Abstract
Background and purpose
Idiopathic multifocal choroiditis (MFC) is part of the group of choriocapillaritis entities. The clinical definition of the disease has evolved with time. The aim of this article was to undertake a review on MFC, on its present-day appraisal and nomenclature and we also report a series of patients with emphasis on the clinical presentation and the importance of vigorous immunosuppressive management.
Methods
A review of the literature and a retrospective case series study which was performed in the Centre for Ophthalmic Specialised care (COS), Lausanne, Switzerland. Patients diagnosed from 1994 to 2020 with idiopathic multifocal choroiditis (MFC) treated with multiple immunosuppressants were included. Exclusion criteria were insufficient follow up and cases not treated with vigorous immunosuppressive therapy. Imaging analysis included spectral domain optical coherence tomography (SD-OCT) / enhanced depth imaging OCT (EDI-OCT), OCT angiography (OCT-A). Fluorescein and Indocyanine angiography (FA, ICGA) before and after the instauration of treatment. Best corrected visual acuity (BCVA), intraocular pressure (IOP), routine ocular examination, laser flare photometry (LFP) were performed at presentation and follow-up. Immunosuppression comprised at minimum two among the following agents: prednisone, cyclosporine, azathioprine, mycophenolic acid or infliximab. Mean duration of therapy was calculated.
Results
26 (52 eyes) of 2102 new patients (1.24%) were diagnosed with MFC. 25 (96%) patients were female and 1 (4%) was male. 43/52 (82%) eyes were myopic with a mean dioptre of − 5.87 ± 2.94, six (12%) eyes were hypermetropic with mean dioptres 2.0 ± 2.68 and three (6%) were emmetropic. 14/52 (27%) eyes had at least 1 anti-VEGF injection because of choroidal neovascularisation (CNVs), 1 eye had a phototherapy laser and 37/52 (71%) had no complication of CNVs during the follow-up. 5/26 (19%) fulfilled the inclusion criteria for our study. Mean age was 26.4 ± 9.3 years. Snellen best corrected visual acuity (BCVA) at presentation was 0.955+/-0.26. Mean follow up was 84+/-55 months. LFP at presentation was 6.34 ± 2.94 ph/ms. None of four patients with prolonged treatment and prolonged follow-up showed disease activity. One patient still under therapy after 4 months’ follow-up still showed an active neovascular membrane.
Conclusion
Treatment with multiple immunosuppressive agents was shown to stop the progression of the disease.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction/historical aspects
Idiopathic Multifocal Choroiditis (MFC) is a choriocapillaritis also called primary inflammatory choriocapillaropathy (PICCP) not linked to a known infectious agent. It predominantly affects healthy myopic white women and is characterised by uni or bilateral chorioretinal lesions which have often a recurrent course with subclinical novel recurrent lesions identified by indocyanine green angiography (ICGA), coexistent with older scarred chorioretinal lesions and frequently complicated by choroidal neovessels (CNVs).
The term of” multifocal choroiditis” with a description corresponding to the present appraisal of the disease was used by Krill et al. in 1969 who named the disease multifocal inner choroiditis [1].
Thereafter the condition was overlapping or confused in different reports with ocular histoplasmosis later called presumed ocular histoplasmosis syndrome (POHS) [2].
In 1973, Nozik and Dorsch described an entity which they called multifocal uveitis and panuveitis [3]. In 1984 Dreyer and Gass published a report entitled “multifocal choroiditis and panuveitis, a syndrome that mimics ocular histoplasmosis syndrome”, reporting 28 additional cases [4]. The panuveitis part in most cases is very minimal and mostly limited to cells in the posterior vitreous and, nowadays, should not be considered as a disease defining sign. The first reports on the utility of ICGA in MFC were published in 1996–97 [5, 6]. The extreme sensitivity of ICGA to follow and monitor active disease was established thereafter [7]. ICGA made it clear that MFC typically affects primarily the choriocapillaris and should therefore be classified in the group of PICCPs [8].
The more severe course of MFC when compared to multiple evanescent white dot syndrome (MEWDS) or acute posterior multifocal placoid pigment epitheliopathy/acute multifocal choriocapillaritis (APMPPE/AMIC) was made clear rapidly as well as its propensity to develop CNVs [9]. In 1984 was published the first report on punctate inner choroidopathy (PIC) difficult to distinguish from MFC [10]. In 1998, a histopathological report on choroidal lesions in MFC showed inflammatory involvement of the choroidal stroma in addition to choriocapillaris involvement [11]. This extension of inflammation to deeper pre-choriocapillaris vessels might be an explanation both for being in the more severe disease spectrum of PICCPs and for the higher frequency of CNVs [11, 12]. Even inner retinal vessels showed scarce perivascular inflammatory infiltration [11].
The boundaries and nosological characteristics of MFC are less well determined and the entity is more heterogeneous than MEWDS or APMPPE/AMIC. When seen by the clinician most of the cases already show chorioretinal scars from preceding silent episodes before the disease becomes symptomatic during a recurrence. The terminology of the different forms that constitute idiopathic multifocal choroiditis will be discussed in the next section. The characteristics of all the subtypes of multifocal choroiditis are the numerous small randomly distributed chorioretinal scars and the recurrent behaviour of the disease as well as the propensity to develop secondary CNVs, which, as said before, is much more frequent than in all other PICCPs. Multifocal choroiditis occurs in the same age groups as other PICCPs, namely in young to middle aged adults with women being predominantly affected [9]. Lesions tend to leave scars, are not spontaneously reversible but seem to respond to corticosteroid therapy and/or non-steroidal immunosuppressive agents which is a recommended treatment.
The aim in this article was to apply pioneering pragmatism in the approach and definition of a disease with many different confusing denominations and present our experience in the appraisal and management of the disease.
Epidemiological aspects and nomenclature
Epidemiology
It is very difficult to establish epidemiological data for MFC as the disease comprises entities that were classified under different terms in the past, which were grouped under the terminology of idiopathic multifocal choroiditis only recently [13]. However, even recent studies still report MFC and PIC, and others as separate entities. When performing a literature search containing the terms of “idiopathic multifocal choroiditis” and “epidemiology” no publication could be found in the PubMed databank. One of the most significant publication reported a series of 41 patients with a mean age of 38.4 years, predominantly women (70,7%) and a bilaterality rate of 51.2% [14].
In a study published in 2015, the frequency of diagnosis of MFC in different US uveitis centres was 1.8%, 6%, 6.5% and 4.9% [15]. In our centre, from 1994 to 2020, MFC was diagnosed in 26/2102 new uveitis patients amounting to a frequency of 1.24% of uveitis diagnoses. Although epidemiological information is scarce and difficult to obtain, it is obvious that MFC is a rare uveitis.
