of 38 amino acid sequences, including 21, 8, and 9 E2Fs from maize, Arabidopsis, and rice, respectively, were used to construct a phylogenetic tree (Fig. 1). It showed that the ZmE2F proteins could be classified into three subfamilies, including E2F, DP, and DEL clades [9]. Eight members, including ZmE2F2, 4, 5, 11, 14, 16, 17, and 21, were clustered into the E2F subscale with AtE2Fs and OsE2Fs. Seven proteins containing ZmE2F1, 7, 10, 12, 13, 15, and 20 were branched into the DEL subfamily with AtDELs and OsDELs. The other 6 ZmE2Fs (ZmE2F3, 6, 8, 9, 18, and 19) belonged to the DP subclade with AtDP and OsDP members. In addition, ZmE2Fs showed a closer phylogenetic relationship with OsE2Fs than with AtE2Fs, indicating more sequence similarity with OsE2Fs.
Conserved motif and domain
Five conserved motifs were identified in the amino acid sequences of ZmE2Fs using the MEME online program (Fig. 2A). Among them, motif 1 was highly conserved in all ZmE2F members and characterized by the RRIYD sequence that was a DNA-binding motif followed by dimerization residues DNVLE sequence [48]. Conserved domain analysis revealed that all ZmE2F members contained at least one DNA-binding domain (DBD) (Pfam ID PF02319, Table S2). ZmE2F7, 12, and 13 possessed two DBDs (Fig. 2B). In addition, ZmE2F5, 6, 9, 18, and 19 also contained a dimerization domain (DD). The coiled coil (CC)-marked box (MB) domain (CC-MB) was found in ZmE2F4, 11, 16, and 17. Furthermore, all ZmE2F members contained a nuclear localization signal.
ZmE2Fs protein-protein Interaction Prediction
Due to the presence of dimerization residues DNVLE sequence within motif 1 in every ZmE2F and DD or CC-MB in some ZmE2Fs, to explore the potential interactions among ZmE2F members, protein-protein interaction (PPI) analysis was performed. As shown in Fig. 3, ten ZmE2Fs were predicted to interact with each other, which generated 23 PPI combinations. Among them, ZmE2F6, 9, 18 and 19 had the largest number of PPIs (6 interactions), while ZmE2F4, 5, 11, 14, 16, and 17 had 4 PPIs. These results imply that the DNVLE sequence, DD, and CC-MB play crucial roles during ZmE2F dimerization.
Gene structure, duplication, synteny, and cis -acting elements
Gene structure analysis revealed that the ZmE2F gene family showed diversity in exon and intron composition (Fig. 4A). The number of exons among ZmE2Fs ranged from six to fourteen. Except for ZmE2F18 and ZmE2F20, the other 19 ZmE2Fs contained 5ʹ or 3ʹ terminal untranslated regions. The ZmE2Fs were unevenly distributed on eight maize chromosomes, excluding chromosomes 3 and 7. Gene duplication analysis showed that there were eight paralogous pairs of ZmE2Fs in the maize genome, including ZmE2F4 and ZmE2F17, ZmE2F6 and ZmE2F18, ZmE2F6 and ZmE2F19, ZmE2F7 and ZmE2F12, ZmE2F7 and ZmE2F13, ZmE2F11 and ZmE2F17, ZmE2F12 and ZmE2F13, and ZmE2F18 and ZmE2F19. Likewise, gene synteny analysis revealed one and twenty-two orthologous pairs between ZmE2F and AtE2F and ZmE2F and OsE2F, respectively (Fig. 4B; Table S3). Meanwhile, the Ka/Ks rates among ZmE2Fs paralogous pairs ranged from 0.19 to 0.35 and their duplication time was estimated to be 16.54–139.30 million years ago (Table S4). The results suggest that gene duplication contributed to E2F expansion during the evolutionary process.
