Abstract
Backgroud
The genus Mesorhizobium is shown by phylogenomics to be paraphyletic and forms part of a complex that includes the genera Aminobacter, Aquamicrobium, Pseudaminobacter and Tianweitania. The relationships for type strains belong to these genera need to be carefully re-evaluated.
Results
The relationships of Mesorhizobium complex are evaluated based on phylogenomic analyses and overall genome relatedness indices (OGRIs) of 61 type strains. According to the maximum likelihood phylogenetic tree based on concatenated sequences of 539 core proteins and the tree constructed using the bac120 bacterial marker set from Genome Taxonomy Database, 65 type strains were grouped into 9 clusters. Moreover, 10 subclusters were identified based on the OGRIs including average nucleotide identity (ANI), average amino acid identity (AAI) and core-proteome average amino acid identity (cAAI), with AAI and cAAI showing a clear intra- and inter-(sub)cluster gaps of 77.40–80.91% and 83.98–86.16%, respectively. Combined with the phylogenetic trees and OGRIs, the type strains were reclassified into 15 genera. This list includes five defined genera Mesorhizobium, Aquamicrobium, Pseudaminobacter, Aminobacterand Tianweitania, among which 40/41 Mesorhizobium species and one Aminobacter species are canonical legume microsymbionts. The other nine (sub)clusters are classified as novel genera. Cluster III, comprising symbiotic M. alhagi and M. camelthorni, is classified as Allomesorhizobium gen. nov. Cluster VI harbored a single symbiotic species M. albiziae and is classified as Neomesorhizobium gen. nov. The remaining seven non-symbiotic members were proposed as: Neoaquamicrobium gen. nov., Manganibacter gen. nov., Ollibium gen. nov., Terribium gen. nov., Kumtagia gen. nov., Borborobacter gen. nov., Aerobium gen. nov.. Furthermore, the genus Corticibacterium is restored and two species in Subcluster IX-1 are reclassified as the member of this genus.
Conclusion
The Mesorhizobium complex are classified into 15 genera based on phylogenomic analyses and OGRIs of 65 type strains. This study resolved previously non-monophyletic genera in the Mesorhizobium complex.
Similar content being viewed by others
Introduction
The genus Mesorhizobium belongs to the family Phyllobacteriaceae in the order Hyphomicrobiales and the class Alphaproteobacteria of the phylum Pseudomonadota [1]. The genus Mesorhizobium was established in 1997 and the name reflects the fact that their growth rate was intermediate between that of the genera Rhizobium and Bradyrhizobium [2]. Bacteria in the genus Mesorhizobium were mainly isolated from root nodules of legume hosts, e.g. those belonging to the genera Acacia, Alhagi, Amorpha, Astragalus, Biserrula, Caragana, Cicer, Mimosa, Robinia and Sophora distributed all over the world [2,3,4,5,6,7,8,9,10]. They are characterized by the formation of root nodules and nitrogen fixation. Thus, species from this genus play important roles in the nitrogen cycle of agriculture, prairie and forestry environments. Some strains have been used as efficient inoculants to enhance legume nitrogen fixation, such as M. ciceri bv. biserrulae WSM1271 used as the commercial inoculant for pasture legume Biserrula pelecinus L. in Australia [11]. Nevertheless, type strains of nine of the other Mesorhizobium species were not isolated from legume nodules and they did not present nodulation abilities, i.e. M. comanense 3P27G6T from groundwater [12], M. hankyongi Gsoil 531 T, M. soli JCM 19897 T, M. terrae NIBRBAC000500504T and M. thiogangeticum SJT from soil [13,14,15], M. composti CC-YTH430T from compost [16], M. ephedrae 6GN30T from root of Ephedra przewalskii [17], M. sediminum KCTC 42205 T from deep-sea sediment [18], and M. microcysteis MaA-C15T from xenic culture of Microcystis aeruginosa [19]. Over the years, extensive work has been carried out on classification of Mesorhizobium based on polyphasic taxonomy, leading to the description of 71 (63 validly named) species in this genus, making it the largest genus in the family Phyllobacteriaceae (https://www.bacterio.net/genus/mesorhizobium/). Among the published species, 37 (accounting for 58%) of them were published in the last ten years (from 2013 till now).
Since the first description of Mesorhizobium, its taxonomy has been continuously revised and improved. According to phylogenetic trees constructed using 16S rRNA gene and housekeeping gene (recA) sequences, the genus was reported to be polyphyletic, with species Mesorhizobium camelthorni and Mesorhizobium alhagi forming a distinct lineage distantly with most Mesorhizobium species [1]; while Mesorhizobium albiziae and Mesorhizobium thiogangeticum formed distinct lineages in the recA phylogenetic tree [1]. Similar phylogenomic relationships were also apparent in the genome BLAST distance phylogeny (GBDP) tree constructed using the whole genome sequences, since several Mesorhizobium species, including M. camelthorni, M. alhagi and M. soli, were intermixed with species from the genera Aquamicrobium and Pseudaminobacter [20]. Moreover, these three genera together with Aminobacter and Tianweitania formed an intricate complex that is causing confusion of the taxonomy in the family Phyllobacteriaceae [20]. The genus Aquamicrobium was established in 1998 [21]. At the present time, the genus comprises 8 validly published species: A. aerolatum [22], A. aestuarii [23], A. ahrensii [24], A. defluvii [21], A. lusatiense [22], A. segne [24], A. soli [25] and A. terrae [26], and a not validly named species: “A. zhengzhouense” [27]. The type strains of this genus were isolated from diverse habitats e.g. sewage [21], tidal flat [23], wastewater-treatment plant [22], air in a duck shed [22], biofilter for the treatment of animal rendering waste gas [24], and contaminated soils [25, 26]. The genus Pseudaminobacter was established in 1999 [28]. It comprises five validly published species: P. arsenicus [29], P. defluvii [28], P. granuli [30], P. manganicus [31], and P. salicylatoxidans [28] and a not validly named species “P. soli" [32]. They were isolated from various aquatic environments e.g. arsenic-rich aquifers [29], sludge [28], wastewater treatment plant [30], and river [28]. The genus Aminobacter was established in 1992 [33]. It comprises six defined species: A. aganoensis [33], A. aminovorans [33], A. anthyllidis [34], A. carboxidus [20], A. ciceronei [35] and A. niigataensis [33]. They were isolated from soil [33, 35, 36] and root nodule [34]. The genus Tianweitania were established in 2016 [37]. It is composed three species, which were isolated from terrestrial sediment, bark tissue and coastal sand respectively [37,38,39]. As described above, the available species list of this intricate complex is over-represented by Mesorhizobium and it is therefore named as the Mesorhizobium complex in this work for simplification. Defined genera should be monophyletic [40], so the taxonomy of the species belonging to the Mesorhizobium complex needs to be carefully re-evaluated at the genus level.
While the earlier studies on these bacteria were mainly based upon physiochemical, biochemical and chemotaxonomic features in combination with the phylogenetic analysis of 16S rRNA gene [41], later studies used multilocus sequence analysis (MLSA) based on concatenated sequences of several housekeeping genes, such as recA, atpD, and glnII [42, 43] to define the Mesorhizobium species. With the rapid progress in genome sequencing technology, genome sequences for the type strains of 50 Mesorhizobium species, three of Aquamicrobium, three of Pseudaminobacter, six of Aminobacter, and three for Tianweitania are now available in the public database (https://www.ncbi.nlm.nih.gov/genome/). The development of modern bioinformatics makes genome-based studies a promising approach for delineation of species, genera and even higher ranks of bacteria [44]. The average nucleotide identity (ANI) value threshold 95–96% combining with digital DNA-DNA hybridization threshold (dDDH) value of 70% have been suggested as appropriate criteria for species delineation [45, 46]. At the genus and higher rank levels, phylogenetic trees constructed based on the whole genome sequence provides sufficiently precise phylogenetic relationships for bacteria [47,48,49], however, there is no agreed standard for genus delineation on the basis of genome similarity. A recent study carried out on 3500 type strain genomes of bacteria and archaea found that the threshold between genera was at a mean ANI of 73.98% (25% quartile, 70.85%; 75% quartile, 76.56%) for specific groups [49]. There is still no clear genus ANI demarcation boundary or estimated genus inflection point for all bacteria [49], and measures based on protein sequences have proved more discriminatory than ANI at the genus level [47, 50]. The average amino acid identity (AAI) has been evaluated for genus delineation, and most bacterial intra-genus AAI values were above 68% [51]. Since the phylogenetic relationships or the similarity between the two genomes could be affected by horizontal gene transfer (HGT) [52], it is more reasonable to use the core-proteome average amino acid identity (cAAI) in genus classification to minimize the impact of HGT [48, 53]. The 86% cAAI threshold had effectively improved the delineation of some species belong to equivocal genera of Rhizobiaceae [47, 54].