Nomenclature considerations (Table 1)
Idiopathic multifocal choroiditis (MFC), the eponym that should be used today for the disease, is a non-infectious primary inflammatory choriocapillaropathy (PICCP) for which no infectious agent has been identified. It should be distinguished from presumed ocular histoplasmosis syndrome (POHS) which is an infectious entity caused by histoplasma capsulatum that used to be called ocular histoplasmosis syndrome [26]. The term of presumed was quickly added because, despite a positive histoplasmin skin test, as the presence of the infectious agent has not been specifically identified in the eye [16, 27, 28]. POHS can be applied to characteristic ocular findings with a positive histoplasmin skin test and/or patients living in an endemic area.
POHS or even ocular histoplasmosis have unfortunately been used in non-endemic areas and/or in histoplasmin negative patients [18, 21, 29] and was sometimes called pseudo-POHS [25, 30]. This is at the origin of part of the confusion in the nomenclature of MFC.
MFC was named by a record number of terminologies, including multifocal inner choroiditis being probably the first description, by Krill et al. in 1969 [1], punctate inner choroidopathy (PIC) [10], POHS (in non-endemic areas or with a negative histoplasmin skin test) [25] or pseudo-POHS [31]. Other alternative names given to the disease in the past were recurrent multifocal choroiditis [22], multifocal choroidopathy or disseminated inner choroiditis [21].
Some reports called the disease multifocal choroiditis with panuveitis [3, 4]. Although some cases may present panuveitis, these unusual forms are seemingly sufficiently rare that the term of panuveitis does not characterise MFC and cannot be considered as a disease defining sign. Indeed, the fact that a study was able to publish 41 patients with MFC without panuveitis supports this position [14]. In our 26 MFC patients none presented panuveitis.
In 2013 a salutary editorial was published to group the differently termed conditions under the eponym of idiopathic multifocal choroiditis [13]. While a very large series of MFC without panuveitis is available [14] and a very clear and comprehensive definition is available [13], it is unfortunate that a recent classification continued to call the disease “multifocal choroiditis with panuveitis”, instead of using the consecrated term of idiopathic multifocal choroiditis, panuveitis not being the hallmark of the condition [32].
Anecdotally, the confusion in terminology became apparent to one of the authors back in 2003, when a fellow from a neighbouring country was sent to analyse ICG angiographies of European patients with so-called POHS. Looking at the angiographies the cases corresponded to what was considered then by us and is now commonly called idiopathic multifocal choroiditis.
Up to date, despite the comprehensive grouping of sub-entities into MFC, there are still reports that distinguish PIC from MFC. When looking at the definition of one and the other of these two” entities” on the tables of an extensive work on sub-entities of MFC, it appears impossible to distinguish the two forms when the titles of the tables are hidden (Fig. 1) [33].
MFC and PIC are undistinguishable. Two tables defining MFC and PIC for which the titles have been hidden. The two “entities” cannot be distinguished and statistical differences among items do not appear to be significant. This is one more argument that the two “sub-entities” are one and the same disease [33].
Table 1. Shows the impact of presumed ocular histoplasmosis syndrome (POHS) on the appraisal of MFC, by slowing the progressive distinction of the specific MFC entity and the confusion on the terminology in the early years such as the European publication by Notting and Deutman in 1975 [30], speaking of presumed histoplasmosis in patients outside endemic areas and with negative histoplasmin skin tests. (Non-exhaustive listing performed on available articles and left to the fair appreciation and careful choice of the authors).
Pathology and clinicopathology
The trigger for MFC is still unknown. Like for other PICCPs, it might be an unidentified infectious agent, such as viruses, as many patients in this group can present flu-like symptoms before the onset of the ocular disease. Several cases of multifocal choroiditis with a similar presentation to the idiopathic form have been linked to infectious agents, including herpes zoster virus [34, 35], Epstein-Barr virus [36,37,38], West Nile virus [39] and Zika virus [40]. The clinicopathology/disease process, however, seems to be immune-mediated non-perfusion of the choriocapillaris, as in other entities of the group, including MEWDS, APMPPE/AMIC and serpiginous choroiditis. This is very well shown by ICGA that characterises the process, identifying choriocapillaris non-perfusion in new developing lesions. Additional arguments for an immune mediated process are (1) several reports indicating that multifocal choroiditis developed after different types of vaccinations [41,42,43], and (2) the fact that the disease responds well to immunosuppression. Choriocapillaris non-perfusion is causing secondary ischaemia of the outer retina damaging photoreceptor outer segments well identified by spectral domain optical coherence tomography (SD-OCT) and by blue-light fundus autofluorescence (BL-FAF) showing hyperautofluorescence in the affected areas due to the loss of the photoreceptor screen to retinal pigment epithelium autofluorescence.
Anatomopathological reports are understandably scarce. In a case of clinicopathological correlation, the clinical lesions corresponded to non-granulomatous perivascular choroidal infiltrates, consisting mainly of B lymphocytes supporting the fact that choroidal vessels are at the origin of the clinicopathology of MFC [11]. The fact that lesions tend to evolve to chorioretinal scars in MFC suggests that larger pre-choriocapillaris vessels are involved. Another report on excised CNVs from MFC also showed infiltration by B lymphocytes [44]. The different grades of severity of PICCPs might be explained by the choroidal vessel calibre involved and the severity of the process. (Table 2).
The clinicopathology appears to be identical in the two main sub-entities, MFC and PIC, described separately before the unifying terminology of idiopathic multifocal choroiditis was adopted. Distinguishing and comparing MFC and PIC has often been attempted in the past but corresponded to a vain exercise because they should be considered as part of the same disease, as is apparent on Fig. 1. Moreover, MFC and PIC were shown to have similar genetic associations another argument for a unique disease [45].
Clinical presentation
Symptoms
The symptoms that connect multifocal choroiditis to all other PICCPs are the photopsias and subjective scotomas. Photopsia is usually much more disturbing for the MFC patient than for other PICCPs and their duration is protracted, being present also when there is no clinical evidence of reactivation of the disease. The patients also report more frequent subjective scotomas. Multifocal choroiditis can be bilateral with involvement being usually asymmetric or sequential, first detected in one eye and months or even years later in the fellow eye. When it is unilateral it has often been included in the subtype of PIC, a terminology that should now be abandoned. Recurrences are usual and can be documented with fundoscopy and ICGA [23].
Clinical signs
Visual acuity impairment is variable and depends on the area of involvement and the rapidity of immunosuppressive treatment implementation.