Gene structure (A) and duplication (B) of ZmE2Fs. Yellow and blue boxes represent exons and untranslated regions, respectively. Black lines represent introns. Yellow, blue, and red boxes with a number represent chromosomes of maize, Arabidopsis, and rice, respectively. The green, blue, and red lines indicate duplicated E2F gene pairs among maize, as well as between maize and Arabidopsis and rice, respectively
Furthermore, cis-acting element analysis revealed that abundant stress- or hormone-responsive elements were found in the promoter regions of ZmE2Fs, such as ABREs, MBSs, AREs, and LTR elements (Table S5), which were responsive to abscisic acid, drought, anaerobic induction, and low temperature. For instance, ABREs were found in all ZmE2F promoters. MBS elements, the MYB binding site involved in drought response, were found in 15 ZmE2F promoters. This finding implies that the ZmE2F genes may be involved in abiotic stress responses.
ZmE2F6 interacts with ZmPP2C26
In our previous study, ZmE2F6 was identified as a potential target of two ZmPP2C26 splicing variants (ZmPP2C26L/S) via Y2H library screening. Hence, to verify whether ZmE2F6 interacts with ZmPP2C26, Y2H, and GST pull-down assays were performed. As shown in Fig. 5, the yeast cells transformed with AD-ZmE2F6 and BD-ZmPP2C26L/S exhibited normal growth and turned blue on SD/-Leu/-Trp/-His/-Ade plates containing X-α-gal. Moreover, the GST-ZmE2F6 protein could be pulled down by His-ZmPP2C26 L/S in the GST pull-down assay. These findings confirmed the interaction between ZmE2F6 and ZmPP2C26L/S. Likewise, there were 7 potential phosphorylated sites in ZmE2F6 amino acid sequences (Table S6) were predicted using NetPhos-3.1 (https://services.healthtech.dtu.dk/services/NetPhos-3.1/).
ZmE2F6 localized to the nucleus
To further validate the cellular localization of the ZmE2F6 protein, the ORF of ZmE2F6 was cloned and inserted into the 35 S-eGFP plasmid to fuse with eGFP. The results of transient expression in tobacco leaves revealed that fluorescent signals were observed in whole cells, including the nucleus and cytoplasm, transformed by the 35 S-eGFP empty vector. However, in tobacco leaves transformed with 35 S-ZmE2F6-eGFP, fluorescent signals were exclusively detected in the nucleus (Fig. 6). This observation is consistent with the bioinformatic prediction, confirming that the ZmE2F6 transcription factor is solely localized in the nucleus.
The expression of ZmE2F6 was induced by drought stress
The qRT-PCR results demonstrated that the expression of the ZmE2F6 gene was responsive to drought stress (Fig. 7). After drought treatment, the expression of ZmE2F6 in maize shoots was significantly upregulated and reached approximately 11-, 8-, 100-, and 47-fold that of the control at 3, 6, 12, and 24 h of treatment. In roots, the expression of ZmE2F6 was significantly upregulated after 3 h of drought treatment and then downregulated at 6, 12, and 24 h of treatment. These results suggest that ZmE2F6 responds to drought stress.
Expression of ZmE2F6 enhanced drought tolerance in Arabidopsis thaliana
To assess the function of ZmE2F6 in regulating drought tolerance, we generated transgenic Arabidopsis lines overexpressing ZmE2F6. In the T1 generation, ten positive transgenic lines were screened by kanamycin on 1/2 MS plates, identified by PCR, and harvested to produce the next generation. Finally, in the T3 generation, two homozygous lines (OE6-5 and OE6-10) were selected, identified by GUS staining, and used for phenotyping (Fig. 8A). It was found that the root length of the OE6-5 and OE6-10 lines was significantly longer than that of the WT under 1/2 MS plates or supplemented with 150 mM mannitol (Fig. 8B, C). Furthermore, natural drought treatment in soil was performed to monitor the drought tolerance of the OE6-5 and OE6-10 lines. The results showed that there was no difference between the transgenic lines and WT before treatment. Subsequently, after two weeks of drought treatment, WT plants were seriously wilting, but OE6-5 and OE6-10 showed slightly inhibited phenotypes. After 3 days of rewatering, the OE-65 and OE6-10 lines exhibited significantly higher survival rates and biomass, indicating that overexpression of ZmE2F6 contributes to enhancing drought tolerance in transgenic Arabidopsis and that ZmE2F6 positively regulates drought tolerance.