This work aimed to clarify the phylogenetic relationships within the Mesorhizobium complex. All type strains of the defined Phyllobacteriaceae species with whole genome sequences available in the public databases were used to compare the phylogenomic relationships, and the overall genome relatedness indices (OGRIs), including ANI, AAI and cAAI, were characterized. Furthermore, the phyletic distribution of key nodulation and nitrogen fixation genes was also analyzed. By combining the above results, taxonomic positions were re-evaluated and modified for some ambiguous Mesorhizobium complex species.
Material and methods
Genome download and dataset
A complete list of all validly published species in the family Phyllobacteriaceae was retrieved from LPSN (https://lpsn.dsmz.de/family/phyllobacteriaceae). One genome sequence from each type strain of the corresponding species was selected and downloaded from GenBank, JGI or GCM (http://gctype.wdcm.org/). For the species with more than one genome sequence, the most complete genome sequence was used. The genome quality was evaluated by CheckM v1.2.1 and the genomes with completeness > 95% and contamination < 5% were deemed competent [55]. The genome characteristics including genome size, G + C%, contig numbers and N50 were analyzed by QUAST v4.0 [56].
Phylogenomic analyses based on core genes
Genes in each qualified genome sequence were predicted and annotated using Prokka v1.13 [57]. Orthologous clusters (OCs) and the core genome sequences were inferred by OrthoFinder v2.5.4 [58]. The single copy ortholog core protein sequences were selected to perform the following analyses: all proteins were aligned using Mafft version 7.471 [59]; the aligned sequences were trimmed by trimAl v1.4 [60]; and the trimmed sequences were concatenated and the maximum likelihood (ML) tree was reconstructed using the best recommended model with the command ‘-m MFP’ by IQ-TREE 2.0.3, with the bootstrap value of 1000 replicates [61]. Shinella granuli DSM 18401 T from Rhizobiaceae was selected as an outgroup. The tree was visualized and decorated with iTOL v6 online program [62]. The bac120 marker set of 120 genes was selected from Genome Taxonomy Database (GTDB) to infer the phylogenetic relationships using the GTDB-Tk v2.1.0 [63]. The phylogenetic tree based on 16S rRNA gene sequences was also reconstructed using IQ-TREE 2.0.3 [61], with the best recommended model “TPM3 + I + G4” and the bootstrap value was set as 1000 replications.
Calculation of overall genome relatedness indices (OGRIs)
ANI values between all genome pairs were calculated by using the orthologous average nucleotide identity tool (OrthoANI, v0.93.1) implemented with the blast + + algorithm [45]. When ANI was larger than 95%, the corresponding genome pair files were further selected to calculate digital DNA-DNA hybridization (dDDH) values by using the Genome-to-Genome Distance Calculator (GGDC, version 3.0) online (https://ggdc.dsmz.de/ggdc.php#) [46]. Genome ANI values more than 95–96% and dDDH values greater than 70% were used as the threshold to determine that strains belonged to the same species [64]. The AAI was calculated from genome sequences between each pair of type strains using CompareM v0.1.2 [53]. For cAAI calculation, the common shared ortholog genes among the 99 Phyllobacteriaceae genomes were defined, then the cAAIs between each pair of type strains were calculated by using CompareM v0.1.2 [48, 53].
Symbiotic nitrogen fixation prediction
Since nitrogen fixation in symbiosis with legume hosts is a prominent feature of most Mesorhizobium species [2, 65], the symbiotic nitrogen fixation abilities for the tested type strains were predicted by the presence of nod genes (nodABC) and nitrogenase cassette (nifHDK) in the genome. The nodABC and nifHDK sequences were extracted from each genome sequence by BLAST + + software with BLASTN (E-value 1E-5) program [66]. Strains with both nod and nif genes were potentially bacteria with symbiotic nitrogen fixation abilities.
Results and discussion
Genome characteristics of the Mesorhizobium complex
A total of 96 genome sequences of the corresponding Phyllobacteriaceae type strains were obtained, 87 were downloaded from GenBank and 9 from GCM (Table S1). The completeness for each genome is greater than 95% and the contamination less than 5% detected by checkM (Table S1), indicating that all the genome sequences meet the requirement [55]. Within the Mesorhizobium complex, 65 genomes (6 complete and 59 draft) were obtained, the genome size ranges between 3.64 and 8.58 Mb, the G + C% is 60.06–66.43%, the complete sequence with 1–6 replicons and the draft genomes with 7–493 contigs.
Phylogenetic analyses for Mesorhizobium complex species
As in previous studies mentioned in the introduction [1], the genus Mesorhizobium is not monophyletic and is intermingled with Aquamicrobium and Pseudaminobacter in the phylogenetic trees constructed using either 16S rRNA genes or complete genome sequences. In the phylogenetic tree constructed using 16S rRNA gene sequences, all the Mesorhizobium type strains are grouped into 6 clusters (Fig. S1) with low bootstrap values (15/94 nodes < 50%). Cluster A includes 31 Mesorhizobium species; Cluster B includes three Mesorhizobium species: M. alhagi, M. camelthorni, M. terrae and Chelativorans multitrophicus; Cluster C contains 12 Mesorhizobium species; Cluster D is composed of M. sediminum and Nitratireductor indicus; Cluster E contains two Mesorhizobium species: M. soli and M. ephedrae; in Cluster F, M. composti intermingles with three Pseudaminobacter species including P. manganicus, P. arsenicus and P. salicylatoxidans (Fig. S1).
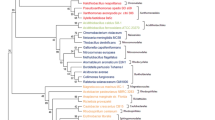
To provide a more robust phylogeny, the phylogenetic relationships among 50 Mesorhizobium species with whole genome sequences were evaluated using two distinct sets of core genes (Figs. 1 and S2). A total of 825 single-copy ortholog sequences are shared by the Mesorhizobium, Aminobacter, Aquamicrobium, Pseudaminobacter and Tianweitania type strains, and 539 are shared by all Phyllobacteriaceae type strains and the outgroup strain Shinella granuli DSM 18401 T through OrthoFinder analyses. The OrthoFinder ML phylogenetic tree is reconstructed by using the concatenated protein sequence of all the 539 core ortholog genes, in which species belonging to the genera Mesorhizobium, Aminobacter, Aquamicrobium, Pseudaminobacter and Tianweitania form a Mesorhizobium complex and are further reclassified as 9 phylogenomic clusters (Fig. 1) strongly supported by high bootstrap values (58/65 were 100%). The Mesorhizobium type strains are grouped into 7 clusters (Cluster I-VII) (Fig. 1). Furthermore, the phylogenetic relationships of the OrthoFinder tree are consistent with the GTDB tree (Fig. S2). Cluster I comprises the type strains representing 41 Mesorhizobium species, including the type species Mesorhizobium loti [1]. Cluster II consists of two Mesorhizobium species (M. composti and M. terrae), two Aquamicrobium species (A. defluvii and A. lusatiense) and Pseudaminobacter manganicus. Cluster III includes two Mesorhizobium species (M. alhagi and M. camelthorni). Cluster V contains the type strains of Mesorhizobium soli and two Pseudaminobacter species (P. salicylatoxidans and P. arsenicus). Cluster VII is composed of two Mesorhizobium species (M. microcysteis and M. sediminum) and Aquamicrobium aerolatum (Figs. 1 and S2). Clusters IV and VI each cover only a single strain of Mesorhizobium ephedrae and Mesorhizobium albiziae, respectively. In short, the phylogenetic relationships of both the 16S rRNA gene and the whole genome sequences indicate that Mesorhizobium is not monophyletic and the taxonomy of Mesorhizobium complex should be re-evaluated.