Only slight non granulomatous anterior segment inflammation can be seen. Therefore, if anterior granulomatous uveitis is present a specific diagnosis, such as sarcoidosis, syphilis or tuberculosis has to be looked for. Cells in the posterior vitreous can be found most of the time when the disease is active but can be absent in quiet disease. In none of our patients did we note what can be termed panuveitis, except for slight signs of posterior vitreous cells. Typically, when present, aqueous flare, measured by laser flare photometry (LFP) is below 20 ph/ms (normal values 3–5 ph/ms).
On fundus examination, the typical lesions are small punched-out randomly distributed choroidal mostly atrophic yellow-white foci with pigment spots that sometimes can become adjacent to each other and form a ribbon of pearls (Fig. 2). These lesions involve predominantly the posterior pole with a size around 70–100 μm, but peripheral lesions can also be seen, reported by several authors as peripheral linear streaks or Schlaegel lines [30, 46, 47]. In the active phases of disease new lesions are not always visible and can be very discreet on FA, whereas ICGA is the most sensitive method to detect new lesions [6, 7, 48]. Fundus lesions may be of different ages, signs of different episodes of recurrence. One particular feature of multifocal choroiditis is the high proportion of CNVs complicating the disease [12, 49, 50].
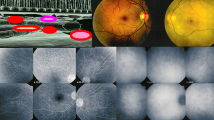
Multifocal choroiditis (MFC) Fundus pictures (top two images): on the left picture, multiple yellow punched-out foci typically seen in MFC (inactive stage); on the right picture, additional new more fluffy lesions during recurrence. ICGA pictures (bottom), during inactive cicatricial stage (1), during reactivation (2), and after treatment (3); almost no additional scars. (Partially reprinted from Diagnostics (Basel). 2021 May 24;11 (6):939. doi:https://doi.org/10.3390/diagnostics11060939)
Imaging and investigations
Indocyanine green angiography (ICGA)
ICGA represented a breakthrough in understanding the clinicopathological process of MFC as well as its appraisal and management [6]. It allowed to detect early signs of incipient episodes showing choriocapillaris hypo or non-perfusion.
The first set of signs identifies old scarred chorioretinal lesions and consists of hypofluorescent areas persisting up to the late angiographic phase, distributed at random in the fundus, corresponding to late hyperfluorescence on fluorescein angiography. This constellation is typical for chorioretinal atrophy from scars of previous inflammatory episodes seen on fundus examination [Figs. 3a & b]. The second set of signs can be seen in addition to the previously described signs when choroiditis recurs or can be seen in their absence when it is the first episode of MFC. The signs consist of hypofluorescent areas, either silent on fluorescein angiography or slightly hyperfluorescent in the late angiographic phase and usually not visible on fundus examination, representing areas of new inflammatory involvement /choriocapillaris non-perfusion (Figs. 3a & b). As in MEWDS, some cases may present peripapillary hypofluorescence translating functionally into an enlarged blind spot [33, 51] (Fig. 2, middle bottom picture) The latter set of signs responds to systemic corticosteroids with or without immunosuppressants and can regress completely if therapy is started early. In a substantial proportion of cases the extent of ICGA hypofluorescence reflecting choriocapillaris hypoperfusion or non-perfusion is far more widespread than visible lesions let suspect, showing widespread areas of late hypofluorescence with absolutely no signs visible on fundus examination or on fluorescein angiography (Figs. 3a & b). It is probably such undetected chronic choriocapillaritis that explains the high proportion of CNVs, as inflammation can be the trigger of CNVs [12]. Indeed, ICGA is useful in distinguishing inflammatory lesions from CNVs, as the latter are hyperfluorescent (Figs. 4a & b). Moreover ICGA, together with OCT angiography (OCT-A) can monitor the evolution of CNVs after anti-vascular endothelial growth factor (anti-VEGF) treatment.
Multifocal choroiditis (MFC) Fundus pictures ODS. Typical chorioretinal scars OD in inactive stage. OS minimal number of scars in an eye with photopsias and subjective scotomas with widespread occult choriocapillaritis (see Fig. 3b). b. Multifocal choroiditis (MFC) Occult choriocapillaritis. ICGA and FA in same patient as Fig. 3a. OS: vast areas of ICGA hypofluorescence indicating choriocapillaris hypo or non-perfusion. These zones indicate that in some cases the inflammatory process is involving vast areas of choriocapillaris non-perfusion that can be a trigger for the development of CNVs. In OD ICGA only shows inactive hypofluorescent scars without occult activity. FA (3 top frames) shows no more than slight late hyperfluorescence (middle and right frames)
Multifocal choroiditis (MFC): CNVs. Small parafoveal CNV brightly hyperfluorescent on FA (left frame) and also hyperfluorescent on ICGA (right frame). On the other hand, the two paramacular atrophic scars are hyperfluorescent on FA (left frame) but are hypofluorescent on ICGA (right frame). b. Multifocal choroiditis (MFC) - CNVs. Shown by OCTA (same patient as Fig. 4a)
Furthermore, ICGA was shown to be a precious modality to distinguish between POHS that presented hyperfluorescent spots and MFC characterised only by hypofluorescent dots [52].
Fluorescein angiography (FA)
FA is marginally useful in the active inflammatory phase of MFC, as indicated by Slakter et al. [6]. It shows mainly signs of chorioretinal scarred lesions associating window effects (late sclera hyperfluorescence due to staining) to masking effects where there is pigment clumping. In the active phase FA may show faint late hyperfluorescence (Fig. 3b, top 3 frames) in areas corresponding to ICGA hypofluorescent dark dots, the ICGA expression of new lesions. In case of severe hypoperfusion of the choriocapillaris, bright late hyperfluorescence (retinal and subretinal staining and even pooling) can occur as for APMPPE/AMIC (see hereunder). The use of fluorescein angiography is however of little contribution to assess and follow active lesions, as FA angiographic signs are often absent or faint in new areas of inflammatory involvement that are, in contrast, clearly shown by ICGA (Fig. 3b). In case of late hyperfluorescence, FA responds in a delayed fashion following the introduction of corticosteroid/immunosuppressive treatment, accounting less precisely for the resolution of new lesions than ICGA. In case of CNV, FA shows the classical signs of leakage and late hyperfluorescence.
Optical coherence tomography (OCT)
Spectral domain optical coherence tomography (SD-OCT) is very helpful in MFC, like for all other PICCPs. In MFC, OCT pictures show that the degree of repercussion of choriocapillaris non-perfusion on the outer retina (and sometimes even inner retina) can be pronounced in the active phases of MFC when compared to the more benign choriocapillaritis entities such as MEWDS. It clearly shows damage and/or loss of the outer segments of photoreceptor cells co-localising with areas of hyperautofluorescence on BL-FAF and areas of hypofluorescence on ICGA (Fig. 5).