The phenotype of transgenic Arabidopsis under drought stress. (A) PCR detection and GUS staining of transgenic lines. (B) The phenotype of transgenic lines on 1/2 MS plates with mannitol. (C) Root length. (D) The phenotype of transgenic lines in soil. (E) Survival rate and biomass of each line. OE6-1 to OE6-10 represent transgenic lines overexpressing ZmE2F6. WT, wild type
To investigate the impact of ZmE2F6 overexpression on endogenous genes in Arabidopsis thaliana, RNA-Seq was conducted on four transgenic lines, OE6-5, OE6-10, and WT. The results showed that there were 657 DEGs in transgenic lines compared to WT. Totally, 19 DEGs were identified in two transgenic lines. Among them, 15 DEGs were upregulated in transgenic lines, including AtMYB44 (AT5G67300), AtB1L (AT1G18740), AtJAZ7 (AT2G34600), AtEXS (AT1G35350), AtIP5PII (AT4G18010), AtPATL2 (AT1G22530), AtAZI1 (AT4G12470), AtXTH23 (AT4G25810), and AtEXL5 (AT2G17230) (Fig. 9). GO analysis showed that these DEGs were associated with stress responses (Figure S1).
Discussion
In eukaryotes, the ZmE2F family is characterized as a TF that plays crucial roles in cell division, DNA repair, and differentiation [11, 13,14,15,16]. However, the E2F family is only genome-wide identified in a few plants, including Arabidopsis, rice, wheat, Moso bamboo, Medicago truncatula, and Phaseolus vulgaris [9, 24,25,26]. In the present study, 21 ZmE2F members were identified in the maize genome (Table 1) and classified into E2F, DP, and DEL subclades (Fig. 1), which was higher than the number of E2F members identified in Arabidopsis (8), rice (9), Medicago truncatula (5), and Phaseolus vulgaris (7) but close to the number in wheat (27) and Moso bamboo (23), owing to their comparable genome size and gene duplication being a major driving force of gene families [25, 49]. Likewise, eight paralogous pairs of ZmE2Fs and twenty-three orthologous pairs of E2Fs among maize, Arabidopsis, and rice were found (Fig. 4). All the Ka/Ks ratios of the ZmE2Fs paralogous pairs were < 1 (Table S4), indicating their duplication evolved under purifying selection [50]. A similar phenomenon was consistently observed in the PheE2F/DP gene family [25]. A previous study showed that there are 12 ZmE2F genes in maize [51], which is much lower than the number identified in this study. This may be due to their preliminary BLAST search using maize genome release 5b.60 and not a comprehensive analysis [51].
All ZmE2F proteins possess at least one DBD characterized by motif 1 containing the RRIYD sequence and dimerization residue DNVLE sequence (Fig. 2), which contributes to the binding of E2F and DNA as a homodimer or as a heterodimer with its dimerization partner DP. In addition, some ZmE2F proteins also have DD and CC-MB domains, which promotes their formation of heterodimers to regulate downstream genes [52]. As a result, ten ZmE2Fs are predicted to interact with each other and generate 23 PPI combinations (Fig. 3), owing to the presence of the DNVLE residues, DD, or CC-MB domain in these proteins.
To date, the function of E2F in regulating plant stress tolerance remains unknown, although few reports have shown that the expression of some E2F genes is responsive to stress [9, 24,25,26]. In our study, abundant stress-responsive acting elements were found in ZmE2F promoter regions, such as ABREs and MBSs, suggesting the response of ZmE2Fs to stress and the potential roles of ZmE2Fs. In our previous study, the ZmPP2C26 gene, a B clade of maize PP2C members, was found to be responsive to drought stress and negatively regulated drought tolerance in Arabidopsis, rice, and maize [36] and targeted on maize ZmE2F6 (Zm00001d048412), indicating its function in the drought response. Hence, the ZmE2F6 gene was cloned and functionally validated. It is found that the ZmE2F6 interacts with two splicing variants of ZmPP2C26 but localized in the nucleus (Figs. 5 and 6), suggesting that ZmPP2C26 physically targets ZmE2F6. In Arabidopsis, it has been previously reported that B clade PP2C (AP2C1) dephosphorylates the autophosphorylated form of CBL-interacting protein kinase 9 (CIPK9) to regulate root growth, seedling development, and stress tolerance (low-K+) [53]. Previous studies showed that BES1/BZR1 TFs are phosphorylated and degraded but moved to the nucleus after dephosphorylation in the cytoplasm [54, 55]. It is proposed that ZmPP2C26 might dephosphorylate ZmE2F6 in the cytoplasm, which needs to be further revealed in our next study.