Maximum likelihood (ML) phylogenomic tree based on 539 concatenated core protein sequences of Phyllobacteriaceae species with genome sequences. The tree was reconstructed using IQ-TREE 2.0.3 with the best model. Type strains with blue asterisk marks represent type species of the indicated genus. Strain Shinella granuli DSM 18401 T was selected as an outgroup
Determination of the overall genome relatedness indices among the Mesorhizobium complex species
All the type strains belonging to the Mesorhizobium complex were selected to compare the genome pair OGRIs. The ANI, AAI and cAAI (using the complex common shared 853 single copy protein sequences) values of the genome pairs are 71.58–96.42%, 66.70–96.85% and 71.41–98.67% respectively (Figs. 2, S3, S4 and S5). Moreover, a notable gap can be found for the each of the OGRIs, i.e. 79.64–81.80% for ANI, 77.40–80.91% AAI and 83.98–86.16% for cAAI (Fig. S3). The inter-cluster and intra-cluster ANI values are 71.58–79.64% and 75.41–96.42% respectively (Figs. 3, S3 and Table S2), while the inter-cluster AAI values range from 66.70% to 77.40%, and the intra-cluster AAI values vary from 73.35% to 96.85% (Fig. S4 and Table S2). The inter-cluster and intra-cluster cAAI values are 71.41–83.98% and 78.59–98.67% (Fig. 2 and Table S2). The overlap between intra-cluster and inter-cluster values is attributable to Clusters II, V, VII and IX, which have intra-cluster values of 75.41–79.52% for ANI, 73.35–77.40% for AAI, and 78.61–82.09% for cAAI (Fig. 3).
Distribution graphs showing the a ANI, b AAI and c cAAI density generated by pairwise comparisons among all 61 genomes belonging to the Mesorhizobium complex. The red lines indicate comparisons between genomes in the same cluster or subcluster, blue lines are for genomes in different (sub)clusters, and green lines are for overlapped data between inter- and intra-clusters
Combining the phylogenetic trees and the OGRIs, a total of 10 subclusters in the Mesorhizobium complex are further classified within the four clusters (Cluster II, V, VII and IX) (Figs. 1 and S2). Furthermore, the classification of 15 (sub)clusters including 5 undivided clusters (Cluster I, III, IV, VI, and VIII) and 10 subclusters is perfectly in line with the density distribution pattern of OGRIs (Fig. 3). The only exception is the intra-subcluster ANI value of 79.62%, between M. microcysteis MaA-C15T and M. sediminum KCTC 42205 T which overlaps the inter-(sub)cluster ANI values (Figs. 3 and S3). The other intra-(sub)cluster ANI values are 81.80–96.42%, the intra-(sub)cluster AAI and cAAI values are 80.91–96.85% and 86.16–98.67% respectively (Fig. 3). The 15 inter-(sub)cluster ANI, AAI and cAAI are 71.58–79.64%, 66.70–77.40% and 71.41–83.98% (Fig. 3), respectively. Hence, our study supports previous findings that AAI and cAAI are more suitable to define genus rank than ANI [48, 50, 51]. In our study, all the cAAI values within the 15 (sub)clusters are higher than 86.16%, which is slightly higher than the proposed 86% cAAI threshold for genus delimitation [47], and the inter-(sub)cluster cAAI values are lower than 83.98% (Fig. 3). Thus, each of the 5 independent clusters and 10 subclusters (Figs. 1 and S2) may represent a different genus. The AAI (77.40–80.91%) and cAAI (83.98–86.16%) gaps between intra- and inter-(sub)cluster values also support this reclassification of genera in the Mesorhizobium complex.
Prediction of symbiotic nitrogen fixation abilities for Mesorhizobium species
The prominent feature of most species in the genus Mesorhizobium is that they nodulate with legume hosts; however, there are Mesorhizobium species isolated from soil or even deep-sea sediment [13, 18]. Mesorhizobium species in Cluster I, Cluster III and Cluster VI harbored both nod and nif genes (Fig. 1), consistent with the fact that most of them were isolated from root nodules [3, 67, 68] (Table S3). The few exceptions among them were M. comanense 3P27G6T and M. huakuii NBRC 15243 T in Cluster I, in which nod and nif genes are absent. Strain M. comanense 3P27G6T was isolated from ground water [12]; while M. huakuii NBRC 15243 T was originally isolated from root nodule of Astragalus sinicus and its nodulation ability was confirmed when the species was described [2, 69]. Since nod genes have been located on the symbiotic plasmids in other strains of this species, such as M. huakuii 7653R [70], it is possible that the type strain M. huakuii NBRC 15243 T had lost its symbiotic plasmid during the subculture procedures in laboratory [71]. Another two Mesorhizobium strains M. camelthorni CCNWXJ 40-4 T (Cluster III) and M. albiziae DSM 21822 T (Cluster VI) harbor the complete nod and nif genes. However, strain M. alhagi CCNWXJ12-2 T (Cluster III) possesses only the nod genes but not the nif genes, which may be due to loss in subculture or defects of sequencing and assembly, since a nifH sequence was reported in the original publication [68]. The above results indicate that strains from the clusters I, III and VI possess the potential of symbiotic nitrogen fixation with the corresponding legume hosts. In this study, all the Mesorhizobium type strains in Cluster II, IV, V and VII lacked nod and nif genes, and they were isolated from compost (M. composti CC-YTH430T) [16], soil (M. terrae NIBRBAC000500504T) [14], root endosphere (M. ephedrae 6GN30T) [17], rhizosphere (Mesorhizobium soli JCM 19897 T) [15], and aquatic environments (M. sediminum KCTC 42205 T and M. microcysteis MaA-C15T) [18, 19].
Genus and species level reconsiderations for Mesorhizobium complex
The combined evidence of the Orthofinder and GTDB phylogenetic trees that are reconstructed using the genome sequences (Figs. 1 and S2) and the intra-(sub) cluster and inter-(sub)cluster OGRIs (Figs. 2, 3, Table 1, Figs. S3, S4) indicates that each of the 5 independent clusters and the 10 subclusters in the Mesorhizobium complex defined in the present study merit the rank of a genus. Four legume-nodulating clusters belong to two defined (Cluster I and VIII) and two novel (Cluster III and VI) genera. The 41 species in Cluster I should be maintained as genus Mesorhizobium, since the type species M. loti represented by strain DSM 2626 T (Fig. 1) is in this cluster. The genome size for the type strains in this cluster varies from 6.20 to 8.58 Mb; the G + C content of genome DNA is between 61.84% and 64.00%; the intra-cluster ANI, AAI and cAAI are 81.80–96.42%, 81.29–96.85% and 88.18–98.67%, respectively (Table 1, Figs. 2, and S4), which are clearly above the gap between intra- and inter-genus values. In this cluster (genus), the ANI and dDDH between M. sophorae ICMP 19535 T and M. waitakense ICMP 19523 T are 96.42% and 72.2% (Fig. S3 and Table S4), which exceed the species threshold of 95–96% and 70% [45, 46, 72], respectively. Based on the facts that both species were isolated from nodules of Sophora microphylla and could nodulate with it [10], and they presented very similar genome size, G + C% (Table S1), physiochemical characteristics, major fatty acids and symbiotic phenotypes [10], they should be combined into a single species. They were published simultaneously, but the species name M. sophorae would be preferred for the combined species (reflecting the original host). Although the ANI values between three species pairs of M. escarrei STM 5069 T and M. ventifaucium STM 4922 T, M. delmotii STM 4623 T and M. temperatum SDW018T, M. delmotii STM 4623 T and M. onobrychidis OM4T are greater than 95% (95.02–95.52%), the DDH values between each pair were less than 70% (62.50–64.30%) (Table S4), thus they can be maintained as independent, but closely related species, like some species in the Rhizobium leguminosarum complex [73]. The 6 species in Cluster VIII should be maintained as genus Aminobacter, since the type species Aminobacter aminovorans represented by strain DSM 7048 T (Figs. 1 and S2) is in this cluster. The genome size for the type strains in this cluster varies from 5.29 to 6.78 Mb; the G + C content of genome DNA is between 62.58% and 63.89%; the intra-cluster ANI, AAI and cAAI are 85.21–91.11%, 86.75–93.93% and 91.81–96.55% respectively (Table 1, Figs. 2, and S4), which are clearly above the gap between intra- and inter-genus values. Strain Aminobacter anthyllidis LMG 26462 T is the only nodulating bacterium with both nod and nif related genes in the cluster [34]. Cluster III includes two symbiotic Mesorhizobium species M. alhagi and M. camelthorni possessing nod and nif genes, both were isolated from root nodules of Alhagi sparsifolia and could nodulate with their original host [3, 68] (Table S3). Their genome sizes are 6.97 and 7.3 Mb, with DNA G + C% 62.65 and 62.41%, the intra-cluster ANI, AAI and cAAI are 91.13%, 91.75% and 96.04%, respectively (Fig. 1 and Table S1). As an independent cluster in both phylogenetic trees, Cluster VI included only M. albiziae DSM 21822 T that is a symbiont of Albizia kalkora [67], harboring both nod and nif genes (Fig. 1). It has a genome size of 6.27 Mb and DNA G + C% of 62.08%. Clusters III and VI represent novel genera, as they do not include any type species, but the type strains had canonical symbiotic ability like those of Cluster I species (Mesorhizobium), so we propose the names Allomesorhizobium and Neomesorhizobium for the two novel genera.