Similar to APMPPE/AMIC, the consequences of choriocapillaris nonperfusion can involve the whole thickness of the retina in the areas of choroiditis with pooling of fluid under the retina around the focus of chorioretinitis. This severe ischaemia might indicate that larger choroidal vessels seem to be involved as suggested by the only histopathological report available [11]. In a case of APMPPE/AMIC with similar features, the authors concluded that the retinal changes were due to ischaemia of the outer retina [53]. Severe outer retinal ischaemia can produce compensatory dilatation and permeability of the inner retinal vessels producing accumulation of intraretinal and subretinal fluid [54] (Fig. 6a & b).
Multifocal choroiditis (MFC). ICGA & FA of acute episode. Areas of ICGA hypofluorescence around optic disc indicating severe choriocapillaris non-perfusion (left picture). On the right image, late FA frame showing diffuse hyperfluorescence due to pooling of dye in the retinal and subretinal space due to probable exudation from retinal vessels due to severe outer retinal ischaemia, as shown in another case by OCT on Fig. 6b. (Partially reprinted from Diagnostics (Basel). 2021 May 24;11 (6):939. https://doi.org/10.3390/diagnostics11060939.). b. Multifocal choroiditis (MFC) patient with outer retina ischaemia and photoreceptor outer segment damage. Intra and subretinal fluid probably coming from retinal reactive permeability increase and exudation induced by outer retinal ischaemia due to choriocapillaris non-perfusion
OCT reports on MFC show disruption of the outer segments of the photoreceptors which correspond to hyperautofluorescent plaques on BL-FAF [55]. It is understandable that OCT findings in MFC and APMPPE/AMIC are similar as it is the same mechanism of choriocapillaris closure with more or less severe consequences on the retina.
Optical coherence tomography angiography (OCT-A)
In contrast to MEWDS where small end-capillary vessels with low flow are not detected by OCT-A, in MFC OCT-A can show choriocapillaris drop-out, as the vessels involved are larger precapillary vessels. However, with presently used conventional OCT-A instruments, ICGA is still more precise (Fig. 7), while swept-source (SS)-OCT may represent a promising and useful tool for the upcoming future.
Case of Multifocal choroiditis (MFC). Comprehensive multimodal imaging. Multiple yellow foci in fundus typically seen in MFC (top left). Circum-papillary hyperautofluorescence on BL-FAF picture (bottom left) without FA signs (top middle) and blind spot enlargement on visual field (bottom middle). ICGA hypofluorescence clearly shows occult choriocapillaris non-perfusion, corresponding to BL-FAF hyperfluorescence. Lesions are barely seen on OCT-A (top right), while OCT shows loss or damage to photoreceptor outer segments (extreme right picture)
Beside choriocapillaris drop-out, OCT-A is especially useful to detect CNVs and detect their evolution after anti-VEGF treatment [56] (Fig. 8).
Fundus autofluorescence (FAF)
Blue-light fundus autofluorescence (BL-FAF) is a recent imaging modality based on the capacity of a scanning laser ophthalmoscope (HRA2, Heidelberg) to detect fluorophores within the RPE. In case of impaired cellular metabolism, lipofuscin accumulates within the RPE cells increasing the amount of autofluorescence. On the other hand, when the cells’ metabolism is completely arrested or in case of cell death there is no more emission autofluorescence [57, 58].
Fundus autofluorescence in MFC shows increased autofluorescence in those areas that have silent (meaning absent FA signs), corresponding to ICGA hypofluorescent areas. The hyperautofluorescence is not explained by the usual mechanism but by the absence of the usual screen of photoreceptor outer segments allowing to better “see” the normal lipofuscin content of RPE cells (Fig. 9).
BL-FAF is hypoautofluorescent in the cicatricial areas. After corticosteroid / immunosuppressive therapy hyperautofluorescence of active disease areas disappears in parallel with resolution of ICGA hypofluorescence. The areas showing hyperautofluorescence go beyond the ICGA hypofluorescent areas indicating that dysfunction of cells and inflammatory involvement go even beyond the areas detected by ICGA. A recent study showed that BL- FAF allowed to detect, like ICGA, widespread occult inflammatory lesions in idiopathic CNVs, confirming the effect of inflammatory choriocapillaris non-perfusion on the outer retina, although this was not the interpretation by the authors of FAF signs of this report [59].
Multimodal imaging is the key to understanding choriocapillaritis entities including MFC
Multimodal imaging has contributed essentially to the understanding of PICCPs such as MFC. However, the term multimodal is not having the same signification from one article to another. Unfortunately, in many such ”to multimodal imaging” reports, ICGA is not included, albeit it is crucial in the imaging appraisal choriocapillaritis diseases [60] (Figs. 7, 8, 9, 10 & 11).
Multifocal choroiditis (MFC) Multimodal imaging monitoring of evolution of lesions following corticosteroid/immunosuppressive treatment. At presentation FA only shows old punctiform cicatricial hyperfluorescent pinpoints nasal to disc. ICGA (late frame) shows the numerous new lesions corresponding to BL-FAF hyperautofluorescence. After 2 months of treatment, BL-FAF, ICGA and visual field have recovered
Case of MFC during acute episode (top 3 images) and after treatment (bottom 3 images. b. Case of MFC during acute episode (top 3 images) and after treatment (bottom 3 images. Figures 11a & b: Spectralis - ICGA/FA/ and OCT images in two cases of MFC. During the active phase of the disease (top lines of pictures), OCT findings disclose the presence of two inflammatory lesions showing damaged and clumped photoreceptor outer segments; RPE layer appears interrupted at its peak with, in the second case (11b), material infiltrating the subretinal space without the involvement of the inner retina. In the quiescent phase (bottom lines of pictures), OCT shows the resolution of the agglomerated material. It is also interesting to note that the FA images (right frames) do not permit to distinguish the active phase versus the quiescent phase of the disease
Visual field testing
Visual field testing can show small scotomas corresponding to chorioretinal scars. In the active phase however, scotomas are larger and correspond to choriocapillaris non perfusion shown on ICGA. Visual field recovery is well correlated with the regression of ICGA hypofluorescent areas that occurs following sub-Tenon’s or systemic corticosteroid therapy (Fig. 12). On the other hand, recovery is not well correlated to FA.