The qRT-PCR results showed that the ZmE2F6 gene was induced by drought stress (Fig. 7), which could be explained by the presence of three MBS and two ABREs in the ZmE2F6 promoter (Table S5). It’s confirmed that MYB transcription factors bind to the MYB binding sites (MBS) of nuclear gene promoters to adjust their transcription and regulate drought tolerance in plants [56, 57]. Likewise, ABRE (ABA-responsive element), the major cis-element for ABA-responsive gene expression, is targeted by ABRE-binding protein (AREB) or ABRE-binding factor (ABF) TFs to regulate the drought response via the ABA signaling pathway [58]. These findings further imply the regulation of ZmE2F6 in drought tolerance.
After overexpressing ZmE2F6 in Arabidopsis, the transgenic lines showed longer root lengths than the WT (Fig. 8B, C), which is consistent with the fact that E2Fs regulate root growth in Arabidopsis [19, 22, 59]. The elevated root growth of ZmE2F6-overexpressing lines may confer osmotic tolerance because roots can respond to moisture and coordinate responses to drought [1]. The ectopic expression of ZmE2F6 enhances drought tolerance in transgenic Arabidopsis (Fig. 8). We found that some stress-related genes were significantly upregulated in the transgenic lines, such as AtMYB44, AtB1L, AtJAZ7, AtEXS, AtIP5PII, AtPATL2, AtAZI1, AtXTH23, and AtEXL5 (Fig. 9), which are well known to regulate abiotic stresses [60,61,62,63,64,65,66,67,68]. The ZmE2F6 protein acts as a TF and localizes in the nucleus to regulate the expression of these genes (Fig. 6). However, the molecular mechanism regulated by ZmE2F6 in maize remains unknown.
Conclusion
Overall, in this study, a comprehensive analysis of the ZmE2F gene family was performed. In total, 21 ZmE2F TFs were identified in the maize genome and divided into three subclades. All ZmE2F proteins possessed at least one DBD characterized by the RRIYD (DNA binding motif) and DNVLE (dimerization residues) sequences. The ZmE2F genes showed diversity in gene structure, expanded by gene duplication, and contained abundant stress-responsive elements in their promoter regions. Then, the ZmE2F6 gene was cloned and functionally verified in the drought response. The ZmE2F6 protein interacted with ZmPP2C26, localized in the nucleus, and responded to drought treatment. The overexpression of ZmE2F6 enhanced drought tolerance in transgenic Arabidopsis by upregulating stress-related gene transcription. This study sheds light on the role of ZmE2F in the drought response and provides novel insights into a greater understanding of the E2F family in crops.
Data availability
The RNA-seq datasets generated during the current study are available in the NCBI repository under BioProject accession number PRJNA1028664.
References
Gupta A, Rico-Medina A, Caño-Delgado AI. The physiology of plant responses to drought. Science. 2020;368:266–9.
Zhang H, Zhu J, Gong Z, Zhu J. Abiotic stress responses in plants. Nat Rev Genet. 2022;23:104–19.
Zhu JK. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–24.
Hrmova M, Hussain SS. Plant transcription factors involved in drought and associated stresses. Int J Mol Sci. 2021;22:5662.
Manna M, Thakur T, Chirom O, Mandlik R, Deshmukh R, Salvi P. Transcription factors as key molecular target to strengthen the drought stress tolerance in plants. Physiol Plant. 2021;172:847–68.
Saharan BS, Brar B, Duhan JS, Kumar R, Marwaha S, Rajput VD, et al. Molecular and physiological mechanisms to mitigate abiotic stress conditions in plants. Life (Basel). 2022;12:1634.
Nevins JR. E2F: a link between the rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–9.
Van Den Heuvel S, Dyson NJ. Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol. 2008;9:713–24.
Ma TY, Li ZW, Zhang SY, Liang GT, Guo J. Identification and expression analysis of the E2F/DP genes under salt stress in Medicago truncatula. Genes Genomics. 2014;36:819–28.