The other 11 members with neither nod nor nif genes belong to four defined genera (Subcluster II-1, Subcluster V-1, Sucluster IX-1 and Subcluster IX-2) and seven novel genera (Cluster IV, Subclusters II-2, 3, 4, Subcluser V-2, Subcluser VII-1, 2). Subcluster II-1 should be maintained as genus Aquamicrobium, since the type species Aquamicrobium defluvii represented by strain DSM 11603 T is included in the subcluster; it is composed of two Aquamicrobium species (A. defluvii and A. lusatiense) (Figs. 1, and S2, Table 1). Their genome sizes are 4.39 and 4.52 Mb, with G + C content of 62.60% and 63.15%, and the ranges of values of intra-cluster ANI, AAI and cAAI are 84.14%, 86.77% and 89.61%, which are clearly higher than the gap between intra- and inter-genus values (Figs. 2, S3, S4 and Table 1). Both type strains were isolated from sludge (Table S3) [21]. Subcluster V-1 includes Pseudaminobacter salicylatoxidans and Mesorhizobium soli (Figs. 1 and S2). Their genome sizes are 4.84 and 6.27 Mb, with DNA G + C% 62.68% and 62.57%, and their intra-subcluster ANI, AAI and cAAI are 82.7%, 82.96% and 87.50%, respectively (Figs. 2, S3, and S4), which are also higher than the gap between intra- and inter-genus values. They were isolated from river [28] and rhizosphere [15] (Table S3). Since Pseudaminobacter salicylatoxidans DSM 6986 T represented the only type species in this subcluster, both species in this cluster should be reclassified as members of the genus Pseudaminobacter. Cluster IX including three Tianweitania strains, they are further classified into two subclusters. The AAI and cAAI intra-Subcluster IX-1 is 88.82% and 92.14%, which obviously higher than the gap between intra- and inter-genus values (Figs. 2, S3 and S4 and Table 1). But the AAI and cAAI between Subcluster IX-2 (T. sediminis) and Subcluster IX-1 are: 76.55% and 76.30%, 77.09% and 76.85% respectively, which are clearly lower than the gap between intra- and inter-genus values (Figs. 2, S3 and S4 and Table 1). Combined with the substrate utilization characteristics [39] and ORGIs of our study, the Cluster IX class into two genera is reasonable. Subcluster IX-1 includes T. aestuarii and T. populi (Figs. 1 and S2), their genome sizes are 3.83 and 4.29 Mb, they were isolated from bark tissue and coastal sand (Table S3). For T. populi is the type species of previos Corticibacterium, and the genus name should be restored and the both strains should be reclassified as Corticibacterium. And the species T. sediminis in Subcluster IX-2 should be maintained as Tianweitania. Subcluster VII-1 includes two former Mesorhizobium species, M. sediminum KCTC 42205 T and M. microcysteis MaA-C15T (Figs. 1 and S2). Their genome sizes are 6.14 and 4.84 Mb, with G + C% 63.27% and 64.14%, and with AAI and cAAI values of 80.91% and 85.99% between them, respectively (Table 1, Fig. S3 and S4), which are clearly higher than the gaps between intra- and inter-genus values. Thus, they should be classified as the same genus. They were isolated from aquatic environments, including xenic culture of Microcystis aeruginosa [19] and sediment [18] (Table S3). As there is no type species in this subcluster and both strains were isolated from aquatic environments, we propose Neoaquamicrobium as the name for this novel genus.
Each of the other six (sub)clusters, Clusters IV, Subclusters II-2, 3, 4, V-2 and VII-2, includes only one species, and each forms an independent branch in the phylogenomic trees (Figs. 1 and S2). The OGRIs between these type strains and other type strains belong to the Mesorhizobium complex are lower than the gap between intra- and inter-genus values (Figs. 2, S3 and S4). As none of these species is the type species of a genus, it is reasonable to reclassify each of them as a new genus. For Subcluster II-2, Pseudaminobacter manganicus JH-7 T was isolated from sludge of a manganese mine [31], and we propose Manganibacter as the genus name. For Subcluster II-3, Mesorhizobium composti CC-YTH430T was isolated from compost and lacks nod and nif genes [16], so a name based on Mesorhizobium would be inappropriate and we propose Ollibium as the genus name. For Subcluster II-4, Mesorhizobium terrae NIBRBAC000500504T was isolated from soil and lacks nod and nif genes [14], and we propose Terribium as the genus name. For Cluster IV, Mesorhizobium ephedrae 6GN30T was isolated from root endosphere of Ephedra przewalskii and lacks nod and nif genes [17], and we propose Kumtagia as the genus name. For Subcluster V-2, Pseudaminobacter arsenicus CB3T was isolated from arsenic-rich aquifers [29], and we propose Borborobacter as the genus name. For Subcluster VII-2, Aquamicrobium aerolatum DSM 21857 T was isolated from air sampled in a duck shed [22], thus we propose Aerobium as the genus name.
In conclusion, the taxonomy of species in Mesorhizobium complex should be revised based upon the analyses of whole genome sequences. Both the phylogenomic results and OGRIs support the division of the species belonging to the complex into 15 genera including 5 defined (Cluster I, Subcluster II-1, Subcluster V-1, Cluster VIII and Cluster IX-2 corresponding to Mesorhizobium, Aquamicrobium, Pseudaminobacter, Aminobacter and Tianweitania) and 9 novel genera (Cluster III, IV, VI, Subclusters II-2, 3, 4, V-2, VII-1, VII-2). Clusters I, III, VI and VIII include symbiotic strains harboring both nod and nif genes.
Taxonomic consequences
-
1.
Description of Manganibacter gen. nov.
Manganibacter (Man.ga.ni.bac′ter, N.L. neut. n. manganicum, manganese; N.L. masc. n. bacter, rod; N.L. masc. n. Manganibacter, a rod-shaped bacterium isolated from a manganese mine).
Cells are Gram-stain-negative, anaerobic, non-motile, capsule-forming and rod-shaped bacterium. The genus represents a distinct branch in the family Phyllobacteriaceae of the class Alphaproteobacteria based on the core-genomic ML phylogeny. The genome size of the type strain is 4.84 Mb and the DNA G + C content is 61.2%. The type species is Manganibacter manganicus.
Description of Manganibacter manganicus comb. nov.
Manganibacter manganicus (man.ga′ni.cus. N.L. masc. adj. manganicus, referring to its association with a manganese mine).
Basonym: Pseudaminobacter manganicus Li et al. 2017.
The description is the same as P. manganicus [31]. The type strain is JH-7 T (= KCTC 52258 T = CCTCC AB 2016107 T) isolated from sludge of a manganese mine near Tongren city, Guizhou Province of China. The DNA genome size is 4.84 Mb, the G + C content of the type strain is 61.2% (by genome).
-
2.
Description of Ollibium gen. nov.
Ollibium (Ol.li′bi.um, L. fem. n. olla, plant pot; Gr. masc. n. bios, life; N.L. neut. n. Ollibium, a bacterium that lives in a plant pot).