Correlation between FA, ICGA and visual field during corticosteroid treatment of MFC. This myopic patient presents with left eye photopsias, left decrease of visual acuity (VA) and the presence of a subjective left scotoma. FA (top left) only shows cicatricial FA lesions corresponding to cicatricial fundus lesions seen on fundus examination. ICGA (top middle) shows widespread hypofluorescent areas indicating fresh choriocapillaris lesions with corresponding visual field defects (top right). During corticosteroid therapy, practically no change is seen on FA, whereas ICGA signs of active disease progressively resolve together with visual field improvement (right column). (Reprinted from Diagnostics (Basel). 2021 May 24;11 (6):939. https://doi.org/10.3390/diagnostics11060939)
As for all PICCPs, enlargement of the blind spot can be seen in MFC and is explained by peripapillary choriocapillaris non-perfusion and consecutive damage to the photoreceptor outer segments [33, 51].
Diagnosis and differential diagnosis
As for all PICCPs an infectious cause should be excluded as a first step. Many infectious causes can produce a clinical picture resembling MFC, including tuberculous choroiditis, West-Nile virus choroiditis, Candida choroiditis, bacterial emboli and more rare diseases such as pneumocystis choroiditis and choroidal coccidiomycosis. Among the non-infectious entities, ocular sarcoidosis has to be excluded.
Finally, when evidence for histoplasma capsulatum infection is found the diagnosis of Histoplasma positive “Presumed Ocular Histoplasmosis Syndrome” (POHS) can be made.
Treatment
Reports on management of MFC are very scarce. Most reports concern the treatment of MFC associated CNVs. There was, however, anecdotal evidence to favour corticosteroid therapy that in most cases should be associated to non-steroidal immunosuppressive therapy in case of newly diagnosed active disease or reactivation of multifocal choroiditis [38, 61].
A recent well conducted study including 32 patients confirmed that immunosuppressive therapy significantly decreased not only the number of recurrences of MFC but also the number of anti-VEGF injections in the cases with CNVs [62]. Monitoring of therapy is best done by ICGA which is equally or more sensitive than visual field testing to detect actively involved areas and response to therapy showing regression of hypofluorescent areas. BL - FAF is equally useful as ICGA to follow and monitor the regression of new active lesions and has the advantage to be non-invasive. It was also shown that after corticosteroid treatment recovery, choroidal blood flow velocity increased, and choroidal thickness decreased indicating that the choroid is involved beyond the choriocapillaris [63].
Corticosteroids can be given initially by sub-Tenon’s injections if the reactivation is unilateral but have to be given systemically if there is no response or if the involvement is bilateral. For rapid action, corticosteroids are recommended. In most cases they are however not sufficient and bear with them deleterious side-effects. In severe cases Immunosuppressive agents should be added. We usually use cyclosporine (2.5–4.5 mg/kg) or tacrolimus (0.05–0.2 mg/kg), rapidly acting agents, together with Mycophenolate mofetil 1.5–3 g, a cytostatic agent that needs several weeks to reach efficient action. Azathioprine (2–2.5 mg/kg) has a similar mode of action as mycophenolate but is slightly less well tolerated. Biologic agents can be used in cases insufficiently well controlled by classical immunosuppression (Fig. 13). In case of MFC complicated by CNVs, the approach has to be more aggressive by combining systemic steroids and systemic immunosuppressive with intravitreal injection of anti-VEGF agents. The well-known role of immunosuppressive agents in the treatment of inflammatory CNVs was confirmed by Neri et al. [64]. A prompt and appropriate use of systemic immunosuppression associated with steroids and anti-VEGF agents leads to better visual outcome as well as long term control of CNV activity compared to steroids and anti-VEGF only [65].
MFC: Diagram of Imaging appraisal in practice; diagnosis and management following the logical sequence of exams. PP: posterior pole. OCT: optical coherence tomography. iCNV: inflammatory choroidal neovascularization. BL-FAF: blue light autofluorescence. OCT-A: OCT-angiography. FA: fluorescence angiography. ICGA: indocyanine green angiography. HDDs: hypofluorescent dark dots. TB: tuberculosis. MEWDS: multiple evanescent white dots syndrome. APMPPE: acute posterior multifocal placoid pigment epitheliopathy. MFC: idiopathic multifocal choroiditis. IS: immunosuppressors. CsA: Cyclosporine. AZA: Azathioprine
Case series
Aim
As mentioned before, MFC belongs to the more severe end of the spectrum of PICCPs, and treatment should be appropriate and sustained. The purpose of our study was to demonstrate the benefit of aggressive multiple immunosuppressive therapy, in order to stop the deleterious course of the disease.
Patients and methods
This retrospective case series was performed in the Centre for Ophthalmic Specialised care (COS), Lausanne, Switzerland. Patients diagnosed from 1994 to 2020 with idiopathic multifocal choroiditis (MFC) treated with multiple immunosuppressants were included. Patients not treated with multiple immunosuppressants or with insufficient follow up were the exclusion criteria. Imaging analysis included spectral domain optical coherence tomography (SD-OCT) and enhanced depth imaging OCT (EDI-OCT) (Heidelberg Engineering GmbH, Heidelberg, Germany), OCT angiography (OCT-A) (AngioVue®, Optovue, Fremont, CA, USA) Fluorescein and Indocyanine angiography (FA, ICGA) (Heidelberg Engineering GmbH, Heidelberg, Germany) before and after the instauration of the treatment. Best corrected visual acuity (BCVA), intraocular pressure (IOP) and routine ocular examination, as well as laser flare photometry (LFP) were performed at presentation and during the follow up of patients. Immunosuppression comprised at minimum two among the following agents: prednisone, cyclosporine, azathioprine, mycophenolic acid and infliximab. Mean duration of therapy was calculated.
Results
From 1994 to 2020, 26 of 2102 new patients (1.24%) were diagnosed with MFC. Most patients were sent for a second opinion. 25 (96%) patients were women and only 1 (4%) was man. Mean age was 35.3 ± 12.7 years. Among the 52 eyes, 43/52 (82%) were myopic with a mean dioptre value of − 5.87 ± 2.94, six (12%) eyes were hypermetropic with mean dioptre value of 2.0 ± 2.68 and 3 (6%) were emmetropic. 14/52 (27%) eyes had at least 1 anti-VEGF injection because of choroidal neovascularisation (CNVs), 1 eye had a phototherapy laser treatment and 37/52 (71%) had no complication of CNVs during the follow-up. 5/26 (19%) patients had a long-term follow-up and fulfilled the inclusion criteria, having been treated with at least two immunosuppressants with sequential monitoring. Mean age was 26.4 ± 9.3 years. Snellen best corrected visual acuity (BCVA) at presentation was 0.955 ± 0.26 and 0.9 ± 0.24. Mean follow up was 84 ± 55 months and mean treatment duration was 52 ± 18 months. LFP at presentation was 6.34 ± 2.94 ph/ms and at last follow-up it was 6.2 ± 3 ph/ms. Visual field mean defect was 4.64 ± 3.1 at presentation and 3.9 ± 1.9 at last follow-up. Treatment with multiple immunosuppressive agents was shown to stop the progression of the disease in the 4 cases with prolonged follow-up and prolonged treatment. In the last patient follow-up and treatment were less than 4 months and there was still activity of the CNV but no occult active disease areas on ICGA and BL-FAF.