Vandepoele K, Raes J, De Veylder L, Rouzé P, Rombauts S, Inzé D. Genome-wide analysis of core cell cycle genes in Arabidopsis. Plant Cell. 2002;14:903–16.
Gómez MS, Sheridan ML, Casati P. E2Fb and E2Fa transcription factors independently regulate the DNA damage response after ultraviolet B exposure in Arabidopsis. Plant J. 2022;109:1098–115.
Liu Y, Lai J, Yu M, Wang F, Zhang J, Jiang J, et al. The Arabidopsis SUMO E3 ligase AtMMS21 dissociates the E2Fa/DPa complex in cell cycle regulation. Plant Cell. 2016;28:2225–37.
Manickavinayaham S, Dennehey BK, Johnson DG. Direct regulation of DNA repair by E2F and RB in mammals and plants: core function or convergent evolution? Cancers (Basel). 2021;13:1–14.
Perrotta L, Giordo R, Francis D, Rogers HJ, Albani D. Molecular analysis of the E2F/DP gene family of daucus carota and involvement of the DcE2F1 factor in cell proliferation. Front Plant Sci. 2021;12:652570.
Wang L, Chen H, Wang C, Hu Z, Yan S. Negative regulator of E2F transcription factors links cell cycle checkpoint and DNA damage repair. Proc Natl Acad Sci U S A. 2018;115:E3837–45.
Yuan R, Liu Q, Segeren HA, Yuniati L, Guardavaccaro D, Lebbink RJ, et al. Cyclin F-dependent degradation of E2F7 is critical for DNA repair and G2-phase progression. EMBO J. 2019;38:e101430.
Del Pozo JC, Boniotti MB, Gutierrez C. Arabidopsis E2Fc functions in cell division and is degraded by the ubiquitin-SCFAtSKP2 pathway in response to light. Plant Cell. 2002;14:3057–71.
Del Pozo JC, Diaz-Trivino S, Cisneros N, Gutierrez C. The balance between cell division and endoreplication depends on E2FC-DPB, transcription factors regulated by the ubiquitin-SCFSKP2A pathway in Arabidopsis. Plant Cell. 2006;18:2224–35.
Sozzani R, Maggio C, Varotto S, Canova S, Bergounioux C, Albani D, et al. Interplay between Arabidopsis activating factors E2Fb and E2Fa in cell cycle progression and development. Plant Physiol. 2006;140:1355–66.
De Veylder L, Beeckman T, Beemster GTS, De Almeida Engler J, Ormenese S, Maes S, et al. Control of proliferation, endoreduplication and differentiation by the Arabidopsis E2Fa-DPa transcription factor. EMBO J. 2002;21:1360–8.
Chandran D, Rickert J, Huang Y, Steinwand MA, Marr SK, Wildermuth MC. Atypical E2F transcriptional repressor DEL1 acts at the intersection of plant growth and immunity by controlling the hormone salicylic acid. Cell Host Microbe. 2014;15:506–13.
Nakagami S, Saeki K, Toda K, Ishida T, Sawa S. The atypical E2F transcription factor DEL1 modulates growth-defense tradeoffs of host plants during root-knot nematode infection. Sci Rep. 2020;10:8836.
Guo XY, Wang Y, Zhao PX, Xu P, Yu GH, Zhang LY, et al. AtEDT1/HDG11 regulates stomatal density and water-use efficiency via ERECTA and E2Fa. New Phytol. 2019;223:1478–88.
Zhang H, Jiang W, Xia P, Yin J, Chen H, Li W, et al. Genome-wide identification transcriptional expression analysis of E2F-DP transcription factor family in wheat. Plant Mol Biol Rep. 2022;40:339–58.
Li L, Shi Q, Li Z, Gao J. Genome-wide identification and functional characterization of the PheE2F/DP gene family in Moso bamboo. BMC Plant Biol. 2021;21:1–15.
Okay A, Amirinia K, Buyuk I. E2F/DP protein family in beans: identification, evolution and expression analysis within the genome. South Afr J Bot. 2023;157:122–34.