Cells are Gram-stain-negative, facultative anaerobic rod-shaped bacterium, that formed yellow-colored colonies on nutrient agar. The genus represents a distinct branch in the family Phyllobacteriaceae of the class Alphaproteobacteria based on the core-genomic ML phylogeny. The genome size of the type strain is 4.65 Mb and the DNA G + C content is 65.18%. The type species is Ollibium composti.
Description of Ollibium composti comb. nov.
Ollibium composti (com.posAAti. N.L. gen. n. composti of compost).
Basonym: Mesorhizobium composti Lin et al. 2020.
The description is the same as M. composti [16]. The type strain is CC-YTH430T (= BCRC 81024 T = JCM 31762 T), and was isolated from a glasshouse compost sample in Taiwan. The genome size is 4.65 M and the DNA G + C content of the type strain is 65.18% (by genome).
-
3.
Description of Terribium gen. nov.
Terribium (Ter.ri′bi.um, L. fem. n. terra, soil; Gr. masc. n. bios, life; N.L. neut. n. Terribium, a bacterium isolated from soil).
Cells are Gram-stain-negative, white-pigmented, aerobic, rod-shaped bacterium. The genus represents a distinct branch in the family Phyllobacteriaceae of the class Alphaproteobacteria based on the core-genomic ML phylogeny. The genome size of the type strain is 6.02 Mb and the DNA G + C content is 63.17%. The type species is Terribium terrae.
Description of Terribium terrae comb. nov.
Terribium terrae (ter′rae. L. gen. n. terrae indicating soil as the source of isolation).
Basonym: Mesorhizobium terrae Jung et al. 2021.
The description is the same as M. terrae [14]. The type strain is NIBRBAC000500504T (= KCTC72278T = JCM33432T) isolated from soil in Jangsu in Jeollabukdo, Korea. The DNA genome size is 6.02 Mb, the G + C content of the type strain is 63.17% (by genome).
-
4.
Description of Allomesorhizobium gen. nov.
Allomesorhizobium (Al.lo.me.so.rhi.zo′bi.um. Gr.masc. allos, other; N.L. neut. n. Mesorhizobium, a bacterial genus name. N.L. neut. n. Allomesorhizobium, a new group phylogenetically separated from the genus Mesorhizobium).
Cells are Gram-staining-negative, aerobic, motile, rod-shaped bacterium. The genus represents a distinct branch in the family Phyllobacteriaceae of the class Alphaproteobacteria based on the core-genomic ML phylogeny. The genome size of the type strains is 6.97–7.30 M and the DNA G + C content is 62.41–62.65%. The type species is Allomesorhizobium alhagi.
Description of Allomesorhizobium alhagi comb. nov.
Allomesorhizobium alhagi (al.ha'gi. N.L. gen. n. alhagi of Alhagi, a genus of leguminous plants, referring to the host from which the type strain was isolated).
Basonym: Mesorhizobium alhagi Chen et al. 2010.
The description is the same as M. alhagi [68]. The type strain, CCNWXJ12-2 T (= ACCC 15461 T = HAMBI 3019 T), was isolated from a root nodule of Alhagi sparsifolia in Alaer, Xinjiang, China. The genome size of the type strain is 6.97 Mb and the DNA G + C content is 62.65% (by genome).
Description of Allomesorhizobium camelthorni comb. nov.
Allomesorhizobium camelthorni (ca.mel.thor′ni. N.L. neut. n. camelthornum camelthorn, a common name for leguminous plants of the genus Alhagi in China; N.L. gen. n. camelthorni of camelthorn, from which the type strain was isolated).
Basonym: Mesorhizobium camelthorni Chen et al. 2011.
The description is the same as M. camelthorni [3]. The type strain is CCNWXJ 40-4 T (= HAMBI 3020 T = ACCC 14549 T), was isolated from a root nodule of Alhagi sparsifolia in Alaer, Xinjiang Province, China. The genome size of the type strain is 7.30 Mb and the DNA G + C content is 62.41% (by genome).
-
5.
Description of Kumtagia gen. nov.
Kumtagia (Kum.ta′gia. N.L. fem. n. Kumtagia, pertaining to the Kumtag Desert in northwest China, where the type strain was isolated).
Cells are Gram-stain-negative, non-spore-forming, facultative, rod-shaped bacterium. The genus represents a distinct branch in the family Phyllobacteriaceae of the class Alphaproteobacteria based on the core-genomic ML phylogeny. The genome size of the type strain is 6.11 Mb and the DNA G + C content is 66.43%. The type species is Kumtagia ephedrae.
Description of Kumtagia ephedrae comb. nov.
Kumtagia ephedrae (eph.e′drae. N.L. gen. n. ephedrae of Ephedra, referring to the generic name of Ephedra przewalskii from which the strain was isolated).
Basonym: Mesorhizobium ephedrae Liu et al. 2018.
The description is the same as M. ephedrae [17]. The type strain 6GN-30 T (= ACCC 60073 T = KCTC 62410 T) was isolated from root of E. przewalskii in Kumtag Desert, Xinjiang, PR China. The genome size of the type strain is 6.11 M and the DNA G + C content 66.43% (by genome).
-
6.
Description of Borborobacter gen. nov.
Borborobacter (Bor.bo.ro.bac.ter. Gr. masc. n. borboros, mud, dirt; N.L. masc. n. bacter, rod; N.L masc. n. Borborobacter, a rod-shaped bacterium isolated from mud).
Cells are Gram-stain-negative, rod-shaped bacterium. The genus represents a distinct branch in the family Phyllobacteriaceae of the class Alphaproteobacteria based on the core-genomic ML phylogeny. The genome size of the type strain is 5.21 Mb and the DNA G + C content is 61.42%. The type species is Pseudaminobacter arsenicus.
Description of Borborobacter arsenicus comb. nov.
Borborobacter arsenicus (ar.se′ni.cus. N.L. masc. adj. arsenicus, pertaining to arsenic).
Basonym: Pseudaminobacter arsenicus Mu et al. 2019.
The description is the same as P. arsenicus [29]. The type strain is CB3T (= CCTCC AB2016116T = KCTC 52625 T) isolated from arsenic-rich aquifers at the Jianghan Plain in Hubei, China. The genome size is 5.21 Mb and the DNA G + C content is 61.42%.
-
7.
Description of Neomesorhizobium gen. nov.
Neomesorhizobium (Ne.o.me.so.rhi.zo′bi.um. Gr. masc. adj. neos, new; N.L. neut. n. Mesorhizobium, a bacterial genus name. N.L. neut. n. Neomesorhizobium, a new genus separated from the genus Mesorhizobium).
Cells are Gram-negative, aerobic, motile, non-spore-forming rods. The genus represents a distinct branch in the family Phyllobacteriaceae of the class Alphaproteobacteria based on the core-genomic ML phylogeny. The genome size of the type strain is 6.27 Mb and the DNA G + C content is 62.08%. The type species is Neomesorhizobium albiziae.
Description of Neomesorhizobium albiziae comb. nov
Neomesorhizobium albiziae (al.bi'zi.ae. N.L. gen. fem. n. albiziae of Albizia, a genus of leguminous plants, referring to the isolation of the first strains from Albizia kalkora).
Basonym: Mesorhizobium albiziae Wang et al. 2007.
The description is the same as M. albiziae Wang et al. 2007 [67]. The type strain is CCBAU 61158 T (= LMG 23507 T = USDA 4964 T), and was isolated from root nodules of Albizia kalkora. The genome size is 6.27 M and the DNA G + C content is 62.08% (by genome).
-
8.
Description of Neoaquamicrobium gen. nov.
Neoaquamicrobium (Ne.o.a.qua.mi.cro′bi.um. Gr. masc. adj. neos, new; N.L. neut. n. Aquamicrobium, a bacterial genus name. N.L. neut. n. Neoaquamicrobium, a new genus which bacteria also living in water).
Cells are Gram-negative, aerobic, motile, non-spore-forming rods. The genus represents a distinct branch in the family Phyllobacteriaceae of the class Alphaproteobacteria based on the core-genomic ML phylogeny. The genome size of the type strains is 4.84–6.14 Mb and the DNA G + C content is 63.27–64.14%. The type species is Neoaquamicrobium sediminum.
Description of Neoaquamicrobium sediminum comb. nov.
Neoaquamicrobium sediminum (se.di.mi′num. L. gen. pl. n. sediminum of sediments, pertaining to source of the isolate).