Summary of findings
Data on patients are summarised on Table 3.
Conclusion
Idiopathic multifocal choroiditis is part of the group of primary inflammatory choriocapillaropathies (PICCPs). It groups the different entities that were considered separately such as PIC or POHS in non-endemic areas with negative search for Histoplasma capsulatum. It is on the more aggressive side of the spectrum of PICCPs and most of the time needs systemic corticosteroids associated with non-steroidal immunosuppression.
Abbreviations
- MFC:
-
Idiopathic multifocal choroiditis
- PICCPs:
-
Primary Inflammatory Choriocapillaropathies
- SD-OCT:
-
Spectral domain optical coherence tomography
- EDI-OCT:
-
Enhanced depth imaging OCT
- OCT-A:
-
OCT angiography
- FA:
-
Fluorescein angiography
- ICGA:
-
Indocyanine angiography
- BCVA:
-
Best corrected visual acuity
- IOP:
-
Intraocular pressure
- LFP:
-
Laser flare photometry
- CNVs:
-
Choroidal neovascularisation
- MEWDS:
-
Multiple evanescent white dot syndrome
- APMPPE:
-
Acute posterior multifocal placoid pigment epitheliopathy
- AMIC:
-
Acute multifocal choriocapillaritis
- PIC:
-
Punctate inner choroidopathy
- POHS:
-
Presumed ocular histoplasmosis syndrome
- BL-FAF:
-
Blue-light fundus autofluorescence
- anti-VEGF:
-
Anti-vascular endothelial growth factor
- SS-OCT:
-
Swept-source-OCT
References
Krill AE, Chishti MI, Klien BA, Newell FW, Potts AM (1969) Multifocal inner choroiditis. Trans Am Acad Ophthalmol Otolaryngol 73(2):222–245 PMID: 5771008
Krill AE, Archer D (1970) Choroidal neovascularization in multifocal (presumed histoplasmosis) choroiditis. Arch Ophthalmol 84(5):595–604. https://doi.org/10.1001/archopht.1970.00990040597007
Nozik RA, Dorsch W (1973) A new chorioretinopathy associated with anterior uveitis. Am J Ophthalmol 76(5):758–762. https://doi.org/10.1016/0002-9394(73)90573-4
Dreyer RF, Gass DJ (1984) Multifocal choroiditis and panuveitis. A syndrome that mimics ocular histoplasmosis. Arch Ophthalmol 102(12):1776–1784. https://doi.org/10.1001/archopht.1984.01040031440019
Herbort CP, Borruat FX, de Courten C, Jaccard L (1996) Angiographie au vert d'indocyanine dans les uvéites postérieures. Klin Monatsbl Augenheilk 208(05):321–326. https://doi.org/10.1055/s-2008-1035227
Slakter JA, Giovannini A, Yannuzzi LA, Scassellati-Sforzolini B, Guyer DR, Sorenson JA, Spaide RF, Orlock D (1997) Indocyanine green angiography in multifocal choroiditis. Ophthalmology 104(11):1813–1819. https://doi.org/10.1016/S0161-6420(97)30022-0
Cimino L, Auer C, Herbort CP (2000) Sensitivity of indocyanine green angiography for the follow-up of active inflammatory choriocapillaropathies. Ocul Immunol Inflamm 8(4):275–283. https://doi.org/10.1076/ocii.8.4.275.6462
Cimino L, Mantovani A, Herbort CP (2004) Primary inflammatory choriocapillaropathies. In: Essentials in Ophthalmology: Uveitis and Immunological Disorders. In: Pleyer U, Mondino B (eds) . Springer, Berlin, pp 209–231
Brown J, Folk JC, Reddy CV, Kimura AE (1996) Visual prognosis of multifocal choroiditis, punctate inner choroidopathy, and diffuse subretinal fibrosis syndrome. Ophthalmology 103(7):1100–1105. https://doi.org/10.1016/S0161-6420(96)30561-7
Watzke RC, Parker AJ, Folk JC, Benson WE, Burgess D, Ober RR (1984) Punctate inner choroidopathy. Am J Ophthalmol 98(5):572–584. https://doi.org/10.1016/0002-9394(84)90243-5
Dunlop AAS, Cree IA, Hage S, Luthert PJ, Lightman S (1998) Multifocal choroiditis, clinicopathologic correlation. Arch Ophthalmol 116(6):801–803. https://doi.org/10.1001/archopht.116.6.801
Neri P, Lettieri M, Fortuna C, Manoni M, Giovannini A (2009) Inflammatory choroidal neovascularization. Middle East Afr J Ophthalmol 16(4):245–251. https://doi.org/10.4103/0974-9233.58422
Essex RW, Wong J, Jampol LM, Dowler J, Bird AC (2013) Idiopathic multifocal choroiditis: a comment on present and past nomenclature. Retina 33:1–4
Fung AT, Pal S, Yannuzzi NA, Christos P, Cooney M, Slakter JS, Klancnik JM Jr, Freund KB, Cunningham ET Jr, Yannuzzi LA (2014 Jan) Multifocal choroiditis without panuveitis: clinical characteristics and progression. Retina. 34(1):98–107. https://doi.org/10.1097/IAE.0b013e31829234cb
Bajwa A, Osmanzada D, Osmanzada S, Khan I, Patrie J, Xin W, Reddy AK (2015) Epidemiology of uveitis in mid-Atlantic United States. Clin Ophthalmol 9:889–901. https://doi.org/10.2147/OPTH.S80972 eCollection 2015
Woods AC, Wahlen HE (1960) The probable role of benign histoplasmosis in the etiology of granulomatous uveitis. Am J Ophthalmol 49(2):205–220. https://doi.org/10.1016/0002-9394(60)91515-4
Archer DB, Maguire CJ (1975) Multifocal choroiditis. Trans Ophthalmol Soc U K 95(1):184–191 PMID: 1064202
Notting JG, Deutman AF, der Werf v (1975) Diagnosis and treatment of macular lesions in multifocal choroiditis (presumed histoplasmosis). Klim Monbl Augenheilkd 166:629–636
Miller SA, Stevens TS, de Venecia G (1976) De novo lesions in presumed ocular histoplasmosis-like syndrome. Br J Ophthalmol 60(10):700–712. https://doi.org/10.1136/bjo.60.10.700 PMID: 1009042; PMCID: PMC1042812
Deutsch TA, Tessler HH (1985) Inflammatory pseudohistoplasmosis. Ann Ophthalmol 17(8):461–465 PMID: 4051378
Saraux H, Pelosse B, Guigui A (1986) Multifocal inner choroiditis : pseudohistoplasmosis. The European form of the presumed American histoplasmosis. J Fr Ophtalmol 9(10):645–651