Singh A, Pandey H, Pandey S, Lal D, Chauhan D, Aparna, Antre SH, Kumar BS. Drought stress in maize: stress perception to molecular response and strategies for its improvement. Funct Integr Genomics. 2023;23:296.
Lobell DB, Deines JM, Tommaso S, Di. Changes in the drought sensitivity of US maize yields. Nat Food. 2020;1:729–35.
Simpkins G. Maize sensitivity to drought. Nat Rev Earth Environ. 2020;1:625–625.
Yang Z, Cao Y, Shi Y, Qin F, Jiang C, Yang S. Genetic and molecular exploration of maize environmental stress resilience: toward sustainable agriculture. Mol Plant. 2023;16:1–22.
Liu B, Zhang B, Yang Z, Liu Y, Yang S, Shi Y, et al. Manipulating ZmEXPA4 expression ameliorates the drought-induced prolonged anthesis and silking interval in maize. Plant Cell. 2021;33:2058–71.
Nuccio ML, Wu J, Mowers R, Zhou HP, Meghji M, Primavesi LF, et al. Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nat Biotechnol. 2015;33:862–9.
Tian T, Wang S, Yang S, Yang Z, Liu S, Wang Y, et al. Genome assembly and genetic dissection of a prominent drought-resistant maize germplasm. Nat Genet. 2023;55:496–506.
Xiang Y, Sun X, Bian X, Wei T, Han T, Yan J, et al. The transcription factor ZmNAC49 reduces stomatal density and improves drought tolerance in maize. J Exp Bot. 2021;72:1399–410.
Singh A, Pandey H, Pandey S, Lal D, Chauhan D, Aparna, et al. Drought stress in maize: stress perception to molecular response and strategies for its improvement. Funct Integr Genomics. 2023;23:1–19.
Lu F, Li W, Peng Y, Cao Y, Qu J, Sun F, et al. ZmPP2C26 alternative splicing variants negatively regulate drought tolerance in maize. Front Plant Sci. 2022;13:1–13.
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13:1194–202.
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–13.
Gaut BS, Morton BR, Mccaig BC, Clegg MT. Substitution rate comparisons between grasses and palms: synonymous rate differences at the nuclear gene adh parallel rate differences at the plastid gene rbcL. Proc Natl Acad Sci. 1996;93:10274–9.
Sun F, Yu H, Qu J, Cao Y, Ding L, Feng W, et al. Maize ZmBES1/BZR1-5 decreases ABA sensitivity and confers tolerance to osmotic stress in transgenic Arabidopsis. Int J Mol Sci. 2020;21:996.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆Ct method. Methods. 2001;25:402–8.
Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–43.
Kim D, Langmead B, Salzberg SL. HISAT: A fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–60.
Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016;11:1650–67.
Love MI, Huber W, Anders S. Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:1–21.
Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39:W316–22.
Guo J, Song J, Wang F, Zhang XS. Genome-wide identification and expression analysis of rice cell cycle genes. Plant Mol Biol. 2007;64:349–60.
Lammens T, Li J, Leone G, De Veylder L. Atypical E2Fs: new players in the E2F transcription factor family. Trends Cell Biol. 2009;19:111–8.
Edger PP, Pires JC. Gene and genome duplications: the impact of dosage-sensitivity on the fate of nuclear genes. Chromosom Res. 2009;17:699–717.
Kondrashov F, Rogozin I, Wolf Y, Koonin E. Selection in the evolution of gene duplications. Genome Biol. 2002;3:RESEARCH0008.
Sánchez-Camargo VA, Suárez-Espinoza C, Romero-Rodríguez S, Garza-Aguilar SM, Stam M, García-Ramírez E, et al. Maize E2F transcription factors. Expression, association to promoters of S-phase genes and interaction with the RBR1 protein in chromatin during seed germination. Plant Sci. 2020;296:110491.
Rubin SM, Gall AL, Zheng N, Pavletich NP. Structure of the rb C-terminal domain bound to E2F1-DP1: a mechanism for phosphorylation-induced E2F release. Cell. 2005;123:1093–106.
Singh A, Yadav AK, Kaur K, Sanyal SK, Jha SK, Fernandes JL, et al. A protein phosphatase 2 C, AP2C1, interacts with and negatively regulates the function of CIPK9 under potassium-deficient conditions in Arabidopsis. J Exp Bot. 2018;69:4003–15.