Basonym: Mesorhizobium sediminum Yuan et al. 2016.
The description is the same as M. sediminum [18]. The type strain is YIM M12096T (= CCTCC AB 2014219 T = KCTC 42205 T), isolated from deep-sea sediment collected from the Indian Ocean. The genome size is 6.14 M and the DNA G + C content of the type strain is 63.27% (by genome).
Description of Neoaquamicrobium microcysteis comb. nov.
Neoaquamicrobium microcysteis (mi.cro.cys'te.is. N.L. gen. n. microcysteis, of the cyanobacterial genus Microcystis).
Basonym: Mesorhizobium microcysteis Jung et al. 2021.
The description is the same as M. microcysteis [19]. The type strain is MaA-C15T (= KACC 21226 T = JCM 33503 T), isolated from a xenic culture of Microcystis aeruginosa in the Republic of Korea. The genome size is 4.84 M and the DNA G + C content of the type strain is 64.14% (by genome).
-
9.
Description of Aerobium gen. nov.
Aerobium (Ae.ro'bi.um, Gr. masc. n. aér, air; Gr. masc. n. bios, life; N.L. neut. n. Aerobium, a bacterium isolated from air).
Cells are Gram-negative, aerobic, motile, rod-shaped. The genus represents a distinct branch in the family Phyllobacteriaceae of the class Alphaproteobacteria based on the core-genomic ML phylogeny. The genome size of the type strains is 3.64 Mb and the DNA G + C content is 60.6%. The type species is Aerobium aerolatum.
Description of Aerobium aerolatum comb. nov.
Aerobium aerolatum (ae.ro.la′tum. Gr. Masc. n. aer air; L. part. adj. latus -a -um carried; N.L. neut. part. adj. aerolatum airborne).
Basonym: Aquamicrobium aerolatum Kämpfer et al. 2009.
The description is the same as Aquamicrobium aerolatum [22]. The type strain is DSM 21857 T (= CCUG 57044 T = Sa14T), isolated from air sampled in a duck shed. The genome size is 3.64 Mb and the DNA G + C content of the type strain is 60.06% (by genome).
-
10.
Emended description of genus Mesorhizobium Jarvis et al. 1997
The description is as given by Jarvis et al. 1997 [2], with the following modifications: the genome sizes of the type strains are 6.20–8.58 Mb, and the genome G + C content varied from 61.84 to 64.00%.
Emended description of Mesorhizobium sophorae [10].
Mesorhizobium sophorae (so.pho′rae. N.L. fem. n. Sophora, botanical name of a genus of leguminous plants; N.L. gen. n. sophorae, of Sophora, referring to the host from which the type strain was isolated).
Basonym: Mesorhizobium sophorae De Meyer et al. 2016.
The description is as before [10] with the following addition. The genome size of the type strain is 8.49 Mb, and the DNA G + C content is 62.22% (by genome).
-
11.
Emended description of genus Pseudaminobacter Kämpfer et al. 1999
Pseudaminobacter (Pseud.ami.no.bac.ter. Gr. adj. pseudos false; N.L. Aminobacter, generic name of a bacterium, N.L. masc. n. Pseudaminobacter, false Aminobacter).
The description is as given by Kämpfer et al. [28] with the following amendment: the genomic size is 4.84–6.27 Mb, the G + C content is 61.42–62.68% (by genome). The type species is Pseudaminobacter salicylatoxidans.
Description of Pseudaminobacter soli comb. nov.
Pseudaminobacter soli (so′li. L. gen. neut. n. soli, of the soil, the source of the type strain).
Basonym: Mesorhizobium soli Nguyen et al. 2015.
The description is the same as M. soli Nguyen et al. 2015 [15]. The type strain, NHI-8 T (= KEMB 9005-153 T = KACC 17916 T = JCM 19897 T), was isolated from rhizosphere of legume tree Robinia pseudoacacia L. at Kyonggi University in Suwon, South Korea. The genomic size is 6.27 Mb, the G + C content is 62.57% (by genome).
-
12.
Emended description of genus Corticibacterium Li et al. 2016
Corticibacterium (Cor.ti.ci.bac.te′ri.um. L. n. cortex bark; L. neut. n. bacterium, a rod; N. L. masc. n. Corticibacterium a rod from bark).
The description is as given by Li et al. [38] with the following emendations: the genome size is 3.83–4.29 Mb, the G + C content is 61.30–6.39% (by genome). The type species is Corticibacterium populi.
Emended description of Corticibacterium populi Li et al. 2016
Corticibacterium populi (po′pu.li. L. fem. gen. n. populi of Populus, the poplar tree).
Homotypic synonym: Tianweitania populi (Li et al., 2016) Song et al. 2023.
The description is the same as Corticibacterium populi Li et al. 2016 [38] and Tianweitania populi Song et al. 2023. [39]. The type strain is 16B10-2-7 T (= CFCC 12884 T = KCTC 42249 T), isolated from bark tissue of Populus × euramericana.
Emended description of Corticibacterium aestuarii comb. nov
Corticibacterium aestuarii (a. es. tu.a’ri.i. L. gen. n. aestuarii of the tidal flat, from where the type strain was isolated).
Basonym: Tianweitania aestuarii Song et al. 2023.
The description is the same as given by Song et al. 2023 [39]. The type strain is BSSL-BM11T (= KACC 21634 T = NBRC 114503 T), isolated from sand of a coastal dune at Boryeong on the Yellow Sea, Republic of Korea.
Availability of data and materials
All the genome sequences used in this study were downloaded from GenBank (https://ftp.ncbi.nlm.nih.gov/genomes/genbank/bacteria/) or GCM (https://gctype.wdcm.org/), and the deposit numbers are listed in Table S1.
Abbreviations
- ANI:
-
Average nucleotide identity
- AAI:
-
Average amino acid identity
- cAAI:
-
Core-proteome average amino acid identity
- GBDP:
-
Genome BLAST distance phylogeny
- GTDB:
-
Genome taxonomy database
- ML:
-
Maximum likelihood
- OGRIs:
-
Overall genome relatedness indices
References
Willems A. “The family Phyllobacteriaceae,” in The Prokaryotes. Eds. Rosenberg E., DeLong E. F., Lory S., Stackebrandt E., Thompson F. (Berlin, Heidelberg: Springer Berlin Heidelberg), 2014;355–418. https://doi.org/10.1007/978-3-642-30197-1_298
Jarvis BDW, Van Berkum P, Chen WX, Nour SM, Fernandez MP, Cleyet-Marel JC, Gillis M. Transfer of Rhizobium loti, Rhizobium huakuii, Rhizobium ciceri, Rhizobium mediterraneum, and Rhizobium tianshanense to Mesorhizobium gen. nov. Int J Syst Evol Microbiol. 1997;47:895–8.
Chen W, Zhu W, Bontemps C, Young JPW, Wei G. Mesorhizobium camelthorni sp nov, isolated from Alhagi sparsifolia. Int J Syst Evol Microbiol. 2011;61(3):574–9.
Guan SH, Chen WF, Wang ET, Lu YL, Yan XR, Zhang XX, Chen WX. Mesorhizobium caraganae sp. nov., a novel rhizobial species nodulated with Caragana spp in China. Int J Syst Evol Microbiol. 2008;58(11):2646–53.
Nandasena KG, Apos O, Hara GW, Tiwari RP, Willems A, Howieson JG. Mesorhizobium australicum sp nov and Mesorhizobium opportunistum sp. nov., isolated from Biserrula pelecinus L. in Australia. Int J Syst Evol Microbiol. 2009;59(9):2140–7.
Wang ET, van Berkum P, Sui XH, Beyene D, Chen WX, Martínez-Romero E. Diversity of rhizobia associated with Amorpha fruticosa isolated from Chinese soils and description of Mesorhizobium amorphae sp. nov. Int J Syst Evol Microbiol. 1999;49(1):51–65.
Zheng WT, Li Y, Wang R, Sui XH, Zhang XX, Zhang JJ, Wang ET, Chen WX. Mesorhizobium qingshengii sp. nov., isolated from effective nodules of Astragalus sinicus. Int J Syst Evol Microbiol. 2013;63(6):2002–7.
Zhou PF, Chen WM, Wei GH. Mesorhizobium robiniae sp. nov., isolated from root nodules of Robinia pseudoacacia. Int J Syst Evol Microbiol. 2010;60(11):2552–6.