Morgan CM, Schatz H (1986) Recurrent multifocal choroiditis. Ophthalmology 93(9):1138–1147. https://doi.org/10.1016/S0161-6420(86)33611-X
Joondeph BC, Tessler HH (1991) Clinical course of multifocal choroiditis: photographic and angiographic evidence of disease recurrence. Ann Ophthalmol 23(11):424–429
Ben Ezra D, Forrester JV (1995) Fundal white dots: the spectrum of a similar pathological process. Br J Ophthalmol 79(9):856–860. https://doi.org/10.1136/bjo.79.9.856 PMID: 7488606; PMCID: PMC505273
Watzke RC, Klein ML, Wener MH (1998) Histoplasmosis-like choroiditis in a non-endemic area: the Northwest United States. Retina 18(3):204–212. https://doi.org/10.1097/00006982-199803000-00003
Jarvis GJ, McCulloch C (1963) Ocular histoplasmosis. Canad Med Ass J 89:1270–1273
Gutman FA (1979) The natural course of active choroidal lesions in the presumed ocular histoplasmosis syndrome. Trans Am Ophth Soc 77:515–541
Gass JDM, Wilkinson CP (1972) Follow-up study of presumed ocular histoplasmosis. Trans Am Acad Ophthalmol Otolaryngol 76(3):672–694
Braunstein RA, Rosen DA, Bird AC (1974) Ocular histoplasmosis syndrome in the united Kingdome. Br J Ophthalmol 58(11):893–898. https://doi.org/10.1136/bjo.58.11.893
Bottoni FG, Deutman AF, Aandekerk AL (1989) Presumed ocular histoplasmosis syndrome and linear streak lesions. Br J Ophthalmol 73(7):528–535. https://doi.org/10.1136/bjo.73.7.528
Callanan D, Gass JD (1992) Multifocal choroiditis and choroidal neovascularization associated with the multiple evanescent white dot and acute idiopathic blind spot enlargement syndrome. Ophthalmology 99(11):1678–1685. https://doi.org/10.1016/S0161-6420(92)31755-5
Standardization of Uveitis Nomenclature (SUN) Working Group (2021) Classification criteria for multifocal choroiditis with panuveitis. Am J Ophthalmol 228:152–158. https://doi.org/10.1016/j.ajo.2021.03.043
Reddy CV, Brown J, Folk JC, Kimura AE, Gupta S, Walker J (1996) Enlarged blind spots in chorioretinal inflammatory disorders. Ophthalmology 103(4):606–617. https://doi.org/10.1016/S0161-6420(96)30645-3
Bloom SM, Snady-McCoy L (1989) Multifocal choroiditis uveitis occurring after herpes zoster ophthalmicus. Am J Ophthalmol 108(6):733–735. https://doi.org/10.1016/0002-9394(89)90873-8
Chiquet C, Germain P, Burillon C, Coudry-Vergne PM, Paupert-Ravault M, Denis P (1996) Multifocal choroiditis associated with herpes zoster ophthalmicus. Apropos of a case. J Fr Ophthalmol 19:712–715
Bhende PS, Biswas J, Madhavan HN (1997) Serological evidence for active Epstein-Barr virus infection in multifocal choroiditis. Indian J Pathol Microbiol 40(2):129–131
Schaal S, Tong MG, Purkiss TJ, Kaplan HJ (2011) Multifocal choroiditis secondary to the reactivation of Epstein-Barr virus. Retin Cases Brief Rep 5(4):360–362. https://doi.org/10.1097/ICB.0b013e3182160939
Borkar PP, Grassi MA (2020) Unilateral multifocal choroiditis following EBV-positive mononucleoisis responsive to immunosuppression: a case report. BMC Ophthalmol 20(1):273. https://doi.org/10.1186/s12886-020-01525-7
Vandenbelt S, Shaikh S, Capone A Jr, Williams GA (2003) Multifocal choroiditis associated with West Nile virus encephalitis. Retina 23:97–99
Jimenez P, Kestelman E, Kestelman B, Gomes Vizzoni A, Cerbino-Nrto J, Land Curi AL (2020) Multifocal choroiditis secondary to acute Zika virus infection. Ocul Immunol Inflamm 28:952–955
Escott S, Tarabishi AD, Davidorf FH (2013) Multifocal choroiditis following simultaneous hepatitis a, typhoid, and yellow fever vaccination. Clin Ophthalmol 7:363–365. https://doi.org/10.2147/OPTH.S37443 Epub 2013 Feb 18
Goyal M, Murthy SI, Annum S (2021) Bilateral multifocal choroiditis following COVI-19 vaccination. Ocul Immunol Inflamm 3(4):1–5. https://doi.org/10.1080/09273948.2021.1957123
Mungmunpuntipantip R, Wiwanitkit V (2021) COVID-19 vaccination and bilateral multifocal chroiditis. Ocul Immunol Inflamm 18:1. https://doi.org/10.1080/09273948.2021.1968003
Shimada H, Yuzawa M, Hirose T, Nakashizuka H, Hattori T, Kazato Y (2008) Pathological findings of multifocal choroiditis with panuveitis and punctate inner choroidopathy. Jpn J Ophthalmol 52(4):282–288. https://doi.org/10.1007/s10384-008-0566-2
Atan D, Faser-Bell S, Plskova J, Kuffova L, Hogan A, Tfall A, Kilmartin DJ, Forrester JV, Bidwell JL, Dick AD, Churchill AJ (2011) Punctate inner choroidopathy and multifocal choroiditis with panuveitis share haplotypic associations with IL-10 and TNF loci. Invest Ophthalmol Vis Sci 52(6):3573–3581. https://doi.org/10.1167/iovs.10-6743
Borodoker N, Cunningham ET Jr, Yannuzzi LA, Nicoletti R (2002) Peripheral curvilinear pigmentary streaks in multifocal choroiditis. Arch Ophthalmol 120(4):520–521. https://doi.org/10.1001/archopht.120.4.520
Somkijrungroj T, Pearlman JA, Gonzales JA (2015) Multifocal choroiditis and panuveitis presenting with progressive equatorial linear streaks: evolution of Schlaegel lines documented with multimodal imaging. Retin Cases Brief Rep 9(3):214–217. https://doi.org/10.1097/ICB.0000000000000141
Weinberger AWA, Kube T, Wolf S (1999) Dark spots in late phase indocyanine green angiographic studies in a patient with presumed ocular histoplasmosis syndrome. Graefes Arch Clin Exp Ophthalmol 237(6):524–526. https://doi.org/10.1007/s004170050274