He G, Liu J, Dong H, Sun J. The Blue-Light receptor CRY1 interacts with BZR1 and BIN2 to modulate the phosphorylation and nuclear function of BZR1 in repressing BR signaling in Arabidopsis. Mol Plant. 2019;12:689–703.
Khan TA, Kappachery S, Karumannil S, AlHosani M, Almansoori N, Almansoori H, et al. Brassinosteroid signaling pathways: insights into plant responses under abiotic stress. Int J Mol Sci. 2023;24:17246.
Prouse MB, Campbell MM. The interaction between MYB proteins and their target DNA binding sites. Biochim Biophys Acta. 2012;1819:67–77.
Wang X, Niu Y, Zheng Y. Multiple functions of MYB transcription factors in abiotic stress responses. Int J Mol Sci. 2021;22:6125.
Nakashima K, Yamaguchi-Shinozaki K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013;32:959–70.
He SS, Liu J, Xie Z, O’Neill D, Dotson S. Arabidopsis E2Fa plays a bimodal role in regulating cell division and cell growth. Plant Mol Biol. 2004;56:171–84.
Jaradat MR, Feurtado JA, Huang D, Lu Y, Cutler AJ. Multiple roles of the transcription factor AtMYBR1/AtMYB44 in ABA signaling, stress responses, and leaf senescence. BMC Plant Biol. 2013;13:192.
Chen T, Zhang W, Yang G, Chen JH, Chen BX, Sun R, et al. TRANSTHYRETIN-LIKE and BYPASS1-LIKE co-regulate growth and cold tolerance in Arabidopsis. BMC Plant Biol. 2020;20:332.
Tellström V, Usadel B, Thimm O, Stitt M, Küster H, Niehaus K. The lipopolysaccharide of Sinorhizobium meliloti suppresses defense-associated gene expression in cell cultures of the host plant Medicago truncatula. Plant Physiol. 2007;143:825–37.
Meng L, Zhang T, Geng S, Scott PB, Li H, Chen S. Comparative proteomics and metabolomics of JAZ7-mediated drought tolerance in Arabidopsis. J Proteom. 2019;196:81–91.
Maathuis FJM, Filatov V, Herzyk P, Krijger GC, Axelsen KB, Chen S, et al. Transcriptome analysis of root transporters reveals participation of multiple gene families in the response to cation stress. Plant J. 2003;35:675–92.
Kaye Y, Golani Y, Singer Y, Leshem Y, Cohen G, Ercetin M, et al. Inositol polyphosphate 5-phosphatase7 regulates the production of reactive oxygen species and salt tolerance in Arabidopsis. Plant Physiol. 2011;157:229–41.
Wege S, Ugalde JM. Metal health: PATELLIN2 reduces iron-induced toxicity in Arabidopsis. Plant Physiol. 2023;192:15–6.
Atkinson NJ, Lilley CJ, Urwin PE. Identification of genes involved in the response of Arabidopsis to simultaneous biotic and abiotic stresses. Plant Physiol. 2013;162:2028–41.
Xu P, Fang S, Chen H, Cai W. The brassinosteroid-responsive xyloglucan endotransglucosylase/hydrolase 19 (XTH19) and XTH23 genes are involved in lateral root development under salt stress in Arabidopsis. Plant J. 2020;104:59–75.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Key R&D Program of China (2021YFF1000303), the Sichuan Science and Technology Program (2022YFH0067) and the National Natural Science Foundation of China (32102226).
Author information
Authors and Affiliations
Contributions
Y.C., K.W. and F.L. designed and carried out the experiments; Y.C., K.W., B.L., H.M. and Y.W. carried out all bioinformatics analysis and wrote the manuscript; Q. Y. provided technical support. F.F., W.L. and H.Y supervised the experiments; H.Y. directed and revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cao, Y., Wang, K., Lu, F. et al. Comprehensive identification of maize ZmE2F transcription factors and the positive role of ZmE2F6 in response to drought stress. BMC Genomics 25, 465 (2024). https://doi.org/10.1186/s12864-024-10369-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-024-10369-0