Zhu YJ, Lu JK, Chen YL, Wang SK, Sui XH, Kang LH. Mesorhizobium acaciae sp. nov., isolated from root nodules of Acacia melanoxylon R Br. Int J Syst Evol Microbiol. 2015;65(10):3558–63.
De Meyer SE, Tan HW, Andrews M, Heenan PB, Willems A. Mesorhizobium calcicola sp nov, Mesorhizobium waitakense sp. nov., Mesorhizobium sophorae sp. nov., Mesorhizobium newzealandense sp nov and Mesorhizobium kowhaii sp. nov. isolated from Sophora root nodules. Int J Syst Evol Microbiol. 2016;66(2):786–95.
Nandasena KG, O’Hara GW, Tiwari RP, Sezmiş E, Howieson JG. In situ lateral transfer of symbiosis islands results in rapid evolution of diverse competitive strains of mesorhizobia suboptimal in symbiotic nitrogen fixation on the pasture legume Biserrula pelecinus L. Environ Microbiol. 2007;9(10):2496–511.
Pedron R, Luchi E, Albiac MA, Di Cagno R, Catorci D, Esposito A, Bianconi I, Losa D, Cristofolini M, Guella G, Jousson O. Mesorhizobium comanense sp. nov., isolated from groundwater. Int J Syst Evol Microbiol. 2021;71(12). https://doi.org/10.1099/ijsem.0.005131.
Ferraz Helene LC, Dall Agnol RF, Delamuta JRM, Hungria M. Mesorhizobium atlanticum sp. nov., a new nitrogen-fixing species from soils of the Brazilian Atlantic Forest biome. Int J Syst Evol Microbiol. 2019;69(6):1800–6.
Jung Y, Kim H, Hur M. Mesorhizobium terrae sp. nov., a novel species isolated from soil in Jangsu Korea. Antonie Van Leeuwenhoek. 2020;113(9):1279–87.
Nguyen TM, Pham VHT, Kim J. Mesorhizobium soli sp. nov., a novel species isolated from the rhizosphere of Robinia pseudoacacia L. in South Korea by using a modified culture method. Antonie Van Leeuwenhoek. 2015;108(2):301–10.
Lin S, Hameed A, Hsieh Y, Young C. Mesorhizobium composti sp nov, isolated from compost. Antonie Van Leeuwenhoek. 2019;112(9):1387–98.
Liu L, Liang L, Zhang X, Li L, Sun Q. Mesorhizobium ephedrae sp. nov. isolated from the roots of Ephedra przewalskii in Kumtag desert. Int J Syst Evol Microbiol. 2018;68(11):3615–20.
Yuan C, Jiang Z, Xiao M, Zhou E, Kim C, Hozzein WN, Park D, Zhi X, Li W. Mesorhizobium sediminum sp nov, isolated from deep-sea sediment. Int J Syst Evol Microbiol. 2016;66(11):4797–802.
Jung J, Seo YL, Kim KR, Park HY, Jeon CO. Mesorhizobium microcysteis sp. nov., isolated from a culture of Microcystis aeruginosa. Int J Syst Evol Microbiol. 2021;71(7). doi: https://doi.org/10.1099/ijsem.0.004847.
Hördt A, López M, Meier-Kolthoff J, Schleuning M, Weinhold L, Tindall B, Gronow S, Kyrpides N, Woyke T, Göker M. Analysis of 1,000+ type-strain genomes substantially improves taxonomic classification of Alphaproteobacteria. Front Microbiol. 2020;11:468.
Bambauer A, Rainey FA, Stackebrandt E, Winter J. Characterization of Aquamicrobium defluvii gen. nov. sp. nov., a thiophene-2-carboxylate-metabolizing bacterium from activated sludge. Arch Microbiol. 1998;169(4):293–302.
Kämpfer P, Martin E, Lodders N, Jäckel U. Transfer of Defluvibacter lusatiensis to the genus Aquamicrobium as Aquamicrobium lusatiense comb. Nov. and description of Aquamicrobium aerolatum sp. nov. Int J Syst Evol Microbiol. 2009;59(10):2468–70.
Jin HM, Kim JM, Jeon CO. Aquamicrobium aestuarii sp. nov., a marine bacterium isolated from a tidal flat. Int J Syst Evol Microbiol. 2013;63(1):4012–7.
Lipski A, Kämpfer P. Aquamicrobium ahrensii sp nov and Aquamicrobium segne sp. nov., isolated from experimental biofilters. Int J Syst Evol Microbiol. 2012;62(10):2511–6.
Xu C, Zhang L, Huang J, Chen K, Li S, Jiang J. Aquamicrobium soli sp. nov., a bacterium isolated from a chlorobenzoate-contaminated soil. Antonie Van Leeuwenhoek. 2017;110(3):305–12.
Wu Z, Wang F, Gu C, Zhang Y, Yang Z, Wu X, Jiang X. Aquamicrobium terrae sp. nov., isolated from the polluted soil near a chemical factory. Antonie Van Leeuwenhoek. 2014;105(6):1131–7.
Wang X, Zhou C, Cheng J, Cao S, Yang H, Zhao J. Aquamicrobium zhengzhouense sp. nov., a bacterium isolated from farmland soil applied with amino acid fertilizer. Curr Microbiol. 2021;78(10):3798–803.
Kämpfer P, Müller C, Mau M, Neef A, Auling G, Busse HJ, Osborn AM, Stolz A. Description of Pseudaminobacter gen nov with two new species, Pseudaminobacter salicylatoxidans sp. nov. and Pseudaminobacter defluvii sp. nov. Int J Syst Evol Microbiol. 1999;49(2):887–97.
Mu Y, Zhou X, Liu L, Zhou X, Zeng X, Li W. Pseudaminobacter arsenicus sp. nov., an arsenic-resistant bacterium isolated from arsenic-rich aquifers. Int J Syst Evol Microbiol. 2019;69(3):791–7.
Hahn YK, Kim MS, Im W. Pseudaminobacter granuli sp. nov., isolated from granules used in a wastewater treatment plant. J Microbiol. 2017;55(8):607–11.
Li J, Huang J, Liao S, Wang G. Pseudaminobacter manganicus sp. nov., isolated from sludge of a manganese mine. Int J Syst Evol Microbiol. 2017;67(5):1589–94.
Zhang K, Tang S, Jiang Y, Zhang F, Zhang J, Qiu J, He J. Pseudaminobacter soli sp nov, isolated from paddy soil contaminated with heavy metals. Curr Microbiol. 2021;79(1):19.
Urakami T, Araki H, Oyanagi H, Suzuki K, Komagata K. Transfer of Pseudomonas aminovorans (den Dooren de Jong 1926) to Aminobacter gen. nov. as Aminobacter aminovorans comb. nov. and description of Aminobacter aganoensis sp. nov. and Aminobacter niigataensis sp. nov. Int J Syst Evol Microbiol. 1992;42(1):84-92.
Maynaud G, Willems A, Soussou S, Vidal C, Mauré L, Moulin L, Cleyet-Marel J, Brunel B. Molecular and phenotypic characterization of strains nodulating Anthyllis vulneraria in mine tailings, and proposal of Aminobacter anthyllidis sp. nov., the first definition of Aminobacter as legume-nodulating bacteria. Syst Appl Microbiol. 2012;35(2):65–72.
Mcdonald IR, Kämpfer P, Topp E, Warner KL, Cox MJ, Hancock TLC, Miller LG, Larkin MJ, Ducrocq V, Coulter C, Harper DB, Murrell JC, Oremland RS. Aminobacter ciceronei sp. nov. and Aminobacter lissarensis sp. nov., isolated from various terrestrial environments. Int J Syst Evol Microbiol. 2005;55(5):1827–32.
Meyer O, Stackebrandt E, Auling G. Reclassification of ubiquinone Q-10 containing carboxidotrophic bacteria: transfer of “[Pseudomonas] carboxydovorans” OM5T to Oligotropha, gen. nov., as Oligotropha carboxidovorans, comb. nov., transfer of “[Alcaligenes] carboxydus” DSM 1086T to Carbophilus, gen. nov., as Carbophilus carboxidus, comb. nov., transfer of “[Pseudomonas] compransoris” DSM 1231T to Zavarzinia, gen. nov., as Zavarzinia compransoris, comb. nov., and amended descriptions of the new genera. Syst Appl Microbiol. 1993;16(3):390–395.