Fine HF, Zhitomrsky I, Freund KB, Barile GR, Shirkey BL, Samsin CM, Yannuzzi LA (2009) Bevacizumab (avastin) and ranibizumab (lucentis) for choroidal neovascularization in multifocal choroiditis. Retina 29(1):8–12. https://doi.org/10.1097/IAE.0b013e318187aff9
Agarwal A, Invernizzi A, Singh RB, Foulsham W, Aggarwal K, Handa S, Agrawal R, Pavesio C, Gupta V (2018) An update on inflammatory choroidal neovascularization: epidemiology, multimodal imaging, and management. J Ophthalmic Inflamm Infect 8(1):13. https://doi.org/10.1186/s12348-018-0155-6 PMID: 30209691; PMCID: PMC6135736
Khorram KD, Jampol LM, Rosenberg MA (1991) Blind spot enlargement as manifestation of multifocal choroiditis. Arch Ophthalmol 109(10):1403–1407. https://doi.org/10.1001/archopht.1991.01080100083047
Slakter JS, Giovannini A (1997) Multifocal and the presumed ocular histoplasmosissyndrome. In: Yannuzzi LA, Flowr RW, Slakter JS (eds) Indocyanine green angiography, vol 20. Mosby, St-Louis, pp 271–278
Lofoco G, Ciucci F, Bardocci A, Quercioli P, Steigerwalt RD Jr, De Gaetano C (2005) Optical coherence tomography findings in a case of acute multifocal poaterior placoid pigment epitheliopathy (APMPPE). Eur J Ophthalmol 15(1):143–147. https://doi.org/10.1177/112067210501500125
Herbort CP Jr, Papasavvas I, Mantovani A (2020) Choriocapillaris involvement in Acute Syphilis Posterior Placoid Chorioretinitis is responsible for functional impairment and points towards an immunologic mechanism: a comprehensive clinicopathological approach. J Curr Ophthalmol 32:381–389. https://doi.org/10.4103/JOCO.JOCO_184_20 eCollection 2020 Oct-Dec
Kramer M, Priel E (2014) Fundus autofluorescence imaging in multifocal choroiditis. Ocul Immunol Inflamm 22(5):349–355. https://doi.org/10.3109/09273948.2013.855797
Zahid S, Chen KC, Jung JJ, Balaratnasingam C, Ghadiali Q, Sorenson J, Rofagha S, Freund KB, Yannuzzi LA (2017) Optical coherence tomography angiography of chorioretinal lesions due to idiopathic multifocal choroiditis. Retina 37(8):1451–1463. https://doi.org/10.1097/IAE.0000000000001381
Schmitz-Valckenberg S, Fitzke FW (2007) Fundus autofluorescence imaging with the confocal scanning laser ophthalmoscope. In: Holz FG, Schmitz-Valckenberg S, Spaide RF, Brid AC (eds) Atlas of fundus autofluorescence imaging. Springer, Heidelberg, pp 31–36
Sparrow JR (2007) Lipofuscin of the retinal pigment epithelium. In: Holz FG, Schmitz-Valckenberg S, Spaide RF, Brid AC (eds) Atlas of fundus autofluorescence imaging. Springer, Heidelberg, pp 3–16
Lima LH, Zett C, Casella MB, Pereira F, Rodrigues EB, Parameswarappa DC, Chabblani J (2020) Occult inflammation detected by autofluorescence May Be the cause of idiopathic choroidal neovascularization. Am J Ophthalmol Case Rep 20:100965. https://doi.org/10.1016/j.ajoc.2020.100965
Spaide RF, Godberg N, Freund KB (2013) Redefining multifocal choroiditis and panuveitis and punctate inner choroidopathy through multimodal imaging. Retina 33(7):1315–1324. https://doi.org/10.1097/IAE.0b013e318286cc77
Michel SS, Ekong A, Baltatzis S, Foster CS (2002) Multifocal choroiditis and panuveitis: immunomodulatory therapy. Ophthalmology 109(2):378–383. https://doi.org/10.1016/S0161-6420(01)00901-0
De Groot EL, ten Dam-van Loon NH, de Boer JH, Ossewaarde-van NJ (2020) The efficacy of corticoid-sparing immunomodulatory therapy in treating patients with central multifocal choroiditis. Acta Ophthalmol 98:816–821
Hirooka K, Saito W, Hashimoto Y, Saito M, Ishida S (2014) Increased macular choroidal blood flow velocity and decreased choroidal thickness with regression of punctate inner choroidopathy. BMC Ophthalmol 14:73. https://doi.org/10.1186/1471-2415-14-73
Neri P, Manoni M, Fortuna C, Lettieri M, Mariotti C, Giovannini A (2010) Association of systemic steroids and mycophenolate mofetil as rescue therapy for uveitic choroidal neovascularization unresponsive to the traditional immunosuppressants: interventional case series. Int Ophthalmol 30(5):583–590. https://doi.org/10.1007/s10792-009-9323-x Epub 2009 Sep 2. PMID: 19727566
Neri P, Pichi F, Pirani V, Arapi I (2020) Systemic immunosuppression is highly effective in the long-term control of inflammatory non-infectious Uveitic choroidal neovascularization: a comparative study. Ocul Immunol Inflamm 7:1–5. https://doi.org/10.1080/09273948.2020.1727530 Epub ahead of print. PMID: 32255394
Acknowledgments
Not applicable.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
“Conceptualization, CPH.; methodology, CPH, PN, AM; software.; validation, CPH, PN, AM, IP; formal analysis, CPH, PN, AM, IP; resources, CPH,; data curation, CPH, PN, IP; writing—original draft preparation, CPH.; writing—review and editing, CPH, PN, AM, IP; visualization, CPH, PN, AM, IP; supervision, CPH.; project administration, CPH, PN, IP; All authors have read and agreed to the submitted version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Papasavvas, I., Neri, P., Mantovani, A. et al. Idiopathic multifocal choroiditis (MFC): aggressive and prolonged therapy with multiple immunosuppressive agents is needed to halt the progression of active disease. An offbeat review and a case series. J Ophthal Inflamm Infect 12, 2 (2022). https://doi.org/10.1186/s12348-021-00278-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12348-021-00278-8