Han L, Mo Y, Feng Q, Zhang R, Zhao X, Lv J, Xie B. Tianweitania sediminis gen. nov., sp. nov., a member of the family Phyllobacteriaceae, isolated from subsurface sediment core. Int J Syst Evol Microbiol. 2016;66(2):719–24.
Li Y, Guo L, Chang J, Xie S, Piao C, Li X. Corticibacterium populi gen nov., sp. nov., a member of the family Phyllobacteriaceae, isolated from bark of Populus euramericana. Int J Syst Evol Microbiol. 2016;66(7):2617–22.
Song JH, Park S, Lee J, Kim W, Yoon J. Tianweitania aestuarii sp. nov., isolated from a coastal dune, reclassification of Corticibacterium populi as Tianweitania populi comb. Nov., and emended description of the genus Tianweitania. Int J Syst Evol Microbiol. 2023;73(12):006193.
de Lajudie PM, Andrews M, Ardley J, Eardly B, Jumas-Bilak E, Kuzmanović N, Lassalle F, Lindström K, Mhamdi R, Martínez-Romero E, et al. Minimal standards for the description of new genera and species of rhizobia and agrobacteria. Int J Syst Evol Microbiol. 2019;69(7):1852–63.
Velázquez E, Igual JM, Willems A, Fernández MP, Muñoz E, Mateos PF, Abril A, Toro N, Normand P. Cervantes E Mesorhizobium chacoense sp. nov., a novel species that nodulates Prosopis alba in the Chaco Arido region (Argentina). Int J Syst Evol Microbiol. 2001;51(3):1011–21.
Han TX, Han LL, Wu LJ, Chen WF, Sui XH, Gu JG, Wang ET, Chen WX. Mesorhizobium gobiense sp. nov. and Mesorhizobium tarimense sp. nov., isolated from wild legumes growing in desert soils of Xinjiang. China Int J Syst Evol Microbiol. 2008;58(11):2610–8.
Zhang JJ, Liu TY, Chen WF, Wang ET, Sui XH, Zhang XX, Li Y, Li Y, Chen WX. Mesorhizobium muleiense sp. nov., nodulating with Cicer arietinum L. Int J Syst Evol Microbiol. 2012;62(11):2737–42.
Chun J, Oren A, Ventosa A, Christensen H, Arahal D, Da Costa M, Rooney A, Yi H, Xu X, De Meyer S, Trujillo ME. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol. 2018;68:461–6.
Lee I, Ouk Kim Y, Park S, Chun J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol. 2016;66(2):1100–3.
Meier-Kolthoff J, Auch A, Klenk H, Göker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics. 2013;14:60.
Kuzmanović N, Fagorzi C, Mengoni A, Lassalle F, Dicenzo GC. Taxonomy of Rhizobiaceae revisited: proposal of a new framework for genus delimitation. Int J Syst Evol Microbiol. 2022;72(3). https://doi.org/10.1099/ijsem.0.005243
Zheng J, Wittouck S, Salvetti E, Franz CMAP, Harris HMB, Mattarelli P, O Toole PW, Pot B, Vandamme P, Walter J, et al. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70(4):2782–2858.
Barco RA, Garrity GM, Scott JJ, Amend JP, Nealson KH, Emerson D. A genus definition for Bacteria and Archaea based on a standard genome relatedness index. mBio. 2020;11(1):10–1128.
Xu L, Sun C, Fang C, Oren A, Xu X. Genomic-based taxonomic classification of the family Erythrobacteraceae. Int J Syst Evol Microbiol. 2020;70(8):4470–95.
Konstantinidis KT, Tiedje JM. Towards a genome-based taxonomy for prokaryotes. J Bacteriol. 2005;187(18):6258–64.
Novichkov P, Omelchenko M, Gelfand M, Mironov A, Wolf Y, Koonin E. Genome-wide molecular clock and horizontal gene transfer in bacterial evolution. J Bacteriol. 2004;186:6575–85.
Buchfink B, Xie C, Huson D. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2014;12(1):59–60.
Frolov EN, Lebedinsky AV, Elcheninov AG, Kublanov IV. Taxonomic proposal for a deep branching bacterial phylogenetic lineage: transfer of the family Thermodesulfobiaceae to Thermodesulfobiales ord. nov. Thermodesulfobiia classis nov and Thermodesulfobiota phyl nov Syst Appl Microbiol. 2023;46(1):126388.
Parks D, Imelfort M, Skennerton C, Philip H, Tyson G. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25(7):1043–55.
Gurevich A, Saveliev V, Vyahhi N, Tesler G. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013;29(8):1072–5.
Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–9.
Emms DM, Kelly S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 2019;20(1):238.
Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–80.
Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25(15):1972–3.
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 2020;37(5):1530–4.
Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(1):293–6.
Parks DH, Chuvochina M, Rinke C, Mussig AJ, Chaumeil P, Hugenholtz P. GTDB: an ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 2021;50(1):D785–94.
Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Nat Acad Sci. 2009;106(45):19126–31.
León-Barrios M, Flores-Félix J, Pérez-Yépez J, Ramirez-Bahena M, Pulido-Suárez L, Igual JM, Velázquez E, Peix Á. Definition of the novel symbiovar canariense within Mesorhizobium neociceri sp. nov., a new species of genus Mesorhizobium nodulating Cicer canariense in the “Caldera de Taburiente” National Park (La Palma, Canary Islands). Syst Appl Microbiol. 2021;44(5):126237.
Wang H, Ooi BC, Tan K, Ong T, Zhou L. BLAST++: BLASTing queries in batches. Bioinformatics. 2003;19(17):2323–4.
Wang FQ, Wang ET, Liu J, Chen Q, Sui XH, Chen WF, Chen WX. Mesorhizobium albiziae sp. nov., a novel bacterium that nodulates Albizia kalkora in a subtropical region of China. Int J Syst Evol Microbiol. 2007;57(6):1192–9.
Chen W, Zhu W, Bontemps C, Young JPW, Wei GH. Mesorhizobium alhagi sp. nov., isolated from wild Alhagi sparsifolia in north-western China. Int J Syst Evol Microbiol. 2010;60(4):958–62.
Chen WX, Li GS, Qi YL, Wang ET, Yuan HL, Li JL. Rhizobium huakuii sp. nov. isolated from the root nodules of Astragalus sinicus. Int J Syst Evol Microbiol. 1991;41(2):275–80.
Wang S, Hao B, Li J, Gu H, Peng J, Xie F, Zhao X, Frech C, Chen N, Ma B, Li YG. Whole-genome sequencing of Mesorhizobium huakuii 7653R provides molecular insights into host specificity and symbiosis island dynamics. BMC Genomics. 2014;15(1):440.
Ji ZJ, Wu ZY, Chen WF, Wang ET, Yan H, Cui QG, Zhang JX, Wang L, Ma SJ. Physiological and symbiotic variation of a long-term evolved Rhizobium strain under alkaline condition. Syst Appl Microbiol. 2020;43(5): 126125.
Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A, Chaumeil P, Hugenholtz P. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol. 2018;36(10):996–1004.
Young JPW, Moeskjær S, Afonin A, Rahi P, Maluk M, James EK, Cavassim MIA, Rashid MH, Aserse AA, Perry BJ, et al. Defining the Rhizobium leguminosarum species complex. Genes. 2021;12(1):111.
Acknowledgements
Not applicable.
Funding
This work was supported by Natural Science Foundation of Shandong Province (ZR202102280248, ZR2020MC043).
Author information
Authors and Affiliations
Contributions
All authors participated in the design, analysis of the data and interpretation of the studies. YL and CFT conceived and designed the study; TYG analyzed the data; YL and TYG drafted the manuscript; LQS, ETW, YJPW and CFT reviewed the manuscript. All authors critically read and approved the final manuscripts.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Y., Guo, T., Sun, L. et al. Phylogenomic analyses and reclassification of the Mesorhizobium complex: proposal for 9 novel genera and reclassification of 15 species. BMC Genomics 25, 419 (2024). https://doi.org/10.1186/s12864-024-10333-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-024-10333